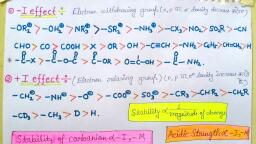Page 1 :
“~, , Po, , QseD phe, , PIB asec, , , , Adsorption: A Surface, Phenomenon, , INTRODUCTION, , , , For a solid or a liquid, the molecules at the surface behave differently from, those in the interior of the phase. A surface has different thermodynamic, properties than a bulk material has. This is because a surface molecule is, weakly bound compared to the one in the bulk. The energy of a surface, molecule is therefore higher than that of a molecule in the bulk of a solid, or liquid. The surface can be as thin as a single molecule, called a film,, the behaviour of which is intimately related to the properties of surfaces, in general. Currently there is an explosive growth in research on surface, structure of solids and substances adsorbed on them. The use of solid surfaces, as catalysts in chemical technology is of tremendous practical importance., The surface effects play important roles in various fields in chemistry such as, heterogeneous catalysis, detergents, colloids, electrochemical cells, corrosion, inhibition, biological cell membrane functions, vapour-phase chromatography,, lubrication, dyeing and humidity control. Thus surface chemistry deals, with all such systems where the surface effects are of great industrial and, biological significance. Heterogeneous catalysis will be discussed in chapter, 6. In this chapter we shall discuss only the theories related to adsorption., , Adsorption is a process of accumulation of any substance on the, surface of another substance. A more general definition is: Adsorption is, a phenomenon of higher concentration of any molecular species at the, surface than in the bulk of a solid or a liquid. Remember that adsorption, is a different phenomenon than absorption. Absorption involves diffusion, of one substance into the bulk of another. If adsorption and absorption go, simultaneously the process is sometimes called sorption (McBain)., , The process of adsorption of gases on solids is generally exothermic,, that is, heat is evolved in the process. This is because the surface energy, 295
Page 2 :
296 Modern Approach to Physical Chemistry Vol. II, , decreases during adsorption, which appears as heat. This can also be, , explained thermodynamically using the expression, , AG = AH - TAS., , We know that for a spontaneous process, AG must be negative. As, the gas molecules (adsorbates) change from a more random state to a less, random one, the adsorption is accompanied with decrease in entropy. AS is, thus negative. For AG to be negative (for a spontaneous process), AHads has, to be sufficiently (highly) negative so that (AH — TAS) becomes negative., , The heat of adsorption in chemisorption is much higher than that in, physical adsorption. The AH,as value for chemisorption ranges from —40 kJ, mol” to -800 kJ mol", whereas for physical adsorption it is between -4 kJ, mol and -40 k] mol”, Hence, according to Le Chatelier's principle, when, the temperature is increased, the extent of adsorption should decrease more, in chemisorption than in physical adsorption., , However, as the chemical bonds of the adsorbates may be broken as, well as formed in the process of chemisorption, one might expect the heat, of adsorption to show both positive and negative values similar to the heat, of reaction values for ordinary chemical reactions. As said earlier, AHgas is, generally negative but there is an exception in the case of chemisorption of, , Hb) gas on glass for which AH,¢s is slightly positive., , PHYSICAL AND CHEMICAL ADSORPTION, , The distinction between physical adsorption and chemisorption may be, understood as follows. Here we focus on the adsorption of gases (adsorbates), , on the surface of solids (adsorbents)., , Physical adsorption Chemisorption, , 1. The adsorption is due to the} 1. It is due to the chemical, interplay of weak van der Waals combination and so the adsorbate, forces and so the adsorbate- is held tightly with the adsorbent., , adsorbent binding is weak., 2. AHggs is low. This also suggests] 2. AHags is usually high. This also, , weak attraction between the suggests strong attraction between, adsorbate and the adsorbent. the adsorbate and the adsorbent., , , , 3,, It is highly reversible. 3. It is usually irreversible.
Page 3 :
Adsorption: A Surface Phenomenon, , , , , , Chemisorption, , , , , , Physical adsorption, , However, easily, are adsorbed, , , , , , , , , , 4. It is specific because adsorption, depends on the possibility of a, reaction between the molecules of, , E more., the adsorbate and the adsorbent., , Adsorption may take place at any, , 85. Adsorption takes place at low} 5., temperature., , temperatures., It has a relatively higher energy of, activation and hence adsorption is, relatively slower., , Tt has almost zero energy of} 6., activation and hence adsorption is, usually fast, , , Adsorption —_ increases with| 7, Adsorption increases with, the increase in pressure, and the increase in pressure but as, , multilayer adsorption occurs. monolayer adsorption occurs,, further increase in p has no effect, , on adsorption., , , , , , , , , , Adsorption, in general, is more effective when the adsorbents possess, large surface area such as silica gel and charcoal which have porous, ctures. The surface area of such porous solids can be as large as several, , undred square metres per gram of the solid., , The plot of the amount of a gas adsorbed per gram of an adsorbent, versus the pressure (or concentration) of the gas at constant temperature is, called the adsorption isotherm. Different types of adsorption isotherms have, en observed, mainly the type I and type Il isotherms as shown below., , Amount of O2 Amount of No, adsorbed adsorbed, , U A, , Dir p—, Fig. 5.1 Fig. 5.2, , Figure 5.1 shows type I isotherm depicting chemisorption. The amount, of gas adsorbed on the solid's surface increases with pressure until a limiting, value is reached. This is because the surface is covered by a monolayer, f the gas and no second layer gets adsorbed. An example of this type is, sorption of O, on charcoal at 90 K.
Page 4 :
298 Modern Approach to Physical Chemistry Vol, 11, , , , , , Figure 5.2 shows type II isotherm represe, , nting, physical adsorptiy, In this, , ‘ase the amount of the gas adsorbed increases gradually with t, Pressure with no limiting value, This is because of multilayer adsorptior, An example of this type is adsorption of Ny on silica gel at 77 K., , , , FREUNDLICH ADSORPTION ISOTHERM, , Various factors such as pressure, temperature, the nature of the adsorbates, and that of the adsorbents influence adsorption. Freundlich first observed, that the decrease in temperature and increase if 4, in pressure, both tend to cause increase in ¢, the magnitude of adsorption of a gas ona Ta, solid as shown in Figure 5.3, representing, the Freundlich adsorption isotherm. The TsTot, dependence of x/m, that is, the amount of ie, the gas per gram of the adsorbent, on the, , pressure at different temperatures is shown for 2, , , , Bhs, , Ty, , ——, a monolayer chemisorption (type I isotherm). Fig. 5.3, It was observed that at constant temperature, adsorption is proportional, , to pressure at a low pressure range while at a relatively higher pressure,, , saturation of adsorbent-surface occurs and then the magnitude of adsorption, does not depend on the pressure., , Mathematically,, Je p -. at low pressure <cei(Od), m, x Yo, and mP -.at high pressure. =o8.2), In general,, ae, m, or 2 kp", (a), m, , where n lies between 0 and 1., In case of adsorption of a liquid ona solid surface, the pressure p of the, , as is replaced by concentration c of the liquid on the surface of the solid., So,, , Boone, 6A) |, m
Page 5 :
Adsorption: A Surface Phenomenon 299, , Thus the log forms are, , , , log =nlogp tlogk » (5.5) |, m, ( log ir, and Jog~ =nloge +logk. (5.6) Oe, m, Equations 5.3 and 5.6 are known, Freundlich isotherm. Hence the plot “Jog p (or log c) ——>, , ween log and logp or loge should ng: 5/4, m, , a straight line which is found to be only roughly true in most cases at, V pressures or concentrations. Figures 5.3 and 5.4 represent Freundlich, sorption isotherm., , LANGMUIR ADSORPTION ISOTHERM, , 3e first quantitative theory of adsorption of gases on solids was given by, ng Langmuir in 1918 which is based on the following assumptions. One, yy be surprised to know that most of the Langmuir’s assumptions are, , , , , , se. However, they were considered as approximations., , - 1. Asolid has a uniform surface and a fixed number of adsorption sites., Each site can hold only one molecule or atom of the adsorbate., , 2. The adsorbed molecules are localised at specific sites and only a, monolayer can be adsorbed as a result of chemisorption., , 3. The adsorbed gas molecules behave ideally and there is no interaction, between the adsorbed molecules., , 4. At equilibrium, the rate of adsorption (condensation) and the rate, of desorption (evaporation) from the surface are equal., , , , Based on these assumptions, the adsorption isotherm may be deduced, as follows., Let @ be the fraction of the adsorbent surface area covered by the, dsorbate molecules at any instant. Then, : the rate of desorption « 6, or Rg «kg: 9, ee (Ol), here kg is the rate constant for the desorption process., As (1 — 8) would be the fraction of the surface free for adsorption,, the rate of adsorption « (1 — 6), the rate at which the gaseous molecules, strike the unit area of the surface.