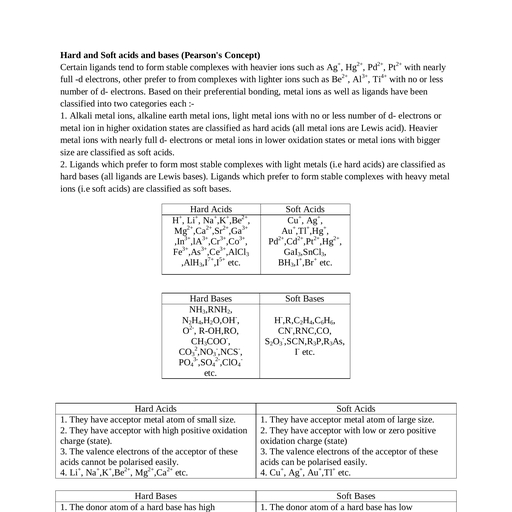
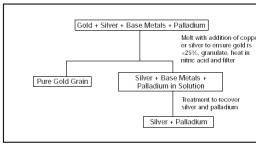
Page 1 :
Solvent Levelling : A solvent in which various strong acids appear equally strong is known as a levelling solvent or solvent, levelling and the phenomenon is called levelling effect., The capacity of an acid to ionise also depends upon the basic strength of the solvent which act as a base., Levelling effect of Water : When a strong acid like Perchloric acid (HClO4), Hydrochloric acid (HCl) and nitric acid (HNO3) is, dissolved in water, it reacts to form hydronium ion, HClO4, , +, , H2O, , H3O+ + ClO4-, , HCl, , +, , H2O, , H3O+ +, , Cl-, , HNO3, , +, , H2O, , H3O+ +, , NO3-, , All the above acids are strong acids than the characteristic cation of solvent (water) i.e. the h3o+ ion. these, acid would therefore, be completely levelled (or converted) to the characteristic cation H3O+ of the solvent, and thus behave as equally strong., Levelling effect of Ammonia : When acids such as HCl, HF and CH3COOH is dissolved in liquid ammonia (it has strong tendency to, take up protons), the acids ionize completely to give NH4+ ion and all the acid behave as a strong acid., HCl, , +, , NH3, , NH4+ + Cl-, , HF, , +, , NH3, , NH4+, , +, , NH4+, , + CH3COO-, , CH3COOH, , +, , NH3, , F-, , It has been found all acids, which in aqueous solution behave stronger than acetic acid appear to be about, the same strength when dissolve in liquid ammonia., CH3COOH, , +, , NH3, , CH3COO-, , +, , NH4+, , *When NH3 is added, it combines with the H+ ion to form a stable NH4+ ion. The equilibrium shifts to the, right as concentration of H+ decreases (Le-Chatelier's Principle). Hence, even weak acid like CH3COOH, behaves as strong acid in solvent NH3.