Page 1 :
[ISOMERISM, , Isomerism is the phenomenon in which more than one compounds have the, same chemical formula but different chemical structures. Chemical compounds, that have identical chemical formula but differ in properties and the, arrangement of atoms in the molecule are called isomers. Therefore, the, compounds that exhibit isomerism are known as isomers., , The word “isomer” is derived from the Greek words “isos” and “meros”,, which mean “equal parts”. This term was coined by the Swedish chemist Jacob, Berzelius in the year 1830., , Types, , There are two primary types of isomerism, which can be further categorized, into different’ subtypes. These primary types are Structural, Isomerism and Stereoisomerism. The classification of different types of, isomers is illustrated below.
Page 2 :
6. Ring Chain Isomerism, , Compounds having the same molecular formula but possessing, open chain and cyclic structures are called ring chain isomers and the, phenomenon is called ring-chain isomerism., , For example propene and cyclopropane are ring chain isomers., , CHy-CH=CH, and, , Propene Cyclopropane
Page 3 :
4. Metamerism, , This type of isomerism arises due to the presence of different alkyl chains, on each side of the functional group. It is a rare type of isomerism and ts, generally limited to molecules that contain a divalent atom (such, as sulfur or oxygen), surrounded by alkyl groups., , Example: C,H), 0 can be represented as :, , ethoxyethane (C,H,OC,H,) and methoxy-propane (CH,OC;H,).
Page 4 :
3. Functional Isomerism, , It is also known as functional group isomerism, As the name suggests, it, refers to the compounds that have the same chemical formula but, different functional groups attached to them. An example of functional, , isomerism can be observed in the compound = C,H,O., , propanal propanone (acetone), , H H 0 H H, , | | 4 |, H-C—C-C H-C—C-C-H, , 1 | \y | i |, , H H H OH
Page 5 :
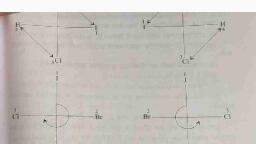
2. Position Isomerism, , The positions of the functional groups or substituent atoms are different in, , position isomers. Typically, this isomerism involves the attachment of the, functional groups to different carbon atoms in the carbon chain. An, example of this type of isomerism can be observed in the compounds, having the formula C,H,Cl., , CH;-CH)-CH) CH;-CH-CH,, | |, cl cl, , |-Chloropropane 2-Chloropropane