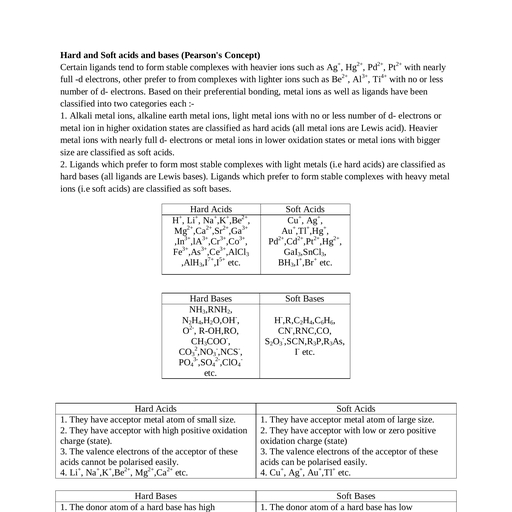
Page 1 :
Relative strength of acid and base : The strength of an acid, according to the concept of Bronsted and Lowry depends upon its tendency to, lose proton and the strength of base depends upon its tendency to gain protons., 1). Example : - The order of basicity of Bronsted bases is F- > Cl- > Br- > I-. Why?, Soln, , :-, , F- > Cl- > Br- > IDecreasing basicity, , Since F- ion has greater power to accept a proton form its conjugate acid HF compared to Cl-, Br- and I-., Hence, F- ion has higher basic strength than Cl-, Br- and I-., 2). Example : - The basicity of hydride anions of the non-metals of the same period decreases CH3->NH2>OH->F-. Why?, Soln : - We know that in a given conjugate acid-base pair, if the acid is strong, its conjugate base will be, weak and vice-versa. Thus, the acidic strength of CH4, NH3,H2O and HF which are conjugate acids of, decreases CH3-,NH2-,OH- and F- ions respectively increases as CH4<NH3<H2O< HF. Thus, the basic, strength og the given base should decrease in the order., , CH3->NH2->OH->FDecreasing basicity, , Example : - Explanation same as above NH2- > PH2- > AsH2- > SbH2- > BiH2- (*Proton accepting, capacity), 3). Example : - CH4 < NH3 < H2O < HF. Explain the acidity order., Soln : - The above trend of increasing acidity can be explained on the basis of electronegativity as well as, Bronsted acids and bases concept. Electronegativity of Fluorine atom in HF results in pulling the shared, electron bond pair towards itself and hence the release of proton (H+) is more easier compared to other, Bronsted acids., 4). Example : - Relative order of the acidic strength of H2O < H2S < H2Se < H2Te. Explain, Soln : - As we move down the group, the size of central atom (A) increases gradually and hence the bond, length A-H also increases. With increase in the A-H bond length, the strength of A-H bond decreases, from O-H bond to Te-H bond. Consequently, the tendency of A-H bond to break to give H+ ion increases, as we go down the group. Therefore, the acidic strength H2O < H2S < H2Se < H2Te., 5). Example : - Relative order of the acidic strength of HF < HCl < HBr < HI. Explain, Explanation can be done using the Bronsted and Lowry concept of acid and base. Strength of conjugate, base F- > Cl- > Br- > I-., 6). Example : - ClO- > ClO2- > ClO3- > ClO4-. Explain the order of decreasing basicity.
Page 2 :
The ClO4- ion has the maximum no. of O-atoms (=4) due to which distribution or spreading of unit, negative charge in this ion is maximum and hence, this ion is the most stable., 7). Example : - HClO < HClO2 < HClO3 < HClO4. Explain the acidity order., Soln : - As the oxidation no. of Cl- - atom increases from +1 to +7, the tendency of Cl - atom to attract the, electron pair of Cl-O bond towards become easier. In other words, we can say that the acidic character of, given acids increase from HClO to HClO4., O, O, O.s = +1, O.s = +3, Cl, , O, , H, , O, , Cl, OH, O.s = +5, , O, , Cl, , O, , H, , O, , Cl, , O, , H, , O, O.s = +7, , 8). Example : - HClO3 > HBrO3 > HIO3 and HNO3 > HPO3 > HAsO3. Explain the acidity order., *explanation is done as in the above example., 9). Example : - BF3 < BCl3 < BBr3 < BI3. Explain the acidity order., All the acids mentioned here are Lewis acids as all the central atom Born has empty p-orbital. All the, given acids has a trigonal planar geometry which arises due to sp2 - hybridisation of Boron atom. One of, the 2p-orbital, say 2pz remains unhybridised and vacant. The filled 2pz-orbital of Fluorine atom makes a, lateral overlap with the vacant 2pz-orbital of Boron atom and gives rise to the formation of an additional, F --------->B bond, called p- p back. Since, orbitals of similar energy and shape overlaps. It results in, an effective overlapping., ppback bond, , F, , F, F, , F, , B, F, , B, F, , However, as in Cl, Br, and I. The effectiveness with which these pair of orbitals decreases due to, difference in energy and shape (i.e. B-Cl ----> 2p-3p, B-Br ----> 2p-4p and B-I ----> 2p-5p). Thus, the, tendency of back bonding in BCl3, BBr3 and BI3 is minimum compared to BF 3. This means, the tendency, of BF3 molecule to accept electron pair is minimum and the acidic character is in the order., BF3 < BCl3 < BBr3 < BI3