Page 2 :
Conformations, The infinite number of arrangements of the atoms or groups of a, molecule in three dimentional space which are interconvertible into, each other by rotation about single bond are called Conformations or, , Rotational Isomers or simply Rotamers., These conformers have different internal dimensions (atom-to-atom, distances, dihedral angles, dipole moment etc.), , ., The energy barrier for rotation of carbon-carbon single bonds, (conversion of different spatial arrangements) is normally small, < 0.6, , kcal/mol and >16 kcal/mol., , 2
Page 3 :
Rotation about Carbon–Carbon Bonds, , 3
Page 4 :
Newman & Sawhorse Projections, , 4
Page 5 :
Staggered conformation:, A conformation about a carbon-carbon single, bond in which the atoms or groups on one, carbon are as far apart as possible from the, atoms or groups on an adjacent carbon, H, H, , H, , H, , H, H, , 5
Page 6 :
Eclipsed conformation:, A conformation about a carbon-carbon single bond, in which the atoms or groups of atoms on one, carbon are as close as possible to the atoms or, groups of atoms on an adjacent carbon, , H, , H, , H, H, , HH, , 6
Page 7 :
Eclipsed conformation, • Each hydrogen on one, carbon as close as, possible to one, hydrogen on the other, carbon, , Staggered conformation, • Hydrogen on one carbon, as far from the hydrogen, from other carbon, , •, , A Staggered conformation is more stable than an, eclipsed conformation, 7
Page 8 :
Types of Strain, Steric - Destabilization due to the repulsion between, the electron clouds of atoms or groups. Groups try to, occupy some common space., Torsional - Destabilization due to the repulsion, between pairs of bonds caused by the electrostatic, repulsion of the electrons in the bonds. Groups are, eclipsed., Angle - Destabilisation due to distortion of a bond, angle from it's optimum value caused by the, electrostatic repulsion of the electrons in the bonds., e.g. cyclopropane, 8
Page 9 :
Torsional strain, Also called eclipsed interaction strain., Strain that results from eclipsed bonds., Strain that arises when non-bonded atoms/groups,, separated by three bonds are forced from a staggered, conformation to an eclipsed conformation., The torsional strain between eclipsed and staggered, ethane is approximately 12.6 kJ (3.0 kcal)/mol, +12.6 kJ/mol, , 9
Page 10 :
60o Rotation Causes Torsional or, Eclipsing Strain, , 10
Page 11 :
Dihedral angle (Ɵ), The angle created by two intersecting planes, , 11
Page 12 :
Conformers of Alkanes, Structures resulting from the free rotation of a C-C, single bond, May differ in energy. The lowest-energy conformer, is most prevalent., Molecules constantly rotate through all the possible, conformations., , 12
Page 13 :
Conformations of Ethane, • Staggered conformer has lowest energy., • Dihedral angle = 600, H, H, , H, , H, , H, H, , Newman projection, , Sawhorse Projection, , 13
Page 14 :
Rotational Conformations of Ethane, , 14
Page 15 :
15
Page 16 :
Ethane as a function of dihedral angle, 16
Page 17 :
17
Page 18 :
The origin of torsional strain in ethane:, Originally thought to be caused by repulsion between eclipsed, hydrogen nuclei, Alternatively, caused by repulsion between electron clouds of, eclipsed C-H bonds, , Theoretical molecular orbital calculations suggest that the energy, difference is not caused by destabilization of the eclipsed, conformation but rather by stabilization of the staggered, conformation, This stabilization arises from the small donor-acceptor interaction, between a C-H bonding MO of one carbon and the C-H, antibonding MO on an adjacent carbon; this stabilization is lost, when a staggered conformation is converted to an eclipsed, 18, conformation
Page 20 :
Conformations of Propane, , 20
Page 21 :
21
Page 22 :
Conformations of Butane
Page 25 :
2 Different Eclipsed Conformations, , 25
Page 26 :
26
Page 27 :
Butane has Steric and Torsional, strain when Eclipsed, , The totally eclipsed conformation is higher in energy, because it forces the two end methyl groups so close, together that their electron clouds experience a, strong repulsion., 27
Page 28 :
2 | 28
Page 29 :
Extra slide, , Three valleys (staggered forms) 120 apart;, , Three hills (eclipsed) 120 apart.
Page 30 :
Draw, staggered, 1-Chloropropane?, , and, , eclipsed, , conformers, , of, , 30
Page 31 :
Draw the Rotational profile of 2-methylbutane about, C2-C3., Eclipsed Structures:, 00, , H, , Me, H, , Me, Me, , 2400, , H, , HH, , HMe, , H, Me, , 1800, , 1200, , Me, H, , HH, , HMe, , H, , Me, , Me, Me, , This was the, high, energy, staggered, structure, 180 0, Now relative energies….., , 3600, , H, Me, , Me, Me, , H, Me, , Me, H
Page 32 :
Staggered Structures:, , 600, , 1200, , H, , H, , Me, , H, , Me, , Me, H, , H, , 3000, H, H, , Me, , Me, Me, , H, , Me, , Me, , Me, H
Page 34 :
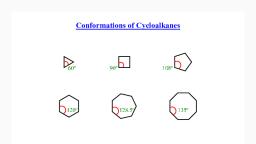
Stability of Cycloalkanes: Ring Strain, Rings larger than 3 atoms are not flat, Cyclic molecules can assume nonplanar conformations, to minimize angle strain and torsional strain by ringpuckering, Larger rings have many more possible conformations, than smaller rings and are more difficult to analyze
Page 35 :
The Baeyer Strain Theory, Baeyer (1885): since carbon, prefers to have bond angles, of approximately 109°, ring, sizes other than five and six, may be too strained to exist, Rings from 3 to 30 C’s do, exist but are strained due to, bond bending distortions and, steric interactions
Page 36 :
Summary: Types of Strain, Angle strain - expansion or compression of bond angles away, from most stable, Torsional strain - eclipsing of bonds on neighboring, atoms/gps, Steric strain - repulsive interactions between nonbonded, atoms in close proximity, , 36
Page 38 :
angle strain: the C-C-C bond angles are, compressed from 109.5° to 60°, torsional strain: there are 6 sets of eclipsed, hydrogen interactions, strain energy is about 116 kJ (27.7 kcal)/mol, , 38
Page 39 :
Cyclobutane, , The ring strain of a planar cyclobutane results from, two factors:, 1.angle strain from the compressing of the bond angles, to 90° rather than the tetrahedral angle of 109.5°, 2. torsional strain from eclipsing of the bonds., 39
Page 40 :
, , , , , , Internal bond angle ~88o (~21o deviated from the normal, 109.5o tetrahedral angle), Cyclobutane ring is not planar but is slightly folded. It is, slightly bent out of plane - one C atom is about 25°, above., If cyclobutane ring were planar, the angle strain would be, somewhat less (the internal angles would be 90o instead, of 88o), but torsional strain would be considerably larger, because all eight C–H bonds would be eclipsed
Page 41 :
puckering from planar cyclobutane reduces torsional, strain but increases angle strain, the conformation of minimum energy is a puckered, “butterfly” conformation, strain energy is about 110 kJ (26.3 kcal)/mol
Page 42 :
Cyclopentane, Planar cyclopentane would have no angle strain but, very high torsional strain, Actual conformations of cyclopentane are nonplanar,, reducing torsional strain. Puckering from planar, cyclopentane reduces torsional strain, but increases, angle stain, , 42
Page 43 :
, , , , , Four carbon atoms are in a plane, The fifth carbon atom is above or below the, plane – looks like an envelope, the conformation of minimum energy is a, puckered “envelope” conformation, strain energy is about 42 kJ (6.5 kcal)/mol
Page 44 :
Measuring Strain in Cycloalkanes, , Heats of combustion can be used to compare stabilities of, alkanes & cycloalkanes., Heats of combustion, of carbon atoms increase., , increase, , as, , the, , number, , Therefore, divide heat of combustion by number, of, C’s, and, compare, heats, of, combustion, on a "per CH2 group" basis.
Page 47 :
Conformations of Cyclohexane, Cyclohexane is by far the most common, cycloalkane in nature and also in organic, chemistry., The cyclohexane ring is free of angle strain and, torsional strain. Zero ring strain implies the bond, angles must be 109.5°. (no angle strain) and also, no eclipsing interactions between the C-H bonds, (no torsional strain).
Page 48 :
Cyclohexane adopts a puckered structure., A planar arrangement of the six methylene groups in cyclohexane, does not give a tetrahedral shape for every carbon atom - this is, achieved by puckering the ring. Cyclohexane does this by, adopting mainly two conformations the CHAIR and the BOAT., , 48
Page 49 :
Chair Conformation, , Most stable conformation. Each carbon is in the, staggered conformation, All the bond angles are 109.5° and all the C-H bonds, are staggered. (Zero ring strain) ., More stable than a boat conformation by 27 kJ (6.549, kcal)/mol.
Page 50 :
Boat Conformation, , 50
Page 51 :
51
Page 52 :
The boat is just a chair with the footrest flipped up., C-1, C-4 are bent toward each other., Four sets of eclipsed C-H interactions & one, flagpole interaction, This also has bond angles of 109.5° and thus avoids, any angle strain, but there is torsional strain., , The two hydrogens at the ends of the boat are in, close contact, causing torsional strain. These flagpole, hydrogens are eclipsed., 52
Page 53 :
Twist-boat conformation, To avoid these unfavorable interactions, the boat, conformation skews slightly, giving a twist boat, conformation. The twist boat conformation has a, lower energy than the pure boat conformation, but is, not as stable as the chair conformations, approximately 41.8 kJ (5.5 kcal)/mol less stable, than a chair conformation, approximately 6.3 kJ (1.5 kcal)/mol more stable, than a boat conformation, , 53
Page 55 :
Half-chair
Page 56 :
Half-chair, , Skew boat
Page 57 :
Half-chair, , Skew-boat
Page 59 :
The chair is the lowest energy conformation, although since, the energy barrier to ring flip is fairly small, there will always, be some other conformations present., The half chair is the point of highest energy, and is not a, 59, stable conformation.
Page 60 :
Axial and Equatorial Bonds in, Cyclohexane, , The chair conformation has two kinds of positions, for substituents on the ring: axial positions and, equatorial positions, Chair cyclohexane has six axial hydrogens, perpendicular to the ring (parallel to the ring axis), and six equatorial hydrogens near the plane of the, ring
Page 61 :
• Each carbon atom in cyclohexane has one axial, and one equatorial hydrogen, • Each face of the ring has three axial and three, equatorial hydrogens in an alternating, arrangement, 61
Page 62 :
How to Draw Cyclohexane, Step 1: Draw two parallel lines slanted, , downward, , Step 2: Draw two lines starting from the, parallel lines slanting upward, and intersecting at a point., , Step 3: Draw two lines downward, starting from the other end of, the parallel lines and intersecting, at another point.
Page 63 :
How to make Axial bonds and, Equatorial bonds, , 63
Page 64 :
Chair–Chair Interconversion/, Ring Flip, , An most important phenomenon in chair, conversion is that any substituent that is axial in, the original conformation becomes equatorial in, the new conformation (exchange of axial and, equatorial positions by a ring-flip ), 64
Page 65 :
All axial bonds become equatorial, All equatorial bonds become axial, All “up” bonds stay up, All “down” bonds stay down, 65
Page 66 :
Example:, , Axial-up becomes Equatorial-up, 66
Page 67 :
Equatorial Conformation is Preferred……WHY????, 67
Page 68 :
A Conformational Analysis of Methyl cyclohexane, , , Substituted cyclohexane, • Exists in two different chair forms, , H, G, , G, , H
Page 70 :
Equatorial Methyl Group, , 70
Page 71 :
Cyclohexane ring rapidly flips between chair, conformations at room temp., Two conformations of monosubstituted cyclohexane, aren’t equally stable., The equatorial conformer of methyl cyclohexane is, more stable than the axial by 7.6 kJ/mol, , 71
Page 73 :
The axial substituent interferes with the axial, hydrogens on C1 and C3. This interference is called, a 1,3-diaxial interaction., , Hydrogen atoms of the axial methyl group on C1 are, too close to the axial hydrogens, three carbons away, on C3 and C5, resulting in 7.6 kJ/mol of steric strain, . Difference between axial and equatorial conformers, is due to steric strain caused by 1,3-diaxial, interactions, , 73
Page 74 :
Mono substituted Cyclohexane, Tert-butylcyclohexane, , Less than 0.01%, , Greater than 99.99%, , Substituents are less crowded in the equatorial, positions., 74
Page 75 :
Fluorocyclohexane, F, , F, 40%, , 60%, , Crowding is less pronounced with a "small", substituent such as fluorine., Size of substituent is related to its branching.
Page 76 :
• The larger the substituent on a cyclohexane ring, the, more the equatorial substituted conformer will be, favored, , Keq = [equatorial conformer]/[axial conformer]
Page 77 :
Substituted cyclohexanes:energy difference, Substituent, , H, OMe, Me, Et, iPr, tBu, 110, , Axial – equatorial energy, difference kJ mol-1, 0, 2.5, 7.3, 7.5, 9.3, >20, 11.7, , % equatorial, , 50, 73, 95, 95, 98, >99.9, 99, 77
Page 78 :
78
Page 79 :
Disubstitued Cycloalkanes, Can exist as pairs of cis-trans stereoisomers, – Cis: groups on same side of ring, – Trans: groups on opposite side of ring, , Chapter 4
Page 80 :
Cis-1,3-dimethylcyclohexane, , Cis-1,3-dimethylcyclohexane can have both methyl, groups in axial positions or both in equatorial positions., The conformation with both methyl groups being, equatorial is more stable. However, both conformations, 80, are equal in energy.
Page 81 :
81
Page 82 :
Trans-1,3-dimethylcyclohexane, , Both conformations have one axial and one equatorial, methyl group so they have the same energy., 82
Page 83 :
Methyl groups are on opposite faces of the ring, Steric strain of 4 3.8 kJ/mol = 15.2 kJ/mol makes the, diaxial conformation 11.4 kJ/mol less favorable than the, diequatorial conformation, trans-1,2-dimethylcyclohexane will exist almost, exclusively (>99%) in the diequatorial conformation, both methyl groups equatorial, •no 1,3-diaxial interactions, , •both methyl groups axial, • four 1,3-diaxial interactions
Page 89 :
Cis-1,4-ditertbutylcyclohexane, , The most stable conformation of cis-1,4-ditertbutylcyclohexane is the twist boat. Both chair, conformations require one of the bulky t-butyl groups, to occupy an axial position., 89
Page 94 :
94
Page 95 :
Cyclohexane Stereochemistry, Cis -Trans Isomers, Position, 1,2, , cis, , trans, , e,a or a,e e,e or a,a, , 1,3, , e,e or a,a, , a,e or e,a, , 1,4, , e,a or a,e, , e,e or a,a, , a = axial; e = equatorial
Page 96 :
Conformations of Polycyclic Molecules, Decalin consists of two cyclohexane rings joined to, share two carbon atoms (the bridgehead carbons, C1, and C6) and a common bond, Two isomeric forms of decalin: trans fused or cis fused, In cis-decalin hydrogen atoms at the bridgehead carbons, are on the same face of the rings, In trans-decalin, the bridgehead hydrogens are on, opposite faces, Both compounds can be represented using chair, cyclohexane conformations, Flips and rotations do not interconvert cis and trans
Page 97 :
97
Page 99 :
Problems, , 99
Page 100 :
• Is this the most stable conformer?
Page 101 :
Problem- 1, A. Draw both chair conformations of cis-1,2dimethylcyclohexane,, and determine which, conformer is more stable?, B. Repeat for the trans isomer., C. Predict which isomer (cis or trans) is more stable., , 101
Page 102 :
A. There are two possible chair conformations for the, cis isomer, and these two conformations interconvert, at room temperature. Each of these conformations, places one methyl group axial and one equatorial,, giving them the same energy., , 102
Page 103 :
B. There are two chair conformations of the trans isomer, , that interconvert at room temperature. Both methyl, groups are axial in one, and both are equatorial in the, other. The diequatorial conformation is more stable, because neither methyl group occupies the more, hindered axial position., , 103
Page 104 :
C. The trans isomer is more stable. The most stable, conformation of the trans isomer is diequatorial and, therefore about 7.6 kJ/mol (1.8 kcal/mol) lower in, energy than either conformation of the cis isomer,, each having one methyl axial and one equatorial., Remember that cis and trans are distinct isomers and, cannot interconvert., , 104