Page 1 :
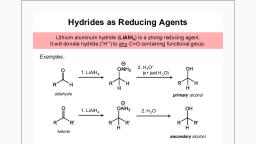
Reactions of Alcohols, , LiAIH4 and NaBH4 Carbonyl Reduction, Mechanism, , Alcohols can be prepared from carbonyl compounds such as aldehydes, ketones,, esters, acid chlorides and even carboxylic acids by hydride reductions. These, reductions are a result of a net addition of two hydrogen atoms tothe C=O bond, , (comes from water or acid), +, , H, ° A 1H, R wh, H a, , (comes from metal hydrides), , The most common hydride reducing agents are the lithium aluminum hydride, (LiALHg) also abbreviated as LAH and sodium borohydride (NaBHg), , NaBH, IH, —_— + Na*B(OEts),, 1H ELOH, , H, 9 H_OH, 1) LiAIHy oS + Lit and Al* salts, 2)H*, , The principle behind the hydride reducing agents is the presence of apolar, covalent bond between a metal and hydrogen Because of higher electronegativity,, the hydrogen bears higher electron densit y which eventually makes it react as a, hydride ion, , , , a polar covalent bond, , For example, the Al-H bond in LiAIHg is so polar that it has nearly ionic character, leaving the hydrogen as a hydride ion which is a very reactive both as a base anda, nucleophile, , ‘Scanned with CamScanner
Page 2 :
=, , H, ARH reactsasif H—Al, H, , —, , covalent ionic, , The hydride ion reacts with the carbonyl group which, in turn, is also a polar, covalent bond and the presence of the m bond makes the H” addition possible, , making C-H, , bond breaking C-O, ' & axbond e, o\., rh § QR, " RR RTR, , electrophilic, , LiALHs is one of them most powerful reducing agents efficiently working for any, carbonyl and some other functional groups as well, , This high reactivity of the hydride ion in LIAIHg makes it incompatible with protic, solvents For example, it reacts violently with water and therefore, LiAIH, reductions, are carried out in dry solvents such as anhydrous ether and THF., , NaBHsg, on the other hand, is not so reactive and can be used, for example, ina, selective reduction of aldehydes and ketones in presence of an ester, , H, AR, 1) LIAIH, OH, —_—_, ste ae, , Notice that LIALH, and NaBH, reduce aldehydes and ketones to primary and, secondary alcohols respectively Esters, on the other hand, are converted to primary, alcohols by LiALHg, , ‘Scanned with CamScanner
Page 3 :
LiAIH, Reduction of Aldehydes and Ketones —, The Mechanism, , As mentioned earlier, both reagents function as a source of hydride (H>) which acts, as a nucleophile attacking the carbon of the carbonyl C=O bond and in the second, step the resulting alkoxide ion is protonated to form an alcohol., , There are, however, some differences depending on the reagent andto address, those, let’s start with the mechanism of LiAIHs Reduction:, , The Mechanism of Ketone and Aldehyde Reduction by LIAIH,, , Catalyzes H addition, , @, @ i, - ge AH, eg" Li AIH, x 3, I ne —_», R R“ER RNR, H H H, wy, H-AI-H. a lithium alkoxide, Ay a tetrahedral intermeduate . Oo, , Nucleophitic attack Proton transfer, , workup, , , , Li* and Al** salts + Alcohol RER, , Simplified version:, , SO NG, , 5, Bs R Rv GS R” 4, H, , The hydride addition tothe carbonyl is also catalyzed by the lithium ion which, serves as a Lewis acid by coordinating tothe carbonyl oxygen. This decreases the, electron density on the oxygen thus making the C=O bond more susceptible toa, nucleophilic attack., , The resulting alkoxide salt can react with the AIH, and convert it to another source, of hydride However, for simplicity, most often we show only one addition tothe, carbonyl followed by a protonation of the alkoxide with water or aqueous acidic, solutions which gives the final product alcohol, , ‘Scanned with CamScanner
Page 4 :
NaBH, Reduction of Aldehydes and Ketones —, The Mechanism, , Sodium borohydride reduces aldehydes and ketones by a similar mechanism with, some important differences that we need to mention., , First, NaBH, is not so reactive and the reaction is usually carried out in protic, solvents such as ethanol or methanol. The solvent has two functions here, , 1) It serves as the source of a proton (H*) once the reduction is complete, , 2) The sodium ion is a weaker Lewis acid than the lithium ion and, inthis case, the, hydrogen bonding between the alcohol and the carbonyl group serves as 2, catalysis to activate the carbonyl group, , The Mechanism of Ketone and Aldchyde Reduction by NaBlly, , Catalyzes H addition, , EtOH NP ,, 50% OH Ht na, Ro. > abr + H-BOoEt, a H, oH Alcohol sodiummethoxy4 borohydride, Nucleophilic attack, Simplified version:, , , , a, , Because NaBH, is not very reactive, it is not strong enough to react with esters, And this also hasto do with the reactivity of the ester as well. In general, aldehydes, and ketones are the most reactive carbonyl compounds (after acid chlorides which, are only used as reagents and not final products because of their reactivity), , We have also seen this in the Grignard reaction. Aldehydes and ketones are more, reactive than esters since the electrophilicity of the carbon atom of the ester is, partially suppressed by the lone pair of the oxygen through resonance stabilization:, , Esters are less reactive than ketones and aldehydes, , 9°, , Je _— electrophilic carbon, , eo, Sigg —~nally, # OEt OEt, less electrophilic, , ‘Scanned with CamScanner
Page 5 :
Because of the resonance stabilization, the C=O carbon atom in the esters is not, electrophilic and NaBHyg being not very reactive is unable to attack it, , That is about the relationship between NaBH, and ester. Let's now see how the, reduction of esters by LiAIH, works., , The Mechanism of LiAIH, Reduction of Esters, , The reduction of an ester to an alcohol requires two hydride additionsto the, carbonyl group and therefore an excess of LiAIH, is used:, , 1) excess LIAIH,, R => R~OH + ROH, 2)H*, , This is because the tetrahedral intermediate formed after the first hydride addition, contains a leaving group which is kicked out re-forming the carbonyl group, , 9 gz, wok — Je + ROH, , The newly formed carbonyl group is an aldehyde and it is more reactive than the, ester, thus is attacked one more time by LIAIHy, , ‘Scanned with CamScanner