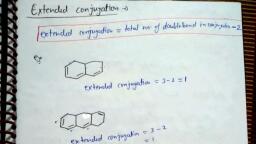
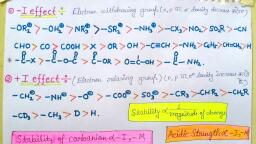Page 1 :
ee eer, , t Ga iN, Q Wer, , \, , , , ' Colloids, , , , INTRODUCTION, , e solutions, which are one-phase homogeneous, , So far we have discussed tru, (For example, when, , other extreme are the suspensions., , mixtures. On the, get a suspension.) On standing, , a handful of sand in water 1s stirred, we, a suspension settle down to give a ¢, es is an intermediate state called colloidal, mixture of a dispersed, (solvent). A colloidal, , the particles of learly heterogeneous, , mixture. Between these two extrem, dispersion, or simply colloid, which is a two-phase, phase (colloidal particles) and a dispersing medium, solution shows a different set of behaviour from that of a true solution., , Thomas Graham observed that solutions of some substances such as, , sugar and urea diffuse quite readily in water through animal and vegetable, membranes. On the other hand, solutions of substances such as starch and, gelatine did not diffuse readily. The former category- of substances was named, , as crystalloid while the latter, colloid’. But a sharp difference between the, , crystalloid and the colloid does not exist and so this classification is not, tenable. The process of separating a crystalloid from a colloid is known as, dialysis. The factors which contribute most to the overall behaviour of a, colloidal system are the particle's size, interactions between the particles, and also between the particle and the solvent, the scattering of visible light, by the colloidal particles and the charge on them., , Because of the lack of osmotic pressure of a colloidal solution, the, colloidal particles are recognised to be much larger than the normal solute, molecules. In fact, many small molecules hold together to form larger single, molecules called aggregates that have a much higher molar mass (ix 10*1 x 10° amu). The suspended colloidal particles may be large molecules (as, aggregates of molecules) or ions, ranging in size from 1 nm to 1000 nm., Note that the sizes of solute particles in true solutions range from 0.1 nm, , * The word ‘colloid’ was derived from the Greek word ‘kolla’, meaning glue., 421
Page 2 :
Fa nceiemnmi - ;, , , , 122 Modern Approach to Physical Chemistry Vol. 1, , to 1 nm and the particles larger than 1000 nm do not mix with the solvent, , to make solutions., , , , , , , , Particle size, , Ci aap, Colloidal dispersion Suspension, ee ta cae a cae, , 1 nm-1000 nm >1000 nm, , (starch in water) (sand in water), [a, , , , , , , , True solution, , , , , , 0.1 nm-1 nm, , , , (sugar in water), A nanometre, a man’s beard grow’, , (nm) is so small that it is difficult to imagine. As an, example, 5 about a nanometre in the time it takes to, raise a razor to his face!, , Based on the sizes of the particles in the range of 1 nm to 100 nm, a, new field of science is fast developing, called nanoscience. As the colloidal, particles in a solution behave in a different manner from those in a true, the nano-sized particles, or nanoparticles, in the form of nanoshells, and nanotubes behave altogether in a different way from the normal-sized, particles. The nanoparticles possess various properties. They have extremely, f which they may be used as efficient catalysts., ows them to penetrate very small spaces., penetrate capillaries to, ion. Carbon nanotubes, , solution,, , large surface area, because oO!, The tiny size of the nanoparticles all, Scientists are working on gold nanoshells which can, kill cancer cells after getting heated by infrared radiati, containing network of carbon atoms in interconnected six-membered rings, , are found to be 50 times stronger than steel wire at one-sixth the weight, and can conduct a thousand times more electric current than a copper wire., , However, the properties of nanoparticles also raise the possibility of unique, , types of toxicity., , Types of Colloids, ‘As said before, the colloids are a two-phase system. They can therefore be, classified according to the physical states of the dispersed phase and that, of the dispersion medium. The following table summarises various types, of colloids., , Table 9.1 Types of colloids, , , , , , , , Dispersing Dispersed Type Examples, medium phase, 1. Gas Liquid Aerosol Cloud, mist, fog, os Gas Solid Aerosol Smoke, gas from volcanic, eruptions, dust
Page 3 :
Colloids, , , , , , Dispersing Dispersed Type Examples, , medium phase, : 3. Liquid is Cas as Ponta 3 Whipped erearn soap iathecl, , 4. | Liquid Liquid Emulsion | Milk, cod-liver oil, mayonnaise, , 5. Liquid Solid Sol Starch, protein, arsenic, sulphide, milk of magnesia, , 6. Solid Gas Solid Plastic foams, pumice stone, , foam, Fs Solid Liquid Gel Gels, jelly, butter, cheese, 8. Solid Solid Solid sol | Coloured precious stones, rock, , salt, steal, ruby glass, , , , There are mainly eight different types of colloidal systems. We shall, mainly deal with those colloidal systems in which the dispersing medium, is liquid or gas and the dispersed phase is a solid. Such colloidal systems, are known as sols. If the dispersing medium is water or air, it is known as, hydrosol or aerosol respectively., , Lyophilic and Lyophobic Colloids, , The colloids are further classified on the basis of the interaction between, the dispersed phase and the dispersing medium, They are lyophilic and, lyophobic colloids. In lyophilic colloids, the substance readily passes into, colloidal state whenever mixed with a suitable solvent (solvent-loving) while, in lyophobic colloids, the substance does not readily pass into colloidal state, when mixed with a solvent (solvent-hating). When the solvent is water they, are known as hydrophilic and hydrophobic colloids respectively. Note that, in association colloids (discussed later), there is occurrence of both the, hydrophilic and hydrophobic ends in the same molecule., , The reasons why colloids do not settle down on standing are Brownian, movement, charge on the particles (for lyophobic colloids) and solvation of, particles (for lyophilic colloids). Table 9.2 shows the distinctions between, the lyophilic and lyophobic colloids,
Page 4 :
EEE, , Modern Approach to Physical Chemistry Vol. IT, , Lyophilic colloids, , sf hard, , Modern Approach to Physical Chemistry Vol. II, , , , , , Lyophobic colloids, , , , 1, These are usually prepared by a} 1. These are prepared by indirect, simple solution process by only methods, shaking the substance with the, solvent., , 2. Such colloids are mostly organic] 2. Such colloids are mostly, , substances (or subtances of, organic origin) such as starch,, proteins, glue, gelatine and high, polymers., , 3. These are stable, that is, not, easily coagulated (precipitated), by electrolytes., , 4. Coagulation is reversible., , 5. These can be stabilised mainly, by the forces of solvation., , 6. They protect lyophobic colloids, (gold number)., , 7. Such particles may or may not, migrate in an electric field. Each, , has an isoelectric point., , 8. Tyndall effect is less distinct., , 9. Viscosity is higher than that of, the solvent., , . Surface tension is generally, lower than that of the solvent., , 12. Such particles are invisible under, ultramicroscope., , , , inorganic substances such as, metal sulphides and oxides,, aqueous solution of silica, Agl,, etc., , . These are unstable, that is, easily, , coagulated by electrolysis., , . Coagulation is irreversible., , . These can be stabilised mainly, , by the charge on the particles., , . These are protected by lyophilic, , colloids., , . Such particles always migrate in, , an electric field., , . Tyndall effect is distinct., , Viscosity is about the same as, that of the solvent., , . Surface tension is similar to that, , of the solvent., , . Such particles are well-defined, , as spots under ultramicroscope, due to Tyndall effect., , Preparation of Colloidal Solutions, , , , As said before, the lyophilic colloids are prepared by a simple solution, process. However, the preparation of lyophobic colloids requires some anirest, methods. These methods are based on the principle of either disintegrating the, , a, , eee eRe ROAR core ee a Ne Sen aes renin Sn ROL NT Ot re
Page 5 :
os, , po, , Colloids 425, , , , , , , , , , , , , , , , , , , , , ubstance prese' cece:, Beuest a pre an in s Massive form to colloidal size (dispersion method), Sor ” ensing the smaiier ec} 5 : ., , or condensing the smaller molecules or ions in the true solution to colloidal, , § size (condensation method)., , * Dispersion method, , E In this method dispersion is carried out by different proc, electrical dispersion, mechanical dispersion and peptisation., , ses, such as, , , , In electrical dispersion process for preparing colloidal solutions of, metals like Au, Ag and Pt, a direct current (DC) electric are is struck between, the said metal electrodes immersed in water. The heat of the arc evaporates, the metal and the vapours are condensed in water to form metal colloidal, i particles. Traces of an electrolyte are usually necessary for stability., , In mechanical dispersion process many substances can be disintergrated, © into particles of colloidal size using a ‘colloid mill’, which consists of two, steel discs fixed very close to each other. The substance and the solvent are, - placed between the discs and the discs are moved mechanically in opposite, directions at a speed of 7000 revolutions per minute. This results in the, formation of a colloidal solution., , , , , Peptisation is a process of dispersing a solid, a coagulum (coagulated, substance) or a precipitate into a colloidal solution by adding a small amount, of an electrolyte. For example, when a trace of an electrolyte, FeCls, is added, to a freshly prepared precipitate, Fe(OH)3, a sol of Fe(OH) is obtained and, is stabilised by preferential adsorption of Fe** ions (from the peptising agent, = FeCl;) on the surface of the colloidal particles of Fe(OH)3- The positively, - charged colloidal particles, [Fe(OH) - Fe®*] repel each other and so do not, coalesce. Peptisation is the reverse process of coagulation. In coagulation,, | the colloidal solution, on the addition of an electrolyte, produces @ coagulum, | whereas in peptisation the electrolyte added transforms a coagulum back, jnto the colloidal form., , Condensation method, nsions. A colloidal, , The true solutions contain particles of molecular dime:, ticles of colloidal, , solution may be obtained by forming aggregates of pari, dimension from the molecules in a true solution. This can be achieved, by employing the chemical reactions such as reduction, oxidation, double, : decomposition, exchange of solvent, etc. In these processes the relative rates, of nucleation and growth must be controlled. i, , The reduction method involves the reduction of soluble salts, by reducing agents such as hydrogen, formaldehyde, hydrazine, hydrogen, etc. As an example, a gold sol is prepared by this method by, , peroxide, f, > reducing chlorauric acid, HAuCly, solution (containing a small amount 0 :, , of metals