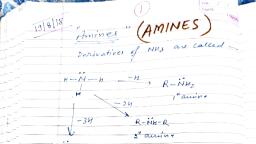
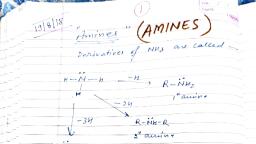
Page 1 :
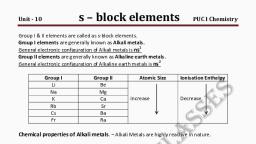
S-Block Elements 161, IMPORTA,, , TERMS AND, , CONCEPTS, , I. Alkali Metals. Group 1 elements are called, , alkali metals, e.g. Li, Na, K, Rb (Rubidium),, , 7., , strongly electropositive in nature due to low, , Cs(Caesium) and Fr (Francium). They are, , Ionisation enthalpy. Electropositive character, , called alkali metals after the Arabic word, , increases down the group due to decrease in, , (al-quis) meaning plant ashes. Such ashes, of plants are rich in carbonates of sodium, and potassium. Na and K are abundant, Li,, , Electropositive Character. Alkali metals are, , ionisation energy., 8. Metallic Character. It goes on increasing, from top to bottom due to decrease in, , Rb and Cs are less abundant. Francium is, , radioactive element. Fr has half life of only, , ionisation enthalpy which is due to increase, in atomic size., , 21 minutes. Their metal hydroxides are very, , 9. Reducing Properties. Alkali metals are, , strong alkalies., , strong reducing agents due to low ionisation, enthalpies. They have strong tendency to, lose electron due to lower standard reduction, potentials which depends upon sublimation,, , 2. Electronic Configuration. All these, elements have one valence electron (ns*). The, outermost s-electron is very well screened, , because they can lose outermost s-electron to, , ionisation and hydration enthalpies., Li is best reducing agent because of lowest, standard reduction potential. It is also due to, , acquire stable electronic configuration. They, show +1 oxidation state., , high hydration enthalpy of smallest Li ion., Reducing power goes on increasing from Na, , Atomic and lonic Size. Alkali metals have, , to Cs due to decrease in ionisation enthalpy, , from the nuclear charge in these elements., , 3. Oxidation State. They readily form M ions, , largest atomic size in respective periods., Atomic and ionic size goes on increasing with, , and decrease in standard reduction potential., , 10. Flame Colouration. Alkali metals and their, salts impart characteristic colour to the flame, , the increase in atomic number due to increase, , because their chlorides are volatile in nature., , in number of shells. Monovalent cations (M), , The valence electron gets excited to higher, energy level. When it comes back to lower, , are smaller than the parent atoms., S. lonisation Enthalpy. They have lowest, , energy level, it radiates energy which belong, , 1Onisation enthalpies in respective periods, , to visible region, e.g. Li imparts crimson red,, , due to larger atomic size, due to which force, , Na imparts golden yellow, K imparts lilac,, Rb imparts pinkish violet and Cs imparts, , of attraction between nucleus and valence, , electrons is less and less energy is required, to, , 6., , remove valence electron., , bluish violet., 11. Photoelectric Effect. Alkali metals (except, , ation energy goes on decreasing down, the group due to increase in atomic size,, , Li), show photoelectric eftect due to low, ionisation enthalpy. K, Rb and Cs are, , CiTective nuclear charge is less and less, , Li and Na in photoelectric, cells because of lower ionisation enthalpies., Alkali metals have low densities due, , preferred, , Y 1S required to remove electrons., , Second lonisation Enthalpy. They have very, Second ionisation enthalpy because after, , losing one electron they acquire noble gas, nfiguration. Therefore, very high, of energyis required to remove seco, electron., , amount, , 12., , over, , Density., to, , larger atomic size., , It goes, , on, , increasing, , atomic, down the group because, volume., increases more than atomic, , Exception., , Na has, , mass, , higher density than, , K.
Page 2 :
In general, , 13. Melting and Boiling Point. Alkali metals, have low, , melting and boiling point, , 2M +2H,O, , due to, , 2M+20OH+ H,, , weak metallic bonds which is due to bigger, , where 'M' is, , atomic size and only one valence electron., , Melting point of alkali metals decrease down, , 17., , Reactivity, , alkali metal, , an, , towards, , decrease in strength of metallic bond., 14. Soft Metals. All alkali metals are soft and, , form hydrides, 2LiH, , can be cut with knife due to weak metallic, , bond., Softness increases down the group due to, decrease in strength of metallic bond., K is softer than Na., , 2Na + H,, , 2K +H2, , 15. Chemical Properties, , tarnish in air due, , melting point. They, , or, , hydroxide on the surface., , form, , Thetemperatureforreactionstooccurdecreases, down the group., 4Li(s)+0,{8) 2Li,0(s), , Li has, , N,, , 18., , 2Li(s), , 19., , 2H,00)> 2LiOH(aq) +H,(g), 2Na(s)+2H,O)> 2NaOH(aq) H,(g), 2K(s), +2H,00)>, +, , 2KOH(aq) + H,(g), , 2NaCl, , 2KCI, , Reducing nature., , Alkali metals are strong, , Lithium is. best reducing agent because ", has lowest standard, Sodium is weakest, , exceptional behaviour. It reacts with, , explosively, , 2Na +Cl, 2K +Cl, reducing agents., , Caesium superoxide, , +, , OH + H,, Reactivity with Halogens. Alkali metas, react directly with, halogens to form ionic, halides., , eroxide, , form lithium nitride., 6Li +N N, 2Li,N, 16. Reaction with, Water. Li reacts, with water, less, vigorously than Na., Na reacts with water, to, produce so much heat, that it melts and, hydrogen produced ignites, in air. Other, metals also react, with H,O., to, , NaOH+H, , HT+H,0, , KO,), , Rbs)+0,g)> RbO,0), Rubidium superoxide, Cs(s)+0,(g) CsO,), , basic in nature and, , hydroxide and H, gas when they react, , lonic equation, , Sodium peroxide, Potassium, , are, , NaH+H,O, , 2Na(s) +O,(g)> Na,0,6), , K(s) +0,(8), , hydrides are ionic solids with hioh, , with H,O., , Reactivity with air increases down the group., , Lithium oxide, , 2KH, , Potassium hydride, , Reactivity towards Air. The alkali metals, formation of oxide, , Lithium hydride, 673K, 2NaH, Sodium, hydrde, 673K, », , All metal, , to, , Hydrogen., , metals react with H, at 673 The :, K kai, exce, which reacts at 1073 K to, 2Li +H 1 1073K, , the group due to increase in atomic size and, , reduction potentia., , reducing agent among, , alkalimetalsduetohigheststandardreductia, potential., , L i has small, , enthalpy, 20., , ration, , size, it has highest hydrau, , which overcomes sublimati, it has, and ionisation, enthalpy that is why, lowest standard reduction potential., , Solution, , of Alkali Metals, , in, , Liquid, , Ammonia. The alkali metals are (1, in liquid ammonia. The solution is, blu in, colour but changes to bronze withincreas, , concentration.
Page 3 :
S-BIOCK EICN, , The blue colour is due to presence of solvated, , The oxides and, , or ammoniated electron., , but superoxides are yellow or orange, , M++, y)N,|M(NH,)+ [etNH,),1, In concentrated olution, the ammoniated, metal, , ions, , electrons, , are, , boundec, , which, , peroxides are colourless, , by the free unpaired, have been describe as, , expanded metals. The blue solutions are, naramagnetic due to presence of unpaired, , electrons whereas bronze coloured solutions, are diamagnetic., Na'(am)+ e (am) + NH,() > NaNH.(am), , +Hg), , ('am' stands for solution, , in ammonia.), 21. Uses of alkali metals. Li is used to make, alloy with Pb to make white metal bearings, , superoxides are also, 1s, paramagnetic. Sodium peroxide, in, widely used as an oxidising agent, , coloured. The, , inorganic chemistry., , The hydroxides which are obtained by, water, , the reaction of the oxides with, are all white crystalline solids., , The alkali metal hydroxides are the, and dissolve, Strongest of all bases, much, freely in water with evolution of, , heat., Basic character of oxides and, , hydroxides increases down the group, , for motor engines, with Al to make aircraft, , due to increase in metallic character., , parts, with Mg to make armour plates., , Solubility, , 22. General Characteristics of Compounds of, , of, , hydroxides, , increases down the group 1., , i) Halides. Alkali metal, , Alkali Metals:, , in water, , halides, , are, , all, , All the common compounds of alkali metals, , high melting, colourless crystalline, , are generally ionic in nature., , solids., , () Oxides and Hydroxides. Lithium in, , They, , can, , be, , prepared by, , reaction of, , excess of air forms monoxide (with, , oxide, hydroxide or carbonate with, , some peroxide, Li,O,). Na forms, peroxide, Na,O,. K, Rb and Cs form, superoxides. Pure M,0, M,0, and, MO, may be prepared under suitable, conditions., The stability of peroxides and, , aqueous hydrochloric acid, e.g.,, , superoxides increases down the group., It is due to the stabilisation of large, cations by large anion through lattice, energy effects., , These oxides are easily hydrolysed by, water to form hydroxides., , Na,0 + H,0> 2Na+ 20H, , K,0 +H,0, , 2K, , +20H, , Na,O, + 2H,O 2Na +20H, , +H,O, , INCT 2018, K,0, +2H,0 > 2K" +20H, , 2KO, + 2H,O > 2K', , +H,O, , +, , 20H+, , H,0,+0,, , Na,O(s)+2HCI(aq) > 2NaCl(s), , +H,O(, KOH(s)+ HCl(aq), , KC{3), , +H,O(), , K,CO () + 2HCl(aq) - 2KC(s), , +H,O() + CO,(g), , All of these halides have high negative, enthalpies of formation (AH°). AH°, values of fluorides become less, negative as we go down the group., AH° values of chlorides, bromides and, , iodides become more negative down, the group., For a given metal, AH° value becomes, , less negative from fluoride to iodide., The melting points of metallic halides, follow this order, MF> MCl> MBr >MI, due to decrease in ionic character from, fluoride to iodide.
Page 4 :
(i) Li is least reactive but, agent., (iii) On combustion, it, , All halides are soluble in water except, LiF which has low solubility due to, , solubility due to small hydration energy, , oflarger Cs' and I., , (iv), , Solubility of lithium salts resembles, ions., , highly electropositive metals., They are generally soluble in water and, , thermally stable. Their carbonates and, in, , most cases, , bicarbonates, , are, , stable to heat., The, , stability, , highly, , (v), , (vi), , 24., , in, , organic solvents., , Differences between Lithium, , Alkali Metals:, , (i) Li is much harder and its, , boiling points, , alkali metals., , are, , higher, , and other, , are, , much less soluble, , decompose at red, , Li,O, heat, , +, , H,O, , Mg(OH)- MgO, , (iv) Both, , react with, , N,, , +H,O, form nitrides,, , to, , e.g., , 6Li +N,, , heat, , (v) Both, , 2Li,N, , heat, , 3Mg +N,, , Anomalous Properties, of ILithium. Lithium, shows anomalous, (abnormal) behaviour, which differs from other alkali, , soluble, , metal, , heat, , 2LiOH, , energy, , compounds, , Mg0, , hot, e.g, , LiHCO, does not exist as solid. It exists, only in solution due to high hydration, , are, , and, , and their hydroxides, , stable, , metals., Reason. It is due to, exceptionally small, size of atom and Li ion. That, is why it has, highest polarising power (i.e., ratio). This results in increasecharge/radius, in covalent, character of Lithium, compounds. Higher the, polarising power, more will be the covalent, character. That is why lithium, , hydrates., , LiHCO, exists only in aqueous, solution, whereas others form solid, Lithium does not form bicarbonates, , (ii) Li,o, , LiCO,0) heatLi,O(s) +CO,(g), , 23., , form, , as, , acetylide on, reaction with ethyne., 25. Similarities between, Lithium and, Magnesium:, ) Both Li and Mg are harder and, (i) Li and Mg react slowly with coldlighter., water., , bicarbonates increases down the group., not so, , 2H,0, whereas other alkali, , chlorides do not, , of carbonates and, , Lithium carbonate is, because it is covalent., , monoxide (Li,0) and lithium, (Li,N) unlike other alkali metalsnitride, LiClis deliquescent solid, (absorbs wate, from atmosphere) and, crystallizes., LiCl., , Other halides of lithium are soluble in, , ethanol. acetone and ethyl acetate. LiCl, is soluble in, pyridine (CH,N)., (ii) Salts of Oxo-acids. Alkali metals form, salts with oxo-acids because they are, , reducin, , forms only lithium, , its high lattice energy. Csl has low, , those of Mg, , best, , cannot, , superoxides., , Mg,N,, form peroxides, , and, , (vi) Nitrates of both form oxides, NO, and, oxygen., , 4LiNO,, , >2Li,0+ 4NO, +0, , 2Mg(NO,), 2Mg0, 26 Alkaline, 6., , Earth Metals., , +4NO, , +0., , Those metals, , whose oxides were known much earlier, , amed, than the metals themselves. They were nan, , alkaline earth metals because (i) they were, alkaline in nature like alkali metal oxides., , meling and, than other, , and, , (ii), , they, , found in earth's cru, e.g. Be (Beryllium), Ca, Mg, Sr (Strontium),, Ba, , (Barium),, , were, , Ra, , (Radium)., , fifth and sixth in abundance, , Ca and Mg, , are, , ectively n
Page 5 :
the earth crust. Sr and Ba have much lower, , abundances. Be is rare and Ra is rarest of, abu, all comprising only 10 per cent of igneous, , (vi) Melting and Boiling Points. They have, fairly higher melting and boiling points, than corresponding alkali metals. The, trend is not systematic. Mg has lowest, , rocks. It is radioactive element with half life, 1600 years., 7 General Characteristics of Alkaline Earth, , melting point., Reason. It is due to small atomic, size and two valence electrons and, , Metals:, , Group 2 elements are called alkaline earth, metals except Be. They include Be, Mg, Ca,, Sr, Ba and Ra. Ra is radioactive., , ()Electronic Configuration. Their, general electronic configu-ration is ns, These have 2 electrons in outermost, shell., , (i) Oxidation State. They, , (ii), , can, , lose two, , (vii), , Electrical and Thermal Conductivity., conductors of heat and, These are, , good, , electricity., , not, (vii) Flame Colour. Be and Mg do, impart any colour to the flame because, electrons are strongly bound, therefore,, , it cannot be excited by flame., , electrons to acquire nearest noble gas, configuration, therefore, their oxidation, , Ca imparts brick red, Sr imparts, , state is +2., , colour because electrons get excited, to, energy levels and when, , Atomic and Ionic Size. The atomic, , and ionic size go on increasing down, the group due to decrease in effective, nuclear charge. Their atomic and ionic, , radii are smaller than corresponding, , alkali metals in corresponding periods, , (iv), , therefore, strong metallic bond., , nuclear, due to increased effective, charge in these elements., first, Ionisation Enthalpies. The, ionisation, , elements, , enthalpies of, , are, , higher than, , group 2, , group, , atomic, elements because of smallest, nuclear, size and higher effective, , charge., , Second ionisation, , enthalpy, , metals, is smaller than that of alkali, noble, because alkali metals acquire, after losing one, gas configuration, electron. lonisation enthalpies group, the group, 2 elements decrease down, size., due to increase in atomic, metals, Appearance. These, , of, , () Physical, in, , silvery white,, harder than the, relatively soft, but, , general, , and, , lustrous, , are, , alkali metals. Be and, greyish., , Mg appear, , to be, , crimson red, Ba gives apple green, , higher, they come back to, , lower energy level,, form of visible, energy is radiated in the, , light., (ix) Density. Group, , 2 elements, , are, , denser, , than corresponding group I elements, which is due to greater strength of, , metallic bond and more closely packed, , crystal structure. However, the variation, in density is irregular. "Ca' has lowest, density., (x) Metallic Character. They are less, electropositive than alkali metals due, to higher ionisation enthalpies. Metallic, character inereases down the group due, to decrease in ionisation enthalpy., , 28. Chemical properties., , Reactivity with Air and Water. The alkaline, earth metals are less reactive than alkali, metals. Be and Mg are kinetically inert, with O, and H,O due to formation, , (passive), of oxide, , layer, , on, , its surface. Be does, , not, , even at red hot and, react with water or steam, 873 K., does not get oxidised in air below
Page 6 :
Powdered Be buns, , on, , to form, , ignition, , They, , Be0, , CaH,, , 2Be+0, Burming, 2Be), 3Be N, Burming Be,N2, is more, , Mg, , to, , readily, , SrO,, , [M(NH,),]" can, , tubes, , Mg(OH), +H, Ca(OH), +H,, , Halogens. Group 2 elements, halogens at elevated temperature, , Be +Cl- heat BeCl,, , Ca is used, , as, , of metals., , heat MgClh, , C a and Ba, vacuum tubes., , healCaCh, , H,. All metals combine with, H, to form hydrides, except Be., Ca+ H >, CaH, (Hydrolith), Calcium bydride, , making windows of x-ray, , reducing agent in extraction, , remove traces, , Radium is used in, treatment of cancer., 30., , of air from, , radiotherapy in, , General Characteristics of Compounds, , of Alkaline Earth Metals. They form, , dipositive, , MgH, , BeH, is prepared by reduction of, , LiAIH, 2BeCl, +LiAlH,, , +2[e(NH,, these, , recovered from, , Mg-Al alloy is used in making aircrafts., Mg powder is used in flash powders and, bulbs, incendiary bombs and signals., Milk of magnesia is used as antacid., MgCO. CaCO, are present in tooth paste, , with, to form halides., , Mg+ H2-, , [M(NH,).P*, , strength springs., , Be is used for, , Reaction with, , Reaction with, , MgS0,, , Copper-beryllium alloys are used in high, , Mg reacts with hot water, Ca, Ba, Sr react, with cold water vigorously., , Ca+Cl,, , MgCl,+H,, Mg(NO,),, +H,, , 29. Uses of Alkali metals, , Barium peroxide, , Mg+Cl, , be, , solutions., , Strontium peroxide, , Ca+2H,0, , metals, , salts and, , solvated, , M+x+y)NH,, , Calcium nitride, , Mg +2H,0 (hot), , earth, , to form, , electron., , > CaN, , Ba +O, B a O ,, , 21,4R, , H,, with NH3., Alkaline earth, metals dissolve in, liquid N to, give, deep blue black solution due to, , 2Ca0, , », , (a, , >BeCl, +H,, , 2HCI, , Mg +HS0,(dil.)-, , Calcium oxide, , Sr +O, , +, , Reaction, , nitrogen to form nitrides., , 3Ca + N,, , acids, , Mg +2HNO,(5%), , Sr and Ba form peroxides. They react with, , 2Ca+0,, , +, , Be+2HCI, , Mg,N,, , react with oxygen, form oxide. Calcium forms oxide whereas, , react, , H,0 >Ca* (ay) 20H, , readily react with, liberate H, gas., , with dazzling light forming MgO and Mg,N, 2Mg+O,, 2MgO, Ca. Sr and Ba, , +, , They are basic in nature., Action with Acids. The alkaline, , air, electropositive and burns in, , 3Mg+ N, , drides except, Bell, which, , ionic, , is covalent,, , and Be,N,, , Mg, , are, , 2BeH,, , BeCl, with, , +LiCl, , +AlCl,, , ions in their compounds., They form less ionic compounds, , tnau, , corresponding alkali compounds due, smaller size and higher charge. Be anu, , form covalent compoune, predominantly, whereas Ba, Ca, Sr form ionic, pounds.
Page 7 :
s-Block Elements 167, Oxides and, , Hydrexides. Be0 is, , anuphoteric whereas, , others, , (ii) Salts of Oxoacids:, Carbonates. BeCO, is, , basic., , are, , ReO is covalent. They are quite, , stable. They, , H,O forming, , react with, , hydroxides., , Ca0+H,O, , Ba(OH),, , Be(OH),+2HCI, BeCl, +2H,0, Be(OH),+2NaOH> Na,[Be(OH),, Sodium beryllate, , Halides. All are ionic except BeX,, in, which is covalent and soluble organic, molecules only exist in, solvent. BeX,, but in condensed phase, gaseous phase,, which Be is four, exist as a polymer in, , has chain, , down the, of carbonates decreases, , stability of carbonates, more, the group. They are, presence, , in solid state, , and stable to heat., , as, , Solubility, , MgBr, and Mgl,, , because, decreases down the group, dominates over hydration, , lattice energy, , energy., BeSO.4H,0, MgSo,.7H,0,, and, 2H,0 are hydrates. SrSO,, , are, , soluble in organic, , CaSO, , BeSO, crystallise, , solvents., decreases, , The tendency to form hydrates, 8H,0,, down the group, e.g. MgCl,., CaC1.6H,0, SrC1,.6H,0, BaCl,.2H,0., , (v), , are, , earth metals, Fluorides of alkaline, water, soluble in, , (partially), , due to high lattice energy., , slightly, , with, , The solubility increases, called, size. CaF, is, Increase in ionic, insoluble in water, fluorospar and is, halides, lonic character of, , increases, , <, , CaCl,, , The oxalates of Ca, Ba and, soluble and solubility, , highly soluble., , (vi), , Nitrates., water., , of, , All nitrates, , They, , are, , carbonates, , are, , soluble in, , by, , reaction, , with dil., , HNO., , obtained, , out., , Mg(NO,).6H,0 is erystallised, on heating, All of them decompose, and O., forming oxides, NO,, , 2Mg(NO,)2MgO+ 4NO, +0,, nitrates, Sr and Ba, , are, , used in, , red and, pyrotechnics for giving, , down the group, , MgCl,, , Oxalates., , increases down the group. Beryllium, oxalate are, oxalate and magnesium, , are covalent, , BeCl, +2H,0, , <, , SrCl,, , of, , Sr are sparingly, , moist air due, and BeCl, fume in, , hydrolysis because they, and form HCI on hydrolysis., 2HCI, Be(OH), +, , out without water, , crystallisation., , to, , BaCl, , MgSO,, , BeSO,, , soluble in water because, are readily, ofsmaller Bes" and, hydration enthalpy, Mg" overcomes lattice enthalpy., 2, of sulphates of group, , CI, , <, , of, , of Na,CO,, extraction of iron., (6) as a flux in, and cement., (c) in manufacture ofglass white solids, are also, (iv) Sulphates. They, and, , Be, , BeCl,, , formation, , CaCO, is used:, for preparation, (a) in Solvay's process, , CI, , C, , sparingly, , of CO, due to, , soluble in, , pure state., , shown below., , MgCl,, , lattice, , in, , in, not obtained, bicarbonates which are, , Beryllium chloride, , structure, , Solubility, , CO,., , increases down, , The solubility, and thermal stability increases down, reacts with acidsas, the group. Be(OH),, well as alkalies and is thus amphoteric., , atoms coordinate., , in atmosphere of, , more, group due to, Thermal, energy., than, hydration, energy, , in water, basic character, , ), , precipitated, hydrolysed. It is, , increase, , > Ca(OH),, , BaO+H,O, , gets, , covalent,, , flames., , green
Page 8 :
31., , Behaviour, , Anonmalous, , ()Be, , has smallest, , which, , are, , of Beryllium:, , size and highest, , It forms, ionisation energy., , predominately, , lent compoun, , covnl., , readily hydrolysed., , while others form basic., and hydroxides, oxides, () It forms amphoteric, of four, others show higher., coordination number, show, (i) Be can, hot., even on red, It does not react with H,O, , (i), , 32. Diagonal Relationship, , between, , Be and Al:, , )They have similar electronegativity., oxides and hydroxides., () Both form amphoteric, (iii) Both become passive due, (), , to formation of oxide, , BeC1, and AICI, have bridged, , structure, , in vapour, , C, C-Be, , Be-Cl, , layer., phase., , CI, , CIA c AKCi, , ()Both form hydroxide ions [Be(OH),F and [AI(OH),, , in aqueous alkali solution., , (vi) Both have similar charge/radius ratio, therefore,have strong tendencies to form complexes, , eg. BeF, , [Be(C,0,),1 .[AIF,J. [AIMC,O,),J*., , NCERT EXERCISES, 10.1. What are the common physical and chemical features of alkali metals?, Ans., , Physical Properties:, , ()Theyare soft metals., (i) They have low melting and boiling points., Chemical Properties:, ) They are highly reactive., (i) They show +1 oxidation state., 10.2. Discuss the, Ans., , general characteristics and gradation, , in, , properties, , () Atomic size goes on, increasing down the group., (i) lonisation enthalpy goes on, decreasing down the group., (iii) They are harder than alkali, metals., (iv) They are less, electropositive than alkali metals but, down, increasing, the group., 10.3. Why are alkali metals not, found in, , of alkaline eartm u s., , sitive character goes, , On, , electropositive cnaa, , nature?, because they are highly, reactive metals. They, the form of compounds., combine with other, substain, , INCT 2017, , Ans. It is, , nces, , and, , exist in