Page 2 :
(with Clinical Concepts & Case Studies), , Dr. U. Satyanarayana, Dr. U. Chakrapani, , Co-published with
Page 3 :
SECTION, , 1
Page 4 :
(with Clinical Concepts & Case Studies), , Dr. U. Satyanarayana, M.Sc., Ph.D., F.I.C., F.A.C.B., , Professor of Biochemistry & Director (Research), Dr. Pinnamaneni Siddhartha Institute of Medical Sciences, (Dr. NTR University of Health Sciences), Chinaoutpalli, Gannavaram (Mdl), Krishna (Dist), A.P., India, , Dr. U. Chakrapani, M.B.B.S., M.S., D.N.B., , Co-published with, , ELSEVIER, , A division of Reed Elsevier India Pvt. Ltd., , Since 1960, Books & Allied Pvt. Ltd.
Page 5 :
Cjpdifnjtusz-!5f, Satyanarayana and Chakrapani, , ELSEVIER, A division of, Reed Elsevier India Private Limited, , Mosby, Saunders, Churchill Livingstone, Butterworth-Heinemann and, Hanley & Belfus are the Health Science imprints of Elsevier., , © 2013 Dr. U. Satyanarayana, First Published: March 1999, Revised Reprint: August 2000, Second Revised Edition: June 2002, Revised Reprint: 2004, 2005, Third Revised Edition (multicolour): 2006, Revised Reprint: 2007, 2010, Fourth Revised Edition: 2013, , All rights are reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by, any means, electronic, mechanical, photocopying, recording, or otherwise without the prior permission of the publishers., , ISBN: 978-81-312-3601-7, , Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment, and the use of drugs become necessary. The author, editors, contributors and the publisher have, as far as it is possible, taken care, to ensure that the information given in this text is accurate and up-to-date. However, readers are strongly advised to confirm that, the information, especially with regard to drug dose/usage, complies with current legislation and standards of practice. Please, consult full prescribing information before issuing prescriptions for any product mentioned in this publication., , This edition of Biochemistry, 4e by Dr. U. Satyanarayana and Dr. U. Chakrapani is co-published by an arrangement with, Elsevier, a division of Reed Elsevier India Private Limited and Books and Allied (P) Ltd., ELSEVIER, A division of Reed Elsevier India Private Limited., Registered Office: 305, Rohit House, 3 Tolstoy Marg, New Delhi-110 001., Corporate Office: 14th Floor, Building No. 10B, DLF Cyber City, Phase II, Gurgaon–122 002, Haryana, India., , BOOKS AND ALLIED (P) Ltd., Registered Office: 8/1 Chintamoni Das Lane, Kolkata 700009., Corporate Office: No. 1-E(1) ‘Shubham Plaza’ (1st Floor), 83/1, Beliaghata Main Road, Kolkata 700 010, West Bengal, India., , Cover Design, Depicts the universal energy currency of the living world—ATP, predominantly synthesized by the mitochondria of the cell, (the functional unit of life), in comparison with the international currencies—$, £, €, `, ¥., Printed and bound at ....., , Copyright.indd i, , 6/7/2013 4:31:26 PM
Page 6 : Preface to the Fourth Edition, , This book ‘Biochemistry’ has undoubtedly become one of the most preferred text books (in India and, many other countries) by the students as well as teachers in medical, biological and other allied sciences., It is certainly a book of choice and a true companion to all learning biochemistry, hence appropriately, regarded by many as ‘Bible of Biochemistry’. This book has undergone three editions, several reprints, and, revised reprints in a span of 13 years., The advances in biochemistry are evergrowing due to exponential growth of the subject. Further, the, critical comments, frank opinions and constructive suggestions by teachers and students need to be, seriously considered. All this necessitates frequent revision of the book., In this fourth edition, a thorough revision and update of each chapter with latest advances has been, done. The main emphasis of this edition is an improved orientation and treatment of human biochemistry, in health and disease. A wide variety of case studies with relevant biochemical profiles (along with diagnosis, and discussion) are newly added as an appendix. In addition, several newer aspects of biochemistry are, covered in this edition, some of them are listed below., l, l, l, l, l, l, l, l, l, l, l, l, l, , Triacylgylcerol/fatty acid cycle, Metabolic syndrome, Glucose toxicity, Estimated average glucose, Peptide nucleic acids, Pseudogenes, Recombinant ribozymes, , l, , Epigenetic regulation of gene expression, Metagenomics, Therapeutic diets, Atkins diet, Dietary antioxidants, High fructose corn syrups, , l, , l, l, l, l, l, l, , l, l, l, l, l, , ω-fatty acid, Soluble and insoluble fiber, Trans fatty acids, Nutrigenomics, Detailed information on antivitamins, Dental caries, Amino acids as neurotransmitters, Disorders of membrane transport, Diagnostic importance of various body fluids and tissues, Enzyme patterns in diseases, Cystatin C, Pleural fluid, High sensitive CRP, , It is a fact that I represent a selected group of individuals authoring books, having some time at, disposal, besides hard work, determination and dedication. I consider myself as an eternal reader and a, regular student of biochemistry. However, it is beyond my capability to keep track of the evergrowing, advances in biochemistry due to exponential growth of the subject. And, this makes me nervous whenever, I think of revising the book. I honestly and frankly admit that I have to depend on mature readers for, subsequent editions of this book., AN INVITATION TO READERS, WELL WISHERS AND SUBJECT EXPERTS, I have to admit that it is not all the time possible for me to meet the readers individually and get their, feedback. I sincerely invite the readers, my well wishers and experts in biochemistry subject to feel free and, write to me (Email ID:
[email protected]) expressing their frank opinions, critical comments and, constructive suggestions. And this will help me to further improve the book in subsequent revisions., , Dr. U. SATYANARAYANA
Page 7 :
Preface to the First Edition, , Biochemistry is perhaps the most fascinating subject as it deals with the chemical language of life, be it, human, animal, plant or microorganism. No other science subject has as much application as biochemistry to, the disciplines of medicine, health, veterinary, agriculture bioengineering and technology. This necessitates a, totally different outlook for the books on biochemistry subject., There are many biochemistry textbooks on the market. Some of them are purely basic while others are, applied, and there are very few books which cover both these aspects together. For this reason, the students, learning biochemistry in their undergraduate courses have to depend on multiple books to acquire a sound, knowledge of the subject., This book, ‘Biochemistry’ is unique with a simultaneous and equal emphasis on basic and applied aspects, of biochemistry. This textbook primarily is an integration of medical and pure sciences, comprehensively written, to meet the curriculum requirements of undergraduate courses in medical, dental, pharmacy, life-sciences and, other categories (agriculture, veterinary, etc.) where students learn biochemistry as one of the subjects., The tendency among the students (particularly medical) is to regard biochemistry as being mostly, concerned with unimportant and complicated metabolic (chemical) pathways. This book gives a new orientation to the subject of biochemistry so that the students appreciate the great importance and significance of, the application of biochemistry to medicine., This book is designed to develop in students a sustained interest and enthusiasm to learn and develop the, concepts in biochemistry in a logical and stepwise manner. It incorporates a variety of pedagogic aids, besides, colour illustrations to help the students understand the subject quickly and to the maximum. The summary, and biomedical/clinical concepts are intended for a rapid absorption and assimilation of the facts and concepts, in biochemistry. The self-assessment exercises will stimulate the students to think rather than merely learn, the subject. In addition, these exercises (essays, short notes, fill in the blanks, multiple choice questions) set, at different difficulty levels, will cater to the needs of all the categories of learners., It will not be out of place to mention here how-and when-the book was born. The entire book was written, in the early morning hours (between 2 AM-6 AM; when the world around is fast asleep), during which period, I carry out my intellectual activities. After a sound sleep, a fresh mind packed with creative ideas and innovative, thoughts, has largely helped me to write this book. My wife pleaded with me that I should not write topics like, diabetes, cancer, AIDS at home. In deference to her sentiment, I made a serious attempt to write those topics, during my leisure time in the Department. But when I went through them in my serene mood of the early, morning hours, I had to discard them in disappointment and rewrite them. Truly, each page of this book was, conceived in darkness and born at daybreak !, This textbook is a distillation of my knowledge and teaching experience in biochemistry, acquired during, the past 25 years. It contains predigested information on biochemistry for good understanding, assimilation, and reproducibility. Each page is crafted with a fine eye. The ultimate purpose of this book is to equip the, reader with comprehensive knowledge in biochemistry with reference to basic as well as applied aspects., Although I have made every effort to make the book error free, I am under no illusion. I welcome, comments, criticism and suggestions from the faculty, students and other readers, and this will help me make, improvements in the next edition., Dr. U. SATYANARAYANA, [ ii ]
Page 8 :
Acknowledgements, , I owe a deep debt of gratitude to my parents, the late Sri U. Venkata Subbaiah, and Smt. Vajramma, for, cultivating in me the habit of early rising. The writing of this book would never have been possible without, this healthy habit. I am grateful to Dr. B. S. Narasinga Rao (former Director, National Institute of Nutrition,, Hyderabad) for disciplining my professional life, and to my eldest brother Dr. U. Gudaru (former Professor of, Power Systems, Walchand College of Engineering, Sangli) for disciplining my personal life., My elder son, U. Chakrapani (MBBS) deserves a special place in this book. He made a significant, contribution at every stage of its preparation—writing, verification, proof-reading and what not. I had the rare, privilege of teaching my son as he happened to be a student of our college. And a major part of this book was, written while he was learning biochemistry. Thus, he was the first person to learn the subject of biochemistry, from my handwritten manuscript. The student-teacher relation (rather than the father-son) has helped me in, receiving constant feedback from him and restructure the book in a way an undergraduate student would, expect a biochemistry textbook to be., Next, I thank Dr. G. Pitcheswara Rao (former Professor of Anatomy, SMC, Vijayawada) for his constructive, criticism and advice, and Dr. B. Sivakumar (Director, National Institute of Nutrition, Hyderabad) for his helpful, suggestions on the microfigures., Last but not least, I thank my wife Krishna Kumari and my younger son, Amrutpani, without whose, cooperation and encouragement this book could never have been written. The manuscript was carefully, nurtured like a new born baby and the book has now become a full-pledged member of our family., ACKNOWLEDGEMENTS TO THE FOURTH EDITION, I am grateful to a large number of faculty members, students, friends and pen friends who directly or, indirectly helped me to revise and improve the content and quality of the book. I have individually and, personally thanked all of them (who number a few hundreds!). I once again express my gratitude to them., I thank Dr (Mrs) U.B. Vijaya Lakshmi, MD, Associate Professor of Biochemistry at our college who, participated to comprehensively prepare case studies with biochemical correlations, besides improving the, biomedical/ clinical aspects in some chapters. My special thanks goes to one student, and an ardent fan of my, books, Mr. Y. Nagendra Sastry (Ph.D), who has been studying my books regularly for over 7-8 years. His, constant feedback and suggestions have certainly contributed to improve this book. I express my gratitude to, Mr. M.S.T. Jagan Mohan (my former colleague), who has helped me with his frequent interactions to revise, the book, and make it more student-friendly., I express my sincere thanks to Mr Arunabha Sen, Director, Books & Allied (P) Ltd, Kolkata for his whole, hearted support and constant encouragement in revising the book, and taking all pains to bring it out to my, satisfaction. I thank Mr. Shyamal Bhattacharya for his excellent page making and graphics-work in the book., I am grateful to Mr. Abhijit Ghosal for his help in the cover design., I thank my wife, Krishna Kumari, my younger son Amrut Pani and my daughter-in law Oohasri for, their constant support and encouragement. My special thanks to my grand daughter Maahe (2 years) whose, ever smiling face, sweet words and deeds infuse energy into my academic activities. I am grateful to, Uppala Author-Publisher interlinks, Vijayawada for sponsoring and supporting me to bring out this edition., , Dr. U. SATYANARAYANA, [ iii ]
Page 9 :
Scope of Biochemistry, , The term Biochemistry was introduced by Carl Neuberg in 1903. Biochemistry broadly deals with the, chemistry of life and living processes. There is no exaggeration in the statement, ‘The scope of biochemistry, is as vast as life itself !’ Every aspect of life-birth, growth, reproduction, aging and death, involves biochemistry., For that matter, every movement of life is packed with hundreds of biochemical reactions. Biochemistry is the, most rapidly developing and most innovative subject in medicine. This becomes evident from the fact that over, the years, the major share of Nobel Prizes earmarked for Medicine and Physiology has gone to researchers, engaged in biochemistry., The discipline of biochemistry serves as a torch light to trace the intricate complexicities of biology,, besides unravelling the chemical mysteries of life. Biochemical research has amply demonstrated that all living, things are closely related at the molecular level. Thus biochemistry is the subject of unity in the diversified, living kingdom., Advances in biochemistry have tremendous impact on human welfare, and have largely benefited mankind, and their living styles. These include the application of biochemistry in the laboratory for the diagnosis of, diseases, the products (insulin, interferon, growth hormone etc.) obtained from genetic engineering, and the, possible use of gene therapy in the near future., Organization of the Book, This textbook, comprising 43 chapters, is organized into seven sections in the heirarchical order of, learning biochemistry., l, , l, , l, l, , l, , l, , l, , Section I deals with the chemical constituents of life—carbohydrates, lipids, proteins and amino acids,, nucleic acids and enzymes., Section II physiological chemistry includes digestion and absorption, plasma proteins, hemoglobin and, prophyrins, and biological oxidation., Section III incorporates all the metabolisms (carbohydrates, lipids, amino acids, nucleotides, minerals), Section IV covers hormones, organ function tests, water, electrolyte and acid-base balance, tissue proteins, and body fluids, and nutrition., Section V is exclusively devoted to molecular biology and biotechnology (DNA-replication, recombination,, and repair, transcription and translation, regulation of gene expression, recombinant DNA and biotechnology), Section VI gives relevant information on current topics such as human genome project, gene therapy,, bioinformatics, prostaglandins, diabetes, cancer, AIDS etc., Section VII deals with the basic aspects for learning and understanding biochemistry (bioorganic, chemistry, biophysical chemistry, tools of biochemistry, genetics, immunology)., , Each chapter in this book is carefully crafted with colour illustrations, headings and subheadings to, facilitate quick understanding. The important applications of biochemistry to human health and disease are put, together as biomedical/clinical concepts. Icons are used at appropriate places to serve as ‘landmarks’., The origins of biochemical words, confusables in biochemistry, practical biochemistry and clinical, biochemistry laboratory, case studies with biochemical correlations, given in the appendix are novel features., The book is so organized as to equip the readers with a comprehensive knowledge of biochemistry., [ iv ]
Page 10 :
Contents, , SECTION ONE, , SECTION, , Chemical Constituents of Life, , Molecular Biology and Biotechnology, 24 ➤ DNA-replication, recombination and repair, 25 ➤ Transcription and translation, 26 ➤ Regulation of gene expression, 27 ➤ Recombinant DNA and biotechnology, , 1, 2, 3, 4, 5, 6, 7, , ➤ Biomolecules and the cell, ➤ Carbohydrates, , 3, 9, , ➤ Lipids, , 28, , ➤ Proteins and amino acids, , 43, , ➤ Nucleic acids and nucleotides, , 69, , SECTION, , ➤ Enzymes, , 85, , ➤ Vitamins, , 116, , Current, 28 ➤, 29 ➤, 30 ➤, 31 ➤, 32 ➤, 33 ➤, 34 ➤, 35 ➤, 36 ➤, , SECTION, , TWO, , Physiological Biochemistry, 8, , ➤ Digestion and absorption, , 165, , 9, , ➤ Plasma proteins, , 182, , 10, , ➤ Hemoglobin and porphyrins, , 196, , 11, , ➤ Biological oxidation, , 221, , SECTION, , THREE, , Metabolisms, 12, , ➤ Introduction to metabolism, , 241, , 13, , ➤ Metabolism of carbohydrates, , 244, , 14, , ➤ Metabolism of lipids, , 285, , 15, , ➤ Metabolism of amino acids, , 330, , 16, , ➤ Integration of metabolism, , 380, , 17, , ➤ Metabolism of nucleotides, , 387, , 18, , ➤ Mineral metabolism, , 403, , Human genome project, Gene therapy, Bioinformatics, Metabolism of xenobiotics (detoxification), Prostaglandins and related compounds, Biological membranes and transport, Free radicals and antioxidants, Environmental biochemistry, Insulin, glucose homeostasis,, and diabetes mellitus, 37 ➤ Cancer, 38 ➤ Acquired immunodeficiency, syndrome (AIDS), , 453, , Water, electrolyte and, acid-base balance, , 468, , 695, , 32, 33, , 745, 751, 756, 759, 763, 769, 772, 779, , INDEX, , Organ function tests, , 669, 685, , 703, 708, 719, 732, 737, , 502, , 427, , 619, 625, 634, 638, 644, 650, 655, 662, , Basics to Learn Biochemistry, 39 ➤ Introduction to bioorganic chemistry, 40 ➤ Overview of biophysical chemistry, 41 ➤ Tools of biochemistry, 42 ➤ Immunology, 43 ➤ Genetics, , ➤ Nutrition, , Hormones, , 578, , SECTION SEVEN, , 487, , FOUR, , Biochemistry and Nutrition, , 566, , Topics, , ➤ Tissue proteins and body fluids, , SECTION, , 523, 542, , SIX, , APPENDICES, Answers to Self-assessment Exercises, I Abbreviations used in this book, II Origins of important biochemical words, III Common confusables in biochemistry, IV Practical biochemistry—principles, V Clinical biochemistry laboratory, VI Case studies with biochemical correlations, , Clinical, 19 ➤, 20 ➤, 21 ➤, 22, 23, , FIVE, , 34, 35, 36, 37, 38
Page 11 :
CHEMICAL CONSTITUENTS OF LIF, LIFEE, 1, ■, 2, ■, 3, ■, 4, ■, 5, ■, 6, ■, 7, ■, , Biomolecules and the Cell, , 3, , Carbohydrates, , 9, , Lipids, , 28, , Proteins and Amino acids, , 43, , Nucleic acids and Nucleotides 69, Enzymes, , 85, , Vitamins, , 116, , Section, , I
Page 12 :
“This page intentionally left blank"
Page 13 :
Section 1, , Chemical Constituents of Life, , Chapter, , Biomolecules and the Cell, , 1, , The cell speaks :, , “I am the unit of biological activity;, Organized into subcellular organelles;, Assigned to each are specific duties;, Thus, I truly represent life!”, , T, , organic compounds. It is believed that man may, contain about 100,000 different types of, molecules although only a few of them have, been characterized., , he living matter is composed of mainly, six elements—carbon, hydrogen, oxygen,, nitrogen, phosphorus and sulfur. These elements, together constitute about 90% of the dry weight, of the human body. Several other functionally, important elements are also found in the cells., These include Ca, K, Na, Cl, Mg, Fe, Cu, Co, I,, Zn, F, Mo and Se., , Complex biomolecules, The organic compounds such as amino acids,, nucleotides and monosaccharides serve as the, monomeric units or building blocks of complex, biomolecules—proteins, nucleic acids (DNA and, RNA) and polysaccharides, respectively. The, important biomolecules (macromolecules) with, their respective building blocks and major, functions are given in Table 1.1. As regards, lipids, it may be noted that they are not, biopolymers in a strict sense, but majority of, them contain fatty acids., , Carbon—a unique element of life, Carbon is the most predominant and versatile, element of life. It possesses a unique property to, form infinite number of compounds. This is, attributed to the ability of carbon to form stable, covalent bonds and C C chains of unlimited, length. It is estimated that about 90% of, compounds found in living system invariably, contain carbon., , Structural heirarchy of an organism, The macromolecules (proteins, lipids, nucleic, acids and polysaccharides) form supramolecular, assemblies (e.g. membranes) which in turn, organize into organelles, cells, tissues, organs, and finally the whole organism., , Chemical molecules of life, Life is composed of lifeless chemical, molecules. A single cell of the bacterium,, Escherichia coli contains about 6,000 different, , 3
Page 14 :
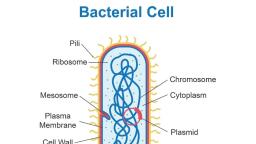
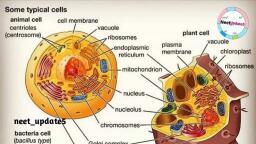
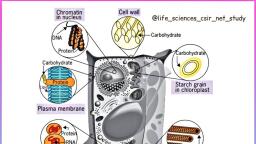
4, , BIOCHEMISTRY, , TABLE 1.1 The major complex biomolecules of cells, , Biomolecule, , Building block, (repeating unit), , Major functions, , 1. Protein, , Amino acids, , Fundamental basis of structure and, function of cell (static and dynamic functions)., , 2. Deoxyribonucleic acid (DNA), , Deoxyribonucleotides, , Repository of hereditary information., , 3. Ribonucleic acid (RNA), , Ribonucleotides, , Essentially required for protein biosynthesis., , 4. Polysaccharide (glycogen), , Monosaccharides (glucose), , Storage form of energy to meet short term, demands., , 5. Lipid, , Fatty acids, glycerol, , Storage form of energy to meet long term, demands; structural components of membranes., , Chemical composition of man, , Prokaryotic and eukaryotic cells, , The chemical composition of a normal man,, weighing 65 kg, is given in Table 1.2. Water is, the solvent of life and contributes to more than, 60% of the weight. This is followed by protein, (mostly in muscle) and lipid (mostly in adipose, tissue). The carbohydrate content is rather low, which is in the form of glycogen., , The cells of the living kingdom may be, divided into two categories, , THE CELL, The cell is the structural and functional unit, of life. It may be also regarded as the basic unit, of biological activity., The concept of cell originated from the, contributions of Schleiden and Schwann (1838)., However, it was only after 1940, the, complexities of cell structure were exposed., , TABLE 1.2 Chemical composition of a normal man, (weight 65 kg), , Constituent, , Percent (%), , Weight (kg), , Water, , 61.6, , 40, , Protein, , 17.0, , 11, , Lipid, , 13.8, , 9, , Carbohydrate, , 1.5, , 1, , Minerals, , 6.1, , 4, , 1. Prokaryotes (Greek : pro – before; karyon –, nucleus) lack a well defined nucleus and possess, relatively simple structure. These include the, various bacteria., 2. Eukaryotes (Greek : eu – true; karyon –, nucleus) possess a well defined nucleus and are, more complex in their structure and function., The higher organisms (animals and plants) are, composed of eukaryotic cells., A comparison of the characteristics between, prokaryotes and eukaryotes is listed in Table 1.3., , EUKARYOTIC CELL, The human body is composed of about 1014, cells. There are about 250 types of specialized, cells in the human body e.g. erythrocytes,, nerve cells, muscle cells, E cells of pancreas., An eukaryotic cell is generally 10 to 100 Pm, in diameter. A diagrammatic representation, of a typical rat liver cell is depicted in, Fig.1.1., The plant cell differs from an animal cell by, possessing a rigid cell wall (mostly composed of, cellulose) and chloroplasts. The latter are the, sites of photosynthesis.
Page 15 :
5, , Chapter 1 : BIOMOLECULES AND THE CELL, , TABLE 1.3 Comparison between prokaryotic and eukaryotic cells, , Characteristic, , Prokaryotic cell, , Eukaryotic cell, , Small (generally 1-10 Pm), , Large (generally 10-100 Pm), , 2. Cell membrane, , Cell is enveloped by a rigid cell wall, , Cell is enveloped by a flexible plasma membrane, , 3. Sub-cellular, organelles, , Absent, , Distinct organelles are found, (e.g. mitochondria, nucleus, lysosomes), , 4. Nucleus, , Not well defined; DNA is found, as nucleoid, histones are absent, , Nucleus is well defined, surrounded by a, membrane; DNA is associated with histones, , 5. Energy metabolism, , Mitochondria absent, enzymes of, energy metabolism bound to, membrane, , Enzymes of energy metabolism are located, in mitochondria, , 6. Cell division, , Usually fission and no mitosis, , Mitosis, , 7. Cytoplasm, , Organelles and cytoskeleton, absent, , Contains organelles and cytoskeleton, (a network of tubules and filaments), , 1. Size, , The cell consists of well defined subcellular, organelles, enveloped by a plasma membrane., By, differential, centrifugation, of, tissue, homogenate, it is possible to isolate each, cellular organelle in a relatively pure form, (Refer Chapter 41). The distribution of major, enzymes and metabolic pathways in different, cellular organelles is given in the chapter, on enzymes (Refer Fig.6.6). The subcellular, organelles are briefly described in the following, pages., , Nucleus, Nucleus is the largest cellular organelle,, surrounded by a double membrane nuclear, envelope. The outer membrane is continuous, with the membranes of endoplasmic reticulum., At certain intervals, the two nuclear membranes, have nuclear pores with a diameter of about 90, nm. These pores permit the free passage of the, products synthesized in the nucleus into the, surrounding cytoplasm., , Mitochondrion, Plasma membrane, Vacuole, , Rough endoplasmic reticulum, , Ribosomes, , Golgi apparatus, , Nucleus, Nucleolus, Smooth endoplasmic reticulum, , Lysosome, , Peroxisome, Cytoskeleton, Cytosol, Coated pits, , Fig. 1.1 : Diagrammatic representation of a rat liver cell.
Page 16 :
6, Nucleus contains DNA, the repository of, genetic information. Eukaryotic DNA is, associated with basic protein (histones) in the, ratio of 1 : 1, to form nucleosomes. An assembly, of nucleosomes constitutes chromatin fibres of, chromosomes (Greek: chroma – colour; soma –, body). Thus, a single human chromosome is, composed of about a million nucleosomes. The, number of chromosomes is a characteristic, feature of the species. Humans have 46, chromosomes, compactly packed in the nucleus., The nucleus of the eukaryotic cell contains a, dense body known as nucleolus. It is rich in, RNA, particularly the ribosomal RNA which, enters the cytosol through nuclear pores., The ground material of the nucleus is often, referred to as nucleoplasm. It is rich in enzymes, such as DNA polymerases and RNA polymerases., , Hutchinson-Gilford, progeria, syndrome, (HGPS) is a rare condition of aging beginning at, birth (incidence I in 5 million births). HGPS, occurs as a result of distortion of nuclear, envelope due to accumulation of abnormal, protein namely lamina A., , Mitochondria, The mitochondria (Greek: mitos – thread;, chondros – granule) are the centres for the, cellular respiration and energy metabolism. They, are regarded as the power houses of the cell, with variable size and shape. Mitochondria are, rod-like or filamentous bodies, usually with, dimensions of 1.0 u 3 Pm. About 2,000, mitochondria, occupying about 1/5th of the total, cell volume, are present in a typical cell., The mitochondria are composed of a double, membrane system (Refer Fig.11.5). The outer, membrane is smooth and completely envelops, the organelle. The inner membrane is folded to, form cristae (Latin – crests) which occupy a, larger surface area. The internal chamber of, mitochondria is referred to as matrix or mitosol., The components of electron transport chain, and oxidative phosphorylation (flavoprotein,, cytochromes b, c1, c, a and a3 and coupling, factors) are buried in the inner mitochondrial, membrane. The matrix contains several enzymes, concerned with the energy metabolism of, , BIOCHEMISTRY, , carbohydrates, lipids and amino acids (e.g., citric, acid cycle, E-oxidation). The matrix enzymes, also participate in the synthesis of heme and, urea. Mitochondria are the principal producers, of ATP in the aerobic cells. ATP, the energy, currency, generated in mitochondria is exported, to all parts of the cell to provide energy for the, cellular work., The mitochondrial matrix contains a circular, double stranded DNA (mtDNA), RNA and, ribosomes. Thus, the mitochondria are equipped, with an independent protein synthesizing, machinery. It is estimated that about 10% of the, mitochondrial proteins are produced in the, mitochondria., The structure and functions of mitochondria, closely resemble prokaryotic cells. It is, hypothesized that mitochondria have evolved, from aerobic bacteria. Further, it is believed that, during evolution, the aerobic bacteria developed, a symbiotic relationship with primordial, anaerobic eukaryotic cells that ultimately led to, the arrival of aerobic eukaryotes., , Endoplasmic reticulum, The network of membrane enclosed spaces, that extends throughout the cytoplasm, constitutes endoplasmic reticulum (ER). Some of, these thread-like structures extend from the, nuclear pores to the plasma membrane., A large portion of the ER is studded with, ribosomes to give a granular appearance which, is referred to as rough endoplasmic reticulum., Ribosomes are the factories of protein, biosynthesis. During the process of cell, fractionation, rough ER is disrupted to form small, vesicles known as microsomes. It may be noted, that microsomes as such do not occur in the cell., The smooth endoplasmic reticulum does not, contain ribosomes. It is involved in the synthesis, of lipids (triacylglycerols, phospholipids, sterols), and metabolism of drugs, besides supplying Ca2+, for the cellular functions., , Golgi apparatus, Eukaryotic cells contain a unique cluster of, membrane vesicles known as dictyosomes
Page 17 :
7, , Chapter 1 : BIOMOLECULES AND THE CELL, , which, in turn, constitute Golgi apparatus (or, Golgi complex). The newly synthesized proteins, are handed over to the Golgi apparatus which, catalyse the addition of carbohydrates, lipids or, sulfate moieties to the proteins. These chemical, modifications are necessary for the transport of, proteins across the plasma membrane., Certain proteins and enzymes are enclosed in, membrane vesicles of Golgi apparatus and, secreted from the cell after the appropriate, signals. The digestive enzymes of pancreas are, produced in this fashion., Golgi apparatus are also involved in the, membrane synthesis, particularly for the, formation of intracellular organelles (e.g., peroxisomes, lysosomes)., , Lysosomes, Lysosomes are spherical vesicles enveloped, by a single membrane. Lysosomes are regarded, as the digestive tract of the cell, since they are, actively involved in digestion of cellular, substances—namely proteins, lipids, carbohydrates and nucleic acids. Lysosomal enzymes, are categorized as hydrolases. These include, the enzymes (with substrate in brackets)—, D-glucosidase (glycogen), cathepsins (proteins),, lipases (lipids), ribonucleases (RNA)., The lysosomal enzymes are responsible for, maintaining the cellular compounds in a dynamic, state, by their degradation and recycling. The, degraded products leave the lysosomes, usually, , by diffusion, for reutilization by the cell., Sometimes, however, certain residual products,, rich in lipids and proteins, collectively known as, lipofuscin accumulate in the cell. Lipofuscin is, the age pigment or wear and tear pigment which, has been implicated in ageing process. As the cell, dies, the lysosomes rupture and release hydrolytic, enzymes that results in post-morteum autolysis., The digestive enzymes of cellular compounds, are confined to the lysosomes in the best interest, of the cell. Escape of these enzymes into cytosol, will destroy the functional macromolecules of the, cell and result in many complications. The, occurrence of several diseases (e.g. arthritis,, muscle diseases, allergic disorders) has been partly, attributed to the release of lysosomal enzymes., , Inclusion cell (I-cell) desease is a rare, condition due to the absence of certain hydrolases, in lysosomes. However, these enzyme are, syntherized and found in the circulation. I-cell, disease is due to a defect in protein targetting, as, the enzymes cannot reach lysosomes., , Peroxisomes, Peroxisomes, also known as microbodies, are, single membrane cellular organelles. They are, spherical or oval in shape and contain the, enzyme catalase. Catalase protects the cell from, the toxic effects of H2O2 by converting it to H2O, and O2. Peroxisomes are also involved in the, oxidation of long chain fatty acids (> C18), and, synthesis of plasmalogens and glycolipids. Plants, contain glyoxysomes, a specialized type of, , + A living cell is a true representative of life with its own organization and specialized, functions., , + Accumulation of lipofuscin, a pigment rich in lipids and proteins, in the cell has been, implicated in ageing process., , + Leakage of lysosomal enzymes into the cell degrades several functional macromolecules, and this may lead to certain disorders (e.g. arthritis)., , + Zellweger syndrome is a rare disease characterized by the absence of functional, peroxisomes.
Page 18 :
8, , BIOCHEMISTRY, , peroxisomes, which, glyoxylate pathway., , are, , involved, , in, , the, , Peroxisome biogenesis disorders (PBDs), are, a group of rare diseases involving the enzyme, activities of peroxisomes. The biochemical, abnormalities associated with PBDs include, increased levels of very long chain fatty acids, (C24 and C26) and decreased concentrations of, plasmalogens. The most severe form of PBDs is, Zellweger syndrome, a condition characterized, by the absence of functional peroxisomes. The, victims of this disease may die within one year, after birth., , Cytosol and cytoskeleton, The cellular matrix is collectively referred to, as cytosol. Cytosol is basically a compartment, containing several enzymes, metabolites and, salts in an aqueous gel like medium. More recent, studies however, indicate that the cytoplasm, actually contains a complex network of protein, filaments, spread throughout, that constitutes, cytoskeleton. The cytoplasmic filaments are of, , three types – microtubules, actin filaments and, intermediate filaments. The filaments which are, polymers of proteins are responsible for the, structure, shape and organization of the cell., , INTEGRATION OF, CELLULAR FUNCTIONS, The eukaryotic cells perform a wide range of, complex reactions/functions to maintain tissues,, and for the ultimate well-being of the whole, organism. For this purpose, the various, intracellular processes and biochemical reactions, are tightly controlled and integrated. Division of, a cell into two daughter cells is good example of, the orderly occurrence of an integrated series of, cellular reactions., , Apoptosis is the programmed cell death or, cell suicide. This occurs when the cell has, fulfilled its biological functions. Apoptosis may, be regarded as a natural cell death and it differs, from the cell death caused by injury due to, radiation, anoxia etc. Programmed cell death is, a highly regulated process., , 1. Life is composed of lifeless chemical molecules. The complex biomolecules, proteins,, nucleic acids (DNA and RNA), polysaccharides and lipids are formed by the monomeric, units amino acids, nucleotides, monosaccharides and fatty acids, respectively., 2. The cell is the structural and functional unit of life. The eukaryotic cell consists of well, defined subcellular organelles, enveloped in a plasma membrane., 3. The nucleus contains DNA, the repository of genetic information. DNA, in association, with proteins (histones), forms nucleosomes which, in turn, make up the chromosomes., 4. The mitochondria are the centres for energy metabolism. They are the principal producers, of ATP which is exported to all parts of the cell to provide energy for cellular work., 5. Endoplasmic reticulum (ER) is the network of membrane enclosed spaces that extends, throughout the cytoplasm. ER studded with ribosomes, the factories of protein, biosynthesis, is referred to as rough ER. Golgi apparatus are a cluster of membrane, vesicles to which the newly synthesized proteins are handed over for further processing, and export., 6. Lysosomes are the digestive bodies of the cell, actively involved in the degradation of, cellular compounds. Peroxisomes contain the enzyme catalase that protects the cell from, the toxic effects of H2O2. The cellular ground matrix is referred to as cytosol which, in, fact, is composed of a network of protein filaments, the cytoskeleton., 7. The eukaryotic cells perform a wide range of complex functions in a well coordinated and, integrated fashion. Apoptosis is the process of programmed cell death or cell suicide.
Page 19 :
Section 1, , Chemical Constituents of Life, , Chapter, , Carbohydrates, , 12, , The carbohydrates speak :, , “We are polyhydroxyaldehydes or ketones;, Classified into mono-, oligo- and polysaccharides;, Held together by glycosidic bonds;, Supply energy and serve as structural constituents.”, , 1. They are the most abundant dietary source, of energy (4 Cal/g) for all organisms., , arbohydrates are the most abundant organic, molecules in nature. They are primarily, composed of the elements carbon, hydrogen and, oxygen. The name carbohydrate literally means, ‘hydrates of carbon’. Some of the carbohydrates, possess the empirical formula (C.H2O)n where, n d 3, satisfying that these carbohydrates are in, fact carbon hydrates. However, there are several, non-carbohydrate compounds (e.g. acetic acid,, C2H4O2; lactic acid, C3H6O3) which also appear, as hydrates of carbon. Further, some of the, genuine carbohydrates (e.g. rhamnohexose,, C6H12O5; deoxyribose, C5H10O4) do not satisfy, the general formula. Hence carbohydrates cannot, be always considered as hydrates of carbon., , C, , 2. Carbohydrates are precursors for many, organic compounds (fats, amino acids)., 3. Carbohydrates (as glycoproteins and glycolipids) participate in the structure of cell, membrane and cellular functions such as cell, growth, adhesion and fertilization., 4. They are structural components of many, organisms. These include the fiber (cellulose) of, plants, exoskeleton of some insects and the cell, wall of microorganisms., 5. Carbohydrates also serve as the storage, form of energy (glycogen) to meet the immediate, energy demands of the body., , Carbohydrates, may, be, defined, as, polyhydroxyaldehydes or ketones or compounds, which produce them on hydrolysis. The term, ‘sugar’ is applied to carbohydrates soluble in, water and sweet to taste., , CLASSIFICATION, OF CARBOHYDRATES, Carbohydrates are often referred to as, saccharides (Greek: sakcharon–sugar). They, are broadly classified into three major groups—, monosaccharides, oligosaccharides and polysaccharides. This categorization is based on the, , Functions of carbohydrates, Carbohydrates participate in a wide range of, functions, , 9
Page 20 :
10, , BIOCHEMISTRY, , TABLE 2.1 Classification of monosaccharides with selected examples, , Monosaccharides (empirical formula), , Aldose, , Ketose, , Trioses (C3H6O3), , Glyceraldehyde, , Dihydroxyacetone, , Tetroses (C4H8O4), , Erythrose, , Erythrulose, , Pentoses (C5H10O5), , Ribose, , Ribulose, , Hexoses (C6H12O6), , Glucose, , Fructose, , Heptoses (C7H14O7), , Glucoheptose, , Sedoheptulose, , number of sugar units. Mono- and oligosaccharides are sweet to taste, crystalline in, character and soluble in water, hence they are, commonly known as sugars., , liberated on hydrolysis. Based on the number of, monosaccharide units present, the oligosaccharides, are, further, subdivided, to, disaccharides, trisaccharides etc., , Monosaccharides, , Polysaccharides, , Monosaccharides (Greek : mono-one) are the, simplest group of carbohydrates and are often, referred to as simple sugars. They have the, general formula Cn(H2O)n, and they cannot be, further hydrolysed. The monosaccharides are, divided into different categories, based on the, functional group and the number of carbon atoms, , Polysaccharides (Greek: poly-many) are polymers of monosaccharide units with high molecular weight (up to a million). They are usually, tasteless (non-sugars) and form colloids with, water. The polysaccharides are of two types –, homopolysaccharides and heteropolysaccharides., , Aldoses : When the functional group in, H, , monosaccharides is an aldehyde, , C O , they, , are known as aldoses e.g. glyceraldehyde,, glucose., Ketoses : When the functional group is a keto, , C O group, they are referred to as ketoses, e.g. dihydroxyacetone, fructose., Based on the number of carbon atoms, the, monosaccharides are regarded as trioses (3C),, tetroses (4C), pentoses (5C), hexoses (6C) and, heptoses (7C). These terms along with functional, groups are used while naming monosaccharides., For instance, glucose is an aldohexose while, fructose is a ketohexose (Table 2.1)., The common monosaccharides and disaccharides of biological importance are given in the, Table 2.2., , Oligosaccharides, Oligosaccharides (Greek: oligo-few) contain, 2-10 monosaccharide molecules which are, , MONOSACCHARIDES—, STRUCTURAL ASPECTS, Stereoisomerism is an important character of, monosaccharides., Stereoisomers, are, the, compounds that have the same structural, formulae but differ in their spatial configuration., A carbon is said to be asymmetric when it is, attached to four different atoms or groups. The, number of asymmetric carbon atoms (n), determines the possible isomers of a given, compound which is equal to 2n. Glucose, contains 4 asymmetric carbons, and thus has 16, isomers., , Glyceraldehyde, —the reference carbohydrate, Glyceraldehyde (triose) is the simplest monosaccharide with one asymmetric carbon atom. It, exists as two stereoisomers and has been chosen, as the reference carbohydrate to represent the, structure of all other carbohydrates.
Page 21 :
11, , Chapter 2 : CARBOHYDRATES, , TABLE 2.2 Monosaccharides and disaccharides of biological importance, , Monosaccharides, , Occurrence, , Biochemical importance, , Trioses, Glyceraldehyde, , Found in cells as phosphate, , Glyceraldehyde 3-phosphate is an intermediate, in glycolysis, , Dihydroxyacetone, , Found in cells as phosphate, , Its 1-phosphate is an intermediate in glycolysis, , Widespread, , Its 4-phosphate is an intermediate in, carbohydrate metabolism, , Widespread as a constituent of, RNA and nucleotides, , For the structure of RNA and nucleotide, coenzymes (ATP, NAD+, NADP+), , Tetroses, D-Erythrose, Pentoses, D-Ribose, D-Deoxyribose, , As a constituent of DNA, , For the structure of DNA, , D-Ribulose, , Produced during metabolism, , It is an important metabolite in hexose, monophosphate shunt, , D-Xylose, , As a constituent of glycoproteins, and gums, , Involved in the function of glycoproteins, , L-Xylulose, , As an intermediate in uronic acid pathway, , Excreted in urine in essential pentosuria, , D-Lyxose, , Heart muscle, , As a constituent of lyxoflavin of heart muscle, , D-Glucose, , As a constituent of polysaccharides, (starch, glycogen, cellulose) and, disaccharides (maltose, lactose,, sucrose). Also found in fruits, , The ‘sugar fuel’ of life; excreted in urine in, diabetes. Structural unit of cellulose in plants, , D-Galactose, , As a constituent of lactose, (milk sugar), , Converted to glucose, failure leads to, galactosemia, , D-Mannose, , Found in plant polysaccharides, and animal glycoproteins, , For the structure of polysaccharides, , D-Fructose, , Fruits and honey, as a constituent, of sucrose and inulin, , Its phosphates are intermediates of glycolysis, , Found in plants, , Its 7-phosphate is an intermediate in hexose, monophosphate shunt, and in photosynthesis, , Hexoses, , Heptoses, D-Sedoheptulose, , Disaccharides, , Occurrence, , Biochemical importance, , Sucrose, , As a constituent of cane sugar and, beet sugar, pineapple, , Most commonly used table sugar supplying, calories, , Lactose, , Milk sugar, , Exclusive carbohydrate source to breast fed, infants. Lactase deficiency (lactose intolerance), leads to diarrhea and flatulence, , Maltose, , Product of starch hydrolysis,, occurs in germinating seeds, , An important intermediate in the digestion of, starch
Page 22 :
12, , BIOCHEMISTRY, , H C O, H C OH, , H C O, HO C H, , CH2OH, D-Glyceraldehyde, , H C O, H C OH, HO C H, , CH2OH, L-Glyceraldehyde, , H C O, HO C H, H C OH, , H C OH, , HO C H, , H C OH, , HO C H, , CH2OH, D-Glucose, , CH2OH, L-Glucose, , Fig. 2.1 : D-and-L- forms of glucose compared with, D- and L- glyceraldehydes (the reference carbohydrate)., , D- and L-isomers, The D and L isomers are mirror images of, each other. The spatial orientation of H and, OH groups on the carbon atom (C5 for, glucose) that is adjacent to the terminal primary, alcohol carbon determines whether the sugar is, D- or L-isomer. If the OH group is on the right, side, the sugar is of D-series, and if on the left, side, it belongs to L-series. The structures of, D- and L-glucose based on the reference monosaccharide, D- and L-glyceraldehyde (glycerose), are depicted in Fig.2.1., It may be noted that the naturally occurring, monosaccharides in the mammalian tissues are, mostly of D-configuration. The enzyme machinery, of cells is specific to metabolise D-series of, monosaccharides., , relation with glyceraldehyde. It may be noted, that the D- and L-configurations of sugars are, primarily, based, on, the, structure, of, glyceraldehyde, the optical activities however,, may be different., Racemic mixture : If d- and l-isomers are, present in equal concentration, it is known as, racemic mixture or dl mixture. Racemic mixture, does not exhibit any optical activity, since the, dextro- and levorotatory activities cancel each, other., In the medical practice, the term dextrose is, used for glucose in solution. This is because of, the dextrorotatory nature of glucose., , Configuration of D-aldoses, The configuration of possible D-aldoses, starting from D-glyceraldehyde is depicted in, Fig.2.2. This is a representation of KillianiFischer synthesis, by increasing the chain length, of an aldose, by one carbon at a time. Thus,, starting with an aldotriose (3C), aldotetroses (4C),, aldopentoses (5C) and aldohexoses (6C) are, formed. Of the 8 aldohexoses, glucose, mannose, and galactose are the most familiar. Among, these, D-glucose is the only aldose monosaccharide that predominantly occurs in, nature., , Configuration of D-ketoses, Starting from dihydroxyacetone (triose), there, are five keto-sugars which are physiologically, important. Their structures are given in Fig.2.3., , Epimers, Optical activity of sugars, Optical activity is a characteristic feature of, compounds with asymmetric carbon atom., When a beam of polarized light is passed, through a solution of an optical isomer, it will be, rotated either to the right or left. The term, dextrorotatory (d+) and levorotatory (l–) are, used to compounds that respectively rotate the, plane of polarized light to the right or to the left., , If two monosaccharides differ from each, other in their configuration around a single, specific carbon (other than anomeric) atom, they, are referred to as epimers to each other (Fig.2.4)., For instance, glucose and galactose are epimers, with regard to carbon 4 (C4-epimers). That is,, they differ in the arrangement of OH group at, C4. Glucose and mannose are epimers with, regard to carbon 2 (C2-epimers)., , An optical isomer may be designated as, D(+), D(–), L(+) and L(–) based on its structural, , The interconversion of epimers (e.g. glucose, to galactose and vice versa) is known as
Page 24 :
14, , BIOCHEMISTRY, , CH2OH, CH2OH, CH2OH, , CH2OH, , C O, , C O, , HOCH, , CH2OH, , HCOH, , C O, CH2OH, , CH2OH, , Dihydroxyacetone, , D-Xylulose, , C O, , C O, HOCH, HCOH, , HOCH, , HCOH, , HCOH, , HCOH, , HCOH, , HCOH, , HCOH, , CH2OH, D-Ribulose, , CH2OH, , CH2OH, , D-Fructose, , D-Sedoheptulose, , Fig. 2.3 : Structures of ketoses of physiological importance., , R1 C, , H, , OR2, + R2 OH, , O, , Aldehyde, , R1 C H, OH, , Alcohol, , Hemiacetal, , The hydroxyl group of monosaccharides can, react with its own aldehyde or keto functional, group to form hemiacetal and hemiketal. Thus,, the aldehyde group of glucose at C1 reacts, with alcohol group at C5 to form two types, of cyclic hemiacetals namely D and E, as depicted, in Fig.2.6. The configuration of glucose is, conveniently represented either by Fischer, formulae or by Haworth projection formulae., , Pyranose and furanose structures, Haworth projection formulae are depicted by, a six-membered ring pyranose (based on pyran), or a five-membered ring furanose (based on, furan). The cyclic forms of glucose are known as, D-D-glucopyranose and, D-D-glucofuranose, (Fig.2.7)., , H C O, , H C O, , H C O, , 2, , 2, , HO C H, , HO C H, , HO C H, , 4, , 4, , H C OH, HO C H, H C OH, CH2OH, D-Galactose, , H C OH HO C H, H C OH, H C OH, , H C OH, H C OH, , Anomers—mutarotation, The D and E cyclic forms of D-glucose are, known as anomers. They differ from each other, in the configuration only around C1 known as, anomeric carbon (hemiacetal carbon). In case of, D anomer, the OH group held by anomeric, carbon is on the opposite side of the group, CH2OH of sugar ring. The reverse is true for, E-anomer. The anomers differ in certain physical, and chemical properties., Mutarotation : The D and E anomers of, glucose have different optical rotations. The, specific optical rotation of a freshly prepared, glucose (D anomer) solution in water is +112.2°, which gradually changes and attains an, equilibrium with a constant value of +52.7°. In, the presence of alkali, the decrease in optical, rotation is rapid. The optical rotation of, E-glucose is +18.7°. Mutarotation is defined as, the change in the specific optical rotation, representing the interconversion of D and E, , H, O C, HO C H, H C OH, , C O, H C OH, HO C H, , HO C H, , H C OH, H C OH, , CH2OH, , CH2OH, , HO C H, , D-Glucose, , D-Mannose, , H C H, OH, , Fig. 2.4 : Structures of epimers (glucose and galactose, are C4-epimers while glucose and mannose are, C2-epimers)., , H, , L-Glucose, , H C H, HO, D-Glucose, , Fig. 2.5 : Enantiomers (mirror images) of glucose.
Page 25 :
15, , Chapter 2 : CARBOHYDRATES, , H, , OH, , 1, , O, , H C OH, , H C OH, , H C OH, , H C, , CH2OH, , H, HO, , CH2OH, , CH2OH, , E-D-Glucose, (+ 18.7q ), , D-Glucose, (aldehyde form), , CH2OH, O, H, , H, , H, , OH, , H, , H, , OH, , CH2OH, OH, H, O C H, OH H, , OH, , HO, , H, , D-D-Glucopyranose, , OH, , D-Glucose, (aldehyde form, acyclic), , O, , 5, , 5, , 5, , D-D-Glucose, (+ 112.2q), , (B), , HO C H, , HO C H, , H C OH, H C, , H, , H C OH, , H C OH, , HO C H, , 1, , C, , H C O, , H C OH, (A), , HO, , 1, , C, , H, HO, , CH2OH, O, H, OH, , H, , H, , OH, , OH, H, , E-D-Glucopyranose, , Fig. 2.6 : Mutarotation of glucose representing D and E anomers (A) Fischer projections (B) Haworth projections., , forms of D-glucose to an equilibrium mixture., Mutarotation depicted in Fig. 2.6, is summarized, below., D-D-Glucose, , E-D-Glucose, , Equilibrium mixture, , + 112.2°, , + 52.7°, , (Specific optical rotation, , [D]20, D, , + 18.7°, , ), , The equilibrium mixture contains 63%, E-anomer and 36% D-anomer of glucose with, O, , O, , Pyran, , H, HO, , CH2OH, O, H, OH, H, , H, , REACTIONS OF MONOSACCHARIDES, , CH2OH, , OH, , OH, , D-D-Glucopyranose, , Mutarotation of fructose : Fructose also, exhibits mutarotation. In case of fructose, the, pyranose ring (six-membered) is converted to, furanose (five-membered) ring, till an equilibrium, is attained. And fructose has a specific optical, rotation of –92° at equilibrium., The conversion of dextrorotatory (+) sucrose, to levorotatory fructose is explained under, inversion of sucrose (see later in this chapter)., , Furan, , H, , 1% open chain form. In aqueous solution, the E, form is more predominant due to its stable, conformation. The D and E forms of glucose are, interconvertible which occurs through a linear, form. The latter, as such, is present in an, insignificant quantity., , H C OH O, , H, , OH, , H, , H, , OH, , H, , OH, , D-D-Glucofuranose, , Fig. 2.7 : Structure of glucose-pyranose, and furanose forms., , Tautomerization or enolization, The process of shifting a hydrogen atom from, one carbon atom to another to produce enediols, is known as tautomerization. Sugars possessing, anomeric carbon atom undergo tautomerization, in alkaline solutions., When glucose is kept in alkaline solution for, several hours, it undergoes isomerization to form
Page 26 :
16, , BIOCHEMISTRY, , H, , Sugar, , H C OH, H C O, H C OH, HO C H, R, , C O, , H C O, , HO C H, , HO C H, , R, D-Fructose, , HO C H, , CuSO4, Enediol, Sugar acid, Cu, , 2+, , Cu, , +, , R, D-Mannose, , D-Glucose, , 2H2O + Cu2O, , H C OH, C OH, HO C H, R, Enediol, (common), , Fig. 2.8 : Formation of a common enediol from, glucose, fructose and mannose, (R corresponds to the end 3 carbon common structure)., , D-fructose and D-mannose. This reaction—, known as the Lobry de Bruyn-von Ekenstein, transformation—results in the formation of a, common intermediate—namely enediol—for all, the three sugars, as depicted in Fig.2.8., The enediols are highly reactive, hence sugars, in alkaline solution are powerful reducing, agents., , Reducing properties, The sugars are classified as reducing or nonreducing. The reducing property is attributed to, the free aldehyde or keto group of anomeric, carbon., In the laboratory, many tests are employed to, identify the reducing action of sugars. These, include Benedict’s test, Fehling’s test, Barfoed’s, test etc. The reduction is much more efficient, in the alkaline medium than in the acid, medium., The enediol forms (explained above) or sugars, reduce cupric ions (Cu2+) of copper sulphate, to cuprous ions (Cu+), which form a yellow, precipitate of cuprous hydroxide or a, red precipitate of cuprous oxide as shown, next., , 2Cu(OH), , It may be noted that the reducing property of, sugars cannot help for a specific identification of, any one sugar, since it is a general reaction., , Oxidation, Depending on the oxidizing agent used, the, terminal aldehyde (or keto) or the terminal, alcohol or both the groups may be oxidized. For, instance, consider glucose :, 1. Oxidation of aldehyde group (CHO o, COOH) results in the formation of gluconic acid., 2. Oxidation of terminal alcohol group, (CH2OH o COOH) leads to the production of, glucuronic acid., , Reduction, When treated with reducing agents such as, sodium amalgam, the aldehyde or keto group of, monosaccharide is reduced to corresponding, alcohol, as indicated by the general formula :, H, H C O, , 2H, , R, , H C OH, R, , The important monosaccharides and their, corresponding alcohols are given below., D-Glucose, D-Galactose, D-Mannose, D-Fructose, D-Ribose, , o, o, o, o, o, , D-Sorbitol, D-Dulcitol, D-Mannitol, D-Mannitol + D-Sorbitol, D-Ribitol, , Sorbitol and dulcitol when accumulate in, tissues in large amounts cause strong osmotic, effects leading to swelling of cells, and certain, pathological conditions. e.g. cataract, peripheral, neuropathy, nephropathy. Mannitol is useful to, reduce intracranial tension by forced diuresis.
Page 27 :
17, , Chapter 2 : CARBOHYDRATES, , H C O, , H C O, C, , H C OH, HO C H, H C OH, H C OH, , Conc. H2SO4, , H C, , 3H2O, , CH2OH, , D-Glucose, , Hydroxymethyl furfural, , H C O, , H C O, C, , H C OH, Conc. H2SO4, , H C, H C, , H C OH, CH2OH, , O, , C, , CH2OH, , H C OH, , H C, , 3H2O, , D-Ribose, , O, , H C, Furfural, , Fig. 2.9 : Dehydration of monosaccharides, with concentrated H2SO4., , configuration on these two carbons give the, same type of osazones, since the difference is, masked by binding with phenylhydrazine. Thus, glucose, fructose and mannose give the same, type (needle-shaped) osazones., Reducing disaccharides also give osazones—, maltose sunflower-shaped, and lactose powderpuff shaped., , Formation of esters, The alcoholic groups of monosaccharides, may be esterified by non-enzymatic or, enzymatic reactions. Esterification of carbohydrate with phosphoric acid is a common, reaction in metabolism. Glucose 6-phosphate, and glucose 1-phosphate are good examples., ATP donates the phosphate moiety in ester, formation., , GLYCOSIDES, Dehydration, When treated with concentrated sulfuric acid,, monosaccharides undergo dehydration with an, elimination of 3 water molecules. Thus hexoses, give hydroxymethyl furfural while pentoses give, furfural on dehydration (Fig.2.9). These furfurals, can condense with phenolic compounds, (D-naphthol) to form coloured products. This is, the chemical basis of the popular Molisch test., In case of oligo- and polysaccharides, they are, first hydrolysed to monosaccharides by acid, and, this is followed by dehydration., Bial’s test : Pentoses react with strong HCl to, form furfural derivatives which in turn react with, orcinol to form green coloured complex. Bial’s, test is useful for detection of xylose in urine in, essential pentosuria., Mucic acid test : Galactose when treated with, nitric acid forms insoluble mucic acid crystals., , Glycosides are formed when the hemiacetal, or hemiketal hydroxyl group (of anomeric, carbon) of a carbohydrate reacts with a hydroxyl, group of another carbohydrate or a noncarbohydrate (e.g. methyl alcohol, phenol,, glycerol). The bond so formed is known as, glycosidic bond and the non-carbohydrate, moiety (when present) is referred to as aglycone., H C O, H C OH, , + H2N NH C6H5, , R, , Glucose, , Phenylhydrazine, , H C N NH C6H5, H C OH, R, Glucohydrazone, , H2N NH C6H5, , Osazone formation, Phenylhydrazine in acetic acid, when boiled, with reducing sugars, forms osazones in a, reaction summarized in Fig.2.10., As is evident from the reaction, the first two, carbons (C1 and C2) are involved in osazone, formation. The sugars that differ in their, , H C N NH C6H5, C N NH C6H5, R, Glucosazone, , Fig. 2.10 : A summary of osazone formation, (R represents C3 to C6 of glucose).
Page 28 :
18, , BIOCHEMISTRY, , The monosaccharides are held together by, glycosidic bonds to result in di-, oligo- or, polysaccharides (see later for structures)., , formed are amino sugars e.g. D-glucosamine,, D-galactosamine. They are present as constituents of heteropolysaccharides., , Naming of glycosidic bond : The, nomenclature of glycosidic bonds is based on, the linkages between the carbon atoms and the, status of the anomeric carbon (D or E). For, instance, lactose—which is formed by a bond, between C1 of E-galactose and C4 of glucose—, is named as E(1 o 4) glycosidic bond. The other, glycosidic bonds are described in the structure, of di- and polysaccharides., , N-Acetylneuraminic acid (NANA) is a, derivative of N-acetylmannose and pyruvic acid., It is an important constituent of glycoproteins, and glycolipids. The term sialic acid is used to, include NANA and its other derivatives., , Physiologically important glycosides, 1. Glucovanillin (vanillin-D-glucoside) is a, natural substance that imparts vanilla flavour., 2. Cardiac glycosides (steroidal glycosides) :, Digoxin and digitoxin contain the aglycone, steroid and they stimulate muscle contraction., 3. Streptomycin, an antibiotic used in the, treatment of tuberculosis is a glycoside., 4. Ouabain inhibits Na+ – K+ ATPase and, blocks the active transport of Na+., 5. Phlorhizin produces renal damage in, experimental animals., , DERIVATIVES OF MONOSACCHARIDES, There are several derivatives of monosaccharides, some of which are physiologically, important (Fig.2.11), 1. Sugar acids : Oxidation of aldehyde or, primary alcohol group in monosaccharide results, in sugar acids. Gluconic acid is produced from, glucose by oxidation of aldehyde (C1 group), whereas glucuronic acid is formed when primary, alcohol group (C6) is oxidized., 2. Sugar alcohols (polyols) : They are, produced by reduction of aldoses or ketoses. For, instance, sorbitol is formed from glucose and, mannitol from mannose., 3. Alditols : The monosaccharides, on, reduction, yield polyhydroxy alcohols, known as, alditols. Ribitol is a constituent of flavin, coenzymes; glycerol and myo-inositol are, components of lipids. Xylitol is a sweetener used, in sugarless gums and candies., 4. Amino sugars : When one or more, hydroxyl groups of the monosaccharides are, replaced by amino groups, the products, , Certain antibiotics contain amino sugars, which may be involved in the antibiotic activity, e.g. erythromycin., 5. Deoxysugars : These are the sugars that, contain one oxygen less than that present in the, parent molecule. The groups, CHOH and, CH2OH become CH2 and CH3 due to the, absence of oxygen. D-2-Deoxyribose is the most, important deoxysugar since it is a structural, constituent of DNA (in contrast to D-ribose in, RNA). Feulgen staining can specifically detect, deoxyribose, and thus DNA in tissues. Fucose is, a deoxy L-galactose found in blood group, antigens, and certain glycoproteins., 6. L-Ascorbic acid (vitamin C) : This is a, water-soluble vitamin, the structure of which, closely resembles that of a monosaccharide., , DISACCHARIDES, Among the oligosaccharides, disaccharides, are the most common (Fig.2.12). As is evident, from the name, a disaccharide consists of two, monosaccharide units (similar or dissimilar) held, together by a glycosidic bond. They are, crystalline, water-soluble and sweet to taste. The, disaccharides are of two types, 1. Reducing disaccharides with free aldehyde, or keto group e.g. maltose, lactose., 2. Non-reducing disaccharides with no free, aldehyde or keto group e.g. sucrose, trehalose., , Maltose, Maltose is composed of two D-D-glucose, units held together by D (1 o 4) glycosidic bond., The free aldehyde group present on C1 of second, glucose answers the reducing reactions, besides
Page 29 :
19, , Chapter 2 : CARBOHYDRATES, , H C O, H C OH, HO C H, , H, , H C OH, , H C OH, , CH2OH, , H C OH, , HO, , Glycerol, , COOH, , O, , H, , OH, , H, , H, , OH, , H, , OH, , H, , H, , OH, , H, , H, , OH, , H, , D-2-Deoxyribose, , OH, H, , myo -Inositol, , D-Glucuronic acid, , HOCH2, , OH, CH2OH, , H, HO, , H, , O, , CH2OH, O, H, , H, , OH, , H, , H, , NH2, , OH, , D-Glucosamine, , H3C C HN, H, , O, H OH, H OH, CH2OH, H, H, HO, , COO–, OH, , H, , N-Acetylneuraminic acid, , Fig. 2.11 : Structures of monosaccharide derivatives (selected examples)., , the osazone formations (sunflower-shaped)., Maltose can be hydrolysed by dilute acid or the, enzyme maltase to liberate two molecules of, D-D-glucose., In isomaltose, the glucose units are held, together by D (1 o 6) glycosidic linkage., , Cellobiose is another disaccharide, identical, in structure with maltose, except that the former, has E (1 o 4) glycosidic linkage. Cellobiose is, formed during the hydrolysis of cellulose., , Sucrose, Sucrose (cane sugar) is the sugar of commerce,, mostly produced by sugar cane and sugar beets., Sucrose is made up of D-D-glucose and ED-fructose. The two monosaccharides are held, together by a glycosidic bond (D1 o E2), between, C1 of D-glucose and C2 of E-fructose. The, reducing groups of glucose and fructose are, involved in glycosidic bond, hence sucrose is a, non-reducing sugar, and it cannot form osazones., Sucrose is an important source of dietary, carbohydrate. It is sweeter than most other, common sugars (except fructose) namely glucose,, lactose and maltose. Sucrose is employed as a, sweetening agent in food industry. The intestinal, enzyme—sucrase—hydrolyses sucrose to glucose, and fructose which are absorbed., , Inversion of sucrose, Sucrose, as such is dextrorotatory (+66.5°)., But, when hydrolysed, sucrose becomes, levorotatory (–28.2°). The process of change, in optical rotation from dextrorotatory (+), to levorotatory (–) is referred to as inversion., The hydrolysed mixture of sucrose, containing, glucose and fructose, is known as invert sugar., The process of inversion is explained below., Hydrolysis of sucrose by the enzyme sucrase, (invertase) or dilute acid liberates one molecule, each of glucose and fructose. It is postulated that, sucrose (dextro) is first split into D-Dglucopyranose (+52.5°) and E-D-fructofuranose,, both being dextrorotatory. However, E-Dfructofuranose is less stable and immediately gets, converted to E-D-fructopyranose which is, strongly levorotatory (–92°). The overall effect is, that dextro sucrose (+66.5°) on inversion is, converted to levo form (–28.2°)., , Lactose, Lactose is more commonly known as milk, sugar since it is the disaccharide found in milk., Lactose is composed of E-D-galactose and E-Dglucose held together by E (1 o 4) glycosidic, bond. The anomeric carbon of C1 glucose is free,, hence lactose exhibits reducing properties and, forms osazones (powder-puff or hedgehog shape).
Page 30 :
20, , H, HO, , BIOCHEMISTRY, , CH2OH, O, H, OH, , H, , H, , OH, , H, , H, , 1, , 4, , O, , Glucose, , CH2OH, O, H, OH, , H, , H, , OH, , POLYSACCHARIDES, H, OH, , Glucose, , Maltose, (D-D-glucosyl (1 o 4) D-D-glucose), , H, HO, , CH2OH, O, H, OH, , H, , H, , OH, , O, , H HOH2C, 1, , H, , 2, , H, , O, , HO, , OH, , Glucose, , CH2OH, , H, , HO, H, , OH, , H, , H, , OH, , H, O, , 1, , H, , Galactose, , 4, , CH2OH, O, H, OH, , H, , H, , OH, , Polysaccharides are linear as well as, branched polymers. This is in contrast to, structure of proteins and nucleic acids which are, only linear polymers. The occurrence of, branches in polysaccharides is due to the fact, that glycosidic linkages can be formed at any, one of the hydroxyl groups of a monosaccharide., Polysaccharides are of two types, 1. Homopolysaccharides on hydrolysis yield, only a single type of monosaccharide. They, are named based on the nature of the, monosaccharide. Thus, glucans are polymers of, glucose whereas fructosans are polymers of, fructose., , Fructose, , Sucrose, (D-D-glucosyl (1 o 2) E-D-fructose), , CH2OH, O, H, , Polysaccharides (or simply glycans) consist of, repeat units of monosaccharides or their, derivatives, held together by glycosidic bonds., They are primarily concerned with two important, functions-structural, and storage of energy., , OH, H, , Glucose, , Lactose, (E-D-galactosyl (1 o 4) E-D-glucose), , Fig. 2.12 : Structures of disaccharides, —maltose, sucrose and lactose., , Lactose of milk is the most important, carbohydrate in the nutrition of young mammals., It is hydrolysed by the intestinal enzyme lactase, to glucose and galactose., , Lactulose, Lactulose is a synthetic dissccharide containing, galactose and fructose. It is neither digested nor, absorbed in the inestine. Lactulose is useful for, the treatment of hepatic encephalopathy, a, disorder characterized by elevated plasma, ammonium levels. Lactulose converts ammonia, (NH3) in the lumen to ammonium ion (NH4+). This, results in a reduction in the plasma NH3, since, +, NH4 ions are not easily absorbed., , 2. Heteropolysaccharides on hydrolysis yield, a mixture of a few monosaccharides or their, derivatives., , HOMOPOLYSACCHARIDES, Starch, Starch is the carbohydrate reserve of plants, which is the most important dietary source for, higher animals, including man. High content of, starch is found in cereals, roots, tubers, vegetables, etc. Starch is a homopolymer composed of, D-glucose units held by D-glycosidic bonds. It is, known as glucosan or glucan., Starch consists of two polysaccharide, components-water soluble amylose (15-20%), and a water insoluble amylopectin (80-85%)., Chemically, amylose is a long unbranched, chain with 200–1,000 D-glucose units held by D, (1 o 4) glycosidic linkages. Amylopectin, on the, other hand, is a branched chain with D (1 o 6), glycosidic bonds at the branching points and D, (1 o 4) linkages everywhere else (Fig.2.13)., Amylopectin molecule containing a few, thousand glucose units looks like a branched, tree (20–30 glucose units per branch).
Page 31 :
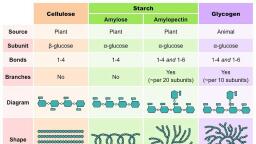
21, , Chapter 2 : CARBOHYDRATES, , H, , CH2OH, O, H, OH, , H, , H, , OH, , H, , H, , 4, , 1, , O, , D-Glucose, , CH2OH, O, H, OH, , H, , H, , OH, , H, O, , n, , D-Glucose, D -Amylose, , H, O, , CH2OH, O, H, OH, , H, , H, , OH, , H, , H, , 1, , 4, , O, , CH2OH, O, H, OH, , H, , H, , OH, , H, 1, , Branch, , (1, , 6) Branch, , O, , H, Main chain, , O, , CH2OH, O, H, OH, , H, , H, , OH, , 6, , H, , H, O, , CH2, O, H, OH, , H, , H, , OH, , H, , H, O, , CH2OH, O, H, OH, , H, , H, , OH, , H, O, , Amylopectin, , Fig. 2.13 : Structure of starch (D-amylose and amylopectin)., , Starches are hydrolysed by amylase, (pancreatic or salivary) to liberate dextrins, and, finally maltose and glucose units. Amylase acts, specifically on D (1 o 4) glycosidic bonds., , Dextrins, Dextrins are the breakdown products of, starch by the enzyme amylase or dilute acids., Starch is sequentially hydrolysed through, different dextrins and, finally, to maltose and, glucose. The various intermediates (identified by, iodine colouration) are soluble starch (blue),, amylodextrin (violet), erythrodextrin (red) and, achrodextrin (no colour)., , Inulin, Inulin is a polymer of fructose i.e., fructosan., It occurs in dahlia bulbs, garlic, onion etc. It is, a low molecular weight (around 5,000) polysaccharide easily soluble in water. Inulin is not, utilized by the body. It is used for assessing, kidney function through measurement of, glomerular filtration rate (GFR)., , Glycogen, , Dextrans, , Glycogen is the carbohydrate reserve in, animals, hence often referred to as animal starch., It is present in high concentration in liver,, followed by muscle, brain etc. Glycogen is also, found in plants that do not possess chlorophyll, (e.g. yeast, fungi)., , Dextrans are polymers of glucose, produced, by microorganisms. They are used as plasma, volume, expanders, in, transfusion,, and, chromatography (e.g. gel filtration)., , The structure of glycogen is similar to that of, amylopectin with more number of branches., Glucose is the repeating unit in glycogen joined, together by D (1 o 4) glycosidic bonds, and
Page 32 :
22, , BIOCHEMISTRY, , Cellulose, though not digested, has great, importance in human nutrition. It is a major, constituent of fiber, the non-digestable carbohydrate. The functions of dietary fiber include, decreasing the absorption of glucose and, cholesterol from the intestine, besides increasing, the bulk of feces. (For details, Chapter 23), CH2OH, , (A), , Chitin, , O, , Chitin is composed of N-acetyl Dglucosamine units held together by E (1 o 4), glycosidic bonds. It is a structural polysaccharide, found in the exoskeleton of some invertebrates, e.g. insects, crustaceans., , 1, , CH2OH, , O, 4, , O, , HETEROPOLYSACCHARIDES, , 1, , (B), , O, 6, , CH2OH, O, O, , 4, , CH2, , CH2OH, O, , O, 1, , O, , 4, , 1, , O, , 4, , 1, , O, , When the polysaccharides are composed of, different types of sugars or their derivatives, they, are referred to as heteropolysaccharides or, heteroglycans., , MUCOPOLYSACCHARIDES, Fig. 2.14 : Structure of glycogen (A) General structure, (B) Enlarged at a branch point., , D (1 o 6) glycosidic bonds at branching points, (Fig.2.14). The molecular weight (up to 1 u 108), and the number of glucose units (up to 25,000), vary in glycogen depending on the source from, which glycogen is obtained., , Cellulose, Cellulose occurs exclusively in plants and it is, the most abundant organic substance in plant, kingdom. It is a predominant constituent of, plant cell wall. Cellulose is totally absent in, animal body., Cellulose is composed of E-D-glucose units, linked by E (1 o 4) glycosidic bonds (Fig.2.15)., Cellulose cannot be digested by mammals—, including man—due to lack of the enzyme that, cleaves E-glycosidic bonds (D amylase breaks D, bonds only). Certain ruminants and herbivorous, animals contain microorganisms in the gut which, produce enzymes that can cleave E-glycosidic, bonds. Hydrolysis of cellulose yields a, disaccharide cellobiose, followed by E-D-glucose., , Mucopolysaccharides are heteroglycans made, up of repeating units of sugar derivatives, namely, amino sugars and uronic acids. These are more, commonly known as glycosaminoglycans, (GAG). Acetylated amino groups, besides sulfate, and carboxyl groups are generally present in, GAG structure. The presence of sulfate and, carboxyl groups contributes to acidity of the, molecules, making them acid mucopolysaccharides., Some of the mucopolysaccharides are found, in combination with proteins to form, mucoproteins or mucoids or proteoglycans, (Fig.2.16). Mucoproteins may contain up to 95%, carbohydrate and 5% protein., , H, , CH2OH, O, H, OH, , H, , H, , OH, , H, O, , 1, , E-D-Glucose, , H, , 4, , CH2OH, O, H, OH, , H, , H, , OH, , E-D-Glucose, , O, H, n, , Fig. 2.15 : Structure of cellulose (The repeating unit ‘n’, may be several thousands).
Page 33 :
23, , Chapter 2 : CARBOHYDRATES, , Hyaluronic acid, , Link protein, Core protein, Chondroitin, sulfate, , Mucopolysaccharides are essential components, of tissue structure. The extracellular spaces of, tissue (particularly connective tissue-cartilage,, skin, blood vessels, tendons) consist of collagen, and elastin fibers embedded in a matrix or ground, substance. The ground substance is predominantly, composed of GAG., The important mucopolysaccharides include, hyaluronic acid, chondroitin 4-sulfate, heparin,, dermatan sulfate and keratan sulfate (Fig.2.17)., , Hyaluronic acid, Keratan sulfate, , Fig. 2.16 : Diagrammatic representation of a, proteoglycan complex., , Hyaluronic acid is an important GAG found, in the ground substance of synovial fluid of joints, and vitreous humor of eyes. It is also present as, a ground substance in connective tissues, and, forms a gel around the ovum. Hyaluronic acid, serves as a lubricant and shock absorbant in, joints., , + Glucose is the most important energy source of carbohydrates to the mammals (except, ruminants). The bulk of dietary carbohydrate (starch) is digested and finally absorbed as, glucose into the body., , + Dextrose (glucose in solution in dextrorotatory form) is frequently used in medical, practice., , + Fructose is abundantly found in the semen which is utilized by the sperms for energy., + Several diseases are associated with carbohydrates e.g., diabetes mellitus, glycogen, storage diseases, galactosemia., , + Accumulation of sorbitol and dulcitol in the tissues may cause certain pathological, conditions e.g. cataract, nephropathy., , + Inulin, a polymer of fructose, is used to assess renal function by measuring glomerular, filtration rate (GFR)., , + The non-digestible carbohydrate cellulose plays a significant role in human nutrition., These include decreasing the intestinal absorption of glucose and cholesterol, and, increasing bulk of feces to avoid constipation., , + The mucopolysaccharide hyaluronic acid serves as a lubricant and shock absorbant in, joints., , + The enzyme hyaluronidase of semen degrades the gel (contains hyaluronic acid) around, the ovum. This allows effective penetration of sperm into the ovum., , + The mucopolysaccharide heparin is an anticoagulant (prevents blood clotting)., + The survival of Antarctic fish below –2°C is attributed to the antifreeze glycoproteins., + Streptomycin is a glycoside employed in the treatment of tuberculosis.
Page 34 :
24, Hyaluronic acid is composed of alternate, units of D-glucuronic acid and N-acetyl, D-glucosamine. These two molecules form, disaccharide units held together by E (1 o 3), glycosidic bond (Fig.2.16). Hyaluronic acid, contains about 250–25,000 disaccharide units, (held by E 1�o 4 bonds) with a molecular weight, up to 4 million., Hyaluronidase is an enzyme that breaks, (E 1 o 4 linkages) hyaluronic acid and other, GAG. This enzyme is present in high, concentration in testes, seminal fluid, and in, certain snake and insect venoms. Hyaluronidase, of semen is assigned an important role in, fertilization as this enzyme clears the gel, (hyaluronic acid) around the ovum allowing a, better penetration of sperm into the ovum., Hyaluronidase of bacteria helps their invasion, into the animal tissues., , Chondroitin sulfates, Chondroitin 4-sulfate (Greek : chondroscartilage) is a major constituent of various, mammalian tissues (bone, cartilage, tendons,, heart, valves, skin, cornea etc.). Structurally, it is, comparable with hyaluronic acid. Chondroitin, 4-sulfate consists of repeating disaccharide units, composed of D-glucuronic acid and N-acetyl, D-galactosamine 4-sulfate (Fig.2.17)., , Heparin, Heparin is an anticoagulant (prevents blood, clotting) that occurs in blood, lung, liver, kidney,, spleen etc. Heparin helps in the release of the, enzyme lipoprotein lipase which helps in, clearing the turbidity of lipemic plasma., Heparin is composed of alternating units of, N-sulfo D-glucosamine 6-sulfate and glucuronate, 2-sulfate (Fig.2.17)., , Dermatan sulfate, Mostly found in skin, dermatan sulfate is, structurally related to chondroitin 4-sulfate. The, only difference is that there is an inversion in the, configuration around C5 of D-glucuronic acid to, form L-iduronic acid (Fig.2.17)., , BIOCHEMISTRY, , COO–, O, H, O 4H, OH H, H, , CH2OH, O, H, H, 1, O, O, H, HO, H, , 1, , H, , 3, , OH, , H, , NH CO CH3, , N-Acetylglucosamine, D-Glucuronic acid, Hyaluronic acid, , COO–, O, H, O 4 H, OH H, H, , n, , SO3– CH2OH, O, O, H, 1, O, O, H, H, H 3, , 1, , H, , OH, , H, , NH CO CH3, , n, , D-Glucuronic acid, , N-Acetylgalactosamine, 4-sulfate, Chondroitin 4-sulfate, , COO–, O H, H, H, 1, OH H, O, H, , O, , CH2 O SO–3, O H, H, H, 4, OH H, H O, , –, , O SO3, , H, , –, , NH SO3, , n, , N-Sulfoglucosamine, 6-sulfate, Heparin, , D-Glucuronate-2-sulfate, , H, O, H, COO–, OH H, H, , H, , SO3– CH2OH, O, O, H, O, O, H, H 3, H, , OH, , H, , NH CO CH3, , N-Acetylgalactosamine, 4-sulfate, Dermatan sulfate, , L-Iduronic acid, , CH2OH, O, HO, H, H, H, H, H, , OH, , D-Galactose, , O, , n, , –, CH2 O SO3, O, H, H, O, H, H, , H, , NH CO CH3, , n, , N-Acetylglucosamine, 6-sulfate, , Keratan sulfate, , Fig. 2.17 : Structures of common glycosaminoglycans –, the disaccharides as repeating units.
Page 35 :
25, , Chapter 2 : CARBOHYDRATES, , TABLE 2.3 A summary of glycosaminoglycans – composition, distribution and functions, , Glycosaminoglycan, , Composition, , Tissue distribution, , Function(s), , Hyaluronic acid, , D-Glucuronic acid,, N-acetylglucosamine, , Connective tissue, synovial fluid,, vitrous humor, , Serves as a lubricant, and, shock absorber. Promotes, wound healing, , Chondroitin sulfate, , D-Glucuronic acid,, N-acetylgalactosamine, 4-sulfate, , Cartilage, bone, skin, blood vessel, walls, , Helps to maintain the structure, and shapes of tissues, , Heparin, , D-Glucuronate 2-sulfate,, N-sulfoglucosamine, 6-sulfate, , Blood, lung, liver, kidney, spleen, , Acts as an anticoagulant, , Dermatan sulfate, , L-Iduronic acid, N-acetylgalactosamine 4-sulfate, , Blood vessel valves, heart valves,, skin, , Maintains the shapes of tissues, , Keratan sulfate, , D-Galactose, N-acetylglucosamine 6-sulfate, , Cartilage, cornea, connective, tissues, , Keeps cornea transparent, , It is a heterogeneous GAG with a variable, sulfate, content, besides small amounts of, mannose, fructose, sialic acid etc. Keratan, sulfate essentially consists of alternating units of, D-galactosamine, and, N-acetylglucosamine, 6-sulfate., , Sometimes the term mucoprotein is used for, glycoprotein with carbohydrate concentration, more than 4%. Glycoproteins are very widely, distributed in the cells and perform variety of, functions. These include their role as enzymes,, hormones, transport proteins, structural proteins, and receptors. A selected list of glycoproteins, and their major functions is given in Table 2.4., , AGAR AND PECTINS, , The carbohydrates found in glycoproteins, include, mannose,, galactose,, N-acetylglucosamine, N-acetylgalactosamine, xylose,, , Keratan sulfate, , Agar, mostly found in sea weeds, is a polymer, of galactose sulfate and glucose. Since agar is, not digested, it serves as a dietary fiber (Refer, Chapter 23). Agarose (with galactose and, anhydrogalactose) is useful in the laboratory as a, major component of microbial culture media,, and in electrophoresis., Pectins, found in apples and citrus fruits,, contain galactouronate and rhamnose. Pectins,, being non-digestible, are useful as dietary fiber., They are also employed in the preparation of, jellies., , GLYCOPROTEINS, Several proteins are covalently bound to, carbohydrates which are referred to as glycoproteins., The, carbohydrate, content, of, glycoprotein varies from 1% to 90% by weight., , TABLE 2.4 A selected list of glycoproteins and, their major functions, , Glycoprotein(s), , Major function(s), , Collagen, , Structure, , Hydrolases, proteases,, glycosidases, , Enzymes, , Ceruloplasmin, , Transport, , Immunoglobulins, , Defense against infection, , Synovial glycoproteins, , Lubrication, , Thyrotropin, erythropoietin, , Hormones, , Blood group substances, , Antigens, , Fibronectin, laminin, , Cell-cell recognition and, adhesion, , Intrinsic factor, , Absorption of vitamin B12, , Fibrinogen, , Blood clotting
Page 36 :
26, , BIOCHEMISTRY, , L-fucose and N-acetylneuraminic acid (NANA)., NANA is an important sialic acid (See Fig.2.11)., , residue is bound to E-galactosyl (1 o 3) D, N-acetylgalactosamine., , Antifreeze glycoproteins : The Antarctic fish, live below –2°C, a temperature at which the, blood would freeze. It is now known that these, fish contain antifreeze glycoprotein which lower, the freezing point of water and interfere with the, crystal formation of ice. Antifreeze glycoproteins, consist of 50 repeating units of the tripeptide,, alanine-alanine-threonine., Each, threonine, , Blood group substances, The blood group antigens (of erythrocyte, membrane) contain carbohydrates as glycoproteins or glycolipids. N-Acetylgalactosamine,, galactose, fucose, sialic acid etc. are found in, the blood group substances. The carbohydrate, content also plays a determinant role in blood, grouping., , 1. Carbohydrates are the polyhydroxyaldehydes or ketones, or compounds which produce, them on hydrolysis. The term sugar is applied to carbohydrates soluble in water and, sweet to taste. Carbohydrates are the major dietary energy sources, besides their, involvement in cell structure and various other functions., 2. Carbohydrates are broadly classified into 3 groups—monosaccharides, oligosaccharides, and polysaccharides. The monosaccharides are further divided into different categories, based on the presence of functional groups (aldoses or ketoses) and the number of, carbon atoms (trioses, tetroses, pentoses, hexoses and heptoses)., 3. Glyceraldehyde (triose) is the simplest carbohydrate and is chosen as a reference to, write the configuration of all other monosaccharides (D- and L- forms). If two, monosaccharides differ in their structure around a single carbon atom, they are known, as epimers. Glucose and galactose are C4 – epimers., 4. D-Glucose is the most important naturally occurring aldose/monosaccharide., Glucose exists as D and E anomers with different optical rotations. The interconversion, of D and E anomeric forms with change in the optical rotation is known as mutarotation., 5. Monosaccharides participate in several reactions. These include oxidation, reduction,, dehydration, osazone formation etc. Formation of esters and glycosides by, monosaccharides is of special significance in biochemical reactions., 6. Among the oligosaccharides, disaccharides are the most common. These include the, reducing disaccharides namely lactose (milk sugar) and maltose (malt sugar) and the, non-reducing sucrose (cane sugar)., 7. Polysaccharides are the polymers of monosaccharides or their derivatives, held together, by glycosidic bonds. Homopolysaccharides are composed of a single monosaccharide, (e.g., starch, glycogen, cellulose, inulin). Heteropolysaccharides contain a mixture of, few monosaccharides or their derivatives (e.g., mucopolysaccharides)., 8. Starch and glycogen are the carbohydrate reserves of plants and animals respectively., Cellulose, exclusively found in plants, is the structural constituent. Inulin is utilized to, assess kidney function by measuring glomerular filtration rate (GFR)., 9. Mucopolysaccharides (glycosaminoglycans) are the essential components of tissue, structure. They provide the matrix or ground substance of extracellular tissue spaces in, which collagen and elastin fibers are embedded. Hyaluronic acid, chondroitin 4-sulfate,, heparin, are among the important glycosaminoglycans., 10. Glycoproteins are a group of biochemically important compounds with a variable, composition of carbohydrate (1-90%), covalently bound to protein. Several enzymes,, hormones, structural proteins and cellular receptors are in fact glycoproteins.
Page 37 :
Chapter 2 : CARBOHYDRATES, , I., , 27, , Essay questions, 1. Define and classify carbohydrates with suitable examples. Add a note on the functions of, carbohydrates., 2. Describe the structure and functions of mucopolysaccharides., 3. Give an account of the structural configuration of monosaccharides, with special reference to, glucose., 4. Discuss the structure and functions of 3 biochemically important disaccharides., 5. Define polysaccharides and describe the structure of 3 homopolysaccharides., , II. Short notes, (a) Epimers, (b) Mutarotation, (c) Osazone formation, (d) Glycosidic bond, (e) Sugar derivatives, (f), Anomers, (g) Enediol, (h) Amino sugars, (i) Inversion of sucrose, (j) Deoxysugars., , III. Fill in the blanks, 1. Name a non-reducing disaccharide ________________________________________., 2. The carbohydrate that is taken as a reference for writing the configuration of others, ____________., 3. If two monosaccharides differ in configuration around a single carbon atom, they are known, as ___________________., 4. The D and E cyclic forms of D-glucose are referred to as ______________________________., 5. The non-carbohydrate moiety found in glycosides is known as __________________________., 6. Give an example of a glycoside antibiotic ___________________________., 7. The glycosidic bonds at the branching points in the structure of starch are ________________., 8. The polysaccharide employed for the assessment of kidney function _____________________., 9. The glycosaminoglycan that serves as a lubricant and shock absorbant of joints ___________., 10. Name the sialic acid, mostly found in the structure of glycoproteins and glycolipids _______., , IV. Multiple choice questions, 11. Ribose and deoxyribose differ in structure around a single carbon, namely, (a) C1 (b) C2 (c) C3 (d) C4., 12. One of the following is not an aldose, (a) Glucose (b) Galactose (c) Mannose (d) Fructose., 13. The glycosaminoglycan that serves as an anticoagulant, (a) Heparin (b) Hyaluronic acid (c) Chondroitin sulfate (d) Dermatan sulfate., 14. The following polysaccharide is composed of E-glycosidic bonds, (a) Starch (b) Glycogen (c) Dextrin (d) Cellulose., 15. The carbon atoms involved in the osazone formation, (a) 1 and 2 (b) 2 and 3 (c) 3 and 4 (d) 5 and 6.
Page 38 :
Section 1, , Chemical Constituents of Life, , Chapter, , Lipids, , 13, , The fat speaks :, O, O, , CH2—O—C—R1, , R2—C—O—CH, , O, , CH2—O—C—R3, , “With water, I say, ‘Touch me not’;, To the tongue, I am tasteful;, Within limits, I am dutiful;, In excess, I am dangerous!”, , L, , ipids (Greek: lipos–fat) are of great, importance to the body as the chief, concentrated storage form of energy, besides, their role in cellular structure and various other, biochemical functions. As such, lipids are a, heterogeneous group of compounds and,, therefore, it is difficult to define them precisely., , (a) Fats and oils (triacylglycerols) : These are, esters of fatty acids with glycerol., The difference between fat and oil, is only physical. Thus, oil is a, liquid while fat is a solid at room, temperature., (b) Waxes : Esters of fatty acids (usually, long chain) with alcohols other than, glycerol. These alcohols may be aliphatic, or alicyclic. Cetyl alcohol is most, commonly found in waxes. Waxes are, used in the preparation of candles,, lubricants, cosmotics, ointments, polishes, etc., , Lipids may be regarded as organic substances, relatively insoluble in water, soluble in organic, solvents (alcohol, ether etc.), actually or, potentially related to fatty acids and utilized by, the living cells., Unlike the polysaccharides, proteins and, nucleic acids, lipids are not polymers. Further,, lipids are mostly small molecules., , Classification of lipids, Lipids are broadly classified (modified from, Bloor) into simple, complex, derived and, miscellaneous lipids, which are further subdivided, into different groups, , 2. Complex (or compound) lipids : These, are esters of fatty acids with alcohols containing, additional, groups, such, as, phosphate,, nitrogenous base, carbohydrate, protein etc., They are further divided as follows, (a) Phospholipids : They contain phosphoric, acid and frequently a nitrogenous base., This is in addition to alcohol and fatty, acids., , 1. Simple lipids : Esters of fatty acids with, alcohols. These are mainly of two types, , 28
Page 39 :
29, , Chapter 3 : LIPIDS, , (i) Glycerophospholipids : These phospholipids contain glycerol as the alcohol, e.g., lecithin, cephalin., , 5. Lipids protect the internal organs, serve as, insulating materials and give shape and smooth, appearance to the body., , (ii) Sphingophospholipids : Sphingosine is, the alcohol in this group of phospholipids e.g., sphingomyelin., , FATTY ACIDS, , (b) Glycolipids : These lipids contain a fatty, acid, carbohydrate and nitrogenous base., The alcohol is sphingosine, hence they, are also called as glycosphingolipids., Glycerol and phosphate are absent e.g.,, cerebrosides, gangliosides., (c) Lipoproteins : Macromolecular complexes, of lipids with proteins., (d) Other complex lipids : Sulfolipids, aminolipids and lipopolysaccharides are among, the other complex lipids., 3. Derived lipids : These are the derivatives, obtained on the hydrolysis of group 1 and group, 2 lipids which possess the characteristics of, lipids. These include glycerol and other alcohols,, fatty acids, mono- and diacylglycerols, lipid (fat), soluble vitamins, steroid hormones, hydrocarbons and ketone bodies., 4. Miscellaneous lipids : These include a, large number of compounds possessing the, characteristics of lipids e.g., carotenoids,, squalene, hydrocarbons such as pentacosane (in, bees wax), terpenes etc., NEUTRAL LIPIDS : The lipids which are, uncharged are referred to as neutral lipids. These, are mono-, di-, and triacylglycerols, cholesterol, and cholesteryl esters., , Functions of lipids, Lipids perform several important functions, 1. They are the concentrated fuel reserve of, the body (triacylglycerols)., 2. Lipids are the constituents of membrane, structure, and, regulate, the, membrane, permeability (phospholipids and cholesterol)., 3. They serve as a source of fat soluble, vitamins (A, D, E and K)., 4. Lipids are important as cellular metabolic, regulators (steroid hormones and prostaglandins)., , Fatty acids are carboxylic acids with, hydrocarbon side chain. They are the simplest, form of lipids., , Occurrence, Fatty acids mainly occur in the esterified form, as major constituents of various lipids. They are, also present as free (unesterified) fatty acids., Fatty acids of animal orgin are much simpler in, structure in contrast to those of plant origin, which often contain groups such as epoxy, keto,, hydroxy and cyclopentane rings., , Even and odd carbon fatty acids, Most of the fatty acids that occur in natural, lipids are of even carbons (usually 14C – 20C)., This is due to the fact that biosynthesis of fatty, acids mainly occurs with the sequential addition, of 2 carbon units. Palmitic acid (16C) and, stearic acid (18C) are the most common. Among, the odd chain fatty acids, propionic acid (3C), and valeric acid (5C) are well known., , Saturated and unsaturated, fatty acids, Saturated fatty acids do not contain double, bonds, while unsaturated fatty acids contain one, or more double bonds. Both saturated and, unsaturated fatty acids almost equally occur in, the natural lipids. Fatty acids with one double, bond are monounsaturated, and those with 2 or, more double bonds are collectively known as, polyunsaturated fatty acids (PUFA)., , Nomenclature of fatty acids, The naming of a fatty acid (systematic name), is based on the hydrocarbon from which it is, derived. The saturated fatty acids end with a, suffix -anoic (e.g., octanoic acid) while the, unsaturated fatty acids end with a suffix -enoic
Page 41 :
31, , Chapter 3 : LIPIDS, , double bonds, starting from the carboxyl end., Thus, saturated fatty acid, palmitic acid is written, as 16 : 0, oleic acid as 18 : 1; 9, arachidonic, acid as 20 : 4; 5, 8, 11, 14., , H, C, H, , C, , (CH2)7COOH, (CH2)7CH3, , Oleic acid, (cis form), , H, C, H3C(H2C)7, , C, , (CH2)7COOH, H, , Elaidic acid, (trans form), , There are other conventions of representing, the double bonds. '9 indicates that the double, bond is between 9 and 10 of the fatty acid. Z 9, represents the double bond position (9 and 10), from the Z end. Naturally occurring unsaturated, fatty acids belong to Z 9, Z� 6 and Z 3 series., Z�3 series, Linolenic acid (18 : 3; 9, 12, 15), Z�6 series, Linoleic acid (18 : 2; 9, 12) and, arachidonic acid (20 : 4; 5, 8,, 11, 14), Z�9 series, Oleic acid (18 : 1; 9), , Isomerism in, unsaturated fatty acids, , The biochemically important saturated and, unsaturated fatty acids are given in the, Table 3.1., , Unsaturated fatty acids exhibit geometric, isomerism depending on the orientation of the, groups around the double bond axis., , ESSENTIAL FATTY ACIDS, The fatty acids that cannot be synthesized by, the body and, therefore, should be supplied in, the diet are known as essential fatty acids (EFA)., Chemically, they are polyunsaturated fatty, acids, namely linoleic acid (18 : 2; 9, 12) and, linolenic acid (18 : 3; 9, 12, 15). Arachidonic, acid (20 : 4; 5, 8, 11, 14) becomes essential, if, its precursor linoleic acid is not provided in the, diet in sufficient amounts. The structures of EFA, are given in the Table 3.1., Biochemical basis for essentiality : Linoleic, acid and linolenic acid are essential since, humans lack the enzymes that can introduce, double bonds beyond carbons 9 to 10., Functions of EFA : Essential fatty acids are, required for the membrane structure and, function, transport of cholesterol, formation of, lipoproteins, prevention of fatty liver etc., (Chapter 23). They are also needed for the, synthesis of another important group of, compounds, namely eicosanoids (Chapter 32)., Deficiency of EFA : The deficiency of EFA, results in phrynoderma or toad skin,, characterized by the presence of horny eruptions, , Fig. 3.1 : Cis-trans isomerism in, unsaturated fatty acids., , on the posterior and lateral parts of limbs, on the, back and buttocks, loss of hair and poor wound, healing., , If the atoms or acyl groups are present on the, same side of the double bond, it is a cis, configuration. On the other hand, if the groups, occur on the opposite side, it is a trans, configuration. Thus oleic acid is a cis isomer, while elaidic acid is a trans isomer, as depicted, in Fig.3.1. Cis isomers are less stable than trans, isomers. Most of the naturally occurring, unsaturated fatty acids exist as cis isomers., In the cis isomeric form, there is a molecular, binding at the double bond. Thus, oleic acid, exists in an L-shape while elaidic acid is a, straight chain. Increase in the number of double, bonds will cause more bends (kinks) and, arachidonic acid with 4 double bonds will have, a U-shape. It is believed that cis isomers of fatty, acids with their characteristic bonds will, compactly pack the membrane structure., Hydroxy fatty acids : Some of the fatty acids, are hydroxylated. E-Hydroxybutyric acid, one of, the ketone bodies produced in metabolism, is a, simple example of hydroxy fatty acids., Cerebronic acid and recinoleic acid are long, chain hydroxy fatty acids., Cyclic fatty acids : Fatty acids with cyclic, structures are rather rare e.g., chaulmoogric acid, found in chaulmoogra oil (used in leprosy, treatment) contains cyclopentenyl ring.
Page 42 :
32, , BIOCHEMISTRY, , O, O, , CH2 O C R1, , R2 C O CH, , O, , O, O, , R2 C O CH, , CH2 O C R3, Triacylglycerol, , CH2 O C R1, CH2OH, , 1,2-Diacylglycerol, , O, CH2 O C R, HO CH, CH2OH, 1-Monoacylglycerol, , O, , CH2 OH, , R C O CH, CH2OH, 2-Monoacylglycerol, , Fig. 3.2 : General structures of acylglycerols, (For palmitoyl R = C15H31; for stearoyl R = C17H35; For linoleoyl R = C17H31), , Eicosanoids : These compounds are related to, eicosapolyenoic fatty acids and include prostaglandins, prostacyclins, leukotrienes and thromboxanes. They are discussed together (Chapter 32)., , TRIACYLGLYCEROLS, Triacylglycerols (formerly triglycerides) are, the esters of glycerol with fatty acids. The fats, and oils that are widely distributed in both plants, and animals are chemically triacylglycerols., They are insoluble in water and non-polar in, character and commonly known as neutral fats., Fats as stored fuel : Triacylglycerols are the, most abundant group of lipids that primarily, function as fuel reserves of animals. The fat, reserve of normal humans (men 20%, women, 25% by weight) is sufficient to meet the body’s, caloric requirements for 2-3 months., Fats primarily occur in adipose tissue :, Adipocytes of adipose tissue—predominantly, found in the subcutaneous layer and in the, abdominal cavity—are specialized for storage of, triacylglycerols. The fat is stored in the form of, globules dispersed in the entire cytoplasm. And, surprisingly, triacylglycerols are not the structural, components of biological membranes., Structures of acylglycerols : Monoacylglycerols, diacylglycerols and triacylglycerols,, respectively consisting of one, two and three, molecules of fatty acids esterified to a molecule, , of glycerol, are known (Fig.3.2). Among these,, triacylglycerols are the most important, biochemically., Simple triacylglycerols contain the same type, of fatty acid residue at all the three carbons e.g.,, tristearoyl glycerol or tristearin., Mixed triacylglycerols are more common., They contain 2 or 3 different types of fatty acid, residues. In general, fatty acid attached to C1 is, saturated, that attached to C2 is unsaturated, while that on C3 can be either. Triacylglycerols, are named according to placement of acyl, radical on glycerol e.g., 1,3-palmitoyl 2-linoleoyl, glycerol., , Triacylglycerols of plants, in general, have, higher content of, unsaturated fatty acids, compared to that of animals., , Stereospecific numbering, of glycerol, The structure of glycerol gives an impression, that carbons 1 and 3 are identical. This is not true, in a 3-dimensional structure. In order to represent, the carbon atoms of glycerol in an unambiguous, manner, biochemists adopt a stereospecific, numbering (sn) and prefix glycerol with sn., 1, , CH2OH, HO, , 2, , C, , H, , 3, , CH2OH, sn-Glycerol
Page 43 :
33, , Chapter 3 : LIPIDS, , It should be noted that C1 and C3 are, different. Cells possess enzymes that can, distinguish, these, two, carbons., Thus, glycerokinase phosphorylates sn-3 (and not sn-1), glycerol to give sn-glycerol 3-phosphate., , PROPERTIES OF TRIACYLGLYCEROLS, A few important properties of triacylglycerols,, which have biochemical relevance, are, discussed below, 1. Hydrolysis : Triacylglycerols undergo, stepwise enzymatic hydrolysis to finally liberate, free fatty acids and glycerol. The process of, hydrolysis, catalysed by lipases is important for, digestion of fat in the gastrointestinal tract and, fat mobilization from the adipose tissues., 2. Saponification : The hydrolysis of triacylglycerols by alkali to produce glycerol and soaps, is known as saponification., Triacylglycerol + 3 NaOH o, Glycerol + 3 R-COONa (soaps), 3. Rancidity : Rancidity is the term used to, represent the deterioration of fats and oils, resulting in an unpleasant taste. Fats containing, unsaturated fatty acids are more susceptible to, rancidity., Rancidity occurs when fats and oils are, exposed to air, moisture, light, bacteria etc., Hydrolytic rancidity occurs due to partial, hydrolysis of triacylglycerols by bacterial, enzymes. Oxidative rancidity is due to oxidation, of unsaturated fatty acids. This results in the, formation of unpleasant products such as, dicarboxylic acids, aldehydes, ketones etc., Rancid fats and oils are unsuitable for human, consumption., Antioxidants : The substances which can, prevent the occurrence of oxidative rancidity are, known as antioxidants. Trace amounts of, antioxidants such as tocopherols (vitamin E),, hydroquinone, gallic acid and D-naphthol are, added to the commercial preparations of fats and, oils to prevent rancidity. Propyl gallate, butylated, hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are the antioxidants used in food, preservation., , 4. Lipid peroxidation in vivo : In the living, cells, lipids undergo oxidation to produce, peroxides and free radicals which can damage, the tissue. The free radicals are believed to cause, inflammatory, diseases,, ageing,, cancer,, atherosclerosis etc. It is fortunate that the cells, possess antioxidants such as vitamin E, urate and, superoxide dismutase to prevent in vivo lipid, peroxidation (Chapter 34)., , Tests to check purity, of fats and oils, Adulteration of fats and oils is increasing day, by day. Several tests are employed in the, laboratory to check the purity of fats and oils., Some of them are discussed hereunder, Iodine number : It is defined as the grams, (number) of iodine absorbed by 100 g of fat or, oil. Iodine number is useful to know the relative, unsaturation of fats, and is directly proportional, to the content of unsaturated fatty acids. Thus, lower is the iodine number, less is the degree of, unsaturation. The iodine numbers of common, oils/fats are given below., Fat/oil, Coconut oil, Butter, Palm oil, Olive oil, Groundnut oil, Cottonseed oil, Sunflower oil, Linseed oil, , Iodine number, 7, 25, 45, 80, 85, 100, 125, 175, , — 10, — 28, — 55, — 85, — 100, — 110, — 135, — 200, , Determination of iodine number will help to, know the degree of adulteration of a given oil., Saponification number : It is defined as the, mg (number) of KOH required to hydrolyse, (saponify) one gram of fat or oil. Saponification, number is a measure of the average molecular, size of the fatty acids present. The value is higher, for fats containing short chain fatty acids. The, saponification numbers of a few fats and oils are, given below, Human fat : 195–200, Butter, : 230–240, Coconut oil : 250–260
Page 44 :
34, , BIOCHEMISTRY, , Reichert-Meissl (RM) number : It is defined as, the number of ml 0.1 N KOH required to, completely neutralize the soluble volatile fatty, acids distilled from 5 g fat. RM number is useful, in testing the purity of butter since it contains a, good concentration of volatile fatty acids (butyric, acid, caproic acid and caprylic acid). This is in, contrast to other fats and oils which have a, negligible amount of volatile fatty acids. Butter, has a RM number in the range 25-30, while it is, less than 1 for most other edible oils. Thus any, adulteration of butter can be easily tested by, this sensitive RM number., Acid number : It is defined as the number of, mg of KOH required to completely neutralize, free fatty acids present in one gram fat or oil., In normal circumstances, refined oils should, be free from any free fatty acids. Oils, on, decomposition—due to chemical or bacterial, contamination—yield free fatty acids. Therefore,, oils with increased acid number are unsafe for, human consumption., , PHOSPHOLIPIDS, These are complex or compound lipids, containing phosphoric acid, in addition to fatty, acids, nitrogenous base and alcohol (Fig.3.3)., , There are two classes of phospholipids, 1. Glycerophospholipids (or phosphoglycerides) that contain glycerol as the alcohol., 2. Sphingophospholipids (or sphingomyelins), that contain sphingosine as the alcohol., , Glycerophospholipids, Glycerophospholipids are the major lipids, that occur in biological membranes. They consist, of glycerol 3-phosphate esterified at its C1 and, C2 with fatty acids. Usually, C1 contains a, saturated fatty acid while C2 contains an, unsaturated fatty acid., 1. Phosphatidic acid : This is the simplest, phospholipid. It does not occur in good, concentration in the tissues. Basically,, phosphatidic acid is an intermediate in the, synthesis of triacylglycerols and phospholipids., The other glycerophospholipids containing, different nitrogenous bases or other groups may, be regarded as the derivatives of phosphatidic, acid., 2. Lecithins (phosphatidylcholine): These are, the most abundant group of phospholipids in the, cell membranes. Chemically, lecithin (Greek :, lecithos—egg yolk) is a phosphatidic acid with, choline as the base. Phosphatidylcholines, represent the storage form of body’s choline., , + Lipids are important to the body as constituents of membranes, source of fat soluble, (A, D, E and K) vitamins and metabolic regulators (steroid hormones and prostaglandins)., , + Triacylglycerols (fats) primarily stored in the adipose tissue are concentrated fuel, reserves of the body. Fats found in the subcutaneous tissue and around certain organs, serve as thermal insulators., , + The unsaturated fatty acids–linoleic and linolenic acid—are essential to humans, the, deficiency of which causes phrynoderma or toad skin., , + The cyclic fatty acid, namely chaulmoogric acid,is employed in the treatment of leprosy., + Fats and oils on exposure to air, moisture, bacteria etc. undergo rancidity (deterioration)., This can be prevented by the addition of certain antioxidants (vitamin E, hydroquinone,, gallic acid)., , + In food preservation, antioxidants—namely propyl gallate, butylated hydroxyanisole, and butylated hydroxytoluene—are commonly used.
Page 46 :
36, , BIOCHEMISTRY, , (a) Dipalmitoyl lecithin is an important, phosphatidylcholine found in lungs. It is a, surface active agent and prevents the, adherence of inner surface of the, lungs due to surface tension. Respiratory, distress syndrome in infants is a disorder, characterized by the absence of dipalmitoyl, lecithin., (b) Lysolecithin is formed by removal of the, fatty acid either at C1 or C2 of lecithin., 3. Cephalins (phosphatidylethanolamine) :, Ethanolamine is the nitrogenous base present in, cephalins. Thus, lecithin and cephalin differ with, regard to the base., 4. Phosphatidylinositol : The steroisomer, myo-inositol is attached to phosphatidic acid to, give phosphatidylinositol(PI). This is an important, component of cell membranes. The action of, certain hormones (e.g. oxytocin, vasopressin) is, mediated through PI., 5. Phosphatidylserine : The amino acid, serine is present in this group of glycerophospholipids. Phosphatidylthreonine is also found in, certain tissues., 6. Plasmalogens : When a fatty acid is, attached by an ether linkage at C1 of glycerol in, the, glycerophospholipids,, the, resultant, compound is plasmalogen. Phosphatidalethanolamine is the most important which is, similar in structure to phosphatidylethanolamine, but for the ether linkage (in place of ester). An, unsaturated fatty acid occurs at C1. Choline,, inositol and serine may substitute ethanolamine, to give other plasmalogens., 7. Cardiolipin : It is so named as it was first, isolated from heart muscle. Structurally, a, cardiolipin consists of two molecules of, phosphatidic acid held by an additional glycerol, through phosphate groups. It is an important, component of inner mitochondrial membrane, and essential for mitrochondrial function., Decreased cardiolipin levels may result in, mitochondrial, dysfunction,, aging,, hypothyroidism, cardioskeletal myopathy (Barth, syndrome). Cardiolipin is the only phosphoglyceride that possesses antigenic properties., , Sphingomyelins, Sphingosine is an amino alcohol present in, sphingomyelins (sphingophospholipids). They do, not contain glycerol at all. Sphingosine is attached, by an amide linkage to a fatty acid to produce, ceramide. The alcohol group of sphingosine is, bound to phosphorylcholine in sphingomyelin, structure (Fig.3.3). Sphingomyelins are important, constituents of myelin and are found in good, quantity in brain and nervous tissues., , Ceramide, acts as a second messenger, (signaling molecule) by regulating programmed, cell death (apoptosis), cell cycle and cell, differentiation. A ceramide containing a, 30-carbon fatty acid is a major component of, skin, and it regulates skin’s water permeability., , Functions of phospholipids, Phospholipids constitute an important group, of compound lipids that perform a wide variety, of functions, 1. In association with proteins, phospholipids, form the structural components of membranes, and regulate membrane permeability., 2. Phospholipids (lecithin, cephalin and, cardiolipin) in the mitochondria maintain the, conformation of electron transport chain, components, and thus cellular respiration., 3. Phospholipids participate in the absorption, of fat from the intestine., 4. Phospholipids are essential for the, synthesis of different lipoproteins, and thus, participate in the transport of lipids., 5. Accumulation of fat in liver (fatty liver) can, be prevented by phospholipids, hence they are, regarded as lipotropic factors., 6. Arachidonic acid, an unsaturated fatty acid, liberated from phospholipids, serves as a, precursor for the synthesis of eicosanoids (prostaglandins, prostacyclins, thromboxanes etc.)., 7. Phospholipids participate in the reverse, cholesterol transport and thus help in the, removal of cholesterol from the body., 8. Phospholipids act as surfactants (agents, lowering surface tension). For instance,, dipalmitoyl phosphatidylcholine is an important
Page 47 :
37, , Chapter 3 : LIPIDS, , Sphingosine, , OH, , O, , CH3 (CH3)12 CH CH CH CH NH C CH(OH) (CH2)21 CH3, , HO, H, , Cerebronic acid, (fatty acid), , CH2OH, O, H, OR, , H, , H, , OH, , O CH2, H, , Fig. 3.4 : Structure of galactosylceramide (R = H). For sulfagalactosylceramide R is a sulfatide (R = SO42–)., , lung surfactant. Respiratory distress syndrome in, infants is associated with insufficient production, of this surfactant., , sequence attached to the ceramide). The, ganglioside, GM2 that accumulates in Tay-Sachs, disease is represented next (outline structure)., , 9. Cephalins, an important group of phospholipids participate in blood clotting., , Ceramide, , 10. Phosphatidylinositol is the source of, second messengers—inositol triphosphate and, diacylglyceol, that are involved in the action of, some horomones., , GLYCOLIPIDS, Glycolipids (glycosphingolipids) are important, constituents of cell membrane and nervous, tissues (particularly the brain). Cerebrosides are, the simplest form of glycolipids. They contain a, ceramide (sphingosine attached to a fatty acid), and one or more sugars. Galactocerebroside, (galactosylceramide) and glucocerebroside are, the most important glycolipids. The structure, of galactosylceramide is given in Fig.3.4. It, contains the fatty acid cerebronic acid., Sulfagalactosylceramide is the sulfatide derived, from galactosylceramide., Gangliosides : These are predominantly found, in ganglions and are the most complex form of, glycosphingolipids. They are the derivatives of, cerebrosides and contain one or more molecules, of N-acetylneuraminic acid (NANA), the most, important sialic acid. The structure of NANA is, given in carbohydrate chemistry (Refer Fig.2.11)., The most important gangliosides present in, the brain are GM1, GM2, GD, and GT,, (G represents ganglioside while M, D and T, indicate mono-, di- or tri- sialic acid residues,, and the number denotes the carbohydrate, , Glucose, Galactose, N-Acetylgalactosamine, , N-Acetylneuraminic acid, , LIPOPROTEINS, Lipoproteins are molecular complexes of, lipids with proteins. They are the transport, vehicles for lipids in the circulation. There are, five types of lipoproteins, namely chylomicrons,, very low density lipoproteins (VLDL), low, density lipoproteins (LDL), high density, lipoproteins (HDL) and free fatty acidalbumin complexes. Their structure, separation,, metabolism and diseases are discussed together, (Chapter 14)., , STEROIDS, Steroids are the compounds containing a, cyclic steroid nucleus (or ring) namely, cyclopentanoperhydrophenanthrene (CPPP). It, consists of a phenanthrene nucleus (rings A, B, and C) to which a cyclopentane ring (D) is, attached., The structure and numbering of CPPP are, shown in Fig.3.5. The steroid nucleus represents, saturated carbons, unless specifically shown as
Page 48 :
38, , BIOCHEMISTRY, , 18, , 12, 11, 1, , 19, , 9, , 13, , C, , A, 3, , 10, , The structure of cholesterol (C27H46O) is, depicted in Fig.3.5. It has one hydroxyl group at, C3 and a double bond between C5 and C6., An 8 carbon aliphatic side chain is attached to, C17. Cholesterol contains a total of 5 methyl, groups., , 8, , B, 7, , 5, 4, , 16, , D, , 15, , 14, , 2, , Structure and occurrence, , 17, , Steroid nucleus, , 6, 22, 21, 20, 18, , 17, , 23, , 26, , 25, 24, , 27, , 19, , Cholesterol, , 3, 5, , HO, , 6, , Cholesterol is found in association with fatty, acids to form cholesteryl esters (esterification, occurs at the OH group of C3)., , 8, 3, , HO, , 7, , 5, , Due to the presence of an –OH group,, cholesterol is weakly amphiphilic. As a structural, component of plasma membranes, cholesterol, is an important determinant of membrane, permeability properties., The occurrence of, cholesterol is much higher in the membranes of, sub-cellular organelles., , Ergosterol, , 6, , Fig. 3.5 : Structures of steroids (A, B, C—Perhydrophenanthrene; D-Cyclopentane)., , double bonds. The methyl side chains (19 & 18), attached to carbons 10 & 13 are shown as single, bonds. At carbon 17, steroids usually contain a, side chain., There are several steroids in the biological, system. These include cholesterol, bile acids,, vitamin D, sex hormones, adrenocortical, hormones , sitosterols, cardiac glycosides and, alkaloids. If the steroid contains one or more, hydroxyl groups it is commonly known as, sterol (means solid alcohol)., , CHOLESTEROL, Cholesterol, exclusively found in animals, is, the most abundant animal sterol. It is widely, distributed in all cells and is a major component, of cell membranes and lipoproteins. Cholesterol, (Greek : chole–bile) was first isolated from bile., Cholesterol literally means ‘solid alcohol from, bile.’, , Properties and reactions : Cholesterol is an, yellowish crystalline solid. The crystals, under, the microscope, show a notched ( ), appearance. Cholesterol is insoluble in water, and soluble in organic solvents such as, chloroform, benzene, ether etc., Several reactions given by cholesterol are, useful for its qualitative identification and, quantitative estimation. These include Salkowski’s, test, Liebermann-Burchard reaction and Zak’s, test., Functions of cholesterol : Cholesterol is a, poor conductor of heat and electricity, since it, has a high dielectric constant. It is present in, abundance in nervous tissues. It appears that, cholesterol functions as an insulating cover for, the transmission of electrical impulses in the, nervous tissue. Cholesterol performs several, other biochemical functions which include its, role in membrane structure and function, in the, synthesis of bile acids, hormones (sex and, cortical) and vitamin D (for details, Refer, Chapters 7 and 19)., , ERGOSTEROL, Ergosterol occurs in plants. It is also found as, a structural constituent of membranes in yeast, and fungi. Ergosterol (Fig.3.5) is an important, precursor for vitamin D. When exposed to light,
Page 49 :
39, , Chapter 3 : LIPIDS, , the ring B of ergosterol opens and it is converted, to ergocalciferol, a compound containing, vitamin D activity., , CH3(CH2)n COO–, Hydrophiic, Hydrophobic, carboxyl group, hydrocarbon chain, (head), (tail), (A) Fatty acid, , The other sterols present in plant cells include, stigmasterol and E-sitosterol., , O, R1 C CH2, O, , AMPHIPATHIC LIPIDS, , R2 C CH, , As per definition, lipids are insoluble (hydrophobic) in water. This is primarily due to the, predominant presence of hydrocarbon groups., However, some of the lipids possess polar or, hydrophilic groups which tend to be soluble in, water., Molecules, which, contain, both, hydrophobic and hydrophilic groups are known, as amphipathic (Greek : amphi-both, pathos—, passion)., , CH2 P choline, Hydrophobic, tail, , Hydrophilic, head, , (B) Phospholipid, , Non-polar, Polar head, tail, (C) Amphipathic lipid, , Examples of amphipathic lipids : Among the, lipids, fatty acids, phospholipids, sphingolipids,, bile salts and cholesterol (to some extent) are, amphipathic in nature., , Aqueous, phase, Non polar, phase, , Phospholipids have a hydrophilic head (phosphate group attached to choline, ethanolamine,, inositol etc.) and a long hydrophobic tail. The, general structure of an amphipathic lipid may be, represented as a polar or hydrophilic head with, a non-polar or hydrophobic tail (Fig.3.6)., Fatty acids contain a hydrocarbon chain with, a carboxyl (COO–) group at physiological pH., The carboxyl group is polar in nature with, affinity to water (hydrophilic) while hydrocarbon, chain of fatty acid is hydrophobic., , (D) Micelle, , Aqueous phase, , Nonpolar phase, , Aqueous phase, (E) Lipid bilayer, , Fig. 3.6 : Summary of amphipathic lipids in the, formation of micelle and lipid bilayer., , Orientation of amphipathic lipids : When the, amphipathic lipids are mixed in water (aqueous, phase), the polar groups (heads) orient, themselves towards aqueous phase while the, non-polar (tails) orient towards the opposite, directions. This leads to the formation of micelles, (Fig.3.6)., Micelles are primarily molecular aggregates, of amphipathic lipids. Micelle formation,, facilitated by bile salts, is very important for lipid, digestion and absorption (Chapter 8).
Page 50 :
40, , BIOCHEMISTRY, , + The phospholipid–dipalmitoyl lecithin—prevents the adherence of inner surface of the, lungs, the absence of which is associated with respiratory distress syndrome in infants., , + Cephalins participate in blood clotting., + The action of certain hormones is mediated through phosphatidylinositol., + Phospholipids are important for the synthesis and transport of lipoproteins and reverse, transport of cholesterol., , + Cholesterol is essential for the synthesis of bile acids, hormones (sex and cortical) and, vitamin D., , + Lipoproteins occur in the membrane structure, besides serving as a means of transport, vehicles for lipids., , + Lipids are associated with certain disorders—obesity, atherosclerosis, and diabetes, mellitus., , + Liposomes are used for administration of a variety of therapeutic substances (drugs,, proteins, nucleic acids) in order to target specific organs or tissues., , Membrane bilayers, In case of biological membranes, a bilayer of, lipids is formed orienting the polar heads to the, outer aqueous phase on either side and the, nonpolar tails into the interior (Fig.3.6). The, formation of a lipid bilayer is the basis of, membrane structure., Liposomes : They are produced when amphipathic lipids in aqueous medium are subjected, to sonification. They have intermittent aqueous, phases in the lipid bilayer. Liposomes, in, combination with tissue specific antigens, are, used as carriers of drugs to target tissues., Emulsions : These are produced when nonpolar lipids (e.g. triacylglycerols) are mixed, with water. The particles are larger in size and, stabilized by emulsifying agents (usually, , amphipathic lipids), such as bile salts and, phospholipids., , SOAPS AND DETERGENTS, Soaps are sodium or potassium salts of, fatty acids. They are produced by saponification, of fats. Sodium soaps are hard that result in, bar soaps. Soaps serve as cleansing agents, since they can emulsify oils and remove the, dirt., , Detergents, Detergents are synthetic cleansing agents e.g., sodium lauryl sulfate. Detergents are superior in, their cleansing action compared to soaps, and, are used in washing clothes, and in tooth, paste.
Page 51 :
Chapter 3 : LIPIDS, , 1. Lipids are the organic substances relatively insoluble in water, soluble in organic, solvents (alcohol, ether), actually or potentially related to fatty acids and are utilized, by the body., 2. Lipids are classified into simple (fats and oils), complex (phospholipids, glycolipids),, derived (fatty acids, steriod hormones) and miscellaneous (carotenoids)., 3. Fatty acids are the major constituents of various lipids. Saturated and unsaturated fatty, acids almost equally occur in natural lipids. The polyunsaturated fatty acids (PUFA), namely linoleic acid and linolenic acid are the essential fatty acids that need to be, supplied in the diet., 4. Triacylglycerols (simply fats) are the esters of glycerol with fatty acids. They are found, in adipose tissue and primarily function as fuel reserve of animals. Several tests (iodine, number, RM number) are employed in the laboratory to test the purity of fats and oils., 5. Phospholipids are complex lipids containing phosphoric acid. Glycerophospholipids, contain glycerol as the alcohol and these include lecithin, cephalin, phosphatidylinositol,, plasmalogen and cardiolipin., 6. Sphingophospholipids (sphingomyelins) contain sphingosine as the alcohol in place of, glycerol (in glycerophospholipids). Phospholipids are the major constituents of plasma, membranes., 7. Cerebrosides are the simplest form of glycolipids which occur in the membranes of, nervous tissue. Gangliosides are predominantly found in the ganglions. They contain, one or more molecules of N-acetylneuraminic acid (NANA)., 8. Steroids contain the ring cyclopentanoperhydrophenanthrene. The steroids of biological, importance include cholesterol, bile acids, vitamin D, sex hormones and cortical, hormones. A steroid containing one or more hydroxyl groups is known as sterol., 9. Cholesterol is the most abundant animal sterol. It contains one hydroxyl group (at C3),, a double bond (C5–C6) and an eight carbon side chain attached to C17. Cholesterol is, a constituent of membrane structure and is involved in the synthesis of bile acids,, hormones (sex and cortical) and vitamin D., 10. The lipids that possess both hydrophobic (non -polar) and hydrophilic (polar) groups are, known as amphipathic. These include fatty acids, phospholipids, sphingolipids and bile, salts. Amphipathic lipids are important constituents in the bilayers of the biological, membranes., , 41
Page 52 :
42, , BIOCHEMISTRY, , I. Essay questions, 1. Write an account of classification of lipids with suitable examples., 2. Describe the structure and functions of phospholipids., 3. Discuss the saturated and unsaturated fatty acids of biological importance, along with their structures., 4. Describe the structure of steroids. Add a note on the functions of cholesterol., 5. Discuss the biological importance of amphipathic lipids., , II. Short notes, (a) Structure of triacylglycerols, (b) Glycolipids, (c) Essential fatty acids, (d) Cis–trans isomerism,, (e) Rancidity, (f) Iodine number, (g) Phosphatidylinositol, (h) Sphingomyelins, (i) Steroid nucleus,, (j) Micelles., , III. Fill in the blanks, 1. The lipids that function as fuel reserve in animals _____________., 2. The isomerism associated with unsaturated fatty acids _____________., 3. The cyclic fatty acid employed in the treatment of leprosy _____________., 4. The lipids that are not the structural components of biological membranes _____________., 5. The prefix sn used to represent glycerol, sn stands for _____________., 6. The number of mg of KOH required to hydrolyse 1 g fat or oil is known as _____________., 7. The phospholipid that prevents the adherence of inner surfaces of lungs _____________., 8. The phospholipid that produces second messengers in hormonal action _____________., 9. Name the glycolipids containing N-acetylneuraminic acid _____________., 10. The steroids contain a cyclic ring known as _____________., , IV. Multiple choice questions, 11. The nitrogenous base present in lecithin, (a) Choline (b) Ethanolamine (c) Inositol (d) Serine., 12. The number of double bonds present in arachidonic acid, (a) 1 (b) 2 (c) 3 (d) 4., 13. One of the following is an amphipathic lipid, (a) Phospholipids (b) Fatty acid (c) Bile salts (d) All of the above., 14. Esterification of cholesterol occurs at carbon position, (a) 1 (b) 2 (c) 3 (d) 4., 15. Name the test employed to check the purity of butter through the estimation of volatile fatty acids, (a) Iodine number (b) Reichert-Meissl number (c) Saponification number (d) Acid number.
Page 53 :
Section 1, , Chemical Constituents of Life, , Chapter, , Proteins and Amino Acids, , 14, , The proteins speak :, , “We are the basis of structure and function of life;, Composed of twenty amino acids, the building blocks;, Organized into primary, secondary, tertiary, and quaternary structure;, Classified as simple, conjugated and derived proteins.”, , P, , roteins are the most abundant organic, molecules of the living system. They occur, in every part of the cell and constitute about, 50% of the cellular dry weight. Proteins form, the fundamental basis of structure and function, of life., , Origin of the word ‘protein’, The term protein is derived from a Greek, word proteios, meaning holding the first place., Berzelius (Swedish chemist) suggested the name, proteins to the group of organic compounds that, are utmost important to life. Mulder (Dutch, chemist) in 1838 used the term proteins for the, high molecular weight nitrogen-rich and most, abundant substances present in animals and, plants., , Functions of proteins, Proteins perform a great variety of specialized, and essential functions in the living cells. These, functions may be broadly grouped as static, (structural) and dynamic., , Structural functions : Certain proteins perform, brick and mortar roles and are primarily, responsible for structure and strength of body., These include collagen and elastin found in bone, matrix, vascular system and other organs and, D-keratin present in epidermal tissues., Dynamic functions : The dynamic functions, of proteins are more diversified in nature. These, include proteins acting as enzymes, hormones,, blood clotting factors, immunoglobulins,, membrane receptors, storage proteins, besides, their function in genetic control, muscle, contraction, respiration etc. Proteins performing, dynamic functions are appropriately regarded as, the working horses of cell., , Elemental composition of proteins, Proteins are predominantly constituted by five, major elements in the following proportion., Carbon, :, 50 – 55%, Hydrogen, :, 6 – 7.3%, Oxygen, :, 19 – 24%, Nitrogen, :, 13 – 19%, Sulfur, :, 0 – 4%, , 43
Page 54 :
44, , BIOCHEMISTRY, , Besides the above, proteins may also contain, other elements such as P, Fe, Cu, I, Mg, Mn, Zn etc., The content of nitrogen, an essential, component of proteins, on an average is 16%., Estimation of nitrogen in the laboratory (mostly, by Kjeldahl’s method) is also used to find out the, amount of protein in biological fluids and foods., , Proteins are polymers of amino acids, Proteins on complete hydrolysis (with concentrated HCl for several hours) yield L-D-amino, acids. This is a common property of all the, proteins. Therefore, proteins are the polymers of, L-D-amino acids., , STANDARD AMINO ACIDS, As many as 300 amino acids occur in nature—, Of these, only 20—known as standard amino, acids are repeatedly found in the structure of, proteins, isolated from different forms of life—, animal, plant and microbial. This is because of, the universal nature of the genetic code available, for the incorporation of only 20 amino acids, when the proteins are synthesized in the cells., The process in turn is controlled by DNA, the, genetic material of the cell. After the synthesis of, proteins, some of the incorporated amino acids, undergo modifications to form their derivatives., , AMINO ACIDS, Amino acids are a group of organic, compounds containing two functional groups—, amino and carboxyl. The amino group (—NH2), is basic while the carboxyl group (—COOH) is, acidic in nature., , General structure of amino acids, The amino acids are termed as D-amino acids,, if both the carboxyl and amino groups are, attached to the same carbon atom, as depicted, below, H, , D, , R C COOH, , H, , D, , –, , R C COO, +, , NH2, , NH3, , General structure, , Exists as ion, , The D-carbon atom binds to a side chain, represented by R which is different for each of, the 20 amino acids found in proteins. The amino, acids mostly exist in the ionized form in the, biological system (shown above)., , Optical isomers of amino acids, If a carbon atom is attached to four different, groups, it is asymmetric and therefore exhibits, optical isomerism. The amino acids (except, glycine) possess four distinct groups (R, H,, +, COO–, NH3 ) held by D-carbon. Thus all the, amino acids (except glycine where R = H) have, optical isomers., The structures of L- and D-amino acids are, written based on the configuration of L- and, D-glyceraldehyde as shown in Fig.4.1. The, proteins are composed of L-D-amino acids., , Classification of amino acids, There are different ways of classifying the, amino acids based on the structure and chemical, nature, nutritional requirement, metabolic fate etc., A. Amino acid classification based on the, structure : A comprehensive classification of, amino acids is based on their structure and, chemical nature. Each amino acid is assigned a, 3 letter or 1 letter symbol. These symbols are, commonly used to represent the amino acids in, protein structure. The 20 amino acids found in, proteins are divided into seven distinct groups., In Table 4.1, the different groups of amino, acids, their symbols and structures are given. The, salient features of different groups are described, next, CHO, H C OH, CH2OH, D-Glyceraldehyde, , R, H C NH2, COOH, D-Amino acid, , CHO, OH C H, CH2OH, L-Glyceraldehyde, , R, H2N C H, COOH, L-Amino acid, , Fig. 4.1 : D- and L-forms of amino acid based on the, structure of glyceraldehyde.
Page 55 :
45, , Chapter 4 : PROTEINS AND AMINO ACIDS, , TABLE 4.1 Structural classification of L-D-amino acids found in proteins, , Name, , Symbol, 3 letters, , I., , Structure, , Special group present, , 1 letter, , Amino acids with aliphatic side chains, , 1. Glycine, , Gly, , G, , H CH COO–, +, NH 3, , 2. Alanine, , Ala, , A, , CH 3 CH COO, +, NH 3, , 3. Valine, , Val, , V, , 4. Leucine, , Leu, , L, , 5. Isoleucine, , Ile, , –, , H3C, , CH CH COO–, +, H3C, NH 3, , Branched chain, , H3C, , CH CH 2 CH COO–, +, H3C, NH 3, , CH 3, CH 2, –, CH CH COO, +, H3C, NH 3, , I, , Branched chain, , Branched chain, , II. Amino acids containing hydroxyl (—OH) groups, , –, , Hydroxyl, , 6. Serine, , Ser, , S, , CH 2 CH COO, +, OH NH 3, , 7. Threonine, , Thr, , T, , H3C CH CH COO, +, OH NH 3, , Tyr, , Y, , Tyrosine, , –, , See under aromatic, , Hydroxyl, , Hydroxyl, Table 4.1 contd. next page
Page 56 :
46, , BIOCHEMISTRY, , Name, , Symbol, 3 letters, , Structure, , Special group present, , 1 letter, , III. Sulfur containing amino acids, 8. Cysteine, , Cys, , C, , –, , Sulfhydryl, , CH 2 CH COO, +, SH NH 3, , –, , CH 2 CH COO, +, S, NH 3, Cystine, , —, , —, , S, , Disulfide, , CH 2 CH COO–, +, NH 3, 9. Methionine, , Met, , M, , –, , Thioether, , CH 2 CH2 CH COO, +, S CH 3, NH 3, , IV. Acidic amino acids and their amides, D, , E, , –, , OOC CH2 CH COO, +, NH 3, , –, , E-Carboxyl, , 10. Aspartic acid, , Asp, , D, , 11. Asparagine, , Asn, , N, , –, H2N C CH 2 CH COO, +, O, NH 3, , 12. Glutamic acid Glu, , E, , –, , 13. Glutamine, , Gln, , Q, , H2N C CH2 CH2 CH COO, +, O, NH 3, , Lys, , K, , CH 2 CH 2 CH 2 CH 2 CH COO, +, +, NH 3, NH 3, , J, , E, , D, , Amide, , –, , J-Carboxyl, , OOC CH2 CH2 CH COO, +, NH 3, , –, , Amide, , V. Basic amino acids, , 14. Lysine, , H, , J, , G, , E, , D, , –, , H-Amino, , –, , 15. Arginine, , Arg, , R, , NH CH 2 CH2 CH 2 CH COO, +, C NH+2, NH 3, , Guanidino, , NH2, CH2 CH COO–, , 16. Histidine, , His, , H, , +, , HN, , N, , NH 3, , Imidazole, Table 4.1 contd. next page
Page 57 :
47, , Chapter 4 : PROTEINS AND AMINO ACIDS, , Name, , Symbol, 3 letters, , Structure, , Special group present, , 1 letter, , VI. Aromatic amino acids, , 17. Phenylalanine Phe, , –, , F, , Benzene or phenyl, , CH2 CH COO, , +, NH, 3, , 18. Tyrosine, , Tyr, , Y, , HO, , CH2, , CH COO–, +, NH 3, , Phenol, , –, , CH 2 CH COO, , 19. Tryptophan, , Trp, , +, , W, , Indole, , NH 3, N, H, , VII. Imino acid, H2C, , 20. Proline, , Pro, , P, , H2C, N, H, , CH 2, H, or, C, –, COO, , –, , N, , COO, , Pyrrolidine, , H, , (Note : R group is shown in red), , 1. Amino acids with aliphatic side chains :, These are monoamino monocarboxylic, acids. This group consists of the most, simple amino acids—glycine, alanine,, valine, leucine and isoleucine. The last, three amino acids (Leu, Ile, Val) contain, branched aliphatic side chains, hence, they are referred to as branched chain, amino acids., 2. Hydroxyl group containing amino acids :, Serine, threonine and tyrosine are, hydroxyl group containing amino acids., Tyrosine—being aromatic in nature—is, usually considered under aromatic amino, acids., 3. Sulfur containing amino acids : Cysteine, with sulfhydryl group and methionine, with thioether group are the two amino, acids incorporated during the course of, , protein synthesis. Cystine, another, importa nt sulfur containing amino acid,, is formed by condensation of two, molecules of cysteine., 4. Acidic amino acids and their amides :, Aspartic acid and glutamic acids are, dicarboxylic monoamino acids while, asparagine and glutamine are their, respective amide derivatives. All these, four amino acids possess distinct codons, for their incorporation into proteins., 5. Basic amino acids : The three amino acids, lysine, arginine (with guanidino group), and histidine (with imidazole ring) are, dibasic monocarboxylic acids. They are, highly basic in character., 6. Aromatic amino acids : Phenylalanine,, tyrosine and tryptophan (with indole ring)
Page 58 :
48, , BIOCHEMISTRY, , are aromatic amino acids. Besides these,, histidine may also be considered under, this category., 7. Imino acids : Proline containing pyrrolidine, ring is a unique amino acid. It has an, imino group ( NH), instead of an amino, group ( NH2) found in other amino acids., Therefore, proline is an D-imino acid., Heterocyclic amino acids : Histidine,, tryptophan and proline., B. Classification of amino acids based on, polarity : Amino acids are classified into 4, groups based on their polarity. Polarity is, important for protein structure., 1. Non-polar amino acids : These amino, acids are also referred to as hydrophobic, (water hating). They have no charge on, the ‘R’ group. The amino acids included, in this group are — alanine, leucine,, isoleucine, valine, methionine, phenylalanine, tryptophan and proline., 2. Polar amino acids with no charge on ‘R’, group : These amino acids, as such, carry, no charge on the ‘R’ group. They however, possess groups such as hydroxyl,, sulfhydryl and amide and participate in, hydrogen bonding of protein structure., The simple amino acid glycine (where, R = H) is also considered in this category., The amino acids in this group are—, glycine, serine, threonine, cysteine,, glutamine, asparagine and tyrosine., 3. Polar amino acids with positive ‘R’ group :, The three amino acids lysine, arginine, and histidine are included in this group., 4. Polar amino acids with negative ‘R’ group :, The dicarboxylic monoamino acids—, aspartic acid and glutamic acid are, considered in this group., C. Nutritional classification of amino acids :, The 20 amino acids (Table 4.1) are required for, the synthesis of variety proteins, besides other, biological functions. However, all these 20, amino acids need not be taken in the diet. Based, on the nutritional requirements, amino acids are, grouped into two classes—essential and nonessential., , 1. Essential or indispensable amino acids :, The amino acids which cannot be, synthesized by the body and, therefore,, need to be supplied through the diet are, called essential amino acids. They are, required for proper growth and, maintenance of the individual. The ten, amino acids listed below are essential for, humans (and also rats) :, Arginine, Valine, Histidine, Isoleucine,, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan., [The code A.V. HILL, MP., T. T. (first letter, of each amino acid) may be memorized, to recall essential amino acids. Other, useful codes are H. VITTAL, LMP; PH., VILLMA, TT, PVT TIM HALL and, MATTVILPhLy.], The two amino acids namely arginine and, histidine can be synthesized by adults, and not by growing children, hence these, are considered as semi–essential amino, acids (remember Ah, to recall). Thus, 8, amino acids are absolutely essential while, 2 are semi-essential., 2. Non-essential or dispensable amino, acids : The body can synthesize about 10, amino acids to meet the biological needs,, hence they need not be consumed in the, diet. These are—glycine, alanine, serine,, cysteine, aspartate, asparagine, glutamate,, glutamine, tyrosine and proline., D. Amino acid classification based on their, metabolic fate : The carbon skeleton of amino, acids can serve as a precursor for the synthesis, of glucose (glycogenic) or fat (ketogenic) or both., From metabolic view point, amino acids are, divided into three groups (for details, Refer, Chapter 15)., 1. Glycogenic amino acids : These amino, acids can serve as precursors for the, formation of glucose or glycogen. e.g., alanine, aspartate, glycine, methionine etc., 2. Ketogenic amino acids : Fat can be, synthesized from these amino acids. Two, amino acids leucine and lysine are, exclusively ketogenic.
Page 59 :
49, , Chapter 4 : PROTEINS AND AMINO ACIDS, , 3. Glycogenic and ketogenic amino acids :, The four amino acids isoleucine, phenylalanine, tryptophan, tyrosine are precursors for synthesis of glucose as well as, fat., , Selenocysteine – the 21st amino acid, As already stated, 20 amino acids are, commonly found in proteins. In recent years, a, 21st amino acid namely selenocysteine has been, added. It is found at the active sites of certain, enzymes/proteins (selenoproteins). e.g. glutathione peroxidase, glycine reductase, 5c-deiodinase, thioredoxin reductase. Selenocysteine is, an unusual amino acid containing the trace, element selenium in place of the sulfur atom of, cysteine., CH2 CH COO–, SH, , +, NH3, , Cysteine, , CH2 CH COO–, +, , SeH NH3, , Selenocysteine, , Incorporation of selenocysteine into the, proteins during translation is carried out by the, codon namely UGA. It is interesting to note that, UGA is normally a stop codon that terminates, protein biosynthesis. Another unique feature is, that selenocysteine is enzymatically generated, from serine directly on the tRNA (selenocysteinetRNA), and then incorporated into proteins., Pyrrolysine – the 22nd amino acid? : In the, year 2002, some researchers have described yet, another amino acid namely pyrrolysine as the, 22nd amino acid present in protein. The stop, codon UAG can code for pyrrolysine., , Properties of amino acids, The amino acids differ in their physico–, chemical properties which ultimately determine, the characteristics of proteins., , A. Physical properties, 1. Solubility : Most of the amino acids are, usually soluble in water and insoluble in organic, solvents., 2. Melting points : Amino acids generally, melt at higher temperatures, often above 200°C., , 3. Taste : Amino acids may be sweet (Gly,, Ala, Val), tasteless (Leu) or bitter (Arg, Ile)., Monosodium glutamate (MSG; ajinomoto) is, used as a flavoring agent in food industry, and, Chinese foods to increase taste and flavor. In, some individuals intolerant to MSG, Chinese, restaurant syndrome (brief and reversible flulike symptoms) is observed., 4. Optical properties : All the amino acids, except glycine possess optical isomers due to, the presence of asymmetric carbon atom. Some, amino acids also have a second asymmetric, carbon e.g. isoleucine, threonine. The structure, of L- and D-amino acids in comparison with, glyceraldehyde has been given (See Fig.4.1)., 5. Amino acids as ampholytes : Amino acids, contain both acidic ( COOH) and basic, ( NH2) groups. They can donate a proton or, accept a proton, hence amino acids are regarded, as ampholytes., Zwitterion or dipolar ion : The name zwitter, is derived from the German word which means, hybrid. Zwitter ion (or dipolar ion) is a hybrid, molecule containing positive and negative ionic, groups., The amino acids rarely exist in a neutral form, with free carboxylic ( COOH) and free amino, ( NH2) groups. In strongly acidic pH (low pH),, the amino acid is positively charged (cation), while in strongly alkaline pH (high pH), it is, negatively charged (anion). Each amino acid has, a characteristic pH (e.g. leucine, pH 6.0) at, which it carries both positive and negative, charges and exists as zwitterion (Fig.4.2)., , Isoelectric pH (symbol pI) is defined as the, pH at which a molecule exists as a zwitterion or, dipolar ion and carries no net charge. Thus, the, molecule is electrically neutral., The pI value can be calculated by taking the, average pKa values corresponding to the ionizable, groups. For instance, leucine has two ionizable, groups, and its pI can be calculated as follows., pH, pI, , pK 1 COO– � pK 2 NH �, 3, , 2.4 � 9.6, 2, , 2, 6.0
Page 60 :
50, , BIOCHEMISTRY, , groups namely carboxyl ( COOH) group and, amino ( NH2) group., , H, R C COOH, , Reactions due to, , NH 2, +, , Amino acid, , 1. Amino acids form salts ( COONa) with, bases and esters ( COORc) with alcohols., , +, , H, , H, , H, , H, , –, , R C COO, , R C COOH, +, , NH 2, , NH 3, , 2. Decarboxylation : Amino acids undergo, decarboxylation to produce corresponding, amines., –, , Anion, (high pH), , Cation, (low pH), , R CH COO, , R C COO–, +, , NH 3, , Zwitterion, (isoelectric pH), , Fig. 4.2 : Existence of an amino acid as cation,, anion and zwitterion., , For the calculation of pI of amino acids with, more than two ionizable groups, the pKas for all, the groups have to be taken into account., Titration of amino acids : The existence of, different ionic forms of amino acids can be more, easily understood by the titration curves. The, graphic representation of leucine titration is, depicted in Fig.4.3. At low pH, leucine exists in, a fully protonated form as cation. As the titration, proceeds with NaOH, leucine loses its protons, and at isoelectric pH (pI), it becomes a, zwitterion. Further titration results in the, formation of anionic form of leucine., Some more details on isoelectric pH are, discussed under the properties of proteins, (p. 60)., , B. Chemical properties, The general reactions of amino acids are, mostly due to the presence of two functional, , +, , NH3, , This reaction assumes significance in the, living cells due to the formation of many, biologically important amines. These, include histamine, tyramine and J-amino, butyric acid (GABA) from the amino acids, histidine,, tyrosine, and, glutamate,, respectively., , H, , Leucine exists as cation at pH below 6, and anion at pH above 6. At the isoelectric pH, (pI = 6.0), leucine is found as zwitterion. Thus, the pH of the medium determines the ionic, nature of amino acids., , R CH2 + CO2, , +, NH3, , H+, H+, , COOH group, , 3. Reaction with ammonia : The carboxyl, group of dicarboxylic amino acids reacts, with NH3 to form amide, Aspartic acid + NH3 o Asparagine, Glutamic acid + NH3 o Glutamine, , 14, 13, 12, 11, 10, 9, 8, pH 7, 6, 5, 3, 2, 1, 0, , R, , pK2, , CH, , COO–, , NH2, , pI, , R, , CH, , COO–, , +, NH3, , pK1, R, , CH, , COOH, , +, NH3, , 0.5, , 1.0, , 1.5, , 2.0, , Equivalents of NaOH, , Fig. 4.3 : Titration curve of an amino acid-leucine, (R = (CH3)2—CH—CH2—;, pK1 = Dissociation constant, for COOH; pI = Isoelectric pH;, +, pK2 = Dissociation constant for NH3 ).
Page 61 :
51, , Chapter 4 : PROTEINS AND AMINO ACIDS, , Reactions due to, , NH2 group, , 4. The amino groups behave as bases and, combine with acids (e.g. HCl) to form, salts ( NH3+ Cl–)., 5. Reaction with ninhydrin : The D-amino, acids react with ninhydrin to form a, purple, blue or pink colour complex, (Ruhemann’s purple)., Amino acid + Ninhydrin o Keto acid +, NH3 + CO2 + Hydrindantin, , acids are very important for protein structure and, functions. Selected examples are given, hereunder., l, , l, , l, , Hydrindantin + NH3 + Ninhydrin o, Ruhemann’s purple, l, , Ninhydrin reaction is effectively used for, the quantitative determination of amino, acids and proteins. (Note : Proline and, hydroxyproline give yellow colour with, ninhydrin)., 6. Colour reactions of amino acids : Amino, acids can be identified by specific colour, reactions (See Table 4.3)., 7. Transamination : Transfer of an amino, group from an amino acid to a keto acid, to form a new amino acid is a very, important reaction in amino acid, metabolism (details given in Chapter 15)., 8. Oxidative deamination : The amino acids, undergo oxidative deamination to liberate, free ammonia (Refer Chapter 15)., , NON-STANDARD AMINO ACIDS, Besides the 20 standard amino acids, (described above) present in the protein, structure, there are several other amino acids, which are biologically important. These include, the amino acid derivatives found in proteins,, non-protein amino acids performing specialized, functions and the D-amino acids., A. Amino acid derivatives in proteins : The, 20 standard amino acids can be incorporated, into proteins due to the presence of universal, genetic code. Some of these amino acids, undergo specific modification after the protein, synthesis occurs. These derivatives of amino, , Collagen—the most abundant protein in, mammals—contains 4-hydroxyproline and, 5-hydroxylysine., Histones—the proteins found in association, with DNA—contain many methylated,, phosphorylated or acetylated amino acids., , J-Carboxyglutamic acid is found in certain, plasma proteins involved in blood clotting., Cystine is formed by combination of two, cysteines. Cystine is also considered as, derived amino acid., , B. Non-protein amino acids : These amino, acids, although never found in proteins, perform, several biologically important functions. They, may be either D-or non-D-amino acids. A, selected list of these amino acids along with their, functions is given in Table 4.2., C. D-Amino acids : The vast majority of, amino acids isolated from animals and plants are, of L-category. Certain D-amino acids are also, found in the antibiotics (actinomycin-D,, valinomycin, gramicidin-S). D-serine and, D-aspartate are found in brain tissue. DGlutamic acid and D-alanine are present in, bacterial cell walls., , Amino acids useful as drugs, There a certain non-standard amino acids that, are used as drugs., l, , l, , l, , D-Penicillamine, (D-dimethylglycine),, a, metabolite of penicillin, is employed in the, chelation therapy of Wilson’s disease. This is, possible since D-penicillamine can effectively, chelate copper., N-Acetylcysteine is used in cystic fibrosis, and, chronic renal insufficiency, as it can function, as an antioxidant., Gabapentin (J-aminobutyrate linked to, cyclohexane) is used as an anticonvulsant.
Page 62 :
52, , BIOCHEMISTRY, , TABLE 4.2 A selected list of important non-protein amino acids along with their functions, , Amino acids, I., , Function(s), , D-Amino acids, Ornithine, , ½, °, ¾, Arginosuccinic acid °¿, Citrulline, , Thyroxine, , Intermediates in the biosynthesis of urea., , ½, ¾, ¿, , Thyroid hormones derived from tyrosine., , Triiodothyronine, S-Adenosylmethionine, Homocysteine, Homoserine, 3, 4-Dihydroxy phenylalanine (DOPA), Creatinine, Ovothiol, Azaserine, Cycloserine, , Methyl donor in biological system., Intermediate in methionine metabolism. A risk factor for coronary heart, diseases, Intermediate in threonine, aspartate and methionine metabolisms., A neurotransmitter, serves as a precursor for melanin pigment., Derived from muscle and excreted in urine, Sulfur containing amino acid found in fertilized eggs, and acts as an, antioxidant, Anticancer drug, Antituberculosis drug, , II. Non-D-amino acids, E-Alanine, E-Aminoisobutyric acid, J-Aminobutyric acid (GABA), G-Aminolevulinic acid (ALA), Taurine, , Component of vitamin pantothenic acid and coenzyme A, End product of pyrimidine metabolism., A neurotransmitter produced from glutamic acid, Intermediate in the synthesis of porphyrin (finally heme), Found in association with bile acids., , STRUCTURE OF PROTEINS, Proteins are the polymers of L-D-amino acids., The structure of proteins is rather complex which, can be divided into 4 levels of organization, (Fig.4.4) :, 1. Primary structure : The linear sequence of, amino acids forming the backbone of proteins, (polypeptides)., 2. Secondary, structure :, The, spatial, arrangement of protein by twisting of the, polypeptide chain., 3. Tertiary structure : The three dimensional, structure of a functional protein., 4. Quaternary structure :, proteins are composed of, , Some of the, two or more, , polypeptide chains referred to as subunits. The, spatial arrangement of these subunits is known, as quaternary structure., [The structural hierarchy of proteins is, comparable with the structure of a building. The, amino acids may be considered as the bricks,, the wall as the primary structure, the twists in a, wall as the secondary structure, a full-fledged, self-contained room as the tertiary structure. A, building with similar and dissimilar rooms will, be the quaternary structure]., The term protein is generally used for a, polypeptide containing more than 50 amino, acids. In recent years, however, some authors, have been using ‘polypeptide’ even if the, number of amino acids is a few hundreds. They, prefer to use protein to an assembly of, polypeptide chains with quaternary structure.
Page 63 :
53, , Chapter 4 : PROTEINS AND AMINO ACIDS, , Primary, structure, , Secondary, structure, , Tertiary, structure, , Quaternary, structure, , Fig. 4.4 : Diagrammatic representation of protein structure, (Note : The four subunits of two types in quaternary structure)., , PRIMARY STRUCTURE OF PROTEIN, Each protein has a unique sequence of amino, acids which is determined by the genes, contained in DNA. The primary structure of a, protein is largely responsible for its function. A, vast majority of genetic diseases are due to, abnormalities in the amino acid sequences of, proteins i.e. changes associated with primary, structure of protein., The amino acid composition of a protein, determines its physical and chemical properties., , double bond in character. It generally exists in, trans configuration. Both, C O and, NH, groups of peptide bonds are polar and are, involved in hydrogen bond formation., Writing of peptide structures : Conventionally,, the peptide chains are written with the free amino, end (N-terminal residue) at the left, and the free, carboxyl end (C-terminal residue) at the right. The, amino acid sequence is read from N-terminal end, to C-terminal end. Incidentally, the protein, biosynthesis also starts from the N-terminal amino, acid., , Peptide bond, The amino acids are held together in a protein, by covalent peptide bonds or linkages. These, bonds are rather strong and serve as the, cementing material between the individual, amino acids (considered as bricks)., Formation of a peptide bond : When the, amino group of an amino acid combines with, the carboxyl group of another amino acid, a, peptide bond is formed (Fig.4.5). Note that a, dipeptide will have two amino acids and one, peptide (not two) bond. Peptides containing, more than 10 amino acids (decapeptide) are, referred to as polypeptides., Characteristics of peptide bonds : The, peptide bond is rigid and planar with partial, , H, , +, , –, , H3N C COO, , +, , H, , +, , H3N C COO–, , R1, , R2, , Amino acid 1, , Amino acid 2, , H2O, , +, , H, , H, , –, , H3N C CO NH C COO, R2, , R1, Dipeptide, , Fig. 4.5 : Formation of a peptide bond.
Page 64 :
54, , BIOCHEMISTRY, , Shorthand to read peptides : The, amino acids in a peptide or protein, are represented by the 3-letter or one, letter abbreviation. This is the, chemical shorthand to write proteins., , +, , H3N —glutamate—cysteine—glycine—COO–, E, , —, , C, , —, , G, , Glu, , —, , Cys, , —, , Gly, , Amino acids in, a peptide, One letter symbols, Three letter symbols, Peptide name, , Glutamyl — cysteinyl — glycine, , Naming of peptides : For naming, peptides, the amino acid suffixes, Fig. 4.6 : Use of symbols in representing a peptide, (Note : A tripeptide with 3 amino acids and two peptide bonds is, -ine (glycine), -an (tryptophan), -ate, shown; Free —NH+3 is on the left while free —COO– is on the right)., (glutamate) are changed to -yl with, the exception of C-terminal amino, 2. Degradation of protein or polypeptide, acid. Thus a tripeptide composed of an Ninto smaller fragments., terminal glutamate, a cysteine and a C-terminal, glycine is called glutamyl-cysteinyl-glycine., 3. Determination of the amino acid sequence., In the Fig.4.6, the naming and representation, 1. Determination of amino acid composition, of a tripeptide are shown., in a protein : The protein or polypeptide is, Dimensions of a peptide chain : The completely hydrolysed to liberate the amino, dimensions of a fully extended polypeptide acids which are quantitatively estimated. The, chain are depicted in Fig.4.7. The two adjacent hydrolysis may be carried out either by acid or, D-carbon atoms are placed at a distance of 0.36 alkali treatment or by enzyme hydrolysis., nm. The interatomic distances and bond angles Treatment with enzymes, however results in, smaller peptides rather than amino acids., are also shown in this figure., , Determination of primary structure, The primary structure comprises the identification of constituent amino acids with regard, to their quality, quantity and sequence in a, protein structure. A pure sample of a protein or, a polypeptide is essential for the determination, of primary structure which involves 3 stages :, 1. Determination of amino acid composition., , O, , Pronase is a mixture of non-specific, proteolytic enzymes that causes complete, hydrolysis of proteins., Separation and estimation of amino acids :, The mixture of amino acids liberated by protein, hydrolysis can be determined by chromatographic techniques. The reader must refer, Chapter 41 for the separation and quantitative, determination of amino acids. Knowledge on, , H, , R1, , O, , H, 0.123 nm, , 121q, , 122q, , C, , 120q, , C, , 117q, , N, , 110q, , 120q, , N, , 0.147 nm, , C, , C, , 0.132 nm, , 0.153 nm, , C, , N, , 120q, 0.1 nm, , H, , O, , H, , R2, , 0.36 nm, , Fig. 4.7 : Dimensions of a fully extended polypeptide chain., (The distance between two adjacent D-carbon atoms is 0.36 nm)., , H
Page 65 :
55, , Chapter 4 : PROTEINS AND AMINO ACIDS, , O2N, , F, , N C S, , NO2, , Edman’s reagent, , Sanger’s reagent, Protein labelling, Hydrolysis, Free amino acids, , R, O2N, , N CH COO–, H, NO2, , Dinitrophenyl (DNP)amino acid, , Protein labelling, Hydrolysis, Polypeptide, (–N–terminal AA), , R1, , O, C, , CH, NH, , N, C, S, Phenylthiohydantoin (PTH)amino acid, , Identified by, chromatography, , Identified by chromatography, , Fig. 4.8 : Sanger’s reagent (1-fluoro 2,4-dinitrobenzene) and Edman’s reagent (Phenyl isothiocyanate) in the, determination of amino acid sequence of a protein (AA-Amino acid)., , primary structure of proteins will be incomplete, without a thorough understanding of chromatography., 2. Degradation of protein into smaller fragments : Protein is a large molecule which is, sometimes composed of individual polypeptide, chains. Separation of polypeptides is essential, before degradation., (a) Liberation of polypeptides : Treatment, with urea or guanidine hydrochloride, disrupts the non-covalent bonds and, dissociates the protein into polypeptide, units. For cleaving the disulfide linkages, between the polypeptide units, treatment, with performic acid is necessary., (b) Number of polypeptides : The number of, polypeptide chains can be identified by, treatment of protein with dansyl chloride., It specifically binds with N-terminal amino, acids to form dansyl polypeptides which, on hydrolysis yield N-terminal dansyl, amino acid. The number of dansyl amino, acids produced is equal to the number of, polypeptide chains in a protein., , (c) Breakdown, of, polypeptides, into, fragments : Polypeptides are degraded, into smaller peptides by enzymatic or, chemical methods., Enzymatic cleavage : The proteolytic enzymes, such as trypsin, chymotrypsin, pepsin and, elastase exhibit specificity in cleaving the, peptide bonds (Refer Fig.8.7). Among these, enzymes, trypsin is most commonly used. It, hydrolyses the peptide bonds containing lysine, or arginine on the carbonyl ( C O) side of, peptide linkage., Chemical cleavage : Cyanogen bromide, (CNBr) is commonly used to split polypeptides, into smaller fragments. CNBr specifically splits, peptide bonds, the carbonyl side of which is, contributed by the amino acid methionine., 3. Determination of amino acid sequence :, The polypeptides or their smaller fragments are, conveniently utilized for the determination of, sequence of amino acids. This is done in a stepwise manner to finally build up the order of, amino acids in a protein. Certain reagents are, employed for sequence determination (Fig.4.8).
Page 66 :
56, , BIOCHEMISTRY, , Sanger’s reagent : Sanger used 1-fluoro 2,, 4-dinitrobenzene (FDNB) to determine insulin, structure. FDNB specifically binds with, N-terminal amino acid to form a dinitrophenyl, (DNP) derivative of peptide. This on hydrolysis, yields DNP-amino acid (N-terminal) and free, amino acids from the rest of the peptide chain., DNP-amino acid can be identified by chromatography., , amino acids in a polypeptide chain. By analysing, the nucleotide sequence of DNA that codes for, protein, it is possible to translate the nucleotide, sequence into amino acid sequence. This, technique, however, fails to identify the disulfide, bonds and changes that occur in the amino acids, after the protein is synthesized (post-translational, modifications)., , Sanger’s reagent has limited use since the, peptide chain is hydrolysed to amino acids., , SECONDARY STRUCTURE OF PROTEIN, , Edman’s reagent : Phenyl isothiocyanate is, the Edman’s reagent. It reacts with the Nterminal amino acid of peptide to form a phenyl, thiocarbamyl derivative. On treatment with mild, acid, phenyl thiohydantoin (PTH)–amino acid, a, cyclic compound is liberated. This can be, identified by chromatography (Fig.4.8)., Edman’s reagent has an advantage since a, peptide can be sequentially degraded liberating, N-terminal amino acids one after another which, can be identified. This is due to the fact that the, peptide as a whole is not hydrolysed but only, releases PTH-amino acid., Sequenator : This is an automatic machine to, determine the amino acid sequence in a, polypeptide (with around 100 residues). It is, based on the principle of Edman’s degradation, (described above). Amino acids are determined, sequentially from N-terminal end. The PTHamino acid liberated is identified by highperformance liquid chromatography (HPLC)., Sequenator takes about 2 hours to determine, each amino acid., , Overlapping peptides, In the determination of primary structure of, protein, several methods (enzymatic or chemical), are simultaneously employed. This results in the, formation of overlapping peptides. This is due to, the specific action of different agents on different, sites in the polypeptide. Overlapping peptides, are very useful in determining the amino acid, sequence., , The conformation of polypeptide chain by, twisting or folding is referred to as secondary, structure. The amino acids are located close to, each other in their sequence. Two types of, secondary structures, D-helix and E-sheet, are, mainly identified., Indian scientist Ramachandran made a, significant contribution in understanding the, spatial arrangement of polypeptide chains., , D-Helix, D-Helix is the most common spiral structure, of protein. It has a rigid arrangement of, polypeptide chain. D-Helical structure was, proposed by Pauling and Corey (1951) which is, regarded as one of the milestones in the, biochemistry research. The salient features of, D-helix (Fig.4.9) are given below, 1. The D-helix is a tightly packed coiled, structure with amino acid side chains extending, outward from the central axis., 2. The D-helix is stabilized by extensive, hydrogen bonding. It is formed between H atom, attached to peptide N, and O atom attached to, peptide C. The hydrogen bonds are individually, weak but collectively, they are strong enough to, stabilize the helix., 3. All the peptide bonds, except the first and, last in a polypeptide chain, participate in, hydrogen bonding., , Reverse sequencing technique, , 4. Each turn of D-helix contains 3.6 amino, acids and travels a distance of 0.54 nm. The, spacing of each amino acid is 0.15 nm., , It is the genetic material (chemically DNA), which ultimately determines the sequence of, , 5. D-Helix is a stable conformation formed, spontaneously with the lowest energy.
Page 67 :
57, , Chapter 4 : PROTEINS AND AMINO ACIDS, , Glu) or basic (Lys, Arg, His) amino acids also, interfere with D-helix structure., , E-Pleated sheet, This is the second type of structure (hence E, after D) proposed by Pauling and Corey., E-Pleated sheets (or simply E-sheets) are, composed of two or more segments of fully, extended peptide chains (Fig.4.10). In the, E-sheets, the hydrogen bonds are formed, between the neighbouring segments of, polypeptide chain(s)., , Parallel and anti-parallel E-sheets, The polypeptide chains in the E-sheets may, be arranged either, in parallel (the same, direction) or anti-parallel (opposite direction)., This is illustrated in Fig.4.10., , O, O, , N, , C, , H, , C, N, H, , N, O, , H, , O, , C, O, N, , O, , H, , C, , N, N, , H, , C, , C, , N, , E-Pleated sheet may be formed either by, separate polypeptide chains (H-bonds are, interchain) or a single polypeptide chain folding, back on to itself (H-bonds are intrachain)., , H, , H, N, , O, , O, , H, , C, , C, , (A), , C, O, , Fig. 4.9 : Diagrammatic representation of secondary, structure of protein—a right handed D-helix, H, (, , -Indicate, , H, , O, , H, , N, , C, , N, , C, , N, , C, , O, , H, , O, , H, N, , H, , O, , N, , C, , C, , N, , O, , H, , H-Bond, between chains, , (B) N-Terminal, C-terminal, , C R groups of amino acids;, , dotted blue lines are hydrogen bonds;, Note that only a few hydrogen bonds shown for clarity)., , 6. The right handed D-helix is more stable, than left handed helix (a right handed helix turns, in the direction that the fingers of right hand curl, when its thumb points in the direction the helix, rises)., 7. Certain amino acids (particularly proline), disrupt the D-helix. Large number of acidic (Asp,, , (C) N-Terminal, , C-terminal, , C-Terminal, , N-terminal, , Fig. 4.10 : Structure of E-pleated sheet (A) Hydrogen, bonds between polypeptide chains (B) Parallel E-sheet, (C) Antiparallel E-sheet. (Note : Red circles in A, , H, represent amino acid skeleton C R ).
Page 68 :
58, , BIOCHEMISTRY, , polypeptides which may be identical or, unrelated. Such proteins are termed as oligomers, and possess quaternary structure. The individual, polypeptide chains are known as monomers,, protomers or subunits. A dimer consits of two, polypeptides while a tetramer has four., , Fig. 4.11 : Diagrammatic representation of a protein, containing D-helix and E-pleated sheet (blue)., , Occurrence of E-sheets : Many proteins, contain E-pleated sheets. As such, the D-helix, and E-sheet are commonly found in the same, protein structure (Fig.4.11). In the globular, proteins, E-sheets form the core structure., Other types of secondary structures : Besides, the D-and E-structures described above, the, E-bends and nonrepetitive (less organised, structures) secondary structures are also found in, proteins., , TERTIARY STRUCTURE OF PROTEIN, The three-dimensional arrangement of, protein structure is referred to as tertiary, structure. It is a compact structure with, hydrophobic side chains held interior while the, hydrophilic groups are on the surface of the, protein molecule. This type of arrangement, ensures stability of the molecule., Bonds of tertiary structure : Besides the, hydrogen bonds, disulfide bonds ( S S), ionic, interactions (electrostatic bonds), hydrophobic, interactions and van der Waals forces also, contribute to the tertiary structure of proteins., Domains : The term domain is used to, represent the basic units of protein structure, (tertiary) and function. A polypeptide with 200, amino acids normally consists of two or more, domains., , QUATERNARY STRUCTURE OF PROTEIN, A great majority of the proteins are composed, of single polypeptide chains. Some of the, proteins, however, consist of two or more, , Bonds in quaternary structure : The, monomeric subunits are held together by nonconvalent bonds namely hydrogen bonds,, hydrophobic interactions and ionic bonds., Importance of oligomeric proteins : These, proteins play a significant role in the regulation, of metabolism and cellular function., Examples of oligomeric proteins : Hemoglobin, aspartate transcarbomylase, lactate, dehydrogenase., , Bonds responsible for, protein structure, Protein structure is stabilized by two types of, bonds—covalent and non-covalent., 1. Covalent bonds : The peptide and disulfide, bonds are the strong bonds in protein structure., The formation of peptide bond and its, chracteristics have been described., Disulfide bonds : A disulfide bond ( S S) is, formed by the sulfhydryl groups ( SH) of two, cysteine, residues,, to, produce, cystine, (Fig.4.12A). The disulfide bonds may be formed, in a single polypeptide chain or between, different polypeptides. These bonds contribute to, the structural conformation and stability of, proteins., 2. Non-covalent bonds : There are, mainly,, four types of non-covalent bonds., (a) Hydrogen bonds : The hydrogen bonds, are formed by sharing of hydrogen atoms, between the nitrogen and carbonyl, oxygen of different peptide bonds, (Fig.4.12B). Each hydrogen bond is weak, but collectively they are strong. A large, number of hydrogen bonds significantly, contribute to the protein structure., (b) Hydrophobic bonds : The non-polar side, chains of neutral amino acids tend to be
Page 69 :
59, , Chapter 4 : PROTEINS AND AMINO ACIDS, , (A), , acidic amino acids with positively, charged groups (e.g., NH3+) of basic, amino acids (Fig.4.12D)., , NH CH CO, CH2, S, , Cystine, , S, , (d) Van der Waals forces : These are the, non-covalent, associations, between, electrically neutral molecules. They are, formed by the electrostatic interactions, due to permanent or induced dipoles., , CH2, NH CH CO, (B), , C CH N, O R1, , H, , Examples of protein structure, , H R2, , O, , Structure of human insulin : Insulin consists, of two polypeptide chains, A and B (Fig.4.13)., The A chain has glycine at the N-terminal end, and asparagine at the C-terminal end. The B, chain has phenylalanine and alanine at the, N- and C-terminal ends, respectively. Originally,, insulin is synthesized as a single polypeptide, preproinsulin which undergoes proteolytic, processing to give proinsulin and finally insulin., , N CH C, (C), , NH CH CO, HC CH3, CH2 Isoleucine, H3C, , CH3, CH, , CH3, , CH2 Leucine, NH CH CO, (D), , NH—CH—CO, CH2, C, O, , Aspartate, –, , O, , +, , NH3, , Lysine, , (CH2)4, NH CH CO, , The structural aspects of hemoglobin and, collagen are respectively given in Chapters 10, and 22., , Methods to determine, protein structure, For the determination of secondary and, tertiary protein structures, X-ray crystallography, is most commonly used. Nuclear magnetic, resonance (NMR) spectra of proteins provides, structural and functional information on the, atoms and groups present in the proteins., , Fig. 4.12 : Major bonds in protein structure (A) Disulfide, bond (B) Hydrogen bonds (C) Hydrophic bonds, (D) Electrostatic bond., (Note : See Fig. 4.5 for peptide bond)., , S, 1, , closely associated with each other in, proteins (Fig.4.12C). As such, these are, not true bonds. The occurrence of, hydrophobic forces is observed in, aqueous environment wherein the, molecules are forced to stay together., (c) Electrostatic bonds : These bonds are, formed, by, interactions, between, negatively charged groups (e.g. COO–) of, , 1, , S, , 7, 6, , 20, 11, , S, , S, , S, , S, , 7, , 19, , 21, , A chain, , 30 B chain, , Fig. 4.13 : Diagrammatic representation of, human insulin structure, (Note : A and B polypeptide chains are held by two, disulfide linkages).
Page 70 :
60, , BIOCHEMISTRY, , Methods for the isolation, and purification of proteins, , Pepsin-1.1; Casein-4.6; Human albumin-4.7;, Urease-5.0; Hemoglobin-6.7; Lysozyme-11.0., , Several methods are employed to isolate and, purify proteins. Initially, proteins are fractionated, by using different concentrations of ammonium, sulfate or sodium sulfate. Protein fractionation, may also be carried out by ultracentrifugation., , 5. Acidic and basic proteins : Proteins in, which the ratio (H Lys + H Arg)/(H Glu + H Asp) is, greater than 1 are referred to as basic proteins., For acidic proteins, the ratio is less than 1., , Protein separation is achieved by utilizing, electrophoresis, isoelectric focussing, immunoelectrophoresis, ion-exchange chromatography,, gel-filtration, high performance liquid chromatography (HPLC) etc. The details of these techniques, are described in Chapter 41., , PROPERTIES OF PROTEINS, 1. Solubility :, Proteins, form, colloidal, solutions instead of true solutions in water. This, is due to huge size of protein molecules., 2. Molecular weight : The proteins vary in, their molecular weights, which, in turn, is, dependent on the number of amino acid, residues. Each amino acid on an average, contributes to a molecular weight of about 110., Majority of proteins/polypeptides may be, composed of 40 to 4,000 amino acids with a, molecular weight ranging from 4,000 to, 440,000. A few proteins with their molecular, weights are listed below :, Insulin-5,700; Myoglobin-17,000; Hemoglobin64,450; Serum albumin-69,000., 3. Shape : There is a wide variation in the, protein shape. It may be globular (insulin), oval, (albumin) fibrous or elongated (fibrinogen)., 4. Isoelectric pH : Isoelectric pH (pI) as a, property of amino acids has been described. The, nature of the amino acids (particularly their, ionizable groups) determines the pI of a protein., The acidic amino acids (Asp, Glu) and basic, amino acids (His, Lys, Arg) strongly influence the, pI. At isoelectric pH, the proteins exist as, zwitterions or dipolar ions. They are electrically, neutral (do not migrate in the electric field) with, minimum solubility, maximum precipitability, and least buffering capacity. The isoelectric, pH(pI) for some proteins are given here, , 6. Precipitation of proteins : Proteins exist in, colloidal solution due to hydration of polar, groups ( COO–, NH3+, OH). Proteins can be, precipitated by dehydration or neutralization of, polar groups., Precipitation at pI : The proteins in general, are least soluble at isoelectric pH. Certain, proteins (e.g. casein) get easily precipitated when, the pH is adjusted to pI (4.6 for casein)., Formation of curd from milk is a marvellous, example of slow precipitation of milk protein,, casein at pI. This occurs due to the lactic acid, produced by fermentation of bacteria which, lowers the pH to the pI of casein., Precipitation by salting out : The process of, protein precipitation by the additional of neutral, salts such as ammonium sulfate or sodium sulfate, is known as salting out. This phenomenon is, explained on the basis of dehydration of protein, molecules by salts. This causes increased proteinprotein interaction, resulting in molecular, aggregation and precipitation., The amount of salt required for protein, precipitation depends on the size (molecular, weight) of the protein molecule. In general, the, higher is the protein molecular weight, the lower, is the salt required for precipitation. Thus, serum, globulins are precipitated by half saturation with, ammonium sulfate while albumin is precipitated, by full saturation. Salting out procedure is, conveniently used for separating serum albumins, from globulins., The addition of small quantities of neutral, salts increases the solubility of proteins. This, process called as salting in is due to the, diminished protein–protein interaction at low salt, concentration., Precipitation by salts of heavy metals : Heavy, metal ions like Pb2+, Hg2+, Fe2+, Zn2+, Cd2+, cause precipitation of proteins. These metals
Page 71 :
61, , Chapter 4 : PROTEINS AND AMINO ACIDS, , being positively charged, when added to protein, solution (negatively charged) in alkaline medium, results in precipitate formation. Based on the, principle of precipitation, raw egg-white, (protein-albumin) is sometimes used to overcome, the toxicity of mercury., Precipitation by anionic or alkaloid reagents :, Proteins can be precipitated by trichloroacetic, acid, sulphosalicylic acid, phosphotungstic acid,, picric acid, tannic acid, phosphomolybdic acid, etc. By the addition of these acids, the, proteins existing as cations are precipitated by the, anionic form of acids to produce proteinsulphosalicylate, protein-tungstate, protein-picrate, etc. Industrial tanning of leather is based on the, principle of protein precipitation by tannic acid., Precipitation by organic solvents : Organic, solvents such as alcohol are good protein, precipitating agents. They dehydrate the protein, molecule by removing the water envelope and, cause precipitation. The use of surgical spirit, (about 20% alcohol) as a disinfectant is based on, the precipitation of proteins and the death of, bacteria., 7. Colour reactions of proteins : The proteins, give several colour reactions which are often, useful to identify the nature of the amino acids, present in them (Table 4.3)., , possessing at least two peptide linkages i.e.,, tripeptides (with 3 amino acids) give positive, biuret test. Histidine is the only amino acid that, answers biuret test. The principle of biuret test is, conveniently used to detect the presence of, proteins in biological fluids. The mechanism of, biuret test is not clearly known. It is believed, that the colour is due to the formation of a, copper co-ordinated complex, as shown below., O, C, , O, NH, , NH, , C, , Cu2+, , HN, C, , NH, , O, , NH, , NH, C, O, , The presence of magnesium and ammonium, ions interfere in the biuret test. This can be, overcome by using excess alkali., , DENATURATION, The phenomenon of disorganization of native, protein structure is known as denaturation., Denaturation results in the loss of secondary,, tertiary and quaternary structure of proteins. This, involves a change in physical, chemical and, biological properties of protein molecules., , Biuret reaction : Biuret is a compound formed, by heating urea to 180°C., TABLE 4.3 Colour reactions of proteins/amino acids, , NH2, C O, , NH2, , NH2, +, NH2, , C O, , Reaction, , Specific group or amino acid, , 1. Biuret reaction, , Two peptide linkages, , 2. Ninhydrin reaction, , D-Amino acids, , 3. Xanthoproteic, reaction, , Benzene ring of aromatic, amino acids (Phe, Tyr, Trp), , 4. Milllons reaction, , Phenolic group (Tyr), , When biuret is treated with dilute copper, sulfate in alkaline medium, a purple colour is, obtained. This is the basis of biuret test, widely used for identification of proteins and, peptides., , 5. Hopkins-Cole reaction, , Indole ring (Trp), , 6. Sakaguchi reaction, , Guanidino group (Arg), , 7. Nitroprusside reaction, , Sulfhydryl groups (Cys), , 8. Sulfur test, , Sulfhydryl groups (Cys), , Biuret test is answered by compounds, containing two or more CO NH groups i.e.,, peptide bonds. All proteins and peptides, , 9. Pauly’s test, , Imidazole ring (His), , C O, , 180qC, , NH, C O, NH2, , NH2, 2 Moles of urea, , Biuret, , 10. Folin–Coicalteau’s test, , Phenolic groups (Tyr)
Page 72 :
62, , BIOCHEMISTRY, , 35, , -SH, , HS-, , 47, , 1, , 1, 112, 82, , 68, Denaturation, , 68, SH, , 47, 35, SH, , Native protein, , 112, , 82, Denatured protein, , Fig. 4.14 : Denaturation of a protein., , Agents of denaturation, Physical agents : Heat,, X-rays, UV radiation., , violent, , shaking,, , Chemical agents : Acids, alkalies, organic, solvents (ether, alcohol), salts of heavy metals, (Pb, Hg), urea, salicylate, detergents (e.g. sodium, dodecyl sulfate)., , Characteristics of denaturation, 1. The native helical structure of protein is, lost (Fig.4.14)., 2. The primary structure of a protein with, peptide linkages remains intact i.e., peptide, bonds are not hydrolysed., 3. The protein loses its biological activity., 4. Denatured protein becomes insoluble in, the solvent in which it was originally soluble., 5 The viscosity of denatured protein, (solution) increases while its surface tension, decreases., 6. Denaturation is associated with increase in, ionizable and sulfhydryl groups of protein. This, is due to loss of hydrogen and disulfide bonds., 7. Denatured protein is more easily digested., This is due to increased exposure of peptide, , bonds to enzymes. Cooking causes protein, denaturation and, therefore, cooked food, (protein) is more easily digested. Further,, denaturation of dietary protein by gastric HCl, enchances protein digestion by pepsin., 8. Denaturation is usually irreversible. For, instance, omelet can be prepared from an egg, (protein-albumin) but the reversal is not possible., 9. Careful denaturation is sometimes reversible (known as renaturation). Hemoglobin, undergoes denaturation in the presence of, salicylate. By removal of salicylate, hemoglobin, is renatured., 10. Denatured protein cannot be crystallized., Coagulation : The term ‘coagulum’ refers to a, semi-solid viscous precipitate of protein., Irreversible denaturation results in coagulation., Coagulation is optimum and requires lowest, temperature at isoelectric pH. Albumins and, globulins (to a lesser extent) are coagulable, proteins. Heat coagulation test is commonly, used to detect the presence of albumin in urine., Flocculation : It is the process of protein, precipitation at isoelectric pH. The precipitate is, referred to as flocculum. Casein (milk protein), can be easily precipitated when adjusted to, isoelectric pH (4.6) by dilute acetic acid.
Page 73 :
63, , Chapter 4 : PROTEINS AND AMINO ACIDS, , Flocculation is reversible. On application of, heat, flocculum can be converted into an, irreversible mass, coagulum., , CLASSIFICATION OF PROTEINS, Proteins are classified in several ways. Three, major types of classifying proteins based on their, function, chemical nature and solubility, properties and nutritional importance are, discussed here., , A. Functional classification of proteins, Based on the functions they perform, proteins, are classified into the following groups (with, examples), 1. Structural proteins : Keratin of hair and, nails, collagen of bone., 2. Enzymes or catalytic proteins : Hexokinase,, pepsin., 3. Transport proteins : Hemoglobin, serum, albumin., 4. Hormonal, hormone., , proteins :, , Insulin,, , growth, , 5. Contractile proteins : Actin, myosin., 6. Storage proteins : Ovalbumin, glutelin., , 7. Genetic proteins : Nucleoproteins., 8. Defense proteins : Snake venoms, Immunoglobulins., 9. Receptor proteins for hormones, viruses., , B. Protein classification based on, chemical nature and solubility, This is a more comprehensive and popular, classification of proteins. It is based on the, amino acid composition, structure, shape and, solubility properties. Proteins are broadly, classified into 3 major groups, 1. Simple proteins : They are composed of, only amino acid residues., 2. Conjugated proteins : Besides the amino, acids, these proteins contain a non-protein, moiety known as prosthetic group or, conjugating group., 3. Derived proteins : These are the denatured, or degraded products of simple and conjugated, proteins., The above three classes are further, subdivided into different groups. The summary, of protein classification is given in the Table 4.4., , + Proteins are the most abundant organic molecules of life. They perform static, (structural) and dynamic functions in the living cells., , + The dynamic functions of proteins are highly diversified such as enzymes, hormones,, clotting factors, immunoglobulins, storage proteins and membrane receptors., , + Half of the amino acids (about 10) that occur in proteins have to be consumed by, humans in the diet, hence they are essential., , + A protein is said to be complete (or first class) protein if all the essential amino acids, are present in the required proportion by the human body e.g. egg albumin., , + Cooking results in protein denaturation exposing more peptide bonds for easy digestion., + Monosodium glutamate (MSG) is used as a flavoring agent in foods to increase taste, and flavour. In some individuals intolerant to MSG, Chinese restaurant syndrome (brief, and reversible flu-like symptoms) is observed.
Page 74 :
64, , BIOCHEMISTRY, , TABLE 4.4 Summary of classification of proteins, PROTEINS, Conjugated, , Simple, Globular proteins, Albumins, , Scleroproteins, , Globulins, , Collagens, , Glutelins, , Elastins, Keratins, , Prolamines, Histones, , Derived, , Nucleoproteins, , Primary, , Glycoproteins, Mucoproteins, , Coagulated, proteins, , Lipoproteins, Phosphoproteins, Chromoproteins, Metalloproteins, , Globins, Protamines, Lectins, , 1. Simple proteins, (a) Globular proteins : These are spherical or, oval in shape, soluble in water or other, solvents and digestible., (i) Albumins : Soluble in water and, dilute salt solutions and coagulated, by heat. e.g. serum albumin,, ovalbumin (egg), lactalbumin (milk)., (ii) Globulins : Soluble in neutral and, dilute salt solutions e.g. serum, globulins, vitelline (egg yolk)., (iii) Glutelins : Soluble in dilute acids and, alkalies and mostly found in plants, e.g. glutelin (wheat), oryzenin (rice)., (iv) Prolamines : Soluble in 70% alcohol, e.g. gliadin (wheat), zein (maize)., (v) Histones : Strongly basic proteins,, soluble in water and dilute acids but, insoluble in dilute ammonium hydroxide e.g. thymus histones., (vi) Globins : These are generally considered along with histones. However,, globins are not basic proteins and are, not precipitated by NH4OH., (vii) Protamines : They are strongly basic, and resemble histones but smaller, in size and soluble in NH4OH., Protamines are also found in, association with nucleic acids e.g., sperm proteins., , Secondary, , Proteans, Metaproteins, , Proteoses, Peptones, Polypeptides, Peptides, , (viii) Lectins are carbohydrate-binding, proteins, and are involved in the, interaction between cells and proteins., They help to maintain tissue and organ, structures. In the laboratory, lectins are, useful for the purification of, carbohydrates by affinity chromatography e.g. concanavalin A, agglutinin., (b) Fibrous proteins : These are fiber like in, shape, insoluble in water and resistant to, digestion. Albuminoids or scleroproteins, are predominant group of fibrous proteins., (i) Collagens are connective tissue, proteins, lacking, tryptophan., Collagens, on boiling with water or, dilute acids, yield gelatin which is, soluble and digestible (Chapter 22)., (ii) Elastins : These proteins are found in, elastic tissues such as tendons and, arteries., (iii) Keratins : These are present in, exoskeletal structures e.g. hair, nails,, horns. Human hair keratin contains as, much as 14% cysteine (Chapter 22)., 2. Conjugated proteins, (a) Nucleoproteins : Nucleic acid (DNA or, RNA) is the prosthetic group e.g. nucleohistones, nucleoprotamines., (b) Glycoproteins : The prosthetic group is, carbohydrate, which is less than 4% of
Page 75 :
65, , Chapter 4 : PROTEINS AND AMINO ACIDS, , protein. The term mucoprotein is used if, the carbohydrate content is more than 4%., e.g. mucin (saliva), ovomucoid (egg white)., (c) Lipoproteins :, Protein, found, in, combination with lipids as the prosthetic, group e.g. serum lipoproteins., (d) Phosphoproteins : Phosphoric acid is the, prosthetic group e.g. casein (milk),, vitelline (egg yolk)., (e) Chromoproteins : The prosthetic group is, coloured in nature e.g. hemoglobins,, cytochromes., (f) Metalloproteins : These proteins contain metal, ions such as Fe, Co, Zn, Cu, Mg etc., e.g., ceruloplasmin (Cu), carbonic anhydrase (Zn)., 3. Derived proteins : The derived proteins, are of two types. The primary derived are the, denatured or coagulated or first hydrolysed, products of proteins. The secondary derived are, the degraded (due to breakdown of peptide, bonds) products of proteins., (a) Primary derived proteins, (i) Coagulated proteins : These are the, denatured, proteins produced by, agents such as heat, acids, alkalies, etc. e.g. cooked proteins, coagulated, albumin (egg white)., (ii) Proteans : These are the earliest, products of protein hydrolysis by, enzymes, dilute acids, alkalies etc., which are insoluble in water. e.g. fibrin, formed from fibrinogen., (iii) Metaproteins : These are the second, stage products of protein hydrolysis, obtained by treatment with slightly, stronger acids and alkalies e.g. acid, and alkali metaproteins., (b) Secondary derived proteins : These are the, progressive hydrolytic products of protein, hydrolysis. These include proteoses,, peptones, polypeptides and peptides., , C. Nutritional classification of proteins, The nutritive value of proteins is determined, by the composition of essential amino acids, , (described already). From the nutritional point of, view, proteins are classified into 3 categories., 1. Complete proteins : These proteins have, all the ten essential amino acids in the required, proportion by the human body to promote good, growth. e.g. egg albumin, milk casein., 2. Partially incomplete proteins : These proteins partially lack one or more essential amino, acids, and can promote moderate growth. e.g., wheat and rice proteins (limiting Lys, Thr)., 3. Incomplete proteins : These proteins, completely lack one or more essential amino, acids. Hence they do not promote growth, at all e.g. gelatin (lacks Trp), zein (lacks Trp,, Lys)., , BIOLOGICALLY IMPORTANT PEPTIDES, Several peptides occur in the living organisms that display a wide spectrum of biological functions. Generally, the term ‘peptide’ is, applied when the number of constituent amino, acids is less than 10. Some examples of biologically active peptides and their functions are, described here., 1. Glutathione : It is a tripeptide composed, of 3 amino acids. Chemically, glutathione is Jglutamyl-cysteinyl-glycine. It is widely distributed, in nature and exists in reduced or oxidized, states., 2G SH, , G S S G, , Reduced, , Oxidized, , Functions : In a steady state, the cells, generally maintain a ratio of about 100/1 of, GSH to G S S G. The reversible oxidationreduction of glutathione is important for many of, its biological functions., l, , l, , Glutathione serves as a coenzyme for certain, enzymes e.g. prostaglandin PGE2 synthetase,, glyoxylase., It prevents the oxidation of sulfhydryl, ( SH) groups of several proteins to, disulfide ( S S ) groups. This is essential for, the protein function, including that of, enzymes.
Page 76 :
66, , l, , l, , BIOCHEMISTRY, , It is believed that glutathione in association, with glutathione reductase participates in the, formation of correct disulfide bonds in several, proteins., , glutathione peroxidase (a selenium containing, enzyme)., , Glutathione (reduced) performs specialized, functions in erythrocytes, , 2. Thyrotropin releasing hormone (TRH) : It, is a tripeptide secreted by hypothalamus. TRH, stimulates pituitary gland to release thyrotropic, hormone., , (i) It maintains RBC membrane structure and, integrity., (ii) It protects hemoglobin from getting, oxidized by agents such as H2O2., l, , l, , l, , Glutathione is involved in the transport of, amino acids in the intestine and kidney, tubules via J-glutamyl cycle or Meister cycle, (Refer Chapter 8)., Glutathione is involved in the detoxication, process. The toxic substances (organophosphates, nitro compounds) are converted, to mercapturic acids., Toxic amounts of peroxides and free radicals, produced in the cells are scavanged by, , 2 GSH + H2O2, , Peroxidase, , G – S – S – G + 2 H2O, , 3. Oxytocin : It is a hormone secreted by, posterior pituitary gland and contains 9 amino, acids (nonapeptide). Oxytocin causes contraction, of uterus., 4. Vasopressin (antidiuretic hormone, ADH) :, ADH is also a nonapeptide produced by posterior, pituitary gland. It stimulates kidneys to retain, water and thus increases the blood pressure., 5. Angiotensins : Angiotensin I is a decapeptide (10 amino acids) which is converted to, angiotensin II (8 amino acids). The later has, more hypertensive effect. Angiotensin II also, stimulates the release of aldosterone from, adrenal gland., , + Collagen is the most abundant protein in mammals. It is rich in hydroxyproline and, hydroxylysine., , + Several biologically important peptides are known in the living organism. These include, glutathione for the maintenance of RBC structure and integrity; oxytocin that causes, uterus contraction; vasopressin that stimulates retention of water by kidneys;, enkephalins that inhibit the sense of pain in the brain., , + Antibiotics such as actinomycin, gramicidin, bacitracin and tyrocidin are peptide in nature., + J-Carboxyglutamic acid is an amino acid derivative found in certain plasma proteins, involved in blood clotting., , + Homocysteine has been implicated as a risk factor in the onset of coronary heart, diseases., , + Several non-protein amino acids of biological importance are known. These include, ornithine, citrulline and arginosuccinic acid (intermediates of urea synthesis), thyroxine, and triiodothyronine (hormones), and E-alanine (of coenzyme A)., , + The protein-free filtrate of blood, required for biochemical investigations (e.g. urea,, sugar) can be obtained by using protein precipitating agents such as phosphotungstic, acid and trichloroacetic acid., , + Heat coagulation test is most commonly employed to detect the presence of albumin in urine.
Page 77 :
67, , Chapter 4 : PROTEINS AND AMINO ACIDS, , 7. Bradykinin and kallidin : They are nonaand decapeptides, respectively. Both of them act, as powerful vasodilators. They are produced, from plasma proteins by snake venom enzymes., , 9. Aspartame : It is a dipeptide (aspartylphenylalanine, methyl, ester),, produced, by a combination of aspartic acid and, phenylalanine. Aspartame is about 200 times, sweeter than sucrose, and is used as a, low-calorie artificial sweetner in softdrink, industry., , 8. Peptide antibiotics : Antibiotics such as, gramicidin,, bacitracin,, tyrocidin, and, actinomycin are peptide in nature., , 10. Gastrointestinal hormones : Gastrin,, secretin etc. are the gastrointestinal peptides, which serve as hormones., , 6. Methionine enkephalin : It is a pentapeptide found in the brain and has opiate like, function. It inhibits the sense of a pain., , 1. Proteins are nitrogen containing, most abundant organic macromolecules widely distributed, in animals and plants. They perform structural and dynamic functions in the organisms., 2. Proteins are polymers composed of L-D-amino acids. They are 20 in number and, classified into different groups based on their structure, chemical nature, nutritional, requirement and metabolic fate. Selenocysteine has been recently identified as the 21st, amino acid, and is found in certain proteins., 3. Amino acids possess two functional groups namely carboxyl ( COOH) and amino ( NH2)., In the physiological system, they exist as dipolar ions commonly referred to as zwitterions., 4. Besides the 20 standard amino acids present in proteins, there are several non-standard, amino acids. These include the amino acid derivatives found in proteins (e.g. hydroxyproline, hydroxylysine) and, non-protein amino acids (e.g. ornithine, citrulline)., 5. The structure of protein is divided into four levels of organization. The primary, structure represents the linear sequence of amino acids. The twisting and spatial, arrangement of polypeptide chain is the secondary structure. Tertiary structure, constitutes the three dimensional structure of a functional protein. The assembly of, similar or dissimilar polypeptide subunits comprises quaternary structure., 6. The determination of primary structure of a protein involves the knowledge of quality,, quantity and the sequence of amino acids in the polypeptide. Chemical and enzymatic, methods are employed for the determination of primary structure., 7. The secondary structure of protein mainly consists of D-helix and/or E-sheet. D-Helix is, stabilized by extensive hydrogen bonding. E-Pleated sheet is composed of two or more, segments of fully extended polypeptide chains., 8. The tertiary and quaternary structures of protein are stabilized by non-covalent bonds, such as hydrogen bonds, hydrophobic interactions, ionic bonds etc., 9. Proteins are classified into three major groups. Simple proteins contain only amino acid, residues (e.g. albumin). Conjugated proteins contain a non-protein moiety known as, prosthetic group, besides the amino acids (e.g. glycoproteins). Derived proteins are, obtained by degradation of simple or conjugated proteins., 10. In addition to proteins, several peptides perform biologically important functions., These include glutathione, oxytocin and vasopressin.
Page 78 :
68, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the classification of amino acids along with their structures., 2. Discuss the organization of protein structure. Give an account of the determination of primary, structure of protein., 3. Describe the classification of proteins with suitable examples., 4. Write an account of non-standard amino acids., 5. Discuss the important biologically active peptides., , II. Short notes, (a) Essential amino acids, (b) Zwitterion, (c) Peptide bond, (d) Edman’s reagent, (e) D-Helix,, (f) E-Pleated sheet, (g) Denaturation, (h) Isoelectric point, (i) Glutathione, (j) Quaternary structure, of protein., , III. Fill in the blanks, 1. The average nitrogen content of proteins, 2. Proteins are the polymers of, , ., , ., ., , 3. Name the sulfur containing essential amino acid, , 4. The charged molecule which is electrically neutral is known as, , ., , ., , 5. The non –D amino acid present in coenzyme A, 6. The bonds forming the backbone of protein structure, , ., , 7. The amino acid that is completely destroyed by acid hydrolysis of protein, 8. The number of peptide bonds present in a decapeptide, 9. The chemical name of Sanger’s reagent, , ., , ., , ., , 10. The phenomenon of disorganization of native protein structure is known as, , ., , IV. Multiple choice questions, 11. The imino acid found in protein structure, (a) Arginine (b) Proline (c) Histidine (d) Lysine., 12. The following is a non-protein amino acid, (a) Ornithine (b) Homocysteine (c) Histamine (d) All of them., 13. The bonds in protein structure that are not broken on denaturation., (a) Hydrogen bonds (b) Peptide bonds (c) Ionic bond (d) Disulfide bonds., 14. Sequenator is an automatic machine to determine amino acid sequence in a polypeptide chain., The reagent used in sequenator is, (a) Sanger’s reagent (b) CNBr (c) Trypsin (d) Edman’s reagent., 15. The reaction given by two or more peptide linkages is, (a) Biuret test (b) Ninhydrin test (c) Xanthoproteic reaction (d) Pauley’s test.
Page 79 :
Section 1, , Chemical Constituents of Life, , Chapter, , Nucleic Acids and Nucleotides, , 15, , DNA, the bank of genetic, information speaks :, , “I am the chemical basis of life and heredity!, Organized into genes that control every function;, Composed of repeating units of deoxyribonucleotides;, Arrranged in a double helix, held by hydrogen bonds.”, , T, , here are two types of nucleic acids,, namely deoxyribonucleic acid (DNA) and, ribonucleic acid (RNA). Primarily, nucleic acids, serve as repositories and transmitters of genetic, information., , Brief history, DNA was discovered in 1869 by Johann, Friedrich Miescher, a Swiss researcher. The, demonstration that DNA contained genetic, information was first made in 1944, by Avery,, Macleod and MacCary., , Functions of nucleic acids, DNA is the chemical basis of heredity and, may be regarded as the reserve bank of genetic, information. DNA is exclusively responsible for, maintaining the identity of different species of, organisms over millions of years. Further, every, aspect of cellular function is under the control of, DNA. The DNA is organized into genes, the, fundamental units of genetic information. The, , genes control the protein synthesis through the, mediation of RNA, as shown below, DNA, , RNA, , Protein, , The interrelationship of these three classes of, biomolecules (DNA, RNA and proteins) constitutes, the central dogma of molecular biology or more, commonly the central dogma of life., , Components of nucleic acids, Nucleic acids are the polymers of nucleotides, (polynucleotides) held by 3c and 5c phosphate, bridges. In other words, nucleic acids are built, up by the monomeric units—nucleotides (It may, be recalled that protein is a polymer of amino, acids)., , NUCLEOTIDES, Nucleotides are composed of a nitrogenous, base, a pentose sugar and a phosphate. Nucleotides perform a wide variety of functions in the, living cells, besides being the building blocks or, , 69
Page 80 :
70, , BIOCHEMISTRY, , H, 6, , C, (A), , N, , C, , H C, , C, , N, , 7, , 5, , 1N, , N, 8, , CH, N, , 2, , N, , N, , 4, , 3, , H, , N9, H, , H, 4, , C, (B), , N, HC, , CH, , 3N, , 5, , CH, , 2, , 6, , N, , N, 1, , Fig. 5.1 : General structure of nitrogen bases, (A) Purine (B) Pyrimidine (The positions are numbered, according to the international system)., , the pyrimidine cytosine (C) is found in both DNA, and RNA. However, the nucleic acids differ, with respect to the second pyrimidine base., DNA contains thymine (T) whereas RNA, contains uracil (U). As is observed in the, Fig.5.2, thymine and uracil differ in structure by, the presence (in T) or absence (in U) of a methyl, group., , Tautomeric forms, of purines and pyrimidines, The existence of a molecule in a keto, (lactam) and enol (lactim) form is known as, tautomerism. The heterocyclic rings of purines, and, , monomeric units in the nucleic acid (DNA and, RNA) structure. These include their role as, structural components of some coenzymes of, B-complex vitamins (e.g. FAD, NAD+), in the, energy reactions of cells (ATP is the energy, currency), and in the control of metabolic, reactions., , pyrimidines, , with, , O, C, , oxo, , functional, , groups exhibit tautomerism as simplified below., OH, , O H, C N, , C N, , Lactam form, , Lactim form, , O, , NH2, N, , N, , N, , HN, , STRUCTURE OF NUCLEOTIDES, As already stated, the nucleotide essentially, consists of nucleobase, sugar and phosphate., The term nucleoside refers to base + sugar. Thus,, nucleotide is nucleoside + phosphate., , H2N, , N, , N, , NH2, N, , Purines and pyrimidines, The nitrogenous bases found in nucleotides, (and, therefore, nucleic acids) are aromatic, heterocyclic compounds. The bases are of two, types—purines and pyrimidines. Their general, structures are depicted in Fig.5.1. Purines are, numbered in the anticlockwise direction while, pyrimidines are numbered in the clockwise, direction. And this is an internationally accepted, system to represent the structure of bases., , Major bases in nucleic acids, The structures of major purines and, pyrimidines found in nucleic acids are shown in, Fig.5.2. DNA and RNA contain the same purines, namely adenine (A) and guanine (G). Further,, , N, , N, , H, Guanine (G), (2-amino 6-oxypurine), , H, Adenine (A), (6-aminopurine), , O, , N, , H, Cytosine (C), (2-oxy 4-aminopyrimidine), O, HN, O, , O, CH3, , N, , H, Thymine (T), (2,4-dioxy-5 methylpyrimidine), , HN, O, , N, , H, Uracil (U), (2,4-dioxypyrimidine), , Fig. 5.2 : Structures of major purines (A, G) and, pyrimidines (C, T, U) found in nucleic acids.
Page 81 :
71, , Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , NH2, , NH2, , C, , C, , 5, , N, , C, , N, , C, , C, , C, , C, , C, , O, , 4, , H, , N, , HO, , N, Lactim form, , The purine—guanine and pyrimidinescytosine, thymine and uracil exhibit tautomerism., The lactam and lactim forms of cytosine are, represented in Fig.5.3., At physiological pH, the lactam (keto) tautomeric forms are predominantly present., Minor bases found in nucleic acids : Besides, the bases described above, several minor and, unusual bases are often found in DNA and RNA., These include 5-methylcytosine, N4-acetylcytosine, N6-methyladenine, N6, N6-dimethyladenine, pseudouracil etc. It is believed that the, unusual bases in nucleic acids will help in the, recognition of specific enzymes., Other biologically important bases : The, bases such as hypoxanthine, xanthine and uric, acid (Fig.5.4) are present in the free state in the, cells. The former two are the intermediates in, purine synthesis while uric acid is the end, product of purine degradation., Purine bases of plants : Plants contain certain, methylated purines which are of pharmacological interest. These include caffeine (of, coffee), theophylline (of tea) and theobromine, (of cocoa)., , HN, N, , O, , O, N, , N, H, , Hypoxanthine, (6-oxypurine), , N, , HN, O, , 3, , H, , 4, , 1, , 2, , O, , HOH2C, , H, , H, , H, 3, , OH, H, 2, , 1, , H, , OH H, D-2-Deoxyribose, , Fig. 5.5 : Structures of sugars present in nucleic acids, (ribose is found in RNA and deoxyribose in DNA; Note, the structural difference at C2 )., , Fig. 5.3 : The tautomeric forms of cytosine., , O, , H, , OH, , OH OH, D-Ribose, , H, Lactam form, , 5, , O, , HOH2C, , HN, , Sugars of nucleic acids, The five carbon monosaccharides (pentoses), are found in the nucleic acid structure. RNA, contains D-ribose while DNA contains, D-deoxyribose. Ribose and deoxyribose differ in, structure at C2. Deoxyribose has one oxygen less, at C2 compared to ribose (Fig.5.5)., , Nomenclature of nucleotides, The addition of a pentose sugar to base, produces a nucleoside. If the sugar is ribose,, ribonucleosides, are, formed., Adenosine,, guanosine, cytidine and uridine are the, ribonucleosides of A, G, C and U respectively. If, the sugar is a deoxyribose, deoxyribonucleosides are produced., The term mononucleotide is used when a, single phosphate moiety is added to a, nucleoside. Thus adenosine monophosphate, (AMP) contains adenine + ribose + phosphate., The principal bases, their respective, nucleosides and nucleotides found in the, structure of nucleic acids are given in Table 5.1., Note that the prefix ‘d’ is used to indicate if the, sugar is deoxyribose (e.g. dAMP)., H, N, , The binding of nucleotide, components, O, , N, , N, , H, H, Xanthine, (2,6-dioxypurine), , O, , N, , N, , H, , H, , Uric acid, (2,6,8-trioxypurine), , Fig. 5.4 : Structures of some biologically important purines., , The atoms in the purine ring are, numbered as 1 to 9 and for, pyrimidine as 1 to 6 (See Fig.5.1). The, carbons of sugars are represented with, an, associated, prime, (c), for, differentiation. Thus the pentose, carbons are 1c to 5c.
Page 83 :
73, , Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , O, , O, HN, , HN, , 5, , F, , N, O, , N, H, 5-Fluorouracil, , NH, N, Allopurinol, , O, , SH, N, , 6, , N, , HN, , N, , 5. Arabinosylcytosine is being used in cancer, therapy as it interferes with DNA replication., 6. The drugs employed in the treatment of, AIDS namely zidovudine or AZT (3-azido, 2c,3c-dideoxythymidine) and didanosine (dideoxyinosine) are sugar modified synthetic nucleotide, analogs (For their structure and more details Refer, Chapter 38)., , 8N, , N, N, H, 6-Mercaptopurine, , H2N, , N, N, H, 8-Azaguanine, , Fig. 5.7 : Structures of selected purine, and pyrimidine analogs., , The anionic properties of nucleotides and, nucleic acids are due to the negative charges, contributed by phosphate groups., , PURINE, PYRIMIDINE, AND NUCLEOTIDE ANALOGS, It is possible to alter heterocyclic ring or, sugar moiety, and produce synthetic analogs, of purines, pyrimidines, nucleosides and, nucleotides. Some of the synthetic analogs are, highly useful in clinical medicine. The structures, of selected purine and pyrimidine analogs are, given in Fig.5.7., The pharmacological applications of certain, analogs are listed below, 1. Allopurinol is used in the treatment of, hyperuricemia and gout (For details, Refer, Chapter 17)., 2. 5-Fluorouracil, 6-mercaptopurine, 8-azaguanine, 3-deoxyuridine, 5- or 6-azauridine,, 5- or 6-azacytidine and 5-idouracil are employed, in the treatment of cancers. These compounds, get incorporated into DNA and block cell, proliferation., 3. Azathioprine (which gets degraded to, 6-mercaptopurine) is used to suppress, immunological rejection during transplantation., 4. Arabinosyladenine is used for the, treatment of neurological disease, viral, encephalitis., , STRUCTURE OF DNA, DNA is a polymer of deoxyribonucleotides, (or simply deoxynucleotides). It is composed of, monomeric units namely deoxyadenylate, (dAMP), deoxyguanylate (dGMP), deoxycytidylate (dCMP) and deoxythymidylate (dTMP), (It may be noted here that some authors prefer to, use TMP for deoxythymidylate, since it is found, only in DNA). The details of the nucleotide, structure are given above., , Schematic representation, of polynucleotides, The monomeric deoxynucleotides in DNA are, held together by 3c,5c-phosphodiester bridges, (Fig.5.8). DNA (or RNA) structure is often, represented in a short-hand form. The horizontal, line indicates the carbon chain of sugar with, base attached to C1c. Near the middle of the, horizontal line is C3c phosphate linkage while at, the other end of the line is C5c phosphate linkage, (Fig.5.8)., , Chargaff’s rule of DNA composition, Erwin Chargaff in late 1940s quantitatively, analysed the DNA hydrolysates from different, species. He observed that in all the species he, studied, DNA had equal numbers of adenine and, thymine residues (A = T) and equal numbers of, guanine and cytosine residues (G = C). This is, known as Chargaff’s rule of molar equivalence, between the purines and pyrimidines in DNA, structure. The significance of Chargaff’s rule was, not immediately realised. The double helical, structure of DNA derives its strength from, Chargaff’s rule (discussed later).
Page 84 :
74, , BIOCHEMISTRY, , 5c end, , DNA structure is considered as a milestone in, the era of modern biology. The structure of, DNA double helix is comparable to a twisted, ladder. The salient features of Watson-Crick, model of DNA (now known as B-DNA) are, described next (Fig.5.9)., , O, O P O–, O, 5c, , O, , H2C, 4c, , H, , 1c, , H, , 3c, , H, , Adenine, 2c, , O, , H, , H, , 5c, , ( A), , O P O–, , 3c, , O, 5c, , O, , H2C, , Guanine, , H, , H, , O, , H, , H, , H, O P O–, , Phosphodiester, bond, Thymine, , O, 5c, , O, , H2C, , H, , H, , O, , H, , 3c, , H, , Minor, groove, , , °, °, Major °, groove ®, °, °, °̄, , H, , O P O–, O, 3c end, , 5c, 5c, 5c, , P, , 3c, , 1c, , P, , 3c, , 1c, , P, , 3c, , 1c T, , , ®, ¯S, , S, P S, A T, P, C, S, P, G, P 3.4 nm, A, S, T, P, S, S, G, P, C, S, P, P, , 0.34 nm, 3c, , 5c, , A, , 2.0 nm, , G, , P, , Fig. 5.8 : Structure of a polydeoxyribonucleotide, segment held by phosphodiester bonds. On the lower, part is the representation of short hand form of, oligonucleotides., , Single-stranded DNA, and RNAs which are, usually single-stranded, do not obey Chargaff’s, rule. However, double-stranded RNA which is, the genetic material in certain viruses satisfies, Chargaff’s rule., , DNA DOUBLE HELIX, The double helical structure of DNA was, proposed by James Watson and Francis Crick in, 1953 (Nobel Prize, 1962). The elucidation of, , ( B), 5c�end, 5c, , P, , 3c, , 3c�end, A, , T, , 3c, , G, , P, T, , P, 5c, , OH, 3c�end, , 3c, , C, , P, 3c, , 5c, P, , P, , 5c, , OH, , 3c, , A, , 5c, , P, C, , G, , 3c, , 5c, , P, 5c�end, , Fig. 5.9 : (A) Watson–Crick model of DNA helix, (B) Complementary base pairing in DNA helix.
Page 85 :
Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , 7. The two strands are held together by, hydrogen bonds formed by complementary base, pairs (Fig.5.10). The A-T pair has 2 hydrogen, bonds while G-C pair has 3 hydrogen, bonds. The G { C is stronger by about 50% than, A = T., , CH 3, O, , (A), , H, N, , N, , To, Chain, , H, N, , H, , N, , N, , O, , N, Adenine, , Thymine, , N, To, Chain, , H, N, , (B), N, To, Chain, , H, O, , N, H, O, , H, Cytosine, , N, , N, , N, N, , H, Guanine, , 75, , N, To, Chain, , Fig. 5.10 : Complementary base pairing in DNA, (A) Thymine pairs with adenine by 2 hydrogen bonds, (B) Cytosine pairs with guanine by 3 hydrogen bonds., , 1. The DNA is a right handed double helix. It, consists of two polydeoxyribonucleotide chains, (strands) twisted around each other on a, common axis., 2. The two strands are antiparallel, i.e., one, strand runs in the 5c to 3c direction while the, other in 3c to 5c direction. This is comparable to, two parallel adjacent roads carrying traffic in, opposite direction., 3. The width (or diameter) of a double helix, is 20 A° (2 nm)., 4. Each turn (pitch) of the helix is 34 A°, (3.4 nm) with 10 pairs of nucleotides, each pair, placed at a distance of about 3.4 A°., 5. Each strand of DNA has a hydrophilic, deoxyribose phosphate backbone (3c-5c phosphodiester bonds) on the outside (periphery) of the, molecule while the hydrophobic bases are, stacked inside (core)., 6. The two polynucleotide chains are not, identical but complementary to each other due, to base pairing., , 8. The hydrogen bonds are formed between a, purine and a pyrimidine only. If two purines, face each other, they would not fit into the, allowable space. And two pyrimidines would, be too far to form hydrogen bonds. The only, base arrangement possible in DNA structure,, from spatial considerations is A-T, T-A, G-C and, C-G., 9. The complementary base pairing in DNA, helix proves Chargaff’s rule. The content of, adenine equals to that of thymine (A = T) and, guanine equals to that of cytosine (G = C)., 10. The genetic information resides on one of, the two strands known as template strand or, sense strand. The opposite strand is antisense, strand. The double helix has (wide) major, grooves and (narrow) minor grooves along the, phosphodiester backbone. Proteins interact with, DNA at these grooves, without disrupting the, base pairs and double helix., , Conformations of DNA double helix, Variation in the conformation of the, nucleotides of DNA is associated with, conformational variants of DNA. The double, helical structure of DNA exists in at least 6, different forms-A to E and Z. Among these, B, A, and Z forms are important (Table 5.2). The, B-form of DNA double helix, described by, Watson and Crick (discussed above), is the most, predominant, form, under, physiological, conditions. Each turn of the B-form has 10 base, pairs spanning a distance of 3.4 nm. The width, of the double helix is 2 nm., The A-form is also a right-handed helix. It, contains 11 base pairs per turn. There is a tilting, of the base pairs by 20° away from the central, axis., The Z-form (Z-DNA) is a left-handed helix, and contains 12 base pairs per turn. The
Page 86 :
76, , BIOCHEMISTRY, , TABLE 5.2 Comparison of structural features of, different conformations of DNA double helix, , Feature, , B-DNA, , A-DNA, , Z-DNA, , Right-handed, , Right-handed, , Left-handed, , Helical, diameter (nm), , 2.37, , 2.55, , 1.84, , Distance per, each complete, turn (nm), , 3.4, , 3.2, , 4.5, , Rise per base, pair (nm), , 0.34, , 0.29, , 0.37, , Helix type, , Number of base, pairs per complete, turn, Base pair tilt, , 10, +19°, , 11, –1.2°, (variable), , 12, –9°, , Helix axis rotation Major groove Through base Minor groove, pairs (variable), , polynucleotide strands of DNA move in a, somewhat ‘zig zag’ fashion, hence the name, Z-DNA., It is believed that transition between different, helical forms of DNA plays a significant role in, regulating gene expression., , Certain antitumor drugs (e.g. cisplatin), produce bent structure in DNA. Such changed, structure can take up proteins that damage the, DNA., , Triple-stranded DNA, Triple-stranded DNA formation may occur, due to additional hydrogen bonds between the, bases. Thus, a thymine can selectively form two, Hoogsteen hydrogen bonds to the adenine of, A-T pair to form T-A-T. Likewise, a protonated, cytosine can also form two hydrogen bonds with, guanine of G–C pairs that results in C–G–C. An, outline of Hoogsteen triple helix is depicted in, Fig.5.11., Triple-helical structure is less stable than, double helix. This is due to the fact that the three, negatively charged backbone strands in triple, helix results in an increased electrostatic, repulsion., , Four-stranded DNA, Polynucleotides with very high contents of, guanine can form a novel tetrameric structure, , OTHER TYPES OF DNA STRUCTURE, It is now recognized that besides double, helical structure, DNA also exists in certain, unusual structures. It is believed that such, structures, are, important, for, molecular, recognition of DNA by proteins and enzymes., This is in fact needed for the DNA to discharge, its functions in an appropriate manner. Some, selected unusual structures of DNA are briefly, described., , Bent DNA, In general, adenine base containing DNA, tracts are rigid and straight. Bent conformation of, DNA occurs when A-tracts are replaced by other, bases or a collapse of the helix into the minor, groove of A-tract. Bending in DNA structure has, also been reported due to photochemical, damage or mispairing of bases., , Fig. 5.11 : An outline of Hoogsteen triple helical, structure of DNA.
Page 87 :
Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , 77, , called G-quartets. These structures are planar, and are connected by Hoogsteen hydrogen, bonds (Fig.5.12A). Antiparallel four-stranded, DNA structures, referred to as G-tetraplexes, have also been reported (Fig.5.12B)., , G-tetraplexes have been implicated in the, recombination of immunoglobulin genes, and, in dimerization of double-stranded genomic, RNA of the human immunodeficiency virus, (HIV)., , The ends of eukaryotic chromosomes namely, telomeres are rich in guanine, and therefore form, G-tetraplexes. In recent years, telomeres have, become the targets for anticancer chemotherapies., , THE SIZE OF DNA MOLECULE, —UNITS OF LENGTH, , (A), , 3c, , 3c, , 3c, , 3c, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , T, , T, , T, , T, , G, , +, , T, , +, , G, , T, G, , +, , G, , G, , G, , T, , G, , G, , G, , G, , 3c, , 3c, , G, G, , 3c, G, G, G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , T, , T, , T, , T, , T, , T, , T, , T, , 5c, , 5c, , 5c, , 5c, , G, G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, G, , +, , G, , G, , G, , G, , G, , 5c, , G, G, , 5c, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , G, , 1 kb = 1000 bp, , G, , 1 Mb = 1000 kb = 1,000,000 bp, , G, , 5c, , G, G, G, G, , G, G, G, , G, , G, , G, 5c, , G, , G, , G, , 3c, , G, , G, , G, , G, , 5c, , G, G, , G, , 5c, , G, , For the measurement of lengths, DNA doublestranded structure is considered, and expresssed, in the form of base pairs (bp). A kilobase pair, (kb) is 103 bp, and a megabase pair (Mb) is 106, bp and a gigabase pair (Gb) is 109 bp. The kb,, Mb and Gb relations may be summarized as, follows :, , 1 Gb = 1000 Mb = 1,000,000,000 bp, , G, , 5c, , G, , 3c, , G, , G, G, , 3c, , G, , G, , G, , (B), , G, , G, , T, G, , G, , G, , 3c, , DNA molecules are huge in size. On an, average, a pair of B-DNA with a thickness, of 0.34 nm has a molecular weight of 660, daltons., , G, G, G, (5c), , 5c, , G, G, G, , The length of DNA varies from species to, species, and is usually expressed in terms of base, pair composition and contour length. Contour, length represents the total length of the genomic, DNA in a cell. Some examples of organisms with, bp and contour lengths are listed., l, , G, G, , It may be noted here that the lengths of RNA, molecules (like DNA molecules) cannot be, expressed in bp, since most of the RNAs are, single-stranded., , l, , G, G, G, (3c), , 3c, , Fig. 5.12 : Four–stranded DNA structure (A) Parallel, G–quartets (B) Antiparallel G–tetraplex., , l, , O phage virus — 4.8 u 104 bp — contour, length 16.5 Pm., , E. coli — 4.6 u 106 bp — contour length, 1.5 Pm., Diploid human cell (46 chromosomes) —, 6.0 u 109 bp — contour length 2 meters., , It may be noted that the genomic DNA size is, usually much larger the size of the cell or, nucleus containing it. For instance, in humans, a, 2-meter long DNA is packed compactly in a, nucleus of about 10Pm diameter.
Page 88 :
78, , BIOCHEMISTRY, , The genomic DNA may exist in linear or, circular forms. Most DNAs in bacteria exist, as closed circles. This includes the DNA, of bacterial chromosomes and the extrachromosomal DNA of plasmids. Mitochondria, and chloroplasts of eukaryotic cells also contain, circular DNA., Chromosomal DNAs in higher organisms are, mostly linear. Individual human chromosomes, contain a single DNA molecule with variable, sizes compactly packed. Thus the smallest, chromosome contains 34 Mb while the largest, one has 263 Mb., , DENATURATION OF DNA STRANDS, The two strands of DNA helix are held, together by hydrogen bonds. Disruption of, hydrogen bonds (by change in pH or increase in, temperature) results in the separation of, polynucleotide strands. This phenomenon of loss, of helical structure of DNA is known as, denaturation (Fig.5.13). The phosphodiester, bonds are not broken by denaturation. Loss of, helical structure can be measured by increase, in absorbance at 260 nm (in a spectrophotometer). The phenomenon of increase in the, absorbance of purines and pyrimidines,, , Denaturation, Renaturation, , Two strands, separated, , DNA helix, , Fig. 5.13 : Diagrammatic representation of denaturation, and renaturation of DNA., , following denaturation, hyperchromicity., , is, , referred, , to, , as, , Melting temperature (Tm) is defined as the, temperature at which half of the helical structure, of DNA is lost. Since G-C base pairs are more, stable (due to 3 hydrogen bonds) than A-T base, pairs (2 hydrogen bonds), the Tm is greater for, DNAs with higher G-C content. Thus, the Tm is, 65°C for 35% G-C content while it is 70°C for, 50% G-C content. Formamide destabilizes, hydrogen bonds of base pairs and, therefore,, lowers Tm. This chemical compound is effectively, used in recombinant DNA experiments., , + DNA is the reserve bank of genetic information, ultimately responsible for the chemical, basis of life and heredity., , + DNA is organized into genes, the fundamental units of genetic information. Genes, control protein biosynthesis through the mediation of RNA., , + Nucleic acids are the polymers of nucleotides. Certain nucleotides serve as B-complex, vitamin coenzymes (FAD, NAD+, CoA), carriers of high energy intermediates (UDP-glucose,, S-adenosylmethionine) and second messengers of hormonal action (cAMP, cGMP)., , + Uric acid is a purine, and the end product of purine metabolism, that has been, implicated in the disorder gout., , + Certain purine bases from plants such as caffeine (of coffee), theophylline (of tea) and, theobromine (of cocoa) are of pharmacological interest., , + Synthetic analogs of bases (5-fluorouracil, 6-mercaptopurine, 6-azauridine) are used to, inhibit the growth of cancer cells., , + Certain antitumor drugs (e.g. cisplatin) can produce bent DNA structure and damage it.
Page 89 :
Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , 79, , Renaturation (or reannealing) is the process, in which the separated complementary DNA, strands can form a double helix., , These 30-nm fibers are further organized into, loops by anchoring the fiber at A/T-rich regions, namely scaffold-associated regions (SARS) to a, protein scafold. During the course of mitosis, the, loops are further coiled, the chromosomes, condense and become visible., , ORGANIZATION OF DNA, IN THE CELL, , STRUCTURE OF RNA, , As already stated, the double-stranded DNA, helix in each chromosome has a length that is, thousands times the diameter of the nucleus. For, instance, in humans, a 2-meter long DNA is, packed in a nucleus of about 10 Pm diameter!, This is made possible by a compact and, marvellous packaging, and organization of DNA, inside in cell., , RNA is a polymer of ribonucleotides, held together by 3c,5c-phosphodiester bridges., Although RNA has certain similarities with DNA, structure, they have specific differences, , Organization of prokaryotic DNA, , 2. Pyrimidine : RNA contains the pyrimidine, uracil in place of thymine (in DNA)., , In prokaryotic cells, the DNA is organized as, a single chromosome in the form of a doublestranded circle. These bacterial chromosomes, are packed in the form of nucleoids, by, interaction with proteins and certain cations, (polyamines)., , 3. Single strand : RNA is usually a singlestranded polynucleotide. However, this strand, may fold at certain places to give a doublestranded structure, if complementary base pairs, are in close proximity., , Organization of eukaryotic DNA, In the eukaryotic cells, the DNA is associated, with various proteins to form chromatin which, then gets organized into compact structures, namely chromosomes (Fig.5.14)., The DNA double helix is wrapped around the, core proteins namely histones which are basic in, nature. The core is composed of two molecules, of histones (H2A, H2B, H3 and H4). Each core, with two turns of DNA wrapped round it, (approximately with 150 bp) is termed as a, nucleosome, the basic unit of chromatin., Nucleosomes are separated by spacer DNA to, which histone H1 is attached (Fig.5.15). This, continuous string of nucleosomes, representing, beads-on-a string form of chromatin is termed as, 10 nm fiber. The length of the DNA is, considerably reduced by the formation of 10 nm, fiber. This 10-nm fiber is further coiled to, produce 30-nm fiber which has a solenoid, structure with six nucleosomes in every turn., , 1. Pentose : The sugar in RNA is ribose in, contrast to deoxyribose in DNA., , 4. Chargaff’s rule—not obeyed : Due to the, single-stranded nature, there is no specific, relation between purine and pyrimidine, contents. Thus the guanine content is not equal, to cytosine (as is the case in DNA)., 5. Susceptibility to alkali hydrolysis : Alkali, can hydrolyse RNA to 2c,3c-cyclic diesters. This, is possible due to the presence of a hydroxyl, group at 2c position. DNA cannot be subjected, to alkali hydrolysis due to lack of this group., 6. Orcinol colour reaction : RNAs can be, histologically identified by orcinol colour, reaction due to the presence of ribose., , TYPES OF RNA, The three major types of RNAs with their, respective cellular composition are given below, 1. Messenger RNA (mRNA) : 5–10%, 2. Transfer RNA (tRNA), , : 10–20%, , 3. Ribosomal RNA (rRNA), , : 50–80%
Page 91 :
81, , Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , TABLE 5.3 Cellular RNAs and their function(s), , Type of RNA, , Abbreviation, , Function(s), , Messenger RNA, , mRNA, , Transfers genetic information from genes to, ribosomes to synthesize proteins., , Heterogeneous nuclear RNA, , hnRNA, , Serves as precursor for mRNA and other RNAs., , Transfer RNA, , tRNA, , Transfers amino acid to mRNA for protein, biosynthesis., , Ribosomal RNA, , rRNA, , Provides structural framework for ribosomes., , Small nuclear RNA, , snRNA, , Involved in mRNA processing., , Small nucleolar RNA, , snoRNA, , Plays a key role in the processing of rRNA, molecules., , Small cytoplasmic RNA, , scRNA, , Involved in the selection of proteins for export., , Transfer–messenger RNA, , tmRNA, , Mostly present in bacteria. Adds short peptide, tags to proteins to facilitate the degradation of, incorrectly synthesized proteins., , Besides the three RNAs referred above, other, RNAs are also present in the cells. These include, heterogeneous nuclear RNA (hnRNA), small, nuclear RNA (snRNA), small nucleolar RNA, (snoRNA) and small cytoplasmic RNA (scRNA)., The major functions of these RNAs are given in, Table 5.3., The RNAs are synthesized from DNA, and are, primarily involved in the process of protein, biosynthesis (Chapter 25). The RNAs vary in, their structure and function. A brief description, on the major RNAs is given., , Messenger RNA (mRNA), The mRNA is synthesized in the nucleus (in, eukaryotes) as heterogeneous nuclear RNA, (hnRNA). hnRNA, on processing, liberates the, functional mRNA which enters the cytoplasm to, participate in protein synthesis. mRNA has high, molecular weight with a short half-life., In general, mRNA of eukaryotes is more stable, with longer half-life, compared to prokaryotic, mRNA., The eukaryotic mRNA is capped at, the 5c-terminal end by 7-methylguanosine, triphosphate. It is believed that this cap helps to, prevent the hydrolysis of mRNA by 5c-exonucleases. Further, the cap may be also involved, in the recognition of mRNA for protein synthesis., , The 3c-terminal end of mRNA contains, a polymer of adenylate residues (20-250, nucleotides) which is known as poly (A) tail., This tail may provide stability to mRNA, besides, preventing it from the attack of 3c-exonucleases., mRNA molecules often contain certain, modified bases such as 6-methyladenylates in, the internal structure., , Transfer RNA (tRNA), Transfer RNA (soluble, , RNA) molecule, contains 71-80 nucleotides (mostly 75) with a, molecular weight of about 25,000. There are at, least 20 species of tRNAs, corresponding to 20, amino acids present in protein structure. The, structure of tRNA (for alanine) was first, elucidated by Holley., The structure of tRNA, depicted in Fig.5.16,, resembles that of a clover leaf. tRNA contains, mainly four arms, each arm with a base paired, stem., 1. The acceptor arm : This arm is capped, with a sequence CCA (5c to 3c). The amino acid, is attached to the acceptor arm., 2. The anticodon arm : This arm, with the, three specific nucleotide bases (anticodon), is, responsible for the recognition of triplet codon, of mRNA. The codon and anticodon are, complementary to each other., 3. The D arm : It is so named due to the, presence of dihydrouridine.
Page 92 :
82, , BIOCHEMISTRY, , 3c, 5c, , Amino acid, , The various other RNAs and their functions, are summarised in Table 5.3., , A, C, C, Acceptor arm, , T\C arm, Variable arm, Anticodon arm, , D arm, Complementary, base pairs, , Other RNAs, , Anticodon, , Fig. 5.16 : Structure of transfer RNA., , 4. The T<C arm : This arm contains a, sequence of T, pseudouridine (represented by, psi, <) and C., 5. The variable arm : This arm is the most, variable in tRNA. Based on this variability,, tRNAs are classified into 2 categories :, (a) Class I tRNAs : The most predominant, (about 75%) form with 3-5 base pairs, length., (b) Class II tRNAs : They contain 13-20 base, pair long arm., Base pairs in tRNA : The structure of tRNA is, maintained due to the complementary base, pairing in the arms. The four arms with their, respective base pairs are given below, The acceptor arm – 7 bp, The T<C arm, – 5 bp, The anticodon arm – 5 bp, The D arm, – 4 bp, , Ribosomal RNA (rRNA), The ribosomes are the factories of protein, synthesis. The eukaryotic ribosomes are, composed of two major nucleoprotein, complexes–60S subunit and 40S subunit. The, 60S subunit contains 28S rRNA, 5S rRNA and, 5.8S rRNA while the 40S subunit contains 18S, rRNA. The function of rRNAs in ribosomes is not, clearly known. It is believed that they play a, significant role in the binding of mRNA to, ribosomes and protein synthesis., , CATALYTIC RNAs—RIBOZYMES, In certain instances, the RNA component of a, ribonucleoprotein (RNA in association with, protein) is catalytically active. Such RNAs are, termed as ribozymes. A selected list of ribozymes, along with their biochemical functions is given, in Table 5.4., Ribonuclease P (RNase P) is a ribozyme, containing protein and RNA component. It, cleaves tRNA precursors to generate mature, tRNA molecules., RNA molecules are known to adapt, tertiary structure just like proteins (i.e. enzymes)., The specific conformation of RNA may be, responsible for its function as biocatalyst. It, is believed that ribozymes (RNAs) were, functioning as catalysts before the occurrence of, protein enzymes, during the course of evolution., , Recombinant ribozymes (rribozymes), It is now possible to design recombinant, ribozymes that will cleave any RNA. These, ribozymes are now being considered as, therapeutic agents to cure diseases. Theoretically, it is possible to selectively degrade faulty RNAs, (mutated or inappropriately expressed RNAs in, diseases) by rribozymes. This way specific RNAs, can be eliminated from the cell that will help to, inhibit the disease process., TABLE 5.4 A selected list of ribozymes and, the corresponding biochemical reactions, , Ribozyme(s), , Biochemical reaction(s), , rRNA, , Peptide bond formation in, protein biosynthesis, , RNase P, Self-splicing RNAs, , RNA clevage and ligation, DNA cleavage, , RNAs of splicesome, , RNA splicing, , In vitro selected RNAs, , RNA polymerization,, RNA phosphorylation, RNA aminoacylation, Glycoside bond formation, Oxidation-reduction reactions, Disulfide exchange
Page 93 :
Chapter 5 : NUCLEIC ACIDS AND NUCLEOTIDES, , 1. DNA is the chemical basis of heredity organized into genes, the basic units of genetic, information., 2. RNAs (mRNA, tRNA and rRNA) are produced by DNA which in turn carry out protein, synthesis., 3. Nucleic acids are the polymers of nucleotides (polynucleotides) held by 3c and 5c, phosphodiester bridges. A nucleotide essentially consists of base + sugar (nucleoside), and phosphate., 4. Besides being the constituents of nucleic acid structure, nucleotides perform a wide variety, of cellular functions (e.g. energy carriers, metabolic regulators, second messengers etc.), 5. Both DNA and RNA contain the purines-adenine (A) and guanine (G) and the pyrimidinecytosine (C). The second pyrimidine is thymine (T) in DNA while it is uracil (U) in RNA., The pentose sugar, D-deoxyribose is found in DNA while it is D-ribose in RNA., 6. The structure of DNA is a double helix (Watson-Crick model) composed of two, antiparallel strands of polydeoxynucleotides twisted around each other. The strands are, held together by 2 or 3 hydrogen bonds formed between the bases i.e. A = T; G { C., DNA structure satisfies Chargaff’s rule that the content of A is equal to T, and that of, G equal to C., 7. Besides the double helical structure, DNA also exists in certain unusual structures —, bent DNA, triple-strand DNA, four-strand DNA., 8. RNA is usually a single stranded polyribonucleotide. mRNA is capped at 5c terminal, end by 7-methylGTP while at the 3c-terminal end, it contains a poly A tail. mRNA, specifies the sequence of amino acids in protein synthesis., 9. The structure of tRNA resembles that of a clover leaf with four arms (acceptor,, anticodon, D-, and T<C) held by complementary base pairs. tRNA delivers amino acids, for protein synthesis., 10. Certain RNAs that can function as enzymes are termed as ribozymes. Ribozymes were, probably functioning as catalysts before the occurrence of protein enzymes during evolution., , 83
Page 94 :
84, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the structure of DNA., 2. Name different RNAs and discuss their structure., 3. Write an account of structure, function and nomenclature of nucleotides., 4. Describe the structure of nitrogenous bases present in nucleic acids. Add a note on tautomerism., 5. “The backbone of nucleic acid structure is 3c-5c phosphodiester bridge.”—justify., , II. Short notes, (a) Chargaff’s rule, (b) Ribose and deoxyribose, (c) Hydrogen bonds in DNA, (d) Nucleoside,, (e) Different forms of DNA, (f) Transfer RNA, (g) Purine bases of plants, (h) Complementary base, pairs, (i) DNA denaturation, (j) hnRNA., , III. Fill in the blanks, 1. The fundamental unit of genetic information is known as _____________., 2. DNA controls protein synthesis through the mediation of _____________., 3. Nucleic acids are the polymers of _____________., 4. The pyrimidine present in DNA but absent in RNA _____________., 5. Ribose and deoxyribose differ in their structure around carbon atom _____________., 6. Nucleotide is composed of _____________., 7. The scientist who observed that there exists a relationship between the contents of purines and, pyrimidines in DNA structure (A = T; G = C) _____________., 8. The base pair G-C is more stable and stronger than A-T due to _____________., 9. Under physiological condition, the DNA structure is predominantly in the form _____________., 10. The acceptor arm of tRNA contains a capped nucleotide sequence _____________., , IV. Multiple choice questions, 11. The nitrogenous base not present in DNA structure, (a) Adenine (b) Guanine (c) Cytosine (d) Uracil., 12. The number of base pairs present in each turn (pitch) of B-form of DNA helix, (a) 9 (b) 10 (c) 11 (d) 12., 13. The backbone of nucleic acid structure is constructed by, (a) Peptide bonds (b) Glycosidic bonds (c) Phosphodiester bridges (d) All of them., 14. The following coenzyme is a nucleotide, (a) FAD (b) NAD+ (c) CoASH (d) All of them., 15. The nucleotide that serves as an intermediate for biosynthetic reaction, (a) UDP-glucose (b) CDP-acylglycerol (c) S-Adenosylmethionine (d) All of them.
Page 95 :
Section 1, , Chemical Constituents of Life, , Chapter, , Enzymes, , 16, , The enzymes speak :, , “We are the catalysts of the living world !, Protein in nature, and in action specific,, rapid and accurate;, Huge in size but with small active centres;, Highly exploited for disease diagnosis in lab centres.”, , E, , nzymes are biocatalysts – the catalysts of life., A catalyst is defined as a substance that, increases the velocity or rate of a chemical, reaction without itself undergoing any change in, the overall process., The student-teacher relationship may be a, good example to understand how a catalyst, works. The students often find it difficult to learn, from a text-book on their own. The teacher, explains the subject to the students and increases, their understanding capability. It is no wonder, that certain difficult things which the students, take days together to understand, and sometimes, do not understand at all – are easily learnt under, the guidance of the teacher. Here, the teacher, acts like a catalyst in enhancing the, understanding ability of students. A good teacher, is always a good catalyst in students’ life!, , Enzymes may be defined as biocatalysts, synthesized by living cells. They are protein in, nature (exception – RNA acting as ribozyme),, colloidal and thermolabile in character, and, specific in their action., , In the laboratory, hydrolysis of proteins by a, strong acid at 100°C takes at least a couple of, days. The same protein is fully digested by the, enzymes in gastrointestinal tract at body, temperature (37°C) within a couple of hours., This remarkable difference in the chemical, reactions taking place in the living system is, exclusively due to enzymes. The very existence, of life is unimaginable without the presence of, enzymes., , HISTORICAL BACKGROUND, Berzelius in 1836 coined the term catalysis, (Greek : to dissolve). In 1878, Kuhne used the, word enzyme (Greek : in yeast) to indicate the, catalysis taking place in the biological systems., Isolation of enzyme system from cell-free extract, of yeast was achieved in 1883 by Buchner., He named the active principle as zymase (later, found to contain a mixture of enzymes),, which could convert sugar to alcohol. In 1926,, , 85
Page 96 :
86, , BIOCHEMISTRY, , James Sumner first achieved the isolation and, crystallization of the enzyme urease from jack, bean and identified it as a protein., , NOMENCLATURE AND, CLASSIFICATION, In the early days, the enzymes were given, names by their discoverers in an arbitrary, manner. For example, the names pepsin, trypsin, and chymotrypsin convey no information about, the function of the enzyme or the nature of the, substrate on which they act. Sometimes, the, suffix-ase was added to the substrate for naming, the enzymes e.g. lipase acts on lipids; nuclease, on nucleic acids; lactase on lactose. These are, known as trivial names of the enzymes which,, however, fail to give complete information of, , enzyme reaction (type of reaction, cofactor, requirement etc.), Enzymes are sometimes considered under two, broad categories : (a) Intracellular enzymes –, They are functional within cells where they are, synthesized. (b) Extracellular enzymes – These, enzymes are active outside the cell; all the, digestive enzymes belong to this group., The International Union of Biochemistry (IUB), appointed an Enzyme Commission in 1961. This, committee made a thorough study of the existing, enzymes and devised some basic principles for, the classification and nomenclature of enzymes., Since 1964, the IUB system of enzyme, classification has been in force. Enzymes are, divided into six major classes (in that order)., Each class on its own represents the general type, of reaction brought about by the enzymes of that, class (Table 6.1)., , TABLE 6.1 Classification of enzymes, , Enzyme class with examples*, , Reaction catalysed, , 1. Oxidoreductases, Alcohol dehydrogenase (alcohol : NAD+ oxidoreductase E.C. 1.1.1.1.),, cytochrome oxidase, L- and D-amino acid oxidases, , Oxidation o� Reduction, AH2 + B o� A + BH2, , 2. Transferases, Hexokinase (ATP : D-hexose 6-phosphotransferase, E.C. 2.7.1.1.),, transaminases, transmethylases, phosphorylase, , Group transfer, A – X + B o� A + B – X, , 3. Hydrolases, Lipase (triacylglycerol acyl hydrolase E.C. 3.1.1.3), choline, esterase, acid and alkaline phosphatases, pepsin, urease, , Hydrolysis, A – B + H2O o� AH + BOH, , 4. Lyases, Aldolase (ketose 1-phosphate aldehyde lyase, E.C. 4.1.2.7),, fumarase, histidase, , Addition o� Elimination, A – B + X – Y o� AX – BY, , 5. Isomerases, Triose phosphate isomerase (D-glyceraldehyde 3-phosphate, ketoisomerase, E.C. 5.3.1.1), retinol isomerase,, phosphohexose isomerase, , Interconversion of isomers, A o� Ac, , 6. Ligases, Glutamine synthetase (L-glutamate ammonia ligase, E.C. 6.3.1.2),, acetyl CoA carboxylase, succinate thiokinase, , Condensation (usually dependent on ATP), A–B, A+B, ATP, , *, , For one enzyme in each class, systematic name along with E.C. number is given in the brackets., , ADP + Pi
Page 97 :
87, , Chapter 6 : ENZYMES, , 1. Oxidoreductases : Enzymes involved in, oxidation-reduction reactions., 2. Transferases : Enzymes that catalyse the, transfer of functional groups., 3. Hydrolases : Enzymes that bring about, hydrolysis of various compounds., 4. Lyases : Enzymes specialised in the, addition or removal of water, ammonia, CO2 etc., 5. Isomerases : Enzymes involved in all the, isomerization reactions., 6. Ligases : Enzymes catalysing the synthetic, reactions (Greek : ligate—to bind) where two, molecules are joined together and ATP is used., [The word OTHLIL (first letter in each class), may be memorised to remember the six classes, of enzymes in the correct order]., Each class in turn is subdivided into many, sub-classes which are further divided. A four, digit Enzyme Commission (E.C.) number is, assigned to each enzyme representing the class, (first digit), sub-class (second digit), sub-sub class, (third digit) and the individual enzyme (fourth, digit). Each enzyme is given a specific name, indicating the substrate, coenzyme (if any) and, the type of the reaction catalysed by the enzyme., Although the IUB names for the enzymes are, specific and unambiguous, they have not been, accepted for general use as they are complex, and cumbersome to remember. Therefore, the, trivial names, along with the E.C. numbers as, and when needed, are commonly used and, widely accepted., , CHEMICAL NATURE AND, PROPERTIES OF ENZYMES, All the enzymes are invariably proteins. In, recent years, however, a few RNA molecules, have been shown to function as enzymes. Each, enzyme has its own tertiary structure and specific, conformation which is very essential for its, catalytic activity. The functional unit of the, enzyme is known as holoenzyme which is often, , made up of apoenzyme (the protein part) and a, coenzyme (non-protein organic part)., Holoenzyme o� Apoenzyme + Coenzyme, (active enzyme), , (protein part), , (non-protein part), , The term prosthetic group is used when the, non-protein moiety tightly (covalently) binds, with the apoenzyme. The coenzyme can be, separated by dialysis from the enzyme while the, prosthetic group cannot be., The word monomeric enzyme is used if it is, made up of a single polypeptide e.g. ribonuclease, trypsin. Some of the enzymes which, possess more than one polypeptide (subunit), chain are known as oligomeric enzymes e.g., lactate, dehydrogenase,, aspartate, transcarbamoylase etc. There are certain multienzyme, complexes possessing specific sites to catalyse, different reactions in a sequence. Only the native, intact multienzyme complex is functionally active, and not the individual units, if they are separated, e.g. pyruvate dehydrogenase, fatty acid synthase,, prostaglandin synthase etc. The enzymes exhibit, all the general properties of proteins (Chapter 4)., , Genetic engineering, and modified enzymes, Recent advances in biotechnology have made, it possible to modify the enzymes with desirable, characters-improved catalytic abilities, activities, under unusual conditions. This approach is, required since enzymes possess enormous, potential for their use in medicine and industry., Hybrid enzymes : It is possible to rearrange, genes and produce fusion proteins. e.g. a hybrid, enzyme (of glucanase and cellulase) that can, more efficiently hydrolyse barley E-glucans in, beer manufacture., Site-directed mutagenesis : This is a, technique used to produce a specified mutation, at a predetermined position in a DNA molecule., The result is incorporation of a desired amino, acid (of one’s choice) in place of the specified, amino acid in the enzyme. By this approach, it, is possible to produce an enzyme with desirable, characteristics. e.g. tissue plasminogen activator, (used to lyse blood clots in myocardial
Page 98 :
88, , BIOCHEMISTRY, , 2. Concentration of substrate, , Enzyme velocity, , Increase in the substrate concentration, gradually increases the velocity of enzyme, reaction within the limited range of substrate, levels. A rectangular hyperbola is obtained when, velocity is plotted against the substrate, concentration (Fig.6.2). Three distinct phases of, the reaction are observed in the graph (A-linear;, B-curve; C-almost unchanged)., , O, , Enzyme concentration, , Fig. 6.1 : Effect of enzyme, concentration on enzyme velociy., , infarction) with increased half-life. This is, achieved by replacing asparagine (at position, 120) by glutamine., In recent years, it has also become possible to, produce hybrid enzymes by rearrangement of, genes. Another innovative approach is the, production of abzymes or catalytic antibodies,, the antibody enzymes., , FACTORS AFFECTING, ENZYME ACTIVITY, The contact between the enzyme and, substrate is the most essential pre-requisite for, enzyme activity. The important factors that, influence the velocity of the enzyme reaction are, discussed hereunder, , Order of reaction : When the velocity of the, reaction is almost proportional to the substrate, concentration (i.e. [S] is less than Km), the rate of, the reaction is said to be first order with respect, to substrate. When the [S] is much greater than, Km, the rate of reaction is independent of, substrate concentration, and the reaction is said, to be zero order., , Enzyme kinetics and Km value, The enzyme (E) and substrate (S) combine, with each other to form an unstable enzymesubstrate complex (ES) for the formation of, product (P)., E+S, , k1, , k2, , ES, , k3, , E+P, , Here k1, k2 and k3 represent the velocity, constants for the respective reactions, as, indicated by arrows., Km, the Michaelis-Menten constant (or Brig’s, and Haldane’s constant), is given by the formula, Km, , k2 � k3, k1, , The following equation is obtained after, suitable algebraic manipulation., v=, , V max [S], Km � [S], , equation (1), , where v = Measured velocity,, , 1. Concentration of enzyme, As the concentration of the enzyme is, increased, the velocity of the reaction, proportionately increases (Fig.6.1). In fact, this, property of enzyme is made use in determining, the serum enzymes for the diagnosis of diseases., By using a known volume of serum, and keeping, all the other factors (substrate, pH, temperature, etc.) at the optimum level, the enzyme could be, assayed in the laboratory., , Vmax, , = Maximum velocity,, , S, , = Substrate concentration,, , Km, , = Michaelis – Menten constant., , Let us assume that the measured velocity (v), 1, is equal to 2 Vmax. Then the equation (1) may be, substituted as follows, 1, V max, 2, , =, , V max [S], Km �[S]
Page 99 :
89, , Chapter 6 : ENZYMES, , since Vmax is approached asymptotically. By, taking the reciprocals of the equation (1), a, straight line graphic representation is obtained., , Vmax, , >@, >@, , C, , B, , Velocity, , 1, 2 Vmax, A, Km, , Substrate concentration, , Fig. 6.2 : Effect of substrate concentration on enzyme, velocity (A-linear; B-curve; C-almost unchanged)., , 2V max [S], , Km � [S], , =, , Km �[S], , = 2 [S], , Km, , = [S], , V max, , K stands for a constant and m stands for, Michaelis (in Km)., , Km or the Michaelis-Menten constant is, defined as the substrate concentration, (expressed in moles/l) to produce half-maximum, velocity in an enzyme catalysed reaction. It, indicates that half of the enzyme molecules (i.e., 50%) are bound with the substrate molecules, when the substrate concentration equals the Km, value., Km value is a constant and a characteristic, feature of a given enzyme (comparable to a, thumb impression or signature). It is a, representative for measuring the strength of ES, complex. A low Km value indicates a strong, affinity between enzyme and substrate, whereas, a high Km value reflects a weak affinity between, them. For majority of enzymes, the Km values, are in the range of 10–5 to 10–2 moles. It may, however, be noted that Km is not dependent on, the concentration of enzyme., Lineweaver-Burk double reciprocal plot : For, the determination of Km value, the substrate, saturation curve (Fig.6.2) is not very accurate, , Km � S, , 1, v, , V max S, Km, , 1, v, , V max, , 1, v, , V max, , Km, , u, u, , >@, >@, , S, 1, �, V max S, S, , >@, , 1, 1, �, V, S, max, , >@, , The above equation is similar to y = ax + b., Therefore, a plot of the reciprocal of the velocity, § 1·, ¨ ¸, © v¹, , vs. the reciprocal of the substrate concen-, , §, ·, tration ¨ 1 ¸ gives a straight line. Here the slope, ©, ¹, [S], , is Km/Vmax and whose y intercept is 1/Vmax., The Lineweaver-Burk plot is shown in, Fig.6.3. It is much easier to calculate the Km, from the intercept on x-axis which is –(1/Km)., Further, the double reciprocal plot is useful in, understanding the effect of various inhibitions, (discussed later)., Enzyme reactions with two or more, substrates : The above discussion is based on, the presumption of a single substrate-enzyme, reaction. In fact, a majority of the enzymecatalysed reactions involve two or more, substrates. Even in case of multisubstrate, , 1, v, , Slope =, 1, Vmax, , – 1, Km, , Km, Vmax, , 1, , >S @, , Fig. 6.3 : Lineweaver-Burk double reciprocal plot.
Page 100 :
90, , BIOCHEMISTRY, , enzymes, despite the complex mathematical, expressions, the fundamental principles conform, to Michaelis-Menten Kinetics., , Velocity of an enzyme reaction increases with, increase in temperature up to a maximum and, then declines. A bell-shaped curve is usually, observed (Fig.6.4)., , Temperature coefficient or Q10 is defined as, increase in enzyme velocity when the, temperature is increased by 10°C. For a majority, of enzymes, Q10 is 2 between 0°C and 40°C., Increase in temperature results in higher, activation energy of the molecules and more, molecular (enzyme and substrate) collision and, interaction for the reaction to proceed faster., The optimum temperature for most of the, enzymes is between 35°C–40°C. However, a few, enzymes (e.g. Taq DNA polymerase, muscle, adenylate kinase) are active even at 100°C. Some, plant enzymes like urease have optimum activity, around 60°C. This may be due to very stable, structure and conformation of these enzymes., In general, when the enzymes are exposed to, a temperature above 50°C, denaturation leading, to derangement in the native (tertiary) structure, of the protein and active site are seen. Majority, of the enzymes become inactive at higher, temperature (above 70°C)., , Enzyme velocity, , 3. Effect of temperature, , Optimum pH, 2, , pH, , Fig. 6.5 : Effect of pH on enzyme velocity., , Clinical significance : Foods can be preserved, in refrigerators (at low temperatures) due to, reduced bacterial enzyme activities. Certain, surgeries are carried out by lowering the patient’s, body temperature (induced hyporthermia), and, thus the matabolic rate., , 4. Effect of pH, Increase in the hydrogen ion concentration, (pH) considerably influences the enzyme activity, and a bell-shaped curve is normally obtained, (Fig.6.5). Each enzyme has an optimum pH at, which the velocity is maximum. Below and, above this pH, the enzyme activity is much, lower and at extreme pH, the enzyme becomes, totally inactive., , Enzyme velocity, , Most of the enzymes of higher organisms, show optimum activity around neutral pH (6-8)., There are, however, many exceptions like pepsin, (1-2), acid phosphatase (4-5) and alkaline, phosphatase (10-11). Enzymes from fungi and, plants are most active in acidic pH (4-6)., , Optimum, �, , �� �� �� �� �� �� �� ��, Temperature (qC), , Fig. 6.4 : Effect of temperature on enzyme velocity., , Hydrogen ions influence the enzyme activity, by altering the ionic charges on the amino acids, (particularly at the active site), substrate, ES, complex etc., , 5. Effect of product concentration, The accumulation of, generally decreases the, , reaction, enzyme, , products, velocity.
Page 101 :
91, , Chapter 6 : ENZYMES, , For certain enzymes, the products combine with, the active site of enzyme and form a loose, complex and, thus, inhibit the enzyme activity., In the living system, this type of inhibition is, generally prevented by a quick removal of, products formed. The end product inhibition by, feedback mechanism is discussed later., , Enzyme, Substrate, Active site, , 6. Effect of activators, Some of the enzymes require certain, inorganic metallic cations like Mg2+, Mn2+,, Zn2+, Ca2+, Co2+, Cu2+, Na+, K+ etc. for their, optimum activity. Rarely, anions are also needed, for enzyme activity e.g. chloride ion (Cl–), for amylase. Metals function as activators of, enzyme velocity through various mechanisms—, combining with the substrate, formation of, ES-metal complex, direct participation in the, reaction and bringing a conformational change, in the enzyme., Two categories of enzymes requiring metals, for their activity are distinguished, l, , Metal-activated enzymes : The metal is not, tightly held by the enzyme and can be, exchanged easily with other ions, e.g. ATPase (Mg2+ and Ca2+), Enolase (Mg2+), , l, , Metalloenzymes : These enzymes hold, the metals rather tightly which are not, readily exchanged. e.g. alcohol dehydrogenase, carbonic anhydrase, alkaline phosphatase, carboxypeptidase and aldolase, contain zinc., Phenol oxidase (copper);, Pyruvate oxidase (manganese);, Xanthine oxidase (molybdenum);, Cytochrome oxidase (iron and copper)., , 7. Effect of time, Under ideal and optimal conditions (like pH,, temperature etc.), the time required for an, enzyme reaction is less. Variations in the time of, the reaction are generally related to the, alterations in pH and temperature., , Fig. 6.6 : A diagrammatic representation of an, enzyme with active site., , 8. Effect of light and radiation, Exposure of enzymes to ultraviolet, beta,, gamma and X-rays inactivates certain enzymes, due to the formation of peroxides. e.g. UV rays, inhibit salivary amylase activity., , ACTIVE SITE, Enzymes are big in size compared to, substrates which are relatively smaller. Evidently,, a small portion of the huge enzyme molecule is, directly involved in the substrate binding and, catalysis (Fig.6.6)., , The active site (or active centre) of an, enzyme represents as the small region at which, the substrate(s) binds and participates in the, catalysis., , Salient features of active site, 1. The existence of active site is due to the, tertiary structure of protein resulting in threedimensional native conformation., 2. The active site is made up of amino acids, (known as catalytic residues) which are far from, each other in the linear sequence of amino acids, (primary structure of protein). For instance, the, enzyme lysozyme has 129 amino acids. The, active site is formed by the contribution of amino, acid residues numbered 35, 52, 62, 63 and 101., 3. Active sites are regarded as clefts or, crevices or pockets occupying a small region in, a big enzyme molecule.
Page 102 :
92, , BIOCHEMISTRY, , 4. The active site is not rigid in structure and (A), shape. It is rather flexible to promote the specific, substrate binding., , Active, site, , 5. Generally, the active site possesses a, substrate binding site and a catalytic site. The, latter is for the catalysis of the specific reaction., , Enzyme, , Substrate, Competitive, Inhibitor, , 6. The coenzymes or cofactors on which, some enzymes depend are present as a part of, (B), the catalytic site., , Enzyme-inhibitor, complex, , 7. The substrate(s) binds at the active site by, weak noncovalent bonds., , Enzyme, , 8. Enzymes are specific in their function due, to the existence of active sites., , Active site, , 9. The commonly found amino acids at the, active sites are serine, aspartate, histidine,, cysteine, lysine, arginine, glutamate, tyrosine etc., Among these amino acids, serine is the most, frequently found., 10. The substrate[S] binds the enzyme (E) at, the active site to form enzyme-substrate complex, (ES). The product (P) is released after the catalysis, and the enzyme is available for reuse., E+S, , ES, , E+P, , ENZYME INHIBITION, Enzyme inhibitor is defined as a substance, which binds with the enzyme and brings about, a decrease in catalytic activity of that enzyme., The inhibitor may be organic or inorganic in, nature. There are three broad categories of, enzyme inhibition, 1. Reversible inhibition., 2. Irreversible inhibition., 3. Allosteric inhibition., , 1. Reversible inhibition, The inhibitor binds non-covalently with, enzyme and the enzyme inhibition can be, reversed if the inhibitor is removed. The, reversible inhibition is further sub-divided into, I. Competitive inhibition (Fig.6.7A), II. Non-competitive inhibition (Fig.6.7B), , Substrate, , Non-competitive, inhibitor, Enzyme-inhibitor, complex, , Other site, , Fig. 6.7 : A diagrammatic representation of, (A) Competitive and (B) Non-competitive inhibition., , I. Competitive inhibition : The inhibitor (I), which closely resembles the real substrate (S) is, regarded as a substrate analogue. The inhibitor, competes with substrate and binds at the active, site of the enzyme but does not undergo any, catalysis. As long as the competitive inhibitor, holds the active site, the enzyme is not available, for the substrate to bind. During the reaction, ES, and EI complexes are formed as shown below, +S, , ES, , E+P, , E +I, EI, , The relative concentration of the substrate and, inhibitor and their respective affinity with the, enzyme determines the degree of competitive, inhibition. The inhibition could be overcome by, a high substrate concentration. In competitive, inhibition, the Km value increases whereas Vmax, remains unchanged (Fig.6.8)., The enzyme succinate dehydrogenase (SDH), is a classical example of competitive inhibition, with succinic acid as its substrate. The, compounds, namely, malonic acid, glutaric acid, and oxalic acid, have structural similarity with, succinic acid and compete with the substrate for, binding at the active site of SDH.
Page 103 :
93, , Chapter 6 : ENZYMES, , COOH, CH2COOH, CH2COOH, Succinic acid, , CH2, COOH, Malonic acid, , Methanol is toxic to the body when it is, converted to formaldehyde by the enzyme, alcohol dehydrogenase (ADH). Ethanol can, compete with methanol for ADH. Thus, ethanol, can be used in the treatment of methanol, poisoning., Some more examples of the enzymes with, substrates and competitive inhibitors (of clinical, and pharmacological significance) are given in, Table 6.2., Antimetabolites : These are the chemical, compounds that block the metabolic reactions, by their inhibitory action on enzymes., Antimetabolites are usually structural analogues, of substrates and thus are competitive inhibitors, (Table 6.2). They are in use for cancer therapy,, gout etc. The term antivitamins is used for the, antimetabolites which block the biochemical, actions of vitamins causing deficiencies, e.g., sulphonilamide, dicumarol., , enzyme surface. This binding impairs the, enzyme function. The inhibitor has no structural, resemblance with the substrate. However, there, usually exists a strong affinity for the inhibitor to, bind at the second site. In fact, the inhibitor does, not interfere with the enzyme-substrate binding., But the catalysis is prevented, possibly due to a, distortion in the enzyme conformation., The inhibitor generally binds with the, enzyme as well as the ES complex. The overall, relation in non-competitive inhibition is, represented below, E+S, +, I, , ES, +, I, , EI + S, , EIS, , E+P, , For non-competitive inhibition, the Km value, is unchanged while Vmax is lowered (Fig.6.9)., Heavy metal ions (Ag+, Pb2+, Hg2+ etc.) can, non-competitively inhibit the enzymes by, binding with cysteinyl sulfhydryl groups. The, general reaction for Hg2+ is shown below., , II. Non-competitive inhibition : The inhibitor, binds at a site other than the active site on the, , E SH + Hg2+, , E S, , Hg2+ + H+, , Vmax, 1, v, , +I, , 1, V, 2 max, , +I, , v, , 1, Vmax, , O, , Km, , Kmc, , [S], (A), , – 1, Km, , – 1, Kmc, , 1, [S], (B), , Fig. 6.8 : Effect of competitive inhibitor (i) on enzyme velocity. (A) Velocity (v) versus substrate (S) plot., (B) Lineweaver-Burk plot (Red lines with inhibitor; competitive inhibitor increases Km, unalters Vmax.).
Page 104 :
94, , BIOCHEMISTRY, , TABLE 6.2 Selected examples of enzymes with their respective substrates and competitive inhibitors, , Enzyme, , Substrate, , Inhibitor(s), , Xanthine oxidase, , Hypoxanthine, xanthine, , Allopurinol, , Used in the control of gout to reduce excess, production of uric acid from hypoxanthine., , Monoamine oxidase, , Catecholamines, (epinephrine, norepinephrine), , Ephedrine,, amphetamine, , Useful for elevating catecholamine levels., , Dihydrofolate reductase Dihydrofolic acid, , Aminopterin,, amethopterin,, methotrexate, , Employed in the treatment of leukemia and, other cancers., , Acetylcholine esterase, , Acetylcholine, , Succinyl choline, , Used in surgery for muscle relaxation, in, anaesthetised patients., , Dihydropteroate, synthase, , Para aminobenzoic acid, (PABA), , Sulfonilamide, , Prevents bacterial synthesis of folic acid., , Vitamin K epoxide, reductase, , Vitamin K, , Dicumarol, , Acts as an anticoagulant., , HMG CoA reductase, , HMG CoA, , Lovastatin,, pravastatin, , Inhibit cholesterol biosynthesis, , Heavy metals also lead to the formation of, covalent bonds with carboxyl groups and, histidine, often resulting in irreversible inhibition., , Significance of inhibitor(s), , irreversible. These inhibitors are usually toxic, substances that poison enzymes., Iodoacetate is an irreversible inhibitor of the, enzymes like papain and glyceraldehyde, 3-phosphate dehydrogenase. Iodoacetate combines, with sulfhydryl ( SH) groups at the active site of, these enzymes and makes them inactive., , 2. Irreversible inhibition, The inhibitors bind covalently with the, enzymes and inactivate them, which is, , �I, , Vmax, , 1, v, +I, 1, Vmaxc, , 1V, 2 max, , v, , 1, Vmax, , O, , Km, , S, (A), , –, , 1, , 1, Km, , >S @, (B), , Fig. 6.9 : Effect of non-competitive inhibitor (I) on enzyme velocity (A) Velocity (v) versus substrate (S) (B) LineweaverBurk plot (Red lines with inhibitor, non-competitive inhibitor does not change Km but decreases Vmax).
Page 105 :
95, , Chapter 6 : ENZYMES, , Diisopropyl fluorophosphate (DFP) is a nerve, gas developed by the Germans during Second, World War. DFP irreversibly binds with enzymes, containing serine at the active site, e.g. serine, proteases, acetylcholine esterase., Many organophosphorus insecticides like, melathion are toxic to animals (including man), as they block the activity of acetylcholine, esterase (essential for nerve conduction),, resulting in paralysis of vital body functions., Disulfiram (Antabuse®) is a drug used in the, treatment of alcoholism. It irreversibly inhibits, the enzyme aldehyde dehydrogenase. Alcohol, addicts, when treated with disulfiram become, sick due to the accumulation of acetaldehyde,, leading to alcohol avoidance. (Note : Alcohol is, metabolized by two enzymes. It is first acted, upon by alcohol dehydrogenase to yield, acetaldehyde. The enzyme aldehyde dehydrogenase converts acetaldehyde to acetic acid.), The penicillin antibiotics act as irreversible, inhibitors of serine – containing enzymes, and, block the bacterial cell wall synthesis., Cyanide inhibits cytochrome oxidase (binds, to iron atom) of electron transport chain., Fluoride inhibits enolase (by removing, manganese), and thus glycolysis., , Suicide inhibition, Suicide inhibition is a specialized form of, irreversible inhibition. In this case, the original, inhibitor (the structural analogue/competitive, inhibitor) is converted to a more potent form by, the same enzyme that ought to be inhibited. The, so formed inhibitor binds irreversibly with the, enzyme. This is in contrast to the original, inhibitor which binds reversibly., A good example of suicide inhibition is, allopurinol (used in the treatment of gout, Refer, Chapter 17). Allopurinol, an inhibitor of, xanthine oxidase, gets converted to alloxanthine,, a more effective inhibitor of this enzyme., The use of certain purine and pyrimidine, analogues in cancer therapy is also explained, on the basis suicide inhibition. For instance,, 5-fluorouracil gets converted to fluorodeoxyuridylate which inhibits the enzyme thymidylate, synthase, and thus nucleotide synthesis., , 3. Allosteric inhibition, The details of this type of inhibition are given, under allosteric regulation as a part of the, regulation of enzyme activity in the living, system., , Enzyme inhibition by drugs, Enzymes are the natural targets for, development of pharmacologic agents. Many of, the drugs used in the treatment of diseases act as, enzyme inhibitors. For example :, l, , l, , l, , Cholesterol loweing statin drugs (lovastatin), inhibit the enzyme HMG CoA reductase., Drugs (tenofovir, emtricitabine) employed to, block HIV replication inhibit the enzyme viral, reverse transcriptase., Hypertension is often treated by the drugs, (captopril, enalapril )which inhibit angiotensin, converting enzyme., , ENZYME SPECIFICITY, Enzymes are highly specific in their action, when compared with the chemical catalysts. The, occurrence of thousands of enzymes in the, biological system might be due to the specific, nature of enzymes. Three types of enzyme, specificity are well-recognised–stereospecificity,, reaction specificity, and substrate specificity., , Specificity is a characteristic property of the, active site., 1. Stereospecificity or optical specificity :, Stereoisomers are the compounds which have, the same molecular formula, but differ in their, structural configuration., The enzymes act only on one isomer and,, therefore, exhibit stereospecificity., e.g. L-amino acid oxidase and D-amino, acid oxidase act on L- and D-amino, acids respectively; hexokinase acts on, D-hexoses; glucokinase on D-glucose;, amylase acts on D-glycosidic linkages;, cellulase cleaves E-glycosidic bonds.
Page 106 :
96, , BIOCHEMISTRY, , cleaves peptide bonds attached to aromatic, amino acids (phenylalanine, tyrosine and, tryptophan). Examples of bond specificityglycosidases acting on glycosidic bonds of, carbohydrates, lipases cleaving ester bonds of, lipids etc., , Substrate, , ac, , cc, , bc, , a, , b, , c, , Enzyme, , Fig. 6.10 : Diagrammatic representation of stereospecificity (ac, bc, cc )—three point attachment of, substrate to the enzyme (a, b, c)., , l, , Broad specificity : Some enzymes act on, closely related substrates which is commonly, known as broad substrate specificity, e.g., hexokinase acts on glucose, fructose, mannose, and glucosamine and not on galactose. It, is possible that some structural similarity, among the first four compounds makes, them a common substrate for the enzyme, hexokinase., , COENZYMES, Stereospecificity is explained by considering, three distinct regions of substrate molecule, specifically binding with three complementary, regions on the surface of the enzyme (Fig.6.10)., The class of enzymes belonging to isomerases, do not exhibit stereospecificity, since they are, specialized in the interconversion of isomers., 2. Reaction specificity : The same substrate, can undergo different types of reactions, each, catalysed by a separate enzyme and this is, referred to as reaction specificity. An amino acid, can undergo transamination, oxidative deamination, decarboxylation, racemization etc. The, enzymes however, are different for each of these, reactions (For details, refer Chapter 15)., 3. Substrate specificity : The substrate, specificity varies from enzyme to enzyme. It may, be either absolute, relative or broad., l, , l, , Absolute substrate specificity : Certain, enzymes act only on one substrate e.g., glucokinase acts on glucose to give glucose 6phosphate, urease cleaves urea to ammonia, and carbon dioxide., Relative substrate specificity : Some enzymes, act on structurally related substances. This, in, turn, may be dependent on the specific group, or a bond present. The action of trypsin is, a good example for group specificity (Refer, Fig.8.7). Trypsin hydrolyses peptide linkage, involving arginine or lysine. Chymotrypsin, , The protein part of the enzyme, on its own, is, not always adequate to bring about the catalytic, activity. Many enzymes require certain nonprotein small additional factors, collectively, referred to as cofactors for catalysis. The, cofactors may be organic or inorganic in nature., , The non-protein, organic, low molecular, weight and dialysable substance associated with, enzyme function is known as coenzyme., The functional enzyme is referred to as, holoenzyme which is made up of a protein part, (apoenzyme) and a non-protein part (coenzyme)., The term prosthetic group is used when a nonprotein moiety is tightly bound to the enzyme, which is not easily separable by dialysis. The term, activator is referred to the inorganic cofactor (like, Ca2+, Mg2+, Mn2+ etc.) necessary to enhance, enzyme activity. It may, however, be noted that, some authors make no distinction between the, terms cofactor, coenzyme and prosthetic group, and use them interchangeably., Coenzymes are second substrates :, Coenzymes are often regarded as the second, substrates or co-substrates, since they have, affinity with the enzyme comparable with that of, the substrates. Coenzymes undergo alterations, during the enzymatic reactions, which are later, regenerated. This is in contrast to the substrate, which is converted to the product.
Page 108 :
98, , MECHANISM OF ENZYME ACTION, Catalysis is the prime function of enzymes., Enzymes are powerful catalysts. The nature of, catalysis taking place in the biological system is, similar to that of non-biological catalysis. For any, chemical reaction to occur, the reactants have to, be in an activated state or transition state., Enzymes lower activation energy : The, energy required by the reactants to undergo the, reaction is known as activation energy. The, reactants when heated attain the activation, energy. The catalyst (or the enzyme in the, biological system) reduces the activation energy, and this causes the reaction to proceed at a, lower temperature. Enzymes do not alter the, equilibrium constants, they only enhance the, velocity of the reaction., The role of catalyst or enzyme is comparable, with a tunnel made in a mountain to reduce the, barrier as illustrated in Fig.6.11. The enzyme, lowers energy barrier of reactants, thereby, making the reaction go faster. The enzymes, reduce the activation energy of the reactants in, such a way that all the biological systems occur, at body temperature (below 40°C)., , Enzyme-substrate complex formation, The prime requisite for enzyme catalysis is, that the substrate (S) must combine with the, enzyme (E) at the active site to form enzymesubstrate complex (ES) which ultimately results, in the product formation (P)., , Activation energy, without enzyme, , Mountain, , A, , Tunnel, , Activation energy, with enzyme, , Energy, , phosphate. Such coenzymes are, therefore,, regarded as nucleotides e.g. NAD+, NADP+,, FMN, FAD, coenzyme A, UDPG etc., Protein coenzymes : Thioredoxin is a protein, that serves as a coenzyme for the enzyme, ribonucleotide reductase (Chapter 17)., Coenzymes do not decide enzyme specificity :, A particular coenzyme may participate in, catalytic reactions along with different enzymes., For instance, NAD+ acts as a coenzyme for lactate, dehydrogenase and alcohol dehydrogenase. In, both the enzymatic reactions, NAD+ is involved, in hydrogen transfer. The specificity of the, enzyme is mostly dependent on the apoenzyme, and not on the coenzyme., , BIOCHEMISTRY, , Energy change, in reaction, , B, , Fig. 6.11 : Effect of enzyme on activation energy, of a reaction (A is the substrate and B is the, product. Enzyme decreases activation energy)., , E+S, , ES, , E+P, , A few theories have been put forth to explain, mechanism of enzyme-substrate complex, formation., , Lock and key model, or Fischer’s template theory, This theory was proposed by a German, biochemist, Emil Fischer. This is in fact the very, first model proposed to explain an enzyme, catalysed reaction., According to this model, the structure or, conformation of the enzyme is rigid. The substrate, fits to the binding site (now active site) just as a, key fits into the proper lock or a hand into the, proper glove. Thus the active site of an enzyme is, a rigid and pre-shaped template where only a, specific substrate can bind. This model does not, give any scope for the flexible nature of enzymes,, hence the model totally fails to explain many, facts of enzymatic reactions, the most important, being the effect of allosteric modulators., , Induced fit theory, or Koshland’s model, Koshland, in 1958, proposed a more, acceptable and realistic model for enzymesubstrate complex formation. As per this model,
Page 109 :
99, , Chapter 6 : ENZYMES, , MECHANISM OF ENZYME CATALYSIS, Substrate, , (A), Enzyme, , ES, , The formation of an enzyme-substrate, complex (ES) is very crucial for the catalysis to, occur, and for the product formation. It is, estimated that an enzyme catalysed reaction, proceeds 106 to 1012 times faster than a noncatalysed reaction. The enhancement in the rate, of the reaction is mainly due to four processes :, 1. Acid-base catalysis;, , (B), Enzyme, , ES, , 2. Substrate strain;, 3. Covalent catalysis;, 4. Entropy effects., , (C), Enzyme, , ES, , Fig. 6.12 : Mechanism of enzyme-substrate (ES), complex formation (A) Lock and key model, (B) Induced fit theory (C) Substrate strain theory., , the active site is not rigid and pre-shaped. The, essential features of the substrate binding site are, present at the nascent active site. The interaction, of the substrate with the enzyme induces a fit or, a conformation change in the enzyme, resulting in, the formation of a strong substrate binding site., Further, due to induced fit, the appropriate amino, acids of the enzyme are repositioned to form the, active site and bring about the catalysis ( Fig.6.12)., Induced fit model has sufficient experimental, evidence from the X-ray diffraction studies., Koshland’s model also explains the action of, allosteric modulators and competitive inhibition, on enzymes., , Substrate strain theory, In this model, the substrate is strained due to, the induced conformation change in the enzyme., It is also possible that when a substrate binds to, the preformed active site, the enzyme induces a, strain to the substrate. The strained substrate, leads to the formation of product., In fact, a combination of the induced fit, model with the substrate strain is considered to, be operative in the enzymatic action., , 1. Acid-base catalysis : Role of acids and, bases is quite important in enzymology. At the, physiological pH, histidine is the most important, amino acid, the protonated form of which, functions as an acid and its corresponding, conjugate as a base. The other acids are OH, group of tyrosine, SH group of cysteine, and, H-amino group of lysine. The conjugates of these, acids and carboxyl ions (COO–) function as, bases., Ribonuclease which cleaves phosphodiester, bonds in a pyrimidine loci in RNA is a classical, example of the role of acid and base in the, catalysis., 2. Substrate strain : Induction of a strain on, the substrate for ES formation is discussed above., During the course of strain induction, the energy, level of the substrate is raised, leading to a, transition state., The mechanism of lysozyme (an enzyme of, tears, that cleaves E-1,4 glycosidic bonds) action, is believed to be due to a combination of, substrate strain and acid-base catalysis., 3. Covalent catalysis : In the covalent, catalysis, the negatively charged (nucleophilic), or positively charged (electrophilic) group is, present at the active site of the enzyme. This, group attacks the substrate that results in the, covalent binding of the substrate to the enzyme., In the serine proteases (so named due to the, presence of serine at active site), covalent, catalysis along with acid-base catalysis occur,, e.g. chymotrypsin, trypsin, thrombin etc.
Page 110 :
100, , BIOCHEMISTRY, , 4. Proximity catalysis : The reactants should, come in close proximity to the enzyme, for, appropriate catalysis to occur. The higher the, concentration of the substrate molecules, the, greater will be the rate of reaction. As the, enzyme binds with substrate molecules at the, active site, the catalysis will increase several fold, (at least a thousand fold), In the actual catalysis of the enzymes, more, than one of the processes – acid-base catalysis,, substrate strain, covalent catalysis and proximity, catalysis are simultaneously operative. This will, help the substrate(s) to attain a transition state, leading to the formation of products., , THERMODYNAMICS OF, ENZYMATIC REACTIONS, , 3. Endothermic (endergonic) reactions :, Energy is consumed in these reactions e.g., glucokinase, Glucose + ATP o Glucose 6-phosphate + ADP, , REGULATION OF ENZYME, ACTIVITY IN THE LIVING SYSTEM, In biological system, regulation of enzyme, activities occurs at different stages in one or, more of the following ways to achieve cellular, economy., 1. Allosteric regulation, 2. Activation of latent enzymes, , The enzyme catalysed reactions may be, broadly grouped into three types based on, thermodynamic (energy) considerations., , 3. Compartmentation of metabolic, pathways, , 1. Isothermic reactions : The energy, exchange between reactants and products is, negligible e.g. glycogen phosphorylase, , 5. Enzyme degradation, , Glycogen + Pi o Glucose 1-phosphate, 2. Exothermic (exergonic) reactions : Energy, is liberated in these reactions e.g. urease, Urea o NH3 + CO2 + energy, , 4. Control of enzyme synthesis, 6. Isoenzymes, , 1. Allosteric regulation, and allosteric inhibition, Some of the enzymes possess additional sites,, known as allosteric sites (Greek : allo–other),, , + The existence of life is unimaginable without the presence of enzymes—the biocatalysts., + Majority of the coenzymes (TPP, NAD+, FAD, CoA) are derived from B-complex, vitamins in which form the latter exert their biochemical functions., , + Competitive inhibitors of certain enzymes are of great biological significance. Allopurinol,, employed in the treatment of gout, inhibits xanthine oxidase to reduce the formation, of uric acid. The other competitive inhibitors include aminopterin used in the treatment, of cancers, sulfanilamide as antibactericidal agent and dicumarol as an anticoagulant., , + The nerve gas (diisopropyl fluorophosphate), first developed by Germans during Second, World War, inhibits acetylcholine esterase, the enzyme essential for nerve conduction, and paralyses the vital body functions. Many organophosphorus insecticides (e.g., melathion) also block the activity of acetylcholine esterase., , + Penicillin antibiotics irreversibly inhibit serine containing enzymes of bacterial cell wall, synthesis.
Page 111 :
101, , Chapter 6 : ENZYMES, , (A), , (B), , (C), , (D), , change in the active site of the enzyme, leading, to the inhibition or activation of the catalytic, activity (Fig.6.13). In the concerted model,, allosteric enzymes exist in two conformational, states – the T (tense or taut) and the R (relaxed)., The T and R states are in equilibrium., Allosteric activator (or) substrate, , T, , besides the active site. Such enzymes are known, as allosteric enzymes. The allosteric sites are, unique places on the enzyme molecule., Allosteric effectors : Certain substances, referred to as allosteric modulators (effectors or, modifiers) bind at the allosteric site and regulate, the enzyme activity. The enzyme activity is, increased when a positive (+) allosteric effector, binds at the allosteric site known as activator, site. On the other hand, a negative (–) allosteric, effector binds at the allosteric site called, inhibitor site and inhibits the enzyme activity., Classes of allosteric enzymes : Enzymes that, are regulated by allosteric mechanism are, referred to as allosteric enzymes. They are, divided into two classes based on the influence, of allosteric effector on Km and Vmax., l, , l, , K-class of allosteric enzymes, the effector, changes the Km and not the Vmax. Double, reciprocal plots, similar to competitive, inhibition are obtained e.g. phosphofructokinase., V-class of allosteric enzymes, the effector alters, the Vmax and not the Km. Double reciprocal, plots resemble that of non-competitive, inhibition e.g. acetyl CoA carboxylase., , Conformational, changes, in, allosteric, enzymes : Most of the allosteric enzymes are, oligomeric in nature. The subunits may be, identical or different. The non-covalent, reversible binding of the effector molecule at the, allosteric site brings about a conformational, , Allosteric inhibitors favour T state whereas, activators and substrates favour R state. The, substrate can bind only with the R form of the, enzyme. The concentration of enzyme molecule, in the R state increases as more substrate is, added, therefore the binding of the substrate to, the allosteric enzyme is said to be cooperative., Allosteric enzymes give a sigmoidal curve (instead, of hyperbola) when the velocity (v) versus, substrate(S) concentration are plotted (Fig.6.14)., The term homotropic effect is used if the, substrate influences the substrate binding, through allosteric mechanism, their effect is, always positive. Heterotropic effect is used, when an allosteric modulator effects the binding, of substrate to the enzyme. Heterotropic, interactions are either positive or negative., Selected examples of allosteric enzymes, responsible for rapid control of biochemical, pathways are given in Table 6.5., , Hyperbolic, curve, Enzyme velocity, , Fig. 6.13 : Diagrammatic representation of an, allosteric enzyme (A) T-form; (B) R-form; (C) Effect of, allosteric inhibitor; (D) Effect of allosteric activator., , R, Allosteric inhibitor, , Sigmoidal, curve, , Substrate concentration, , Fig. 6.14 : Effect of substrate concentration on allosteric enzyme (red line-sigmoidal curve) in comparison, with normal enzyme (blue line-hyperbolic curve).
Page 112 :
102, , BIOCHEMISTRY, , TABLE 6.5 Some enzymes with allosteric effectors, Allosteric, Enzyme, , Metabolic pathway, , Inhibitor, , Activator, , Hexokinase, , Glycolysis, , Glucose 6-phosphate, , Phosphofructokinase, , Glycolysis, , ATP, , AMP, ADP, , Isocitrate dehydrogenase, , Krebs cycle, , ATP, , ADP, NAD+, , Pyruvate carboxylase, , Gluconeogenesis, , —, , Acetyl CoA, , —, , Fructose 1, 6 - bisphosphatase, , Gluconeogenesis, , AMP, , Carbamoyl phosphate synthetase I, , Urea cycle, , —, , N - Acetylglutamate, , Tryptophan oxygenase, , Tryptophan metabolism, , —, , L - Tryptophan, , Acetyl CoA carboxylase, , Fatty acid synthesis, , Palmitate, , Isocitrate, , Carbamoyl phosphate + Aspartate, , Feedback regulation, The process of inhibiting the first step by the, final product, in a series of enzyme catalysed, reactions of a metabolic pathway is referred to, as feedback regulation. Look at the series of, reactions given below, A, , e1, , B, , e2, , C, , e3, , —, , D, , e4, , E, , A is the initial substrate, B, C, and D are the, intermediates and E is the end product, in a, pathway catalysed by four different enzymes, (e1, e2, e3, e4). The very first step (A o B by the, enzyme e1) is the most effective for regulating, the pathway, by the final end product E. This, type of control is often called negative feedback, regulation since increased levels of end product, will result in its (e1) decreased synthesis. This is, a real cellular economy to save the cell from, the wasteful expenditure of synthesizing a, compound which is already available within the, cell., Feedback inhibition or end product inhibition, is a specialised type of allosteric inhibition, necessary to control metabolic pathways for, efficient cellular function., Aspartate transcarbamoylase (ATCase) is, a good example of an allosteric enzyme, inhibited by a feedback mechanism. ATCase, catalyses the very first reaction in pyrimidine, biosynthesis., , Aspartate, transcarbamoylase, Feedback, control, , Carbamoyl aspartate + Pi, , Cytidine triphosphate (CTP), , Carbamoyl phosphate undergoes a sequence, of reactions for synthesis of the end product,, CTP. When CTP accumulates, it allosterically, inhibits the enzyme aspartate transcarbamoylase, by a feedback mechanism., Feedback regulation or feedback inhibition?, Sometimes a distinction is made between these, two usages. Feedback regulation represents a, phenomenon while feedback inhibition involves, the mechanism of regulation. Thus, in a true, sense, they are not synonymous. For instance,, dietary cholesterol decreases hepatic cholesterol, biosynthesis through feedback regulation. This, does not involve feedback inhibition, since, dietary cholesterol does not directly inhibit the, regulatory enzyme HMG CoA reductase., However, the activity of gene encoding this, enzyme is reduced (repression) by cholesterol., , 2. Activation of latent enzymes, Latent enzymes, as such, are inactive. Some, enzymes are synthesized as proenzymes or, zymogens which undergo irreversible covalent
Page 113 :
103, , Chapter 6 : ENZYMES, , activation by the breakdown of one or more, peptide bonds. For instance, proenzymes –namely, chymotrypsinogen, pepsinogen and plasminogen,, are respectively – converted to the active enzymes, chymotrypsin, pepsin and plasmin., Certain enzymes exist in the active and, inactive forms which are interconvertible,, depending on the needs of the body. The, interconversion is brought about by the, reversible covalent modifications, namely, phosphorylation and dephosphorylation, and, oxidation and reduction of disulfide bonds., Glycogen phosphorylase is a muscle enzyme, that breaks down glycogen to provide energy., This enzyme is a homodimer (two identical, subunits) and exists in two interconvertible forms., Phosphorylase b (dephospho enzyme) is inactive, which is converted by phosphorylation of serine, residues to active form phosphorylase a. The, inactive enzyme phosphorylase b is produced on, dephosphorylation as illustrated below., 2 ATP, , cAMP dependent, protein kinase, , 2 ADP, , P, P, Phosphorylase b, (inactive), , Phosphatase, , 2 Pi, , Phosphorylase a, (active), , Other examples of phosphorylated active, enzymes – citrate lyase, fructose 2,6-bisphosphatase., There are some enzymes which are active in, dephosphorylated state and become inactive, when phosphorylated e.g. glycogen synthase,, acetyl CoA carboxylase, HMG CoA reductase., A few enzymes are active only with sulfhydryl, ( SH) groups, e.g. succinate dehydrogenase,, urease. Substances like glutathione bring about, the stability of these enzymes., E S S E, Oxidised, inactive, enzyme, , 2G SH, , E SH + E SH, GS SG, , Reduced, active, enzyme, , 3. Compartmentation, There are certain substances in the body (e.g.,, fatty acids, glycogen) which are synthesized and, also degraded. There is no point for simultaneous, occurrence of both the pathways. Generally, the, synthetic (anabolic) and breakdown (catabolic), pathways are operative in different cellular, organelles to achieve maximum economy. For, instance, enzymes for fatty acid synthesis are, found in the cytosol whereas enzymes for fatty, acid oxidation are present in the mitochondria., The intracellular location of certain enzymes, and metabolic pathways is given in Table 6.6., , TABLE 6.6 Distribution of certain enzymes and metabolic pathways in cellular organelles, Organelle, , Enzyme/metabolic pathway, , Cytoplasm, , Aminotransferases; peptidases; glycolysis; hexose monophosphate shunt; fatty acid, synthesis; purine and pyrimidine catabolism., , Mitochondria, , Fatty acid oxidation; amino acid oxidation; Krebs cycle; urea synthesis; electron, transport chain and oxidative phosphorylation., , Nucleus, , Biosynthesis of DNA and RNA., , Endoplasmic reticulum (microsomes), , Protein biosynthesis; triacylglycerol and phospholipid synthesis; steroid synthesis and, reduction; cytochrome P450; esterase., , Lysosomes, , Lysozyme; phosphatases; phospholipases; hydrolases; proteases; lipases; nucleases., , Golgi apparatus, , Glucose 6-phosphatase; 5c-nucleotidase; glucosyl- and galactosyl-transferases., , Peroxisomes, , Catalase; urate oxidase; D-amino acid oxidase; long chain fatty acid oxidation.
Page 114 :
104, , 4. Control of enzyme synthesis, Most of the enzymes, particularly the rate, limiting ones, are present in very low, concentration. Nevertheless, the amount of the, enzyme directly controls the velocity of the, reaction, catalysed by that enzyme. Many rate, limiting enzymes have short half-lives. This helps, in the efficient regulation of the enzyme levels., , BIOCHEMISTRY, , In general, the key and regulatory enzymes, are most rapidly degraded. If not needed, they, immediately disappear and, as and when, required, they are quickly sysnthesized. Though, not always true, an enzyme with long half-life is, usually sluggish in its catalytic activity., , 6. Isoenzymes, , There are two types of enzymes—(a) Constitutive enzymes (house-keeping enzymes)—the, levels of which are not controlled and remain, fairly constant. (b) Adaptive enzymes—their, concentrations increase or decrease as per body, needs and are well-regulated. The synthesis of, enzymes (proteins) is regulated by the genes, (Refer Chapter 26)., , Multiple forms of the same enzyme will also, help in the regulation of enzyme activity, Many, of the isoenzymes are tissue-specific. Although, isoenzymes of a given enzyme catalyse the same, reaction, they differ in Km, Vmax or both. e.g., isoenzymes of LDH and CPK., , Induction and repression : The term induction, is used to represent increased synthesis of, enzyme while repression indicates its decreased, synthesis. Induction or repression which, ultimately determines the enzyme concentration, at the gene level through the mediation of, hormones or other substances., , UNITS OF ENZYME ACTIVITY, , Examples of enzyme induction : The hormone, insulin induces the synthesis of glycogen, synthetase, glucokinase, phosphofructokinase, and pyruvate kinase. All these enzymes are, involved in the utilization of glucose. The, hormone cortisol induces the synthesis of many, enzymes e.g. pyruvate carboxylase, tryptophan, oxygenase and tyrosine aminotransferase., , Enzymes are never expressed in terms of their, concentration (as mg or Pg etc.), but are, expressed only as activities. Various methods, have been introduced for the estimation of, enzyme activities (particularly for the plasma, enzymes). In fact, the activities have been, expressed in many ways, like King-Armstrong, units, Somogyi units, Reitman-Frankel units,, spectrophotometric units etc., , Katal, , Examples of repression : In many instances,, substrate can repress the synthesis of enzyme., Pyruvate carboxylase is a key enzyme in the, synthesis of glucose from non-carbohydrate, sources like pyruvate and amino acids. If there is, sufficient glucose available, there is no necessity, for its synthesis. This is achieved through, repression of pyruvate carboxylase by glucose., , In order to maintain uniformity in the, expression of enzyme activities (as units), worldover, the Enzyme Commission of IUB has, suggested radical changes. A new unit – namely, katal (abbreviated as kat) – was introduced., One kat denotes the conversion of one mole, substrate per second (mol/sec). Activity may also, be expressed as millikatals (mkat), microkatals, (Pkat) and so on., , 5. Enzyme degradation, , International Units (IU), , Enzymes are not immortal, since it will create, a series of problems. There is a lot of variability, in the half-lives of individual enzymes. For some,, it is in days while for others in hours or in, minutes, e.g. LDH4 — 5 to 6 days; LDH1 — 8 to, 12 hours; amylase — 3 to 5 hours., , Some workers prefer to use standard units or, SI units (System International). One SI unit or, International Unit (IU) is defined as the amount, of enzyme activity that catalyses the conversion, of one micromol of substrate per minute., SI units and katal are interconvertible.
Page 115 :
105, , Chapter 6 : ENZYMES, , 1 IU, 1 kat, , = 16.67 nkat, (or), = 6 u 107 IU, , TABLE 6.7 A selected list of applications, of enzymes, Enzyme, , Laboratory use of enzyme units, In the clinical laboratories, however, the, units— namely katal or SI units—are yet to find, a place. Many investigators still use the old units, like King-Armstrong units, Somogyi units etc., while expressing the enzyme activities. It is, therefore, essential that the units of enzyme, activity, along with the normal values, be, invariably stated while expressing the enzymes, for comparison., , NON-PROTEIN ENZYMES, Ribozymes, Ribozymes are a group of ribonucleic acids, that function as biological catalysts, and they are, regarded as non-protein enzymes., Altman and his coworkers, in 1983, found, that ribonuclease P – an enzyme till then known, to cleave precursors of tRNAs to give tRNAs –, was functional due to RNA component present, in the enzyme and not the protein part of the, enzyme (Refer Chapter 4)., The RNA part isolated from ribonuclease P, exhibited a true enzyme activity and also obeyed, Michaelis-Menten kinetics. Later studies have, proved that RNA, in fact, can function as an, enzyme and bring about the catalysis., RNA molecules are known to adapt a tertiary, structure just as in the case of proteins (i.e., enzymes). The specific conformation of RNA, may be responsible for its function as biocatalyst., It is believed that ribozymes (RNAs) were, functioning as catalysts before the occurrence of, protein enzymes during evolution., , APPLICATIONS OF ENZYMES, Certain enzymes are useful as therapeutic, agents,, analytical, reagents,, in, genetic, manipulations and for industrial applications, (Table 6.7)., , Application, , Therapeutic applications, Streptokinase/urokinase, Asparaginase, Papain, D1-Antitrypsin, Pancreatic enzymes, (trypsin, lipase), , To remove blood clots, In cancer therapy, Anti-inflammatory, To treat emphysema, For digestion (in, pancreatic diseases), , Analytical application reagents (for estimation), Glucose oxidase and peroxidase, Urease, Cholesterol oxidase, Uricase, Lipase, Luciferase, Alkaline phosphatase/, horse radish peroxidase, , Glucose, Urea, Cholesterol, Uric acid, Triacylglycerols, To detect bacterial, contamination of foods, In the analytical technique, ELISA, , Applications in genetic engineering, Restriction endonucleases, Gene transfer, DNA finger, printing, Polymerase chain, Taq DNA polymerase, reaction, Industrial applications, Rennin, Glucose isomerase, , Cheese preparation, Production of high, fructose syrup, In food industry, Washing powder, , D-Amylase, Proteases, , Enzymes as therapeutic agents, 1. Streptokinase prepared from streptococcus, is useful for clearing the blood clots., Streptokinase activates plasma plasminogen to, plasmin which, in turn, attacks fibrin to convert, into soluble products., Plasminogen, Streptokinase, Plasmin, Fibrin, (clot), , Soluble products
Page 116 :
106, , BIOCHEMISTRY, , 2. The enzyme asparaginase is used in the, treatment of leukemias. Tumor cells are dependent, on asparagine of the host’s plasma for their, multiplication. By administering asparaginase, the, host’s plasma levels of asparagine are drastically, reduced. This leads to depression in the viability, of tumor cells., , Enzymes as analytical reagents, Some enzymes are useful in the clinical, laboratory for the measurement of substrates,, drugs, and even the activities of other enzymes., The biochemical compounds (e.g. glucose, urea,, uric acid, cholesterol) can be more accurately, and specifically estimated by enzymatic, procedures compared to the conventional, chemical methods. A good example is the, estimation of plasma glucose by glucose oxidase, and peroxidase method., , Immobilized enzymes, Enzymes can be used as catalytic agents in, industrial and medical applications. Some of, these enzymes are immobilized by binding them, to a solid, insoluble matrix which will not, affect the enzyme stability or its catalytic, activity. Beaded gels and cyanogen bromide, activated sepharose are commonly used for, immobilization of enzymes. The bound enzymes, can be preserved for long periods without loss of, activity., Glucose oxidase and peroxidase, immobilized, and coated on a strip of paper, are used in the, clinical laboratory for the detection of glucose in, urine., Glucose, H2O2, o-Toluidine, (colourless), , Oxidase, Peroxidase, , O, , Gluconic acid + H2O2, H2O, Oxidized toluidine, (blue colour), , The intensity of the blue colour depends on, the concentration of glucose. Hence, the strip, method is useful for semi-quantitative estimation, of glucose in urine., , DIAGNOSTIC IMPORTANCE, OF ENZYMES, Estimation of enzyme activities in biological, fluids (particularly plasma/serum) is of great, clinical importance. Enzymes in the circulation, are divided into two groups – plasma functional, and plasma non-functional., , 1. Plasma specific or plasma, functional enzymes, Certain enzymes are normally present in the, plasma and they have specific functions to, perform. Generally, these enzyme activities are, higher in plasma than in the tissues. They are, mostly synthesized in the liver and enter the, circulation e.g. lipoprotein lipase, plasmin,, thrombin, choline esterase, ceruloplasmin etc., , 2. Non-plasma specific or plasma, non-functional enzymes, These enzymes are either totally absent or, present at a low concentration in plasma, compared to their levels found in the tissues., The digestive enzymes of the gastrointestinal, tract (e.g. amylase, pepsin, trypsin, lipase etc.), present in the plasma are known as secretory, enzymes. All the other plasma enzymes, associated with metabolism of the cell are, collectively referred to as constitutive enzymes, (e.g. lactate dehydrogenase, transaminases, acid, and alkaline phosphatases, creatine phosphokinase)., Estimation of the activities of non-plasma, specific enzymes is very important for the, diagnosis and prognosis of several diseases., The normal serum level of an enzyme, indicates the balance between its synthesis and, release in the routine cell turnover. The raised, enzyme levels could be due to cellular damage,, increased rate of cell turnover, proliferation, of cells, increased synthesis of enzymes etc., Serum enzymes are conveniently used as, markers to detect the cellular damage which, ultimately helps in the diagnosis of diseases., (Note : Ther term biomarker refers to any, laboratory analyte (enzyme, protein, antigen,, antibody, metabolite etc.) that is useful for the
Page 117 :
107, , Chapter 6 : ENZYMES, , TABLE 6.8 Important enzymes in the diagnosis of diseases, , Serum enzyme (elevated), , Disease (most important), , Amylase, , Acute pancreatitis, , Serum glutamate pyruvate transaminase (SGPT), , Liver diseases (hepatitis), , Serum glutamate oxaloacetate transaminase (SGOT), , Heart attacks (myocardial infarction), , Alkaline phosphatase, , Rickets, obstructive jaundice, , Acid phosphatase, , Cancer of prostate gland, , Lactate dehydrogenase (LDH), , Heart attacks, liver diseases, , Creatine phosphokinase (CPK), , Myocardial infarction (early marker), , Aldolase, , Muscular dystrophy, , 5c-Nucleotidase, , Hepatitis, , J-Glutamyl transpeptidase (GGT), , Alcoholism, , diognosis/prognosis of any disease. Biomarker is, a vague term, and less frequently used by, biochemists.), A summary of the important enzymes useful, for the diagnosis of specific diseases is given in, Table 6.8. Detailed information on the diagnostic, enzymes including reference values is provided, in Table 6.9. A brief account of selected, diagnostic enzymes is discussed, , Amylase : The activity of serum amylase is, increased in acute pancreatitis (reference 80-180, Somogyi units/dl). The peak value is observed, within 8-12 hours after the onset of disease, which returns to normal by 3rd or 4th day., Elevated activity of amylase is also found in urine, of the patients of acute pancreatitis. Serum, amylase is also important for the diagnosis of, chronic pancreatitis, acute parotitis (mumps) and, obstruction of pancreatic duct., , It may be noted that SGPT is more specific for, the diagnosis of liver diseases while SGOT is for, heart diseases. This is mainly because of their, cellular distribution – SGPT is a cytosomal, enzyme while SGOT is found in cytosol and, mitochondria., , Alkaline phosphatase (ALP) : It is elevated in, certain bone and liver diseases (reference 3-13, KA units/dl). ALP is useful for the diagnosis of, rickets, hyperparathyroidism, carcinoma of, bone, and obstructive jaundice., Acid phosphatase (ACP) : It is increased in, the cancer of prostate gland (reference 0.5-4 KA, units/dl). The tartarate labile ACP (reference, <1 KA units/dl) is useful for the diagnosis and, prognosis of prostate cancers i.e. ACP is a good, tumor marker., , Alanine transaminase (ALT/SGPT) : SGPT is, elevated in acute hepatitis of viral or toxic, origin, jaundice and cirrhosis of liver (reference, 3-40 IU/l)., , Lactate dehydrogenase (LDH) : LDH is useful, for the diagnosis of myocardial infarction,, infective hepatitis, leukemia and muscular, dystrophy (serum LDH reference 50-200 IU/l)., LDH has five isoenzymes, the details of which, are described later., , Aspartate transaminase (AST/SGOT) : SGOT, activity in serum is increased in myocardial, infarction and also in liver diseases (reference, 4-45 IU/l)., , Creatine kinase (CK) : It is elevated in, myocardial infarction (early detection) and, muscular dystrophy (reference 10-50 IU/l). CK, has three isoenzymes (described later).
Page 119 :
109, , Chapter 6 : ENZYMES, , TABLE 6.10 Decrease in plasma (serum) enzymes in certain diseases, , Enzyme, , Reference values, , Disease(s) in which decreased, , Amylase, , 80–180 Somogyi units/dl Liver diseases, , Pseudocholinesterase (ChE II), , 10–20 IU/dl, , Viral hepatitis, malnutrition, liver cancer,, cirrhosis of liver, , Ceruloplasmin, , 20–50 mg/dl, , Wilson’s disease, (hepatolenticular degeneration), , Glucose 6-phosphate dehydrogenase (G6PD) in RBC 120–260 IU/1012 RBC, , J-Glutamyl transpeptidase (GGT) : It is a, sensitive diagnostic marker for the detection of, alcoholism. GGT is also increased in infective, hepatitis and obstructive jaundice., , Decreased plasma enzyme activities, Sometimes, the plasma activities of the, enzymes may be lower than normal which could, be due to decreased enzyme synthesis or, congenital deficiency. In Table 6.10, the, decreased plasma enzymes in certain disorders, are given., , ISOENZYMES, The multiple forms of an enzyme catalysing, the same reaction are isoenzymes or isozymes., They, however, differ in their physical and, chemical properties which include the structure,, electrophoretic and immunological properties,, Km and Vmax values, pH optimum, relative, susceptibility to inhibitors and degree of, denaturation., , Explanation for the, existence of isoenzymes, Many possible reasons are offered to explain, the presence of isoenzymes in the living systems., 1. Isoenzymes synthesized from different, genes e.g. malate dehydrogenase of cytosol is, different from that found in mitochondria., 2. Oligomeric enzymes consisting of more, than one type of subunits e.g. lactate dehydrogenase and creatine phosphokinase., , Congenital deficiency with hemolytic anemia, , 3. An enzyme may be active as monomer or, oligomer e.g. glutamate dehydrogenase., 4. In glycoprotein enzymes, differences in, carbohydrate content may be responsible for, isoenzymes e.g. alkaline phosphatase., , Isoenzymes of lactate, dehydrogenase (LDH), Among the isoenzymes, LDH has been the, most thoroughly investigated., LDH whose systematic name is L-lactateNAD+ oxidoreductase (E.C. 1.1.1.27) catalyses, the interconversion of lactate and pyruvate as, shown below, O, , LDH, , CH 3 C COOH, , CH 3 CH COOH, OH, Lactic acid, , NAD, , +, , NA DH + H +, Pyruvic acid, , LDH has five distinct isoenzymes LDH1,, LDH2, LDH3, LDH4 and LDH5. They can be, separated by electrophoresis (cellulose or starch, gel or agarose gel). LDH1 has more positive, charge and fastest in electrophoretic mobility, while LDH5 is the slowest., Structure of LDH isoenzymes : LDH is an, oligomeric (tetrameric) enzyme made up of four, polypeptide subunits. Two types of subunits, namely M (for muscle) and H (for heart) are, produced by different genes. M–subunit is basic, while H subunit is acidic. The isoenzymes, contain either one or both the subunits giving, LDH1 to LDH5. The characteristic features of, LDH isoenzymes are given in Table 6.11.
Page 120 :
110, , BIOCHEMISTRY, , TABLE 6.11 Lactate dehydrogenase (LDH) isoenzymes and their characteristics, , Isoenzyme, , Subunit, constitution, , Principal, tissue of origin, , Electrophoretic, mobility, , Whether, Percentage of, destroyed, normal serum, by heat (at 60qC), in humans, , LDH1, , H4, , Heart and RBC, , Fastest, , No, , 25%, , LDH2, , H3M, , Heart and RBC, , Faster, , No, , 35%, , LDH3, , H2M2, , Brain and kidney, , Fast, , Partially, , 27%, , LDH4, , HM3, , Liver and skeletal muscle, , Slow, , Yes, , 8%, , LDH5, , M4, , Skeletal muscle and liver, , Slowest, , Yes, , 5%, , 1, , 2, , 3, , 4, , 5, , (A), , (B), 1, , 2, , (C), 1, , 2, , 3, , 4, , 5, , Fig. 6.15 : Electrophoresis of lactate dehydrogenase, with relative proportions of isoenzymes (A) Normal, serum (B) Serum from a patient of myocardial, infarction (LDH1 and LDH2n) (C) Serum from, a patient of liver disease (LDH5n)., , Significance of differential catalytic activity :, LDH1 (H4) is predominantly found in heart, muscle and is inhibited by pyruvate – the, substrate. Hence, pyruvate is not converted to, lactate in cardiac muscle but is converted to, acetyl CoA which enters citric acid cycle. LDH5, (M4) is mostly present in skeletal muscle and the, inhibition of this enzyme by pyruvate is minimal,, hence pyruvate is converted to lactate. Further,, LDH5 has low Km (high affinity) while LDH1 has, high Km (low affinity) for pyruvate. The, differential catalytic activities of LDH1 and LDH5, in heart and skeletal muscle, respectively, are, well suited for the aerobic (presence of oxygen), and anaerobic (absence of oxygen) conditions,, prevailing in these tissues., Diagnostic importance of LDH : Isoenzymes, of LDH have immense value in the diagnosis of, heart and liver related disorders (Fig.6.15). In, healthy individuals, the activity of LDH2 is, higher than that of LDH1 in serum. In the case of, myocardial infarction, LDH1 is much greater, than LDH2 and this happens within 12 to 24, hours after infarction. Increased activity of LDH5
Page 121 :
111, , Chapter 6 : ENZYMES, , in serum is an indicator of liver diseases. LDH, activity in the RBC is 80–100 times more than, that in the serum. Hence for estimation of LDH, or its isoenzymes, serum should be totally free, from hemolysis or else false positive results will, be obtained., , Isoenzymes of creatine, phosphokinase, Creatine kinase (CK) or creatine phosphokinase, (CPK) catalyses the inter-conversion of phosphocreatine (or creatine phosphate) to creatine., CPK, , Phosphocreatine, ADP, , Creatine, ATP, , CPK exists as three isoenzymes. Each, isoenzyme is a dimer composed of two, subunits—M (muscle) or B (brain) or both., , Isoenzyme, CPK1, CPK2, CPK3, , Subunit, BB, MB, MM, , Tissue of origin, Brain, Heart, Skeletal muscle, , In healthy individuals, the isoenzyme, CPK2 (MB) is almost undetectable in serum, with less than 2% of total CPK. After the, myocardial infarction (MI), within the first 6-18, hours, CPK2 increases in the serum to as high as, 20% (against 2% normal). CPK2 isoenzyme is, not elevated in skeletal muscle disorders., Therefore, estimation of the enzyme CPK2 (MB), is the earliest reliable indication of myocardial, infarction., , Isoenzymes of alkaline phosphatase, As many as six isoenzymes of alkaline, phosphatase (ALP) have been identified. ALP is, a monomer, the isoenzymes are due to, the difference in the carbohydrate content, (sialic acid residues). The most important, ALP isoenzymes are D1-ALP, D2-heat labile, ALP, D2-heat stable ALP, pre-E ALP, J-ALP, etc., Increase in D2-heat labile ALP suggests, hepatitis whereas pre E-ALP indicates bone, diseases., , + In the living system, the regulation of enzyme activities occurs through allosteric, inhibition, activation of latent enzymes, compartmentation of metabolic pathways,, control of enzyme synthesis and degradation., , + Feedback (or end product) inhibition is a specialized form of allosteric inhibition that, controls several metabolic pathways e.g. CTP inhibits aspartate transcarbamoylase;, Cholesterol inhibits HMG CoA reductase. The end product inhibition is utmost, important to cellular economy since a compound is synthesized only when required., , + Certain RNA molecules (ribozymes) function as non-protein enzymes. It is believed that, ribozymes were functioning as biocatalysts before the occurrence of protein enzymes, during evolution., , + Certain enzymes are utilized as therapeutic agents. Streptokinase in used to dissolve, blood clots in circulation while asparaginase is employed in the treatment of leukemias., , + Determination of serum enzyme activities is of great importance for the diagnosis of, several diseases (refer Table 6.8)., , + Lowered body temperature (hypothermia) is accompained by a decrease in enzyme, activities. This principle is exploited to reduce metabolic demand during open heart, surgery or transportation of organs for transplantation surgery.
Page 122 :
112, , Enzyme activity, , BIOCHEMISTRY, , LDH, CP, , K, , 0, , SGOT, , 6 12 18 24 30 36 42 48 54 60 66 72 4, , Hours, , 5, , 6, , 7, , 8, , 9 10 11, , Days, , Fig. 6.16 : Enzyme pattern in myocardial infarction, (CPK-Creatine phosphokinase; SGOT-Serum, glutamate oxaloacetate transaminase;, LDH-Lactate dehydrogenase)., , Isoenzymes of alcohol, dehydrogenase, Alcohol dehydrogenase (ADH) has two, heterodimer isoenzymes. Among the white, Americans and Europeans, DE1 isoenzyme is, predominant whereas in Japanese and Chinese, (Orientals) DE2 is mostly present. The isomer DE2, more rapidly converts alcohol to acetaldehyde., Accumulation of acetaldehyde is associated, with tachycardia (increase in heart rate) and facial, flushing among Orientals which is not commonly, seen in whites. It is believed that Japanese and, Chinese have increased sensitivity to alcohol due, to the presence of DE2–isoenzyme of ADH., , ENZYME PATTERN IN DISEASES, For the right diagnosis of a particular disease,, it is always better to estimate a few (three or, more) serum enzymes, instead of a single, enzyme. Examples of enzyme patterns in, important diseases are given here., , Enzymes in myocardial infarction, The enzymes – namely creatine phosphokinase, (CPK), aspartate transaminase (AST) and lactate, dehydrogenase (LDH)—are important for the, diagnosis of myocardial infarction (MI). The, elevation of these enzymes in serum in relation to, hours/days of MI is given in the Fig.6.16., Creatine phosphokinase (precisely isoenzyme, MB) is the first enzyme to be released into, circulation within 6-18 hours after the infarction., Therefore, CPK estimation is highly useful for the, early diagnosis of MI. This enzyme reaches a, , peak value within 24-30 hours, and returns to, normal level by the 2nd or 3rd day., Aspartate transaminase (AST or SGOT) rises, sharply after CPK, and reaches a peak within 48, hours of the myocardial infarction. AST takes, 4-5 days to return to normal level., Lactate dehydrogenase (LDH1) generally rises, from the second day after infarction, attains a, peak by the 3rd or 4th day and takes about, 10-15 days to reach normal level. Thus, LDH is, the last enzyme to rise and also the last enzyme, to return to normal level in MI., Cardiac troponins (CT) : Although not, enzymes, the proteins cardiac troponins are, highly useful for the early diagnosis of MI. Among, these, troponin I (inhibitory element of actomysin, ATPase) and troponin T (tropomysin binding, element) are important. Cardiac troponin I (CTI) is, released into circulation within four hours after, the onset of MI, reaches a peak value by 12–24, hours, and remains elevated for about a week., The protein myoglobin is also an early marker, for the diagnosis of MI. However, it is not, specific to cardiac diseases. High serum, concentration of brain natriuretic peptide is a, marker for congestive cardiac failure. In the, Table 6.12, a summary of the diagnostic markers, used in MI is given. Table 6.13 gives enzyme, patterns in various diseases., , Enzymes in liver diseases, The following enzymes—when elevated in, serum – are useful for the diagnosis of liver, dysfunction due to viral hepatitis (jaundice),, toxic hepatitis, cirrhosis and hepatic necrosis, 1. Alanine transaminase, 2. Aspartate transaminase, 3. Lactate dehydrogenase., The enzymes that markedly increase in, intrahepatic and extrahepatic cholestasis are :, (1) Alkaline phosphatase, (2) 5’-Nucleotidase, Serum-J-glutamyl transpeptidase is useful in, the diagnosis of alcoholic liver diseases., , Enzymes in muscle diseases, In the muscular dystrophies, serum levels of, certain muscle enzymes are increased. These, include creatine phosphokinase, aldolase and, aspartate transaminase.
Page 123 :
113, , Chapter 6 : ENZYMES, , TABLE 6.12 Summary of diagnostic markers used for the evaluation of acute myocardial infarction, , Diagnostic marker, , Time of peak, elevation, , Time of return, to normal level, , Diagnostic importance, , Myoglobin, , 4-6 hrs, , 20–25 hrs, , Earliest marker, however not cardiac specific., , Cardiac troponin I, , 12-24 hrs, , 5-9 days, , Early marker and cardiac specific., , Cardiac troponin T, , 18-36 hrs, , 5-14 days, , Relatively early marker and cardiac specific., However, elevated in other degenerative diseases., , Creatine phosphokinase (MB), , 20-30 hrs, , 24-48 hrs, , Cardiac specific and early marker., , Lactate dehydrogenase (LDH I), , 48-72 hrs, , 10-15 days, , Relatively late marker and cardiac specific., , Aspartate transaminase, , 30-48 hrs, , 4-6 days, , Not cardiac specific., , Enzymes in cancers, Increase in the serum acid phosphatase, (tartarate labile) is specific for the detection of, prostatic carcinoma., [Note : Prostate specific antigen (PSA; mol, wt. 32 KD), though not an enzyme, is a more, reliable marker for the detection of prostate, cancer. Normal serum concentration of PSA is, 1-4 ng/ml]., , Neuron–specific enolase serves as a marker, for lung cancer, neuroblastoma, pheochromocytoma etc., , DIAGNOSTIC IMPORTANCE OF, ENZYMES IN OTHER BODY FLUIDS, AND TISSUES, Besides serum/plasma enzymes, enzyme, estimations in other body fluids, and tissues also, , TABLE 6.13 Serum enzyme profiles (patterns) in diseases, , Disease/enzyme(s), I, , Significance, , Myocardial infarction (Refer Table 6.12 and Fig. 6.16), , II Hepatic disease, Alanine transaminase (ALT), Aspartate transaminse (AST), J-Glutamyl transpeptidase (GGT), 5c-Nucleotidase, , Marketdly elevated in viral hepatitis, Increased in liver diseases. Significantly elevated in, obstructive jaundice (gall stones)., Marketdly increased in alcoholic liver diseases., Elevated in hapatic cholestasis., , III Muscle disease, Creatine kinase (CK), , Markedly increased in muscle disease (CK-MM more, sensitive)., , Aldolase (ALD), , Early marker (not specific), , Aspartate transaminase (AST), , Significantly increased, although not specific., , IV Bone disease, Alkaline phosphatase (ALP), , Increased in rickets and Paget’s disease., , V Pancreatic disease, Amylase, Lipase, , Significantly elevated in acute pancreatitis., Markedly increased in acute pancreatitis., , VI, , Prostate cancer, Acid phosphatase (ACP), Prostate specific antigen (PSA), , Marker enzyme for prostate cancer., Significantly elvated in prostate cancer (not an enzyme).
Page 124 :
114, , BIOCHEMISTRY, , have some diagnostic, examples are lised., , importance., , A, , few, , Urine : Urinary amylase is increased in, acute, pencreatitis., E-N-Acetylgalactosidase, in urine is elevated in renal graft dysfunction., E-Glucuronidase is increased in the cancers of, urinary bladder, pancreas etc., Cerebrospinal fluid : Lactate dehydrogenase, is increased in CSF in meningitis., Gastric juice : E-Glucuronidase activity is, increased in gastric carcinoma., Feces : Fetal trypsin levels are decreased in, cystic fibrosis., , Liver : Glucose 6-phosphatase in liver is, significantly lower in type I glycogen storage, disease., Muscle : Phosphorylase activity in muscle is, decreased in McArdle’s disease., Erythrocytes, :, Glucose, 6-phosphate, delydrogenase deficiency in RBC causes, hemolytic anemia. Decreased transketolase, activity in erythrocytes is used for diagnosis of, thiamine deficiency., In addition to the above, cultured fibroblasts,, and amniotic cells are frequently used for the, diagnosis of inborn errors of metabolism e.g., phenylalanine, hydroxylase, deficiency, in, phenylkonuria (in cultured amniotic cells)., , 1. Enzymes are the protein biocatalysts synthesized by the living cells. They are classified, into six major classes—oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases., 2. An enzyme is specific in its action, possessing active site, where the substrate binds to, form enzyme-substrate complex, before the product is formed., 3. Factors like concentration of enzyme, substrate, temperature, pH etc. influence enzyme, activity. The substrate concentration to produce half-maximal velocity is known as, Michaelis constant (Km value)., 4. Enzyme activities are inhibited by reversible (competitive, and non-competitive),, irreversible and allosteric manner., 5. Many enzymes require certain non-protein substances called cofactors (coenzymes) for their, action. Most of the coenzymes are derivatives of B-complex vitamins (e.g. NAD+, FAD, TPP etc.), 6. The mechanism of enzyme action is explained by lock and key model (of Fischer), more, recently induced fit model (of Koshland) and substrate strain theory., 7. The enzymes enhance the rate of reaction through acid-base catalysis, covalent catalysis, and/or proximity catalysis., 8. In the living system, there is a constant regulation of enzyme levels, brought about by, allosteric mechanism, activation of proenzymes, synthesis and degradation of enzymes, etc., 9. Estimation of serum enzymes is of great help in the diagnosis of several diseases., Serum amylase and lipase are increased in acute pancreatitis; alanine transaminase in, hepatitis; aspartate transaminase, lactate dehydrogenase (LDH) and creatine, phosphokinase (CPK) in myocardial infarction; alkaline phosphatase in rickets and, hyperparathyroidism; acid phosphatase in prostatic carcinoma; J-glutamyl transpeptidase in alcoholism., 10. Isoenzymes are the multiple forms of an enzyme catalysing the same reaction which, however, differ in their physical and chemical properties. LDH has five isoenzymes, while CPK has three. LDH1 and CPK2 are very important in the diagnosis of MI.
Page 125 :
Chapter 6 : ENZYMES, , 115, , I. Essay questions, 1. What are enzymes? Describe their classification and nomenclature., 2. Write an account of the various factors affecting enzyme activity., 3. Describe the mechanism of enzyme action., 4. What are coenzymes? Write briefly on the role of coenzymes in enzyme action., 5. Write an account of the importance of serum enzymes in the diagnosis of diseases., , II. Short notes, (a) Enzyme specificity, (b) Competitive inhibition, (c) Coenzymes, (d) Allosteric enzymes,, (e) Isoenzymes, (f) Km value, (g) Serum enzymes in myocardial infarction, (h) Lactate dehydrogenase,, (i) Role of metals in enzyme action, (j) Active site., , III. Fill in the blanks, 1. The literal meaning of enzyme is ____________., 2. The class of enzymes involved in synthetic reactions are ____________., 3. The non-protein part of holoenzyme ____________., 4. Enzymes lose the catalytic activity at temperature above 70qC due to ____________., 5. Examples of two enzymes containing zinc are ____________ and ____________., 6. The place at which substrate binds with the enzyme ____________., 7. The enzyme glucose 6-phosphate dehydrogenase requires the coenzyme ____________., 8. The E.C. number for alcohol dehydrogenase is ____________., 9. Phsophofructokinase is allosterically activated by ____________., 10. The very first enzyme elevated in serum in myocardial infarction ____________., , IV. Multiple choice questions, 11. Pepsin is an example for the class of enzymes namely, (a) Oxidoreductases (b) Transferases (c) Hydrolases (d) Ligases., 12. The coenzyme not involved in hydrogen transfer, (a) FMN (b) FAD (c) NADP+ (d) FH4., 13. In the feedback regulation, the end product binds at, (a) Active site (b) Allosteric site (c) E-S complex (d) None of these., 14. J-Glutamyl transpeptidase activity in serum is elevated in, (a) Pancreatitis (b) Muscular dystrophy (c) Myocardial infarction (d) Alcoholism., 15. In recent years, a non-protein compound has been identified to bring about catalysis in, biological system. The name of the compound is, (a) DNA (b) RNA (c) Lipids (d) Carbohydrates.
Page 126 :
Section 1, , Chemical Constituents of Life, , Chapter, , Vitamins, , 17, , The vitamins speak :, Vitamins, Fat, soluble, , Water, soluble, , “We are for growth, health and welfare of organisms;, Discharge our duties directly or through coenzymes;, Deficiency symptoms are our alert signals;, Satisfied we shall be, with additional supplements.”, , I, , t is difficult to define vitamins precisely., Vitamins may be regarded as organic, compounds required in the diet in small, amounts to perform specific biological functions, for normal maintenance of optimum growth and, health of the organism. The bacterium E.coli, does not require any vitamin, as it can synthesize, all of them. It is believed that during the course, of evolution, the ability to synthesize vitamins, was lost. Hence, the higher organisms have to, obtain them from diet. The vitamins are required, in small amounts, since their degradation is, relatively slow., , History and nomenclature, In the beginning of 20th century, it was, clearly understood that the diets containing, purified carbohydrate, protein, fat and minerals, were not adequate to maintain the growth and, health of experimental rats, which the natural, foods (such as milk) could do., Hopkins coined the term accessory factors to, the unknown and essential nutrients present in, , the natural foods. Funk (1913) isolated an active, principle (an amine) from rice polishings and,, later in yeast, which could cure beri-beri in, pigeons. He coined the term vitamine (Greek :, vita-life) to the accessory factors with a belief, that all of them were amines. It was later realised, that only few of them are amines. The term, vitamin, however, is continued without the final, letter ‘e’., The usage of A, B and C to vitamins was, introduced in 1915 by McCollum and Davis., They first felt there were only two vitamins—, fat soluble A and water soluble B (anti-beriberi, factor). Soon another water soluble anti-scurvy, factor named vitamin C was described. Vitamin, A was later found to possess two componentsone that prevents night blindness (vitamin A) and, another anti-ricket factor named as vitamin D. A, fat soluble factor called vitamin E, in the absence, of which rats failed to reproduce properly, was, discovered. Yet another fat soluble vitamin, concerned with coagulation was discovered in, mid 1930s. It was named as vitamin K. In the, , 116
Page 127 :
117, , Chapter 7 : VITAMINS, , hematopoietic (folic acid and B12). Most of the, water soluble vitamins exert the functions, through their respective coenzymes while only, one fat soluble vitamin (K) has been identified to, function as a coenzyme., , sequence of alphabets it should have been F,, but K was preferred to reflect its function, (koagulation)., As regards the water soluble factors, vitamin, C was identified as a pure substance and named, as ascorbic acid. Vitamin B was found to be a, complex mixture and nomenclature also became, complex. B1 was clearly identified as anti-beriberi factor. Many investigators carried out, intensive research between 1920 and 1930 and, went on naming them as the water soluble, vitamins B2, B3, B4, B5, B6, B7, B8, B9, B10, B11, and B12. Some of them were found to be, mixtures of already known vitamins. And for this, reason, a few members (numbers!) of the Bcomplex series disappeared from the scene., Except for B1, B2, B6 and B12, names are more, commonly used for other B-complex vitamins., , Synthesis of vitamins, by intestinal bacteria, Vitamins, as per the definition, are not, synthesized in the body. However, the bacteria, of the gut can produce some of the vitamins,, required by man and animals. The bacteria, mainly live and synthesize vitamins in the colon, region, where the absorption is relatively poor., Some of the animals (e.g. rat, deer etc.) eat, their own feces, a phenomenon known as, coprophagy., As far as humans are concerned, it is believed, that the normal intestinal bacterial synthesis, and, absorption of vitamin K and biotin may be, sufficient to meet the body requirements. For, other B-complex vitamins, the synthesis and, absorption are relatively less. Administration of, anitibiotics often kills the vitamin synthesizing, bacteria present in the gut, hence additional, consumption of vitamins is recommended., , Classification of vitamins, There are about 15 vitamins, essential for, humans. They are classified as fat soluble (A, D,, E and K) and water soluble (C and B-group), vitamins as shown in the Table 7.1. The, B-complex vitamins may be sub-divided into, energy-releasing (B1, B2, B6, biotin etc.) and, , TABLE 7.1 Classification of vitamins, , Vitamins, Fat soluble, Vitamin A, Vitamin D, Vitamin E, Vitamin K, , Water soluble, Non B-complex, Vitamin C, , B-complex, Energy-releasing, , Hematopoietic, , Thiamine (B1), , Folic acid (B9), , Riboflavin (B2), , Vitamin B12, (cyanocobalamin), , Niacin (B3), Pyridoxine (B6), Biotin (B7), Pantothenic acid (B5)
Page 128 :
118, , BIOCHEMISTRY, , Fat soluble vitamins—general, , Vitamers, , The four vitamins, namely vitamin A, D, E,, and K are known as fat or lipid soluble. Their, availability in the diet, absorption and transport, are associated with fat. They are soluble in fats, and oils and also the fat solvents (alcohol,, acetone etc.). Fat soluble vitamins can be stored, in liver and adipose tissue. They are not readily, excreted in urine. Excess consumption of these, vitamins (particularly A and D) leads to their, accumulation and toxic effects., , The term vitamers represents the chemically, similar substances that possess qualitatively, similar vitamin activity. Some good examples of, vitamers are given below, , All the fat soluble vitamins are isoprenoid, compounds, since they are made up of one or, more of five carbon units namely isoprene units, ( CH C.CH3 CH CH ). Fat soluble vitamins, perform diverse functions. Vitamin K has a, specific coenzyme function., , Water soluble vitamins—general, The water soluble vitamins are a, heterogenous group of compounds since they, differ chemically from each other. The only, common character shared by them is their, solubility in water. Most of these vitamins are, readily excreted in urine and they are not toxic, to the body. Water soluble vitamins are not, stored in the body in large quantities (except, B12). For this reason, they must be continuously, supplied in the diet. Generally, vitamin, deficiencies are multiple rather than individual, with overlapping symptoms. It is often difficult, to pinpoint the exact biochemical basis for the, symptoms., The water soluble vitamins form coenzymes, (Refer Table 6.3) that participate in a variety of, biochemical reactions, related to either energy, generation or hematopoiesis. It may be due to, this reason that the deficiency of vitamins results, in a number of overlapping symptoms. The, common symptoms of the deficiency of one or, more vitamins involved in energy metabolism, include dermatitis, glossitis (red and swollen, tongue), cheilitis (rupture at the corners of lips),, diarrhea, mental confusion, depression and, malaise., Deficiency of vitamins B1, B6 and B12 is more, closely associated with neurological manifestations., , l, , l, , Retinol, retinal and retinoic acid are vitamers, of vitamin A., Pyridoxine, pyridoxal and pyridoxamine are, vitamers of vitamin B6., , INDIVIDUAL VITAMINS, In the following pages, the individual, members of the fat soluble and water soluble, vitamins are discussed with regard to the, chemistry, biochemical functions, recommended, dietary/daily allowances (RDA), dietary sources,, deficiency manifestations etc., , VITAMIN A, The fat soluble vitamin A, as such is present, only in foods of animal origin. However, its, provitamins carotenes are found in plants., It is recorded in the history that Hippocrates, (about 500 B.C.) cured night blindness. He, prescribed to the patients ox liver (in honey),, which is now known to contain high quantity of, vitamin A., , Chemistry, In the recent years, the term vitamin A is, collectively used to represent many structurally, related and biologically active molecules, (Fig.7.1). The term retinoids is often used to, include the natural and synthetic forms of, vitamin A. Retinol, retinal and retinoic acid are, regarded as vitamers of vitamin A., 1. Retinol (vitamin A alcohol) : It is a primary, alcohol containing E-ionone ring. The side chain, has two isoprenoid units, four double bonds and, one hydroxyl group. Retinol is present in animal, tissues as retinyl ester with long chain fatty acids.
Page 129 :
119, , Chapter 7 : VITAMINS, , H3C, , CH3, , CH3, , E-Ionone, , H3C, , CH3, , CH3, , CH3, , CH3, , CH3, , CH2OH, , Retinal, , C O, H, , Retinoic acid, Retinol, , C O, OH, , CH3, , CH3, , E-Carotene, , CH3, , H3C, , CH3, , H3C, , Fig. 7.1 : Structures of vitamin A and related compounds (Red colour represents, the substituent groups in the respective compounds)., , 2. Retinal (vitamin A aldehyde) : This is an, aldehyde form obtained by the oxidation of, retinol. Retinal and retinol are interconvertible., Previously, the name retinine was used for, retinal., 3. Retinoic acid (vitamin A acid) : This is, produced by the oxidation of retinal. However,, retinoic acid cannot give rise to the formation of, retinal or retinol., 4. E-Carotene (provitamin A) : This is found, in plant foods. It is cleaved in the intestine to, produce two moles of retinal. In humans, this, conversion is inefficient, hence E-carotene, possesses about one-sixth vitamin A activity, compared to that of retinol., , Absorption, transport, and mobilization, Dietary retinyl esters are hydrolysed by, pancreatic or intestinal brush border hydrolases, in the intestine, releasing retinol and free fatty, acids. Carotenes are hydrolysed by E-carotene, 15-15c-dioxygenase of intestinal cells to release, 2 moles of retinal which is reduced to retinol. In, the intestinal mucosal cells, retinol is reesterified, to long chain fatty acids, incorporated into, chylomicrons and transferred to the lymph. The, retinol esters of chylomicrons are taken up by, the liver and stored (Fig.7.2)., , As and when needed, vitamin A is released, from the liver as free retinol. It is believed that, zinc plays an important role in retinol, mobilization. Retinol is transported in the, circulation by the plasma retinol binding protein, (RBP; mol. wt. 21,000) in association with, pre-albumin. The retinol-RBP complex binds to, specific receptors on the cell membrane of, peripheral tissue and enters the cells. Many, cells of target tissues contain a cellular retinolbinding protein that carries retinol to the, nucleus and binds to the chromatin (DNA)., It is here that retinol exerts its function in, a manner analogous to that of a steroid, hormone., , BIOCHEMICAL FUNCTIONS, Vitamin A is necessary for a variety of, functions such as vision, proper growth and, differentiation, reproduction and maintenance of, epithelial cells. In recent years, each form of, vitamin A has been assigned specific functions, (Fig.7.3)., Vitamin A and vision : The biochemical function of vitamin A in the process of vision was, first elucidated by George Wald (Nobel Prize, 1968). The events occur in a cyclic process, known as Rhodopsin cycle or Wald’s visual, cycle (Fig.7.4).
Page 131 :
121, , Chapter 7 : VITAMINS, E-Carotene, (antioxidant), , Retinol, (steroid hormone—growth and differentiation), , Retinal, (visual cycle), , Retinyl phosphate, (glycoprotein synthesis), , Retinoic acid, (steroid hormone-growth and differentiation), , Fig. 7.3 : Summary of the functions, of vitamin A compounds., , Rods and cones, The retina of the eye possesses two types of, cells—rods and cones. The human eye has about, 10 million rods and 5 million cones. The rods, are in the periphery while cones are at the centre, of retina. Rods are involved in dim light vision, whereas cones are responsible for bright light, and colour vision. Animals—such as owls and, cats for which night vision is more important—, possess mostly rods., , Wald’s visual cycle, Rhodopsin (mol. wt. 35,000) is a conjugated, protein present in rods. It contains 11-cis retinal and, the protein opsin. The aldehyde group (of retinal) is, linked to H-amino group of lysine (of opsin)., The primary event in visual cycle, on, exposure to light, is the isomerization of 11-cisretinal to all-trans retinal. This leads to a, conformational change in opsin which is, responsible for the generation of nerve impulse., The all-trans-retinal is immediately isomerized, by retinal isomerase (of retinal epithelium) to, 11-cis-retinal. This combines with opsin to, regenerate rhodopsin and complete the visual, cycle (Fig.7.4). However, the conversion of all, trans-retinal to 11-cis retinal is incomplete., Therefore, most of the all-trans-retinal is, transported to the liver and converted to all-trans, retinol by alcohol dehydrogenase. The all-transretinol undergoes isomerization to 11-cis retinol, which is then oxidized to 11-cis retinal to, participate in the visual cycle., , Dark adaptation time : When a person shifts, from a bright light to a dim light (e.g. entry into, a dim cine theatre), rhodopsin stores are, depleted and vision is impaired. However,, within a few minutes, known as dark adaptation, time, rhodopsin is resynthesized and vision is, improved. Dark adaptation time is increased in, vitamin A deficient individuals., Bleaching of rhodopsin : When exposed to, light, the colour of rhodopsin changes from red, to yellow, by a process known as bleaching., Bleaching occurs in a few milliseconds and, many unstable intermediates are formed during, this process., Rhodopsin, , Prelumirhodopsin, , All-trans-retinal + Opsin, , Lumirhodopsin, , Metarhodopsin II, , Metarhodopsin I, , Visual cascade and cGMP : When light strikes, the retina, a number of biochemical changes, leading to membrane hyperpolarization occur, resulting in the genesis of nerve impulse. The, hyperpolarization of the membrane is brought, about by a visual cascade involving cyclic GMP., When a photon (from light) is absorbed by, rhodopsin, metarhodopsin II is produced. The, protein transducin is activated by metarhodopsin, II. This involves an exchange of GTP for GDP on, inactive transducin. The activated transducin, activates cyclic GMP phosphodiesterase. This, Rhodopsin, (11-cis-retinal-opsin), , Light, (photon), Nerve, impulse, , Opsin, , 11-cis-retinal, , Retinal, isomerase, , NADH + H+, NAD+, , All-trans-retinal, , NADH + H+, Alcohol, dehydrogenase, , NAD+, , 11-cis-retinol, , Isomerase, (liver), , Alcohol, dehydrogenase, (liver), , All-trans-retinol, , Fig. 7.4 : Wald’s visual cycle.
Page 132 :
122, , BIOCHEMISTRY, , synthesis and thus are involved in the cell growth, and differentiation., , Rhodopsin, Photon, , 2. Vitamin A is essential to maintain healthy, epithelial tissue. This is due to the fact that, retinol and retinoic acid are required to prevent, keratin synthesis (responsible for horny surface)., , Metarhodopsin II, GTP, , GDP, , Transducin, (inactive), , Transducin, (active), , Phosphodiesterase, (inactive), , Phosphodiesterase, (active), , 3c,5c-cGMP, , 5c-GMP, , Fig. 7.5 : The visual cascade involving cyclic guanosine, monophosphate (3c,5c-cGMP)., , enzyme degrades cyclic GMP in the rod cells, (Fig.7.5). A rapid decrease in cyclic GMP closes, the Na+ channels in the membranes of the rod, cells. This results in hyperpolarization which is, an excitatory response transmitted through the, neuron network to the visual cortex of the brain., , Colour vision, Cones are specialized in bright and colour, vision. Visual cycle comparable to that present, in rods is also seen in cones. The colour vision, is governed by colour sensitive pigments–, porphyropsin (red), iodopsin (green) and, cyanopsin (blue). All these pigments are retinalopsin complexes. When bright light strikes the, retina, one or more of these pigments are, bleached, depending on the particular colour of, light. The pigments dissociate to all-trans-retinal, and opsin, as in the case of rhodopsin. And this, reaction passes on a nerve impulse to brain as a, specific colour—red when porphyropsin splits,, green when iodopsin splits or blue for cyanopsin., Splitting of these three pigments in different, proportions results in the perception of different, colours by the brain., , Other biochemical, functions of vitamin A, 1. Retinol and retinoic acid function almost, like steroid hormones. They regulate the protein, , 3. Retinyl phosphate synthesized from retinol, is necessary for the synthesis of certain, glycoproteins, and mucopolysaccharides which, are required for growth and mucus secretion., 4. Retinol, is, necessary, for, normal, reproduction. It acts like a hormone and, regulates gene expression., 5. Vitamin A is considered to be essential for, the maintenance of proper immune system to, fight against various infections., 6. Cholesterol synthesis requires vitamin A., Mevalonate, an intermediate in the cholesterol, biosynthesis, is diverted for the synthesis of, coenzyme Q in vitamin A deficiency. It is, pertinent to note that the discovery of coenzyme, Q was originally made in vitamin A deficient, animals., 7. Carotenoids (most important E-carotene), function as antioxidants and reduce the risk of, cancers initiated by free radicals and strong, oxidants. E-Carotene is found to be beneficial to, prevent heart attacks. This is also attributed to, the antioxidant property., , Recommended dietary, allowance (RDA), The daily requirement of vitamin A is, expressed as retinol equivalents (RE) rather than, International Units (IU)., 1 retinol equivalent =1 Pg retinol, =6 Pg E-carotene, =12 Pg other carotenoids, =3.33 IU of vitamin A, activity from retinol, =10 IU of vitamin A, activity from E-carotene, The RDA of vitamin A for adults is around, 1,000 retinol equivalents (3,500 IU) for man and, 800 retinol equivalents (2,500 IU) for woman.
Page 133 :
123, , Chapter 7 : VITAMINS, , One International Unit (IU) equals to 0.3 mg of, retinol. The requirement increases in pregnant, women and lactating mothers., , Dietary sources, Animal sources contain (preformed) vitamin, A. The best sources are liver, kidney, egg yolk,, milk, cheese, butter. Fish (cod or shark) liver oils, are very rich in vitamin A., , Vegetable sources contain the provitamin, A-carotenes. Yellow and dark green vegetables, and fruits are good sources of carotenes e.g., carrots, spinach, pumpkins, mango, papaya etc., , Vitamin A deficiency, The vitamin A deficiency may be due to, inadequate dietary intake, impaired intestinal, absorption, reduced storage in liver and chronic, alcholism. The deficiency symptoms are not, immediate, since the hepatic stores can meet the, body requirements for quite sometime (2-4, months). The deficiency manifestations are, related to the eyes, skin and growth., Deficiency manifestations of the eyes : Night, blindness (nyctalopia) is one of the earliest, symptoms of vitamin A deficiency. The, individuals have difficulty to see in dim light, since the dark adaptation time is increased., Prolonged deficiency irreversibly damages a, number of visual cells., Severe deficiency of vitamin A leads to, xerophthalmia. This is characterized by dryness, in conjunctiva and cornea, and keratinization of, epithelial cells. In certain areas of conjunctiva,, white triangular plaques known as Bitot’s spots, are seen., If xerophthalmia persisits for a long time,, corneal ulceration and degeneration occur. This, results in the destruction of cornea, a condition, referred to as keratomalacia, causing total, blindness. Therefore, adequate intake of vitamin A, is necessary for the prevention of blindness., , Other deficiency manifestations, Effect on growth : Vitamin A deficiency, results in growth retardation due to impairment, in skeletal formation., , Effect on reproduction : The reproductive, system is adversely affected in vitamin A, deficiency. Degeneration of germinal epithelium, leads to sterility in males., Effect on skin and epithelial cells : The skin, becomes rough and dry. Keratinization of, epithelial cells of gastrointestinal tract, urinary, tract and respiratory tract is noticed. This leads to, increased bacterial infection. Vitamin A deficiency, is associated with formation of urinary stones., The plasma level of retinol binding protein is, decreased in vitamin A deficiency., , Hypervitaminosis A, Excessive consumption of vitamin A leads to, toxicity. The symptoms of hypervitaminosis A, include dermatitis raised intracranial tension,, enlargement of liver, skeletal decalcification,, tenderness of long bones, loss of weight,, irritability, loss of hair, joint pains etc. Elderly, people are more susceptible to vitamin A, toxicity, hence overdoses should be avoided., Total serum vitamin A level (normal 20–50, Pg/dl) is elevated in hypervitaminosis A. Free, retinol or retinol bound to plasma lipoproteins is, actually harmful to the body. It is now believed, that the vitamin A toxicosis symptoms appear, only after retinol binding capacity of retinol, binding protein exceeds., Higher concentration of retinol increases the, synthesis of lysosomal hydrolases. The manifestations of hypervitaminosis A are attributed to, the destructive action of hydrolases, particularly, on the cell membranes., , BENEFICIAL EFFECTS OF E-CAROTENE, Increased consumption of E-carotene is, associated with decreased incidence of heart, attacks, skin and lung cancers. This is attributed, to the antioxidant role of E-carotene which is, independent of its role as a precursor of vitamin, A. Ingestion of high doses of E-carotene for long, periods are not toxic like vitamin A., , VITAMIN D, Vitamin D is a fat soluble vitamin. It, resembles sterols in structure and functions like, a hormone.
Page 134 :
124, , BIOCHEMISTRY, , used for fat soluble crystalline material, which, later turned out to be a mixture)., , Absorption, transport and storage, , Ergosterol (plants), , HO, Sunlight, , Vitamin D is absorbed in the small intestine, for which bile is essential. Through lymph,, vitamin D enters the circulation bound to plasma, D2-globulin and is distributed throughout the, body. Liver and other tissues store small amounts, of vitamin D., , METABOLISM AND, BIOCHEMICAL FUNCTIONS, , H2, C, , HO, Ergocalciferol (D2), , Fig. 7.6 : Formation of ergocalciferol from ergosterol., , The symptoms of rickets and the benefical, effects of sunlight to prevent rickets have been, known for centuries. Hess (1924) reported that, irradiation with ultraviolet light induced antirachitic activity in some foods. Vitamin D was, isolated by Angus (1931) who named it calciferol., , Chemistry, Ergocalciferol (vitamin D2) is formed from, ergosterol and is present in plants (Fig.7.6)., Cholecalciferol (vitamin D3) is found in animals., Both the sterols are similar in structure except, that ergocalciferol has an additional methyl, group and a double bond. Ergocalciferol and, cholecalciferol are sources for vitamin D activity, and are referred to as provitamins., During the course of cholesterol biosynthesis, (Chapter 14), 7-dehydrocholesterol is formed, as an intermediate. On exposure to sunlight,, 7-dehydrocholesterol is converted to cholecalciferol in the skin (Fig.2.7). Vitamin D is, regarded as sun-shine vitamin., The synthesis of vitamin D3 in the skin is, proportional to the exposure to sunlight. Dark, skin pigment (melanin) adversly influences the, synthesis of cholecalciferol. (Note : The term, vitamin D1 is no more in use. It was originally, , Vitamins D2 and D3, as such, are not, biologically active. They are metabolized, identically in the body and converted to active, forms. The metabolism and biochemical, functions of vitamin D are depicted in Fig.7.8., , Synthesis of 1,25-DHCC, Cholecalciferol is first hydroxylated at 25th, position to 25-hydroxycholecalciferol (25-OH, D3) by a specific hydroxylase present in liver., 25-OH D3 is the major storage and circulatory, form of vitamin D. Kidney possesses a specific, enzyme, 25-hydroxycholecalciferol (calcidiol), 1-hydroxylase which hydroxylates 25-hydroxycholecalciferol at position 1 to produce 1,25dihydroxycholecalciferol (1,25-DHCC). 1,25, DHCC contains 3 hydroxyl groups (1,3 and 25, carbon) hence referred to as calcitriol. Both the, hydroxylase enzymes (of liver and kidney), require cytochrome P450, NADPH and O2 for, the hydroxylation process. The synthesis of, calcitriol is depicted in Figs.7.7 and 7.8., , Regulation of the, synthesis of 1,25-DHCC, The concentration of 1,25-DHCC is regulated, by plasma levels of calcium and phosphate., They control hydroxylation reaction at position, 1. Low plasma phosphate increases the activity, of 25-hydroxycholecalciferol 1-hydroxylase. Low, plasma calcium enhances the production of, parathyroid hormone which in turn activates, 1-hydroxylase. Thus the action of phosphate is, direct while that of calcium is indirect on kidney, 1-hydroxylase.
Page 135 :
125, , Chapter 7 : VITAMINS, , Biochemical functions, Calcitriol (1,25-DHCC) is the biologically, active form of vitamin D. It regulates the plasma, levels of calcium and phosphate. Calcitriol acts, at 3 different levels (intestine, kidney and bone), to maintain plasma calcium (normal 9–11 mg/dl)., 3, , 7, , HO, , 1. Action of calcitriol on the intestine :, Calcitriol increases the intestinal absorption of, calcium and phosphate. In the intestinal cells,, calcitriol binds with a cytosolic receptor to form, a calcitriol-receptor complex. This complex then, approaches the nucleus and interacts with a, specific DNA leading to the synthesis of a, specific calcium binding protein. This protein, increases the calcium uptake by the intestine., The mechanism of action of calcitriol on the, target tissue (intestine) is similar to the action of, a steroid hormone., , 7-Dehydrocholesterol, (animals), Sunlight, Skin, , Diet, , H2, C, , HO, , Cholecalciferol, (D3; calciol), Calciol 25-hydroxylase, (liver), , 25, , OH, , H2, C, , 3. Action of calcitriol on the kidney :, Calcitriol is also involved in minimizing the, excretion of calcium and phosphate through the, kidney, by decreasing their excretion and, enhancing reabsorption., , HO, 25-Hydroxycholecalciferol, (Calcidiol), Calcidiol 1D-hydroxylase, (kidney), , 25, , HO, , 2. Action of calcitriol on the bone : In the, osteoblasts of bone, calcitriol stimulates calcium, uptake for deposition as calcium phosphate. Thus, calcitriol is essential for bone formation. The bone, is an important reservoir of calcium and phosphate., Calcitriol along with parathyroid hormone, increases the mobilization of calcium and, phosphate from the bone. This causes elevation in, the plasma calcium and phosphate levels., , OH, , H2, C, , 1, , HO, 1,25-Dihydroxycholecalciferol, (1,25 DHCC or calcitriol), , Fig. 7.7 : Biosynthesis of active form of vitamin, D-calcitriol (1,25 DHCC)., , The sequence of events that take place in, response to low plasma calcium concentration, and the action of calcitriol on intestine, kidney, and bone, ultimately leading to the increase in, plasma calcium is given in Fig.7.9., , 24,25-Dihydroxycholecalciferol (24,25-DHCC), is another metabolite of vitamin D. It is also, synthesized in the kidney by 24-hydroxylase. The, exact function of 24,25-DHCC is not known. It is, believed that when calcitriol concentration is, adequate, 24-hydroxylase acts leading to the, synthesis of a less important compound 24,, 25-DHCC. In this way, to maintain the, homeostasis of calcium, synthesis of 24,25-DHCC, is also important.
Page 137 :
127, , Chapter 7 : VITAMINS, , In countries with good sunlight (like India),, the RDA for vitamin D is 200 IU (or 5 mg, cholecalciferol)., , Plasma calcium, , Parathyroid hormone, , Dietary sources, , Calcitriol, , Bone Ca, mobilization, , Intestinal, Ca absorption, , Renal, Ca absorption, , Good sources of vitamin D include fatty fish,, fish liver oils, egg yolk etc. Milk is not a good, source of vitamin D., Vitamin D can be provided to the body in, three ways, , Plasma calcium, , Fig. 7.9 : Summary of the action of calcitriol, in elevating plasma calcium., , Vitamin D is a hormone and, not a vitamin—justification, Calcitriol (1,25-DHCC) is now considered, as an important calciotropic hormone, while, cholecalciferol is the prohormone. The following, characteristic features of vitamin D (comparable, with hormone) justify its status as a hormone., 1. Vitamin D3 (cholecalciferol) is synthesized, in the skin by ultra-violet rays of sunlight., 2. The biologically active form of vitamin D,, calcitriol is produced in the kidney., 3. Calcitriol has target organs—intestine,, bone and kidney, where it specifically acts., 4. Calcitriol action is similar to steroid, hormones. It binds to a receptor in the cytosol, and the complex acts on DNA to stimulate the, synthesis of calcium binding protein., 5. Actinomycin D inhibits the action of, calcitriol. This supports the view that calcitriol, exerts its effect on DNA leading to the synthesis, of RNA (transcription)., 6. Calcitriol synthesis is self-regulated by a, feedback mechanism i.e., calcitriol decreases its, own synthesis., , Recommended dietary, allowance (RDA), The daily requirement of vitamin D is 400, International Units or 10 mg of cholecalciferol., , 1. Exposure of skin to sunlight for synthesis, of vitamin D;, 2. Consumption of natural foods;, 3. By irradiating foods (like yeast) that, contain precursors of vitamin D and fortification, of foods (milk, butter etc.)., , Deficiency symptoms, Vitamin D deficiency is relatively less, common, since this vitamin can be synthesized, in the body. However, insufficient exposure, to sunlight and consumption of diet lacking, vitamin D results in its deficiency., Vitamin D deficiency occurs in strict, vegetarians, chronic alcoholics, individuals with, liver and kidney diseases or fat malabsorption, syndromes. In some people, who cover the entire, body (purdah) for religious customs, vitamin D, deficiency is also observed, if the requirement is, not met through diet., Deficiency of vitamin D causes rickets in, children and osteomalacia in adults. Rickets is, derived from an old English word ‘wrickken’,, meaning to twist. Osteomalacia is derived, from Greek (osteon-bone; malakia-softness)., Vitamin D is often called as antirachitic vitamin., Rickets in children is characterized by bone, deformities due to incomplete mineralization,, resulting in soft and pliable bones and delay in, teeth formation. The weight-bearing bones are, bent to form bow-legs. In rickets, the plasma, level of calcitriol is decreased and alkaline, phosphatase activity is elevated. Alkaline, phosphatase is concerned with the process of, bone formation. There is an overproduction of
Page 138 :
128, , BIOCHEMISTRY, , alkaline phosphatase related to more cellular, activity of the bone. It is believed to be due, to a vain attempt to result in bone formation., In case of osteomalacia (adult rickets), demineralization of the bones occurs (bones, become softer), increasing their susceptibility, to fractures., , Renal rickets, (renal osteodystrophy), , CH3, H3C, 7, , HO, , 6 5, , O, 1, , 8, 9, 10, , CH3, , 2, , CH3, CH3, CH2 (CH2 CH2 CH CH2 )3 H, , 3, 4, , D-Tocopherol (5,7,8-trimethyltocol), E-Tocopherol (5,8-dimethyltocol), J-Tocopherol (7,8-dimethyltocol), , Fig. 7.10 : Structure of D-tocopherol (Note : The tocopherols, differ in the substitution of methyl groups, represented in red)., , This is seen in patients with chronic renal, failure. Renal rickets is mainly due to decreased, synthesis of calcitriol in kidney. It can be treated, by administration of calcitriol., , Hypervitaminosis D, Vitamin D is stored mostly in liver and slowly, metabolised. Among the vitamins, vitamin D is, the most toxic in overdoses (10-100 times RDA)., Toxic effects of hypervitaminosis D include, demineralization of bone (resorption) and, increased calcium absorption from the intestine,, leading to elevated calcium in plasma, (hypercalcemia). Prolonged hypercalcemia is, associated with deposition of calcium in many, soft tissues such as kidney and arteries. Hypervitaminosis D may lead to formation of stones in, kidneys (renal calculi). High consumption of, vitamin D is associated with loss of appetite,, nausea, increased thirst, loss of weight etc., , VITAMIN E, Vitamin E (tocopherol) is a naturally occurring, antioxidant. It is essential for normal reproduction, in many animals, hence known as anti-sterility, vitamin. Vitamin E is described as a ‘vitamin in, search of a disease.’ This is due to the lack of any, specific vitamin E deficiency disease in humans., Evans and his associates (1936) isolated the, compounds of vitamin E activity and named, them as tocopherols (Greek : tokos-child birth;, pheros-to bear; ol-alcohol)., , tocopherols (vitamin E vitamers) have been, identified–D, E, J, G etc. Among these,, D-tocopherol is the most active. The tocopherols, are derivatives of 6-hydroxy chromane (tocol), ring with isoprenoid (3 units) side chain. The, antioxidant property is due to the hydroxyl group, of chromane ring., , Absorption, transport and storage, Vitamin E is absorbed along with fat in the, small intestine. Bile salts are necessary for the, absorption. In the liver, it is incorporated into, lipoproteins (VLDL and LDL) and transported., Vitamin E is stored in adipose tissue, liver and, muscle. The normal plasma level of tocopherol, is less than 1 mg/dl., , Biochemical functions, Most of the functions of vitamin E are related, to its antioxidant property. It prevents the nonenzymatic oxidations of various cell components, (e.g. unsaturated fatty acids) by molecular, oxygen and free radicals such as superoxide, –, (O2) and hydrogen peroxide (H2O2). The, element selenium helps in these functions., Vitamin E is lipophilic in character and is, found in association with lipoproteins, fat, deposits and cellular membranes. It protects the, polyunsaturated fatty acids (PUFA) from, peroxidation reactions. Vitamin E acts as a, scavenger and gets itself oxidized (to quinone, form) by free radicals (R) and spares PUFA, as, shown below, O, , Chemistry, Vitamin E is the name given to a group of, tocopherols and tocotrienols. About eight, , O, , CH3, , + RH, , +R, HO, , CH3, , O
Page 139 :
129, , Chapter 7 : VITAMINS, , The biochemical functions of vitamin E,, related either directly or indirectly to its, antioxidant property, are given hereunder, 1. Vitamin E is essential for the membrane, structure and integrity of the cell, hence it is, regarded as a membrane antioxidant., 2. It prevents the peroxidation of polyunsaturated fatty acids in various tissues and, membranes. It protects RBC from hemolysis by, oxidizing agents (e.g. H2O2)., 3. It is closely associated with reproductive, functions and prevents sterility. Vitamin E, preserves and maintains germinal epithelium of, gonads for proper reproductive function., 4. It increases the synthesis of heme by, enhancing the activity of enzymes Gaminolevulinic acid (ALA) synthase and ALA, dehydratase., 5. It is required for cellular respiration–, through electron transport chain (believed to, stabilize coenzyme Q)., 6. Vitamin E prevents the oxidation of, vitamin A and carotenes., 7. It is required for proper storage of creatine, in skeletal muscle., 8. Vitamin E is needed for optimal absorption, of amino acids from the intestine., 9. It is involved in proper synthesis of nucleic, acids., 10. Vitamin E protects liver from being, damaged by toxic compounds such as carbon, tetrachloride., 11. It works in association with vitamins A, C, and E-carotene, to delay the onset of cataract., 12. Vitamin E has been recommended for the, prevention of chronic diseases such as cancer, and heart diseases. Clinical trials in this regard, are rather disappointing, hence it is no more, recommended. However, some clinicians, continue to use it particularly in subjects, susceptible to heart attacks. It is believed, that vitamin E prevents the oxidation of LDL., (Note : The oxidized LDL have been implicated, to promote heart diseases.), , Vitamin E and selenium, The element selenium is found in the enzyme, glutathione peroxidase that destroys free, radicals. Thus, Se is also involved in antioxidant, functions like vitamin E, and both of them act, synergistically. To a certain extent, Se can spare, the requirement vitamin E, and vice versa., , Recommended dietary, allowance (RDA), Intake of vitamin E is directly related to the, consumption of polyunsaturated fatty acids, (PUFA) i.e., requirement increases with increased, intake of PUFA. A daily consumption of about, 10 mg (15 IU) of D-tocopherol for man and 8 mg, (12 IU) for woman is recommended. One mg of, D-tocopherol is equal to 1.5 IU. Vitamin E, supplemented diet is advised for pregnant and, lactating women., , Dietary sources, Many vegetable oils are rich sources of, vitamin E. Wheat germ oil, cotton seed oil,, peanut oil, corn oil and sunflower oil are the, good sources of this vitamin. It is also present in, meat, milk, butter and eggs., , Deficiency symptoms, The symptoms of vitamin E deficiency vary, from one animal species to another. In many, animals, the deficiency is associated with, sterility, degenerative changes in muscle,, megaloblastic anaemia and changes in central, nervous system. Severe symptoms of vitamin E, deficiency are not seen in humans except, increased fragility of erythrocytes and minor, neurological symptoms., , Toxicity of vitamin E, Among the fat soluble vitamins (A, D, E, K),, vitamin E is the least toxic. No toxic effect has, been reported even after ingestion of 300 mg/, day for 23 years., , VITAMIN K, Vitamin K is the only fat soluble vitamin with, a specific coenzyme function. It is required for
Page 140 :
130, , BIOCHEMISTRY, , the production of blood clotting, factors, essential for coagulation (in, German–Koagulation; hence the, name K for this vitamin)., , O, , Chemistry, , O, , CH3, CH3, , CH3, , CH2 CH C CH2 (CH2 CH2 CH CH2)3 H, , Vitamin K exists in different, forms (Fig.7.11). Vitamin K1, (phylloquinone) is present in plants., Vitamin K2 (menaquinone) is, produced by the intestinal bacteria, and also found in animals. Vitamin, K3 (menadione) is a synthetic form., All the three vitamins (K1, K2, K3), are naphthoquinone derivatives., Isoprenoid side chain is present in, vitamins K1 and K2. The three, vitamins are stable to heat. Their, activity is, however, lost by, oxidizing agents, irradiation, strong, acids and alkalies., , Absorption, transport, and storage, Vitamin K is taken in the diet or synthesized, by the intestinal bacteria. Its absorption takes, place along with fat (chylomicrons) and is, dependent on bile salts. Vitamin K is transported, along with LDL and is stored mainly in liver and,, to a lesser extent, in other tissues., , Biochemical functions, The functions of vitamin K are concerned with, blood clotting process. It brings about the posttranslational (after protein biosynthesis in the, cell) modification of certain blood clotting, factors. The clotting factors II (prothrombin), VII,, IX and X are synthesized as inactive precursors, (zymogens) in the liver. Vitamin K acts as a, coenzyme for the carboxylation of glutamic acid, residues present in the proteins and this reaction, is catalysed by a carboxylase (microsomal). It, involves the conversion of glutamate (Glu) to, J-carboxyglutamate (Gla) and requires vitamin K,, O2 and CO2 (Fig.7.12). The formation of, J-carboxyglutamate is inhibited by dicumarol,, an anticoagulant found in spoilt sweet clover., Warfarin is a synthetic analogue that can inhibit, vitamin K action (Fig.7.13)., , Vitamin K1 (phylloquinone), , O, CH3, CH3, (CH2 CH C CH2)6 H, O, Vitamin K2 (menaquinone), , O, CH3, , O, Vitamin K3 (menadione), , Fig. 7.11 : Structures of vitamin K., , Vitamin K is also required for the, carboxylation of glutamic acid residues of, osteocalcin, a calcium binding protein present, in the bone., The mechanism of carboxylation is not fully, understood. It is known that a 2,3-epoxide, derivative of vitamin K is formed as an, intermediate during the course of the reaction., Dicumarol inhibits the enzyme (reductase) that, converts epoxide to active vitamin K., Role of Gla in clotting : The J-carboxyglutamic acid (Gla) residues of clotting factors, are negatively charged (COO–) and they, combine with positively charged calcium ions, (Ca2+) to form a complex. The mechanism of, action has been studied for prothrombin. The, prothrombin, Ca complex binds to the, phospholipids on the membrane surface of the, platelets (Fig.7.14). This leads to the increased, conversion of prothrombin to thrombin., , Recommended dietary, allowance (RDA), Strictly speaking, there is no RDA for vitamin, K, since it can be adequately synthesized in the
Page 141 :
131, , Chapter 7 : VITAMINS, , O2, , H, Protein, , Glu, , H, , Carboxylase, , N CH C, , Protein, , Vitamin K, , CH2 O, , N CH C, CH2 O, , CO2, , CH2, , CH, , COOH, Precursors of clotting, factors (II, VII, IX, X), , Gla, , COOH COOH, , Dicumarol,, warfarin, , Clotting factors (II, VII, IX, X), , Fig. 7.12 : Vitamin K dependent carboxylation of the precursors of clotting factors., , gut. It is however, recommended that half of the, body requirement is provided in the diet, while, the other half is met from the bacterial synthesis., Accordingly, the suggested RDA for an adult is, 70-140 Pg/day., , Dietary sources, Cabbage, cauliflower, tomatoes, alfa alfa,, spinach and other green vegetables are good, sources. It is also present in egg yolk, meat, liver,, cheese and dairy products., , Deficiency symptoms, The deficiency of vitamin K is uncommon,, since it is present in the diet in sufficient quantity, and/or is adequately synthesized by the intestinal, bacteria. However, vitamin K deficiency may, occur due to its faulty absorption (lack of bile, salts), loss of vitamin into feces (diarrheal, , (Protein)-Glu, , diseases) and administration of antibiotics (killing, of intestinal flora)., Deficiency of vitamin K leads to the lack of, active prothrombin in the circulation. The result, is that blood coagulation is adversely affected., The individual bleeds profusely even for minor, injuries. The blood clotting time is increased., , Hypervitaminosis K, Administration of large doses of vitamin K, produces hemolytic anaemia and jaundice,, particularly in infants. The toxic effect is due to, increased breakdown of RBC., , Antagonists of vitamin K, The compounds—namely heparin, bishydroxycoumarin—act as anticoagulants and are, antagonists to vitamin K. The salicylates and, dicumarol are also antagonists to vitamin K., , (Protein)-Gla, , H, , Carboxylase, , N CH C, , Prothrombin, , CH2 O, CH, O C, Vitamin K, (hydroquinone form), , C O, , J-Carboxyglutamate, complexed with calcium, , 2,3-Epoxide form, –, , Reductase, , O–, , O, Ca2+, , Reductase, Quinone, form, , Dicumarol,, warfarin, , Fig. 7.13 : Summary of vitamin K, cycle in carboxylation reaction., , Platelet membrane, (with phospholipids), , Fig. 7.14 : Mechanism of action of J-carboxyglutamate, of prothrombin in blood clotting.
Page 142 :
132, , BIOCHEMISTRY, , dehydroascorbic, acid, are, biologically, active., However,, + H2 O, COOH, O C, HO C, –2H, O C, O, O, D-ascorbic acid is inactive. The, +2H, HO C, COOH, O C, O C, plasma, and, tissues, preH C, H C, H C OH, dominantly contain ascorbic, HO C H, HO C H, HO C H, acid in the reduced form. The, ratio of ascorbic acid to, CH2OH, CH2OH, CH2OH, dehydroascorbic acid in many, L-Ascorbic acid, Dehydro L-ascorbic, Diketo L-gulonic, Oxalic, tissues is 15 : 1. On hydration,, (reduced form), acid (oxidized form), acid, acid, dehydroascorbic, acid, is, Fig. 7.15 : Structures of vitamin C (ascorbic acid), irreversibly, converted, to, 2,3and its related compounds., diketogulonic acid which is, inactive. Hydration reaction is, almost, spontaneous,, in alkaline or neutral, Dicumarol is structurally related to vitamin K and, solution., It, is, for, this, reason, that oxidation of, acts as a competitive inhibitor in the synthesis of, vitamin, C, is, regarded, as, biological, inactivation, active prothrombin., (formation of diketogulonic acid). Oxidation of, ascorbic acid is rapid in the presence of copper., VITAMIN C (ASCORBIC ACID), Hence vitamin C becomes inactive if the foods, Vitamin C is a water soluble versatile vitamin. are prepared in copper vessels., O C, , O C, , O C OH, , It plays an important role in human health and, disease. Vitamin C has become the most, controversial vitamin in recent years. This is, because of the claims and counter-claims on the, use of vitamin C in megadoses to cure everything, from common cold to cancer., Scurvy has been known to man for centuries., It was the first disease found to be associated, with diet. In the sixteenth century about 10,000, mariners died of a miraculous disease (scurvy), due to lack of fresh vegetables in their diet., James Lind, a surgeon of the English Navy, in, 1753 published ‘Treatise on Scurvy’. Based on, Lind’s observations, the Royal Navy since 1795, used to supply lime or lemon juice to all the, crews. The English Navy used to carry crates of, lemons, hence they were popularly known as, Limeys., , Chemistry, Ascorbic acid is a hexose (6 carbon), derivative and closely resembles monosaccharides in structure (Fig.7.15). The acidic, property of vitamin C is due to the enolic, hydroxyl groups. It is a strong reducing, agent. L-Ascorbic acid undergoes oxidation, to form dehydroascorbic acid and this, reaction is reversible. Both ascorbic acid and, , Biosynthesis and metabolism, Many animals can synthesize ascorbic, acid from glucose via uronic acid pathway, (Chapter 13). However, man, other primates,, guinea pigs and bats cannot synthesize ascorbic, acid due to the deficiency of a single enzyme, namely L-gulonolactone oxidase., Vitamin C is rapidly absorbed from the, intestine. It is not stored in the body to a, significant extent. Ascorbic acid is excreted in, urine as such, or as its metabolites—, diketogulonic acid and oxalic acid (Fig.7.15)., , Biochemical functions, Most of the functions of vitamin C are related, to its property to undergo reversible oxidation–, reduction i.e., interconversion of ascorbic acid, and dehydroascorbic acid., 1. Collagen formation : Vitamin C plays the, role of a coenzyme in hydroxylation of proline, and lysine while protocollagen is converted to, collagen (i.e. post-translational modification)., The hydroxylation reaction is catalysed by lysyl, hydroxylase (for lysine) and prolyl hydroxylase, (for proline). This reaction is dependent on, vitamin C, molecular oxygen and D-ketoglutarate, (Fig.7.16).
Page 143 :
133, , Chapter 7 : VITAMINS, , H, , O, , N, , CH C, , H2C, , CH2, , Proline, , C, H2, D-Ketoglutarate, , O2, Prolyl hydroxylase, , Ascorbic acid, Succinate + CO2, , H2O, , H, , O, , N, , CH C, , H2C, , CH2, , Hydroxyproline, , C, H, , OH, , Fig. 7.16 : Ascorbic acid dependent, hydroxylation of proline of protocollagen., , Hydroxyproline and hydroxylysine are, essential for the collagen cross-linking and the, strength of the fiber. In this way, vitamin C is, necessary for maintenance of normal connective, tissue and wound healing process., 2. Bone formation : Bone tissues possess an, organic matrix, collagen and the inorganic, calcium, phosphate etc. Vitamin C is required, for bone formation., 3. Iron and hemoglobin metabolism :, Ascorbic acid enhances iron absorption by, keeping it in the ferrous form. This is due to the, reducing property of vitamin C. It helps in the, formation of ferritin (storage form of iron) and, mobilization of iron from ferritin., , 5. Tyrosine metabolism : Ascorbic acid is, required for the oxidation of p-hydroxy, phenylpyruvate (enzyme hydroxylase) to homogentisic acid in tyrosine metabolism., 6. Folic acid metabolism : The active form of, the vitamin folic acid is tetrahydrofolate (FH4)., Vitamin C is needed for the formation of FH4, (enzyme-folic acid reductase). Further, in, association with FH4, ascorbic acid is involved, in the maturation of erythrocytes., 7. Peptide hormone synthesis : Many peptide, hormones contain carboxyl terminal amide, which is derived from terminal glycine., Hydroxylation of glycine is carried out by, peptidylglycine hydroxylase which requires, vitamin C., 8. Synthesis of corticosteroid hormones :, Adrenal gland possesses high levels of ascorbic, acid, particularly in periods of stress. It is, believed that vitamin C is necessary for the, hydroxylation reactions in the synthesis of, corticosteroid hormones., 9. Sparing action of other vitamins :, Ascorbic acid is a strong antioxidant. It spares, vitamin A, vitamin E, and some B-complex, vitamins from oxidation., 10. Immunological function : Vitamin C, enhances the synthesis of immunoglobulins, (antibodies) and increases the phagocytic action, of leucocytes., 11. Preventive action on cataract : Vitamin C, reduces the risk of cataract formation., 12. Preventive action on chronic diseases :, As an antioxidant, vitamin C reduces the risk, of cancer, cataract, and coronary heart, diseases., , Vitamin C is useful in the reconversion of, methemoglobin to hemoglobin. The degradation, of hemoglobin to bile pigments requires ascorbic, acid., , Recommended dietary, allowance (RDA), , 4. Tryptophan metabolism : Vitamin C is, essential for the hydroxylation of tryptophan, (enzyme-hydroxylase) to hydroxytryptophan in, the synthesis of serotonin., , About 60-70 mg vitamin C intake per day will, meet the adult requirement. Additional intakes, (20-40% increase) are recommended for women, during pregnancy and lactation.
Page 144 :
134, , BIOCHEMISTRY, , Dietary sources, Citrus fruits, gooseberry (amla), guava, green, vegetables (cabbage, spinach), tomatoes,, potatoes (particularly skin) are rich in ascorbic, acid. High content of vitamin C is found in, adrenal gland and gonads. Milk is a poor source, of ascorbic acid., , Deficiency symptoms, The deficiency of ascorbic acid results in, scurvy. This disease is characterized by spongy, and sore gums, loose teeth, anemia, swollen, joints, fragile blood vessels, decreased, immunocompetence, delayed wound healing,, sluggish hormonal function of adrenal cortex and, gonads, haemorrhage, osteoporosis etc. Most of, these symptoms are related to impairment in the, synthesis of collagen and/or the antioxidant, property of vitamin C., , Megadoses of vitamin C, and its controversy, Linus Pauling (1970) first advocated the, , consumption of megadoses of ascorbic acid, (even up to 18 g/day, 300 times the daily, requirement!) to prevent and cure common, cold. He is remembered as a scientist who, suggested ‘keep vitamin C in gunny bags and eat, in grams.’ This generated a lot of controversy, worldover. It is now clear that megadose of, vitamin C does not prevent common cold. But, the duration of cold and the severity of, symptoms are reduced. It is believed that, ascorbic acid promotes leukocyte function., Megadoses (1-4 g/day) of vitamin C are still, continued in common cold, wound healing,, trauma etc. As an antioxidant, ascorbic acid, certainly provides some health benefits., Ascorbic acid, as such, has not been found to, be toxic. But, dehydroascorbic acid (oxidized, form of ascorbic acid) is toxic. Further, oxalate is, a major metabolite of vitamin C. Oxalate has, been implicated in the formation of kidney, stones. However, there are controversial reports, on the megadoses of vitamin C leading to urinary, stones., , + It is believed that during the course of evolution, the ability to synthesize vitamins was, lost by the higher organisms, hence they should be supplied through the diet., , + For humans, the normal intestinal bacterial synthesis of vitamin K and biotin is almost, sufficient to meet the body requirements., , + Administration of antibiotics often destroys the vitamin synthesizing bacteria in the gut,, hence additional supplementation of vitamins is recommended during antibiotic therapy., , + Vitamin A deficiency causes night blindness; vitamin D deficiency rickets (in children), or osteomalacia (in adults); vitamin E deficiency minor neurological symptoms; vitamin, K deficiency bleeding., , + Fat soluble vitamins are not readily excreted in urine, hence excess consumption leads, to their accumulation and toxic effects., , + Vitamin C deficiency causes scurvy. The manifestations of scurvy are related to the, impairment in the synthesis of collagen and/or the antioxidant property of vitamin C., , + Megadoses of vitamin C are used in common cold, wound healing, trauma etc., + E-Carotene, vitamin E and ascorbic acid serve as antioxidants and reduce the risk of, heart attacks and cancers.
Page 145 :
135, , Chapter 7 : VITAMINS, , –, , 2. D-Ketoglutarate dehydrogenase, is an enzyme of the citric acid cycle., This enzyme is comparable with, pyruvate dehydrogenase and requires, TPP., , Pyrophosphate, , 3. Transketolase is dependent on, TPP. This is an enzyme of the hexose, monophosphate shunt (HMP shunt)., , Reactive, carbon, , NH2, , S, CH2 N, , N, , –, , +, , O, , O–, , CH2 CH2 O P O P O, H3C, , CH 3, , N, , Pyrimidine Methylene, bridge, , Thiazole, , O, , Thiamine, , Fig. 7.17 : Structures of thiamine and thiamine, pyrophosphate (TPP)., , THIAMINE (VITAMIN B1), Thiamine (anti-beri-beri or antineuritic, vitamin) is water soluble. It has a specific, coenzyme, thiamine pyrophosphate (TPP) which, is mostly associated with carbohydrate, metabolism., , Chemistry, Thiamine contains a pyrimidine ring and a, thiazole ring held by a methylene bridge, (Fig.7.17). Thiamine is the only natural, compound with thiazole ring., The alcohol (OH) group of thiamine is, esterfied with phosphate (2 moles) to form the, coenzyme, thiamine pyrophosphate (TPP or, cocarboxylase). The pyrophosphate moiety is, donated by ATP and the reaction is catalysed, by the enzyme thiamine pyrophosphate, transferase., , Biochemical functions, The coenzyme, thiamine pyrophosphate or, cocarboxylase is intimately connected with the, energy releasing reactions in the carbohydrate, metabolism (Fig.7.18)., 1. The enzyme pyruvate dehydrogenase, catalyses (oxidative decarboxylation) the, irreversible conversion of pyruvate to acetyl, CoA. This reaction is dependent on TPP, besides, the other coenzymes (details given in, carbohydrate metabolism, Chapter 13)., , O, , 4. The branched chain D-keto, acid dehydrogenase (decarboxylase), catalyses the oxidative decarboxylation, of branched chain amino acids (valine,, leucine and isoleucine) to the, respective keto acids. This enzyme also requires, TPP., 5. TPP plays an important role in the, transmission of nerve impulse. It is believed that, TPP is required for acetylcholine synthesis and, the ion translocation of neural tissue., , Recommended dietary, allowance (RDA), The daily requirement of thiamine depends, on the intake of carbohydrate. A dietary supply, of 1-1.5 mg/day is recommended for adults, (about 0.5 mg/1,000 Cals of energy). For children, RDA is 0.7-1.2 mg/day. The requirement, marginally increases in pregnancy and lactation, (2 mg/day), old age and alcoholism., , Dietary sources, Cereals, pulses, oil seeds, nuts and yeast are, good sources. Thiamine is mostly concentrated, in the outer layer (bran) of cereals. Polishing of, rice removes about 80% of thiamine. Vitamin B1, is also present in animal foods like pork, liver,, heart, kidney, milk etc. In the parboiled (boiling, of paddy with husk) and milled rice, thiamine is, not lost in polishing. Since thiamine is a water, soluble vitamin, it is extracted into the water, during cooking process. Such water should not, be discarded., , Deficiency symptoms, The deficiency of vitamin B1 results in a, condition called beri-beri [Sinhalese : I cannot
Page 146 :
136, , BIOCHEMISTRY, , Glucose, , Glucose 6-phosphate, , Pyruvate, TPP, , Ribose 5-phosphate, Xylulose, 5-phosphate, , Pyruvate, dehydrogenase, , CO 2, , Sedoheptulose, 7-phosphate, , Acetyl CoA, , Transketolase, TPP, , Glyceraldehyde, 3-phosphate, Oxaloacetate, , Citrate, Citric acid, cycle, D-Ketoglutarate, TPP, Succinyl, CoA, , D-Ketoglutarate, dehydrogenase, , CO2, , Fig. 7.18 : Summary of the reactions dependent on thiamine pyrophosphate (TPP)., , (said twice)]. Beri-beri is mostly seen in, populations consuming exclusively polished rice, as staple food. The early symptoms of thiamine, deficiency are loss of appetite (anorexia),, weakness,, constipation,, nausea,, mental, depression, peripheral neuropathy, irritability, etc. Numbness in the legs complaints of ‘pins, and needles sensations’ are reported., , Biochemical changes in B1 deficiency, 1. Carbohydrate metabolism is impaired., Accumulation of pyruvate occurs in the tissues, which is harmful. Pyruvate concentration in, plasma is elevated and it is also excreted in urine., 2. Normally, pyruvate does not cross the, blood-brain barrier and enter the brain., However, in thiamine deficiency, an alteration, occurs in the blood-brain barrier permitting the, pyruvate to enter the brain directly. It is believed, that pyruvate accumulation in brain results in, disturbed metabolism that may be responsible, for polyneuritis., , 3. Thiamine deficiency leads to impairment, in nerve impulse transmission due to lack of TPP., 4. The transketolase activity in erythrocytes is, decreased. Measurement of RBC transketolase, activity is a reliable diagnostic test to assess, thiamine deficiency., In adults, two types of beri-beri, namely wet, beri-beri and dry beri-beri occur. Infantile beriberi that differs from adult beri-beri is also seen., Wet beri-beri (cardiovascular beri-beri) : This, is characterized by edema of legs, face, trunk, and serous cavities. Breathlessness and, palpitation are present. The calf muscles are, slightly swollen. The systolic blood pressure is, elevated while diastolic is decreased. Fast and, bouncing pulse is observed. The heart becomes, weak and death may occur due to heart failure., Dry beri-beri (neurological beri-beri) : This is, associated with neurological manifestations, resulting in peripheral neuritis. Edema is, not commonly seen. The muscles become
Page 147 :
137, , Chapter 7 : VITAMINS, , progressively weak and walking becomes, difficult. The affected individuals depend on, support to walk and become bedridden, and may, even die, if not treated., The symptoms of beri-beri are often mixed in, which case it is referred to as mixed beri-beri., Infantile beri-beri : This is seen in infants, born to mothers suffering from thiamine, deficiency. The breast milk of these mothers, contains low thiamine content. Infantile beri-beri, is characterized by sleeplessness, restlessness,, vomiting, convulsions and bouts of screaming, due to cardiac dilatation., , Wernicke-Korsakoff syndrome, This disorder also known as cerebral beriberi, is mostly seen in chronic alcoholics. The, body demands of thiamine increase in, alcoholism. Insufficient intake or impaired, intestinal absorption of thiamine will lead to this, syndrome. It is characterized by loss of memory,, apathy and a rhythmical to and fro motion of the, eye balls., , Thiamine deficiency due to, thiaminase and pyrithiamine, The enzyme thiaminase is present in certain, seafoods. Their inclusion in the diet will destroy, thiamine by a cleavage action (pyrimidine and, thiazole rings split) and lead to deficiency., Incidence of beri-beri in some parts of Japan is, attributed to the consumption of fish (rich in, thiaminase). Pyrithiamine, a structural analogue, and an antimetabolite of thiamine is found in, certain plants like ferns. Horses and cattle often, develop thiamine deficiency (fern poisoning), due to the overconsumption of the plant fern., , Thiamine antagonists, Pyrithiamine and oxythiamine are the two, important antimetabolites of thiamine., , RIBOFLAVIN (VITAMIN B2), Riboflavin through its coenzymes takes part, in a variety of cellular oxidation–reduction, reactions., , Chemistry, Riboflavin contains 6,7-dimethyl isoalloxazine, (a heterocyclic 3 ring structure) attached to, D-ribitol by a nitrogen atom. Ribitol is an open, chain form of sugar ribose with the aldehyde, group (CHO) reduced to alcohol (CH2OH)., Riboflavin is stable to heat but sensitive to, light. When exposed to ultra-violet rays of, sunlight, it is converted to lumiflavin which, exhibits yellow fluorescence. The substances, namely lactoflavin (from milk), hepatoflavin, (from liver) and ovoflavin (from eggs) which, were originally thought to be different are, structurally identical to riboflavin., , Coenzymes of riboflavin, Flavin mononucleotide (FMN) and flavin, adenine dinucleotide (FAD) are the two, coenzyme forms of riboflavin. The ribitol (5, carbon) is linked to a phosphate in FMN. FAD is, formed from FMN by the transfer of an AMP, moiety from ATP (Fig.7.19)., , Biochemical functions, The flavin coenzymes (mostly FAD and to a, lesser extent FMN) participate in many redox, reactions responsible for energy production. The, functional unit of both the coenzymes is, isoalloxazine ring which serves as an acceptor of, two hydrogen atoms (with electrons). FMN or, FAD undergo identical reversible reactions, accepting two hydrogen atoms forming FMNH2, or FADH2 (Fig.7.20)., Enzymes that use flavin coenzymes (FMN or, FAD) are called flavoproteins. The coenzymes, (prosthetic groups) often bind rather tightly, to, the protein (apoenzyme) either by non-covalent, bonds (mostly) or covalent bonds in the, holoenzyme. Many flavoproteins contain metal, atoms (iron, molybdenum etc.) which are known, as metalloflavoproteins., The coenzymes, FAD and FMN are associated, with certain enzymes involved in carbohydrate,, lipid, protein and purine metabolisms, besides, the electron transport chain. A few examples are, listed in Table 7.2. Further details are given in, the respective chapters.
Page 148 :
138, , BIOCHEMISTRY, , O, , Recommended dietary, allowance (RDA), The, daily, requirement, of, riboflavin for an adult is 1.2-1.7 mg., Higher intakes (by 0.2-0.5 mg/day), are, advised for pregnant and, lactating women., , H3C, , N, , H3C, , N, , Isoalloxazine, , CH 2, , NH, N, , O, , H C OH R, I, H C OH B, I, H C OH T, O, CH 2OH L, , Dietary sources, Milk and milk products, meat,, eggs, liver, kidney are rich sources., Cereals, fruits, vegetables and fish, are moderate sources., , Riboflavin, ATP, Flavokinase, ADP, , O, , Deficiency symptoms, Riboflavin deficiency symptoms, include cheilosis (fissures at the, corners of the mouth), glossitis, (tongue smooth and purplish) and, dermatitis. Riboflavin deficiency as, such is uncommon. It is mostly seen, along with other vitamin deficiencies. Chronic alcoholics are, susceptible to B2 deficiency. Assay, of the enzyme glutathione reductase, in erythrocytes will be useful in, assessing riboflavin deficiency., , H3C, , N, , H3C, , N, , N, , O, , CH 2, H C OH, H C OH, H C OH O, CH 2O P O–, O–, Flavin mononucleotide (FMN), ATP, FAD synthase, PPi, , Antimetabolite : Galactoflavin is, an antimetabolite of riboflavin., , O, H3C, , NIACIN, , NH, , H3C, , N, , NH, N, , N, , O, , CH 2, , Niacin or nicotinic acid is also, known as pellagra preventive (P.P.), factor of Goldberg. The coenzymes, of niacin (NAD+ and NADP+) can, be synthesized by the essential, amino acid, tryptophan., The disease pellagra (Italian :, rough skin) has been known for, centuries. However, its relation to, the deficiency of a dietary factor was, first identified by Goldberger., Goldberger and his associates, conducted an interesting experiment, , NH2, , H C OH, , N, N, , H C OH, H C OH O, , O, , N, , CH 2O P O P O CH2, O–, , O–, , N, , O, H, , H, , H, , H, , OH OH, Flavin adenine, dinucleotide (FAD), , Fig. 7.19 : Structures and biosynthesis of flavin mononucleotide, (FMN) and flavin adenine dinucleotide (FAD).
Page 149 :
139, , Chapter 7 : VITAMINS, , H, , O, , +, , –, , 2H , 2e, , O, , H3C, , N, , H3C, , N, , N, , R, , R, , H, , Oxidized flavin, (FMN or FAD), , Reduced flavin, (FMNH2 or FADH2), , H3C, , N, , H3C, , N, , NH, N, , O, , +, , –, , 2H , 2e, , NH, O, , Fig. 7.20 : Participation of FMN or FAD in oxidation-reduction reactions (R-represents the rest of the, structure of FMN or FAD as depicted in Fig. 7.19)., , for this purpose. Twelve convicts were promised, pardon if they consumed diet of pellagrous, families for one year. The diet consisted of corn, meal, corn starch, rice, sweet potato and pork, fat. More than half of the subjects showed, symptoms of pellagra in less than an year, while, no such symptoms were observed in other, prisoners on a regular diet. Administration of, dried meat or liver to the patients cured pellagra, (Goldberger, 1928)., , nicotine (present in tobacco leaves). The term, ‘niacin’ was coined and more commonly used, for nicotinic acid. This was done to emphasize, the role of niacin as a vitamin and avoid the, impression that nicotinic acid is an oxidized, form of nicotine. However, most of the authors, use niacin and nicotinic acid synonymously., , Much before it was recognized as a vitamin,, nicotinic acid was well known as a chemical, compound, produced by the oxidation of, , Niacin is a pyridine derivative. Structurally, it, is pyridine 3-carboxylic acid. The amide form of, niacin is known as niacinamide or nicotinamide., , Chemistry and synthesis, of coenzymes, , TABLE 7.2 Selected examples of FAD and FMN dependent enzymes along with their respective reactions, , Enzyme, , Reaction, , FAD dependent, I. Carbohydrate metabolism, (a) Pyruvate dehydrogenase complex*, , Pyruvate o Acetyl CoA, , (b) D-Ketoglutarate dehydrogenase complex*, , D-Ketoglutarate o Succinyl CoA, , (c) Succinate dehydrogenase, , Succinate o Fumarate, , II. Lipid metabolism, (d) Acyl CoA dehydrogenase, , Acyl CoA o D, E-Unsaturated acyl CoA, , III. Protein metabolism, (e) Glycine oxidase, , Glycine o Glyoxylate + NH3, , (f) D-Amino acid oxidase, , D-Amino acid o D-Keto acid + NH3, , IV. Purine metabolism, (g) Xanthine oxidase, , Xanthine o Uric acid, , FMN dependent, L-Amino acid oxidase, , * Dihydrolipoyl dehydrogenase component requires FAD, , L-Amino acid o D-Keto acid + NH3
Page 151 :
141, , Chapter 7 : VITAMINS, , H, , AH2, , CONH2, , –, , H, , H, , H, CONH2, , +, , H, , + H, , +, , N, , N, , Ribose, P, , +, , P, , Ribose, , Adenine, , P, , Ribose, , +, , NAD (oxidized), , P, , Adenine, Ribose, , NADH + H+ (reduced), , Overall reaction, AH2 + NAD+, , A + NADH + H+, , Fig. 7.22 : Mechanism of oxidation and reduction of, nicotinamide coenzyme—NAD+, (Note : Similar mechanism operates for NADP+ also)., , dependent on NAD+ or NADP+. The coenzymes, are loosely bound to the enzymes and can be, separated easily by dialysis. NAD+ and NADP+, participate in almost all the metabolisms, (carbohydrate, lipid, protein etc.). Some enzymes, are exclusively dependent on NAD+ whereas, some require only NADP+. A few enzymes can, use either NAD+ or NADP+. Selected examples, of enzymes and the reactions they catalyse are, given in Table 7.3., , NADH produced is oxidized in the electron, transport chain to generate ATP. NADPH is also, important for many biosynthetic reactions as it, donates reducing equivalents., , Recommended dietary, allowance (RDA), The daily requirement of niacin for an adult is, 15-20 mg and for children, around 10-15 mg., Very often, the term niacin equivalents (NE) is, used while expressing its RDA. One NE = 1 mg, niacin or 60 mg of tryptophan. Instead of mg,, the daily requirements are known as niacin, equivalents. Pregnancy and lactation in women, impose an additional metabolic burden and, increase the niacin requirement., , Dietary sources, The rich natural sources of niacin include, liver, yeast, whole grains, cereals, pulses like, beans and peanuts. Milk, fish, eggs and, vegetables are moderate sources. The essential, , amino acid tryptophan can serve as a precursor, for the synthesis of nicotinamide coenzymes. On, an average, 1 g of a good quality protein, containing about 60 mg of tryptophan is, equivalent to 1 mg of niacin (conversion ratio, 60 : 1) for the synthesis of nicotinamide, coenzymes. Tryptophan has many other essential, and important functions in the body, hence, dietary tryptophan cannot totally replace niacin., Increased conversion of tryptophan to niacin has, been reported in low protein diet and starvation., Tryptophan can replace niacin to an extent of, 10% for the synthesis of coenzymes. Therefore,, both niacin and tryptophan have to be invariably, provided in the diet., , Deficiency symptoms, Niacin deficiency results in a condition called, pellagra (Italian: rough skin). This disease, involves skin, gastrointestinal tract and central, nervous system. The symptoms of pellagra are, commonly referred to as three Ds. The disease, also progresses in that order dermatitis, diarrhea,, dementia, and if not treated may rarely lead to, death (4th D). Pellagra is frequently observed in, Hartnup’s disease (See p-173)., Dermatitis (inflammation of skin) is usually, found in the areas of the skin exposed to sunlight, (neck, dorsal part of feet, ankle and parts of face)., Diarrhea may be in the form of loose stools, often, with blood and mucus. Prolonged diarrhea leads, to weight loss. Dementia is associated with, degeneration of nervous tissue. The symptoms of, dementia include anxiety, irritability, poor, memory, insomnia (sleeplessness) etc., Pellagra is mostly seen among people whose, staple diet is corn or maize. Niacin present in, maize is unavailable to the body as it is in bound, form, and tryptophan content is low in maize., , Therapeutic uses of niacin, Administration of niacin in pharmacological, doses (2-4 g/day, 200 times the RDA) results in, a number of biochemical effects in the body, not, related to its function as a vitamin. Most of the, effects are believed to be due to the influence of, niacin on cyclic AMP levels.
Page 156 :
146, , BIOCHEMISTRY, , Deficiency symptoms, , Toxic effects of overdose vitamin B6, , Pyridoxine deficiency is associated with, neurological symptoms such as depression,, irritability, nervousness and mental confusion., Convulsions and peripheral neuropathy are, observed in severe deficiency. These symptoms, are, related to the decreased synthesis of, biogenic, amines, (serotonin,, GABA,, norepinephrine and epinephrine). In children, B6, deficiency with a drastically reduced GABA, production results in convulsions (epilepsy)., , Excess use of vitamin B6 (2.5 g/day) in the, women of premenstrual syndrome is associated, with sensory neuropathy. Some workers have, suggested that vitamin B6 more than 200 mg/day, may cause neurological damage., , Decrease in hemoglobin levels, associated, with hypochromic microcytic anaemia, is seen, in B6 deficieny. This is due to a reduction in, heme production., The synthesis of niacin coenzymes (NAD+, and NADP+) from tryptophan is impaired., Xanthurenic acid, produced in high quantities is, excreted in urine, which serves as a reliable, index (particularly after tryptophan load test) for, B6 deficiency., Dietary deficiency of pyridoxine is rather rare, and is mostly observed in women taking oral, contraceptives, alcoholics and infants., , Drug induced B6 deficiency, Isoniazid (isonicotinic acid hydrazide, INH) is, a drug frequently used for the treatment of, tuberculosis. It combines with pyridoxal, phosphate to form inactive hydrazone derivatives, which inhibit PLP dependent enzymes., Tuberculosis patients, on long term use of, isoniazid, develop peripheral neuropathy which, responds to B6 therapy., The drug penicillamine (E-dimethyl cysteine), is used in the treatment of patients with, rheumatoid arthritis, Wilson’s disease and, cystinuria. This drug also reacts with PLP to form, inactive thiazolidine derivative., Administration of drugs namely isoniazid and, penicillamine should be accompanied by pyridoxine supplementation to avoid B6 deficiency., , Pyridoxine antagonists, Isoniazid, deoxypyridoxine and methoxy, pyridoxine are the antagonists of vitamin B6., , BIOTIN, Biotin (formerly known as anti-egg white, injury factor, vitamin B7 or vitamin H) is a sulfur, containing B-complex vitamin. It directly participates as a coenzyme in the carboxylation, reactions., Boas (1927) observed that rats fed huge, quantity of raw egg white developed dermatitis, and nervous manifestations, besides retardation, in growth. She however, found that feeding, cooked egg did not produce any of these, symptoms. It was later shown that the egg white, injury in rats and chicks was due to the presence, of an anti-vitamin in egg white. The egg-white, injury factor was identified as a glycoprotein–, avidin and biotin was called as anti-egg white, injury factor., , Chemistry, Biotin is a heterocyclic sulfur containing, monocarboxylic acid. The structure is formed by, fusion of imidazole and thiophene rings with a, valeric acid side chain (Fig.7.28). Biotin is, covalently bound to H-amino group of lysine to, form biocytin in the enzymes. Biocytin may be, regarded as the coenzyme of biotin., , Site for, CO2 binding, , O, C, , Imidazole, ring, Thiophene, ring, , H N, , NH, , H C, , C H, , Binds with lysine, in enzyme, , CH (CH2)4 COOH, , H2 C, S, , Fig. 7.28 : Structure of biotin with binding sites.
Page 157 :
147, , Chapter 7 : VITAMINS, , Biochemical functions, Biotin serves as a carrier of CO2 in, carboxylation reactions. The reaction catalysed, by pyruvate carboxylase, converting pyruvate to, oxaloacetate has been investigated in detail. This, enzyme has biotin bound to the apoenzyme, linked to the H-amino group of lysine, forming, the active enzyme (holoenzyme). Biotin–enzyme, reacts with CO2 in presence of ATP (provides, energy) to form a carboxybiotin–enzyme, complex. This high energy complex hands over, the CO2 to pyruvate (carboxylation reaction) to, produce oxaloacetate (Fig.7.29)., As a coenzyme, biotin is involved in various, metabolic reactions., 1. Gluconeogenesis and citric acid cycle :, The conversion of pyruvate to oxaloacetate, by biotin dependent pyruvate carboxylase, (described above) is essential for the synthesis of, glucose from many non-carbohydrate sources., Oxaloacetate so formed is also required for the, continuous operation of citric acid cycle., 2. Fatty acid synthesis : Acetyl CoA is the, starting material for the synthesis of fatty acids., The very first step in fatty acid synthesis is a, carboxylation reaction., Acetyl CoA, , are dependent on biotin. This was later proved, to be wrong. There are a few carboxylation, reactions which do not require biotin e.g., formation of carbamoyl phosphate in urea cycle,, incorporation of CO2 in purine synthesis.], , Recommended dietary, allowance (RDA), A daily intake of about 100-300 mg is, recommended for adults. In fact, biotin is, normally synthesized by the intestinal bacteria., However, to what extent the synthesized biotin, contributes to the body requirements is not, clearly known., , Dietary sources, Biotin is widely distributed in both animal and, plant foods. The rich sources are liver, kidney,, egg yolk, milk, tomatoes, grains etc., , Deficiency symptoms, The symptoms of biotin deficiency include, anemia, loss of appetite, nausea, dermatitis,, , Biotin-Enz + CO2, ATP, , Biotin, Acetyl CoA carboxylase, , Malonyl CoA, , ADP + Pi, , 3. Propionyl CoA is produced in the metabolism of certain amino acids (valine, isoleucine, threonine etc.) and degradation of odd, chain fatty acids. Its further metabolism is, dependent on biotin., Biotin, , Propionyl CoA, , Propionyl CoA carboxylase, , O, , O, –, , O C N, H, , H, , Biotin-Enz, , O, CH3 C COO–, Pyruvate, , O, , Biotin, , E-Methyl glutaconyl CoA, , [Note : It was once believed that all the, carboxylation reactions in the biological system, , Enz, , Carboxybiotin-enzyme complex, , 4. In the metabolism of leucine, the following, reaction is dependent on biotin., E-Methylcrotonyl, CoA carboxylase, , NH, , (CH2)4 CO, H, , S, , Methylmalonyl CoA, , E-Methylcrotonyl CoA, , Lys, , NH, , –, , O, –, , O C CH2 C COO, Oxaloacetate, , Fig. 7.29 : Role of biotin in the carboxylation reaction,, catalysed by the enzyme, pyruvate carboxylase (Enz-Enzyme).
Page 158 :
148, , BIOCHEMISTRY, , glossitis etc. Biotin deficiency may also, result in depression, hallucinations,, muscle pain and dermatitis., , CH3 OH O, –, , A HO CH2 C, , CH C NH CH2 CH2 COO, , CH3, , E-Alanine, Pantoic acid, Pantothenic acid, , Biotin deficiency is uncommon, since, it is well distributed in foods and also, supplied by the intestinal bacteria. The, deficiency may however, be associated, with the following two causes., , ATP, ADP, 4c-Phosphopantothenate, , 1. Destruction of intestinal flora due, to prolonged use of drugs such as, sulfonamides., , Cysteine, , ATP, ADP, , 2. High consumption of raw eggs. The, raw egg white contains a glycoprotein–, avidin, which tightly binds with biotin, and blocks its absorption from the, intestine. An intake of about 20 raw eggs, per day is needed to produce biotin, deficiency, symptoms, in, humans., Consumption of an occasional raw egg, will not result in deficiency., , 4c-Phosphopantothenyl cysteine, , 4c-Phosphopantetheine, ATP, PPi, Dephospho-coenzyme A, , Antagonists, , Coenzyme A, , Desthiobiotin, biotin sulphonic acid, are biotin antagonists., CH3 OH O H, , PANTOTHENIC ACID, Pantothenic acid (Greek : pantos–, everywhere), formerly known as chick, anti-dermatitis factor (or filtrate factor) is, widely distributed in nature. It’s, metabolic role as coenzyme A is also, widespread., , Pyrophosphate, , B, , Chemistry and synthesis, of coenzyme A, Pantothenic acid consists of two, components, pantoic acid and E-alanine,, held together by a peptide linkage. Synthesis of, coenzyme A from pantothenate occurs in a series, of reactions (Fig.7.30). Pantothenate is first, phosphorylated to which cysteine is added., Decarboxylation, followed by addition of AMP, moiety and a phosphate (each from ATP) results, in coenzyme A. The structure of coenzyme A, , –O, , CH2, , C, , O, , CH3, , P, , O, , P, , C, , N, , H, –O, , C, , N, , CH2, , CH2, , SH, , NH2, , CH2, , O, , CH2, , N, , Pantoic, acid, O, , O H, CH2, , E-Alanine, , O, , O, –, , CH, , N, , Adenine, , O, , N, , H, , H, , O, , OH, , N, , H, P, , O, , Ribose 3-phosphate, , O–, , Coenzyme A, , Fig. 7.30 : (A) Summary of the synthesis of coenzyme A, from pantothenic acid (B) Structure of coenzyme A., , consists of pantothenic acid joined to, E-mercaptoethanolamine (thioethanolamine) at, one end. On the other side, pantothenic acid is, held by a phosphate bridge to adenylic acid. The, adenylic acid is made up of adenine, and a, phosphate linked to carbon-3 of ribose.
Page 159 :
149, , Chapter 7 : VITAMINS, , O, , Coenzyme A may be regarded, of, metabolic integration, since, CH2 C S CoA, O, O, a, central molecule for a, R C S CoA H3C C S CoA CH2 COOH, of, biochemical reactions, as, Succinyl CoA, Acetyl CoA, Acyl CoA, Fig.7.32., Fig. 7.31 : Selected examples of compounds, bound to coenzyme A., , Biochemical functions, The functions of pantothenic acid are exerted, through coenzyme A or CoA (A for acetylation)., Coenzyme A is a central molecule involved in all, the metabolisms (carbohydrate, lipid and, protein). It plays a unique role in integrating, various metabolic pathways. More than 70, enzymes that depend on coenzyme A are known., Coenzyme A has a terminal thiol or sulfhydryl, group ( SH) which is the reactive site, hence, CoA-SH is also used. Acyl groups (free fatty, acids) are linked to coenzyme A by a thioester, bond, to give acyl CoA. When bound to acetyl, unit, it is called acetyl CoA. With succinate,, succinyl CoA is formed. There are many other, compounds bound to coenzyme A., Coenzyme A serves as a carrier of activated, acetyl or acyl groups (as thiol esters). This is, comparable with ATP which is a carrier of, activated phosphoryl groups., A few examples of enzymes involved the, participation of coenzyme A are given below., CoA, , Pyruvate, , Acetyl CoA, , as a coenzyme, acetyl CoA is, wide variety, illustrated in, , Succinyl CoA is also involved in many, reactions, including the synthesis of porphyrins, of heme., Besides the various functions through, coenzyme A, pantothenic acid itself is a, component of fatty acid synthase complex and, is involved in the formation of fatty acids., , Recommended dietary, allowance (RDA), The requirement of pantothenic acid for, humans is not clearly known. A daily intake of, about 5-10 mg is advised for adults., , Dietary sources, Pantothenic acid is one of the most widely, distributed vitamins found in plants and animals., The rich sources are egg, liver, meat, yeast, milk, etc., , Deficiency symptoms, It is a surprise to biochemists that despite the, involvement of pantothenic acid (as coenzyme A), in a great number of metabolic reactions,, , Carbohydrates, , Amino acids, , Fatty acids, , Pyruvate dehydrogenase, , D-Ketoglutarate, , CoA, D-Ketoglutarate dehydrogenase, Succinyl CoA, , Fatty acid, , CoA, , ACETYL CoA, , Acyl CoA, , Thiokinase, , In some of the metabolic reactions, group, transfer is important which occurs in a coenzyme, A bound form., , TCA, cycle, , Acetyl CoA + Choline o� Acetylcholine + CoA, , Energy, , Acetyl CoA + Oxaloacetate o� Citrate + CoA, Succinyl CoA + Acetoacetate o�Acetoacetyl CoA, + Succinate, , Fatty, acids, , Cholesterol, , TriacylVitamin D,, glycerols steroid hormones, , Ketone Detoxibodies fication, , Energy, , Fig. 7.32 : An overview of formation and, utilization of acetyl CoA.
Page 160 :
150, , BIOCHEMISTRY, , its deficiency manifestations have, not been reported in humans. This, may be due to the widespread, distribution of this vitamin or the, symptoms of pantothenic acid may, be similar to other vitamin, deficiencies. Dr. Gopalan, a world, renowned nutritionist from India,, linked the burning feet syndrome, (pain and numbness in the toes,, sleeplessness, fatigue etc.) with, pantothenic acid deficiency., , 8, 1, , N, , O H, 9, , 3, , 5, , 4, , 6, , N, , 10, , –, , CH2 NH, , C N CH COO, CH2, , OH, , CH2, –, , COO, Para amino, benzoic acid, , Pteridine, , Glutamate, , Pteroic acid, Folic acid, 2NADPH + 2H, , H, N, , H2N, N, , N, 8, , 5, , N, H, , +, , 2NADP+, 7, , OH, , Folic acid or folacin (Latin :, folium-leaf) is abundantly found, in green leafy vegetables. It is, important, for, one, carbon, metabolism and is required for the, synthesis of certain amino acids,, purines and the pyrimidine-thymine., , 7, , 2, , Pantothenic acid deficiency in, experimental animals results in, anemia, fatty liver, decreased, steroid synthesis etc., , FOLIC ACID, , N, , N, , H 2N, , 6, , H, H, , Dihydrofolate, reductase, , O H, , CH2 NH, H, , –, , C N CH COO, CH2, , 5,6,7,8-Tetrahydrofolic acid CH, 2, –, , COO, , Fig. 7.33 : Conversion of folic acid to tetrahydrofolic acid (THF)., , Chemistry, Folic acid consists of three components–, pteridine ring, p-amino benzoic acid (PABA) and, glutamic acid (1 to 7 residues). Folic acid mostly, has one glutamic acid residue and is known as, pteroyl-glutamic acid (PGA)., The active form of folic acid is, tetrahydrofolate (THF or FH4). It is synthesized, from folic acid by the enzyme dihydrofolate, reductase. The reducing equivalents are, provided by 2 moles of NADPH. The hydrogen, atoms are present at positions 5, 6, 7 and 8 of, THF (Fig.7.33)., , Absorption, transport and storage, Most of the dietary folic acid found as, polyglutamate with 3-7 glutamate residues (held, by peptide bonds) is not absorbed in the, , intestine. The enzyme folate conjugase present, in duodenum and jejunum splits the glutamate, residues. Only the monoglutamate of folic acid, is absorbed from the intestine. However, inside, the cells, tetrahydrofolates are found as, polyglutamates (with 5-6 amino acid residues), derivatives, which appear to be biologically most, potent. As polyglutamate, folic acid is stored to, some extent in the liver. The body can store, 10-12 mg of folic acid that will usually last for, 2-3 months. In the circulation, N5-methyl, tetrahydrofolate is abundantly present., , Biochemical functions, Tetrahydrofolate (THF or FH4), the coenzyme, of folic acid, is actively involved in the one, carbon metabolism. THF serves as an acceptor, or donor of one carbon units (formyl, methyl, etc.) in a variety of reactions involving amino, acid and nucleotide metabolism.
Page 161 :
151, , Chapter 7 : VITAMINS, , The one carbon units bind with THF at, position N5 or N10 or on both N5 and N10 of, pteroyl structure. The attachment of formyl, ( CHO) at position 5 of THF gives N5-formyl, tetrahydrofolate which is commonly known as, folinic acid or citrovorum factor. The other, commonly found one carbon moieties and their, binding with THF are given below., H, N, , H2N, , N, CH2, , liberation of free THF and for its repeated use in, one carbon metabolism. In B12 deficiency,, conversion of N5-methyl THF to THF is blocked, (more details given under vitamin B12)., , Recommended dietary, allowance (RDA), The daily requirement of folic acid is around, 200 Pg. In the women, higher intakes are, recommended during pregnancy (400 Pg/day), and lactation (300 Pg/day)., , 10, , N, , CH2 N, , 5, , N, , OH, , H, H(R), , THF-1 carbon derivative, , CH (Glu)n, , H(R), , R group (one carbon unit), , N5-Formyl THF, , CHO, , N10-Formyl THF, , CHO, , N5-Formimino, , CH NH, , THF, , N5, N10-Methenyl THF, , CH, , N5,, , CH2, , N10-Methylene, , THF, , N5 – Methyl THF, , Dietary sources, Folic acid is widely distributed in nature. The, rich sources are green leafy vegetables, whole, grains, cereals, liver, kidney, yeast and eggs., Milk is rather a poor source of folic acid., , Deficiency symptoms, , CH3, , The essential functions of THF in one carbon, metabolism are summarized in Fig.7.34., The interrelationship between the various, 1-carbon THF derivatives along with their, involvement in the synthesis of different, compounds is given in Fig.15.32 (Chapter 15)., Many important compounds are synthesized in, one carbon metabolism., , Folic acid deficiency is probably the most, common vitamin deficiency, observed primarily, in the pregnant women, in both developed, (including USA) and developing countries, (including India). The pregnant women, lactating, women, women on oral contraceptives, and, alcoholics are also susceptible to folate, Glycine, serine, histidine etc., , One carbon (1C) moiety, (methyl, formyl etc.), , 1. Purines (carbon 2, 8) which are incorporated into DNA and RNA., 2. Pyrimidine, nucleotide–deoxythymidylic, acid (dTMP), involved in the synthesis of DNA., , One carbon (1C), donors, , THF, , 1C THF, , 3. Glycine, serine, ethanolamine and choline, are produced., 4. N-Formylmethionine, the, protein biosynthesis is formed., , initiator, , of, , Tetrahydrofolate is mostly trapped as, N5-methyl THF in which form it is present in the, circulation. Vitamin B12 is needed for the, conversion of N5-methyl THF to THF, in a, reaction wherein homocysteine is converted to, methionine. This step is essential for the, , One carbon moiety (1C), accepted for the synthesis of, , Amino acids, Purines, Thymidylate Choline, (glycine, serine) (2, 8 carbons), , Fig. 7.34 : An overview of one carbon metabolism, (THF-Tetrahydrofolate).
Page 162 :
152, deficiency. The folic acid deficiency may be due, to (one or more causes) inadequate dietary, intake,, defective, absorption,, use, of, anticonvulsant drugs (phenobarbitone, dilantin,, phenyltoin), and increased demand., In folic acid deficiency, decreased production, of purines and dTMP is observed which impairs, DNA synthesis. Due to a block in DNA, synthesis, the maturation of erythrocytes is, slowed down leading to macrocytic RBC. The, rapidly dividing cells of bone marrow are, seriously affected. The macrocytic anemia, (abnormally large RBC) associated with, megaloblastic changes in bone marrow is a, characteristic feature of folate deficiency., Folic acid deficiency in pregnant women may, cause neural defects in the fetus. Hence high, doses of folic acid are recommended in, pregnancy to prevent birth defects., Folic acid is associated with the metabolism, of histidine. Formiminoglutamate (FIGLU),, formed in histidine metabolism transfers the one, carbon fragment, formimino group ( CH NH), to tetrahydrofolate to produce N5-formimino, THF. In case of folic acid deficiency, FIGLU, accumulates and is excreted in urine. Histidine, load test utilizing the excretion of FIGLU in urine, is used to assess folic acid deficiency., , Folic acid and, hyperhomocysteinemia, Elevated plasma levels of homocysteine are, associated with increased risk of atherosclerosis,, thrombosis and coronary heart disease., Hyperhomocysteinemia is mostly due to, functional, folate, deficiency, caused, by, impairment to form methyl-tetrahydrofolate by, the enzyme methylene tetrahydrofolate reductase, (See Fig.7.39). This results in a failure to convert, homocysteine to methionine. Folic acid, supplementation reduces hyperhomocysteinemia,, and thereby the risk for various health, complications., , Folic acid antagonists, Aminopterin and amethopterin (also called as, methotrexate) are structural analogues of folic, , BIOCHEMISTRY, , acid. They competitively inhibit dihydrofolate, reductase and block the formation of THF. The, biosynthesis of purines, thymine nucleotides and, hence DNA is impaired. This results in the, blockage of cell proliferation. Aminopterin and, methotrexate are successfully used in the, treatment of many cancers, including leukemia., , Trimethoprim (a component of the drug, septran or bactrim) and pyrimethamine, (antimalarial drug) are structurally related to folic, acid. They inhibit dihydrofolate reductase, and, the formation of THF. Trimethoprim is used to, treat bacterial infections of sore throat, urinary, tract, gastrointestinal tract etc., Sulfonamides are structural analogues of, PABA. They competitively inhibit the enzyme, (dihydropteroate synthase) responsible for the, incorporation of PABA into pteridine to produce, folic acid. For this reason, sulfonamides are used, as antibacterial drugs. Sulfonamides, have no, effect on human body, since folic acid is not, synthesized and supplied through the diet., , COBALAMIN (VITAMIN B12), Vitamin B12 is also known as anti-pernicious, anemia vitamin. It is a unique vitamin,, synthesized by only microorganisms and not by, animals and plants. It was the last vitamin to be, discovered., , Chemistry, Vitamin B12 is the only vitamin with a, complex structure. The empirical formula of, vitamin B12 (cyanocobalamin) is C63H90N14, O14PCo. The structure of vitamin B12 consists of, a corrin ring with a central cobalt atom. The, corrin ring is almost similar to the tetrapyrrole, ring structure found in other porphyrin, compounds e.g. heme (with Fe) and chlorophyll, (with Mg)., The corrin ring has four pyrrole units, just like, a porphyrin. Two of the pyrrole units (A and D), are directly bound to each other whereas the, other two (B and C) are held by methene bridges., The groups namely methyl, acetamide and
Page 164 :
154, , BIOCHEMISTRY, , Plasma, , Gastrointestinal tract, , Tissues, Various other, tissues, , Protein, B12, TC I-B12, , TC II-B12, , Stomach, Protein, IF, , B12, IF B12, , Liver, Methyl B12, , Deoxyadenosyl B12, (storage form), , TC II, B12, , Mucosal cell, IF B12, , TC I, , B12, , TC I-B12, (90%), , Methyl B12, , B12, , TC II-B12, (10%), , B12, , Ca2+ dependent, , Fig. 7.37 : Absorption, transport and storage of vitamin B12 (IF-Intrinsic factor; TC-Transcobalamins (TC–I, TC–II)., , around 50,000. Intrinsic factor is resistant to, proteolytic digestive enzymes. IF generally forms, a dimer, binds strongly with 1 or 2 moles of, vitamin B12. This binding protects vitamin B12, against its uptake and use by bacteria., , Methylcobalamin which is in excess is taken up, by the liver, converted to deoxyadenosyl B12 and, stored in this form. It is believed that liver can, store about 4-5 mg, an amount sufficient to meet, the body requirements of B12 for 4-6 years., , The cobalamin–IF complex travels through, the gut. The complex binds to specific receptors, on the surface of the mucosal cells of the ileum., The binding of the complex and entry of B12 into, the mucosal cells is mediated by Ca2+ ions. In, the mucosal cells, B12 is converted to, methylcobalamin (Fig.7.37). It is then transported, in the circulation in a bound form to proteins, namely, transcobalamins, (TC-I,, TC-II)., Methylcobalamin is mostly bound to TC-I (90%), and to a lesser degree to TC-II (10%). It is, believed that TC-I acts as a repository of B12,, while TC-II mediates the tissue uptake of B12., , Biochemical functions, About ten enzymes requiring vitamin B12, have been identified. Most of them are found in, bacteria (glutamate mutase, ribonucleotide, reductase etc.). There are only two reactions in, mammals that are dependent on vitamin B12., 1. Synthesis of methionine from homocysteine : Vitamin B12, as methylcobalamin is, used in this reaction. This is an important, reaction involving N5-methyl tetrahydrofolate, from which tetrahydrofolate is liberated, (enzyme-homocysteine methyltransferase or
Page 165 :
155, , Chapter 7 : VITAMINS, , methionine synthase). This metabolic step, signifies the interrelation between vitamin B12, and folic acid (details given later), , Amino acids, (Val, Ile, Thr, Met), , Propionyl CoA, , Thymine, uracil, , THF, , N5-Methyl THF, Homocysteine, , Odd chain, fatty acids, , O, , Methylcobalamin, , C S CoA, , Methionine, Homocysteine methyltransferase, , H3C C H, –, , 2. Isomerization of methymalonyl CoA to, succinyl CoA : The degradation of odd chain, fatty acids, certain amino acids (valine,, isoleucine etc.) and pyrimidines (thymine and, uracil) produce directly or through the mediation, of propionyl CoA, an important compound, methylmalonyl CoA. This is converted by the, enzyme methylmalonyl CoA mutase to succinyl, CoA in the presence of B12 coenzyme,, deoxyadenosyl cobalamin (Fig.7.38). This, reaction involves hydrogen transfer and, intramolecular rearrangement. In B12 deficiency,, methylmalonyl CoA accumulates and is excreted, in urine as methylmalonic acid., , COO, , Methylmalonyl CoA, , Methylmalonyl CoA, mutase, Methylmalonic, acid, , Excreted in, urine, , 5-Deoxyadenosylcobalamin (of B12), , O, C S CoA, CH2, CH2, –, , COO, , Succinyl CoA, , Recommended dietary, allowance (RDA), A daily intake of about 3 Pg of vitamin B12 is, adequate to meet the adult requirements. For, children, 0.5-1.5 Pg/day is recommended., During pregnancy and lactation, the requirement, is 4 Pg/day., , Dietary sources, Foods of animal origin are the only sources, for vitamin B12. The rich sources are liver,, kidney, milk, curd, eggs, fish, pork and chicken., Curd is a better source than milk, due to the, synthesis of B12 by Lactobacillus., , Vitamin B12 is synthesized only by microorganisms (anaerobic bacteria). Plants cannot, synthesize, hence B12 is never found in plant, foods. Animals obtain B12 either by eating foods,, derived from other animals or from the intestinal, bacterial synthesis., , Deficiency symptoms, The most important disease associated with, vitamin B12 deficiency is pernicious anemia. It is, , Citric acid cycle, , Porphyrins, , Fig. 7.38 : Role of vitamin B12 in isomerization, of methylmalonyl CoA to succinyl CoA, ( -Blockade in B12 deficiency)., , characterized by low hemoglobin levels,, decreased number of erythrocytes and, neurological manifestations. One or more of the, following causes are attributed to the occurrence, of pernicious anemia., 1. Autoimmune destruction of gastric parietal, cells that secrete intrinsic factor. In the absence, of IF, vitamin B12 cannot be absorbed., 2. Hereditary malabsorption of vitamin B12., 3. Partial or total gastrectomy – these individuals become intrinsic factor deficient., 4. Insufficient production of IF and/or gastric, HCl, occasionally seen in older people., 5. Dietary deficiency of B12 is seen among, the strict vegetarians of low socioeconomic, group in the developing countries (India, Srilanka, etc.).
Page 166 :
156, From the foregoing discussion, it is clear that, pernicious anemia is more a disease of the, stomach than due to the deficiency of vitamin B 12., B12 deficiency is also associated with, neuronal degeneration and demyelination of, nervous system. The symptoms include, paresthesia (numbness and tingling) of fingers, and toes. In advanced stages, confusion, loss of, memory and even psychosis may be observed., The neurological symptoms of pernicious anemia, are believed to be due to the accumulation of, methylmalonyl CoA that interferes in myelin, sheath formation in two possible ways., 1. The biosynthesis of fatty acids, required for, myelin formation, is imparied. This is because,, methylmalonyl CoA acts as a competitive, inhibitor of malonyl CoA in fatty acid synthesis., 2. Methylmalonyl CoA can substitute, malonyl CoA in fatty acid synthesis, resulting in, a new type of branched chain fatty acids. These, fatty acids will disrupt the normal membrane, structure., The excretion of methylmalonic acid, (elevated) in urine and estimation of serum B12, level are used to assess B12 deficiency., , Treatment, Vitamin B12 is administered in therapeutic, doses (100-1000 Pg) intramuscularly. Folic acid, administration can also reverse hematological, abnormalities observed in B12 deficiency., However, the neurological symptoms persist., Therefore, a combined supplementation of B12, and folate is employed to treat the patients with, megaloblastic anemias., , INTERRELATION BETWEEN FOLIC, ACID AND VITAMIN B12, —FOLATE TRAP OR METHYL, TRAP HYPOTHESIS, The deficiency of either folate or vitamin B12, results in a similar type of anemia. This suggests, a probable biochemical interrelation between, these two vitamins. There is only one metabolic, reaction known, common to folate and vitamin, B12 (Fig.7.39)., , BIOCHEMISTRY, , In vitamin B12 deficiency, increased folate, levels are observed in plasma. The activity of the, enzyme, homocysteine, methyltransferase, (methionine synthase) is low in B12 deficiency., As a result, the only major pathway for the, conversion of N5-methyl THF to tetrahydrofolate, is blocked and body THF pool is reduced., Essentially, almost the entire body folate, becomes trapped as N5-methyl THF. This is, known as folate trap or methyl trap. In this, manner, B12 deficiency results in decreased, folate coenzymes that leads to reduced, nucleotide and DNA synthesis., Although the tissue folate levels are adequate, or high, there is a functional folate deficiency, due to the lack of THF pool. The outcome is the, development of megaloblastic anemia. Administration of the amino acid methionine has been, shown to partially correct the symptoms of B12, deficiency. This is due to the fact that the, formation of N5-methyl THF is inhibited by, S-adenosylmethionine. A combined therapy of, vitamin B12 and folic acid is generally employed, to treat the patients with megaloblastic anemia., , VITAMIN LIKE COMPOUNDS, Besides the vitamins described above, there, are many other compounds present in foods as, accessory factors. Earlier workers have described, these factors sometime or the other, as essential, to higher animals. However, their essential, nature and requirement in humans has not been, established. Although not essential in the diet,, they perform many important functions in the, body. Selected examples of such substances, which may be regarded as vitamin like, compounds are described here., , CHOLINE, Choline is trimethylhydroxy ethanolamine., CH3, H3C N+ CH2 CH2OH, CH3
Page 167 :
157, , Chapter 7 : VITAMINS, , N5,N10-Methylene THF, , Methyl donor, , Methylene tetrahydrofolate reductase, , Methionine, , Cobalamin, N5-Methyl THF, , One carbon, metabolism, , Block in, B12 deficiency, , THF, , Homocysteine, methyltransferase, , Methylcobalamin, , Homocysteine, , Fig. 7.39 : Interrelationship between folic acid and vitamin B12., , It can be synthesized in the body (from, serine). It is also available from many dietary, sources (e.g. milk, eggs, liver, cereals etc.)., , Biochemical functions, 1. Choline, as a component of phospholipids, (lecithins), is involved in membrane structure, and lipid transport., 2. Choline prevents the accumulation of fat, in liver (as lipotropic factor). It promotes the, synthesis of phospholipids and lipoproteins and, the disposal of triacylglycerols from liver., 3. Due to the presence of three methyl, groups (one carbon fragments), choline is, actively involved in one carbon metabolism., 4. Choline is a precursor for the synthesis of, acetylcholine which is required for transmission, of nerve impulse., , Choline—an essential nutrient?, As such, choline can be synthesized and, reutilized in humans. This may however, be, insufficient to meet the body needs. Some, workers label choline as an essential dietary, nutrient with RDA in the range of 400–500, mg/day., , INOSITOL, Inositol is hexahydroxy-cyclohexane. It is also, known as myo-inositol or meso-inositol., , H, HO, , OH, , OH, , H, , H, , OH, , H, , H, , OH, , OH, H, , Biochemical functions, 1. Inositol is required for the synthesis of, phosphatidylinositol (lipositol) which is a constituent of cell membrane., 2. It acts as a lipotropic factor (along with, choline) and prevents the accumulation of fat in, liver., 3. For some hormones, inositol acts as a, second messenger at the membrane level for the, release of Ca2+ ions., 4. Inositol concentration in the heart muscle, in high, the significance of which however, is, not known., 5. Phytin is hexaphosphate of inositol found, is plants. It prevents the absorption of iron and, calcium from the intestine., , LIPOIC ACID, Lipoic acid (thioctic acid) is a sulfur, containing fatty acid (6,8-dithiooctanoic acid). It, exists in an oxidized and reduced form. Lipoic, acid is fat as well as water soluble.
Page 168 :
158, , BIOCHEMISTRY, , H2C—CH2—CH—(CH2)4COOH, S, , H2C—CH2—CH—(CH2)4 —COOH, , S, , SH, , Lipoic acid, (oxidized), , SH, , Lipoic acid, (reduced), , lipoic acid) is gaining importance. Being fat and, water soluble, it can comfortably reach various, tissues. The therapeutic applications of lipoic acid, are related to its antioxidant property (regarded as, universal antioxidant), some of them are listed, , Biochemical functions, l, , Lipoic acid is involved in the decarboxylation, reactions along with other vitamins (thiamine,, niacin, riboflavin and pantothenic acid). The, conversion of pyruvate to acetyl CoA (by, pyruvate dehydrogenase) and D-ketoglutarate to, succinyl CoA (by D-ketoglutarate dehydrogenase), requires lipoic acid., , l, , l, , Therapeutic uses of lipoic acid, In recent years, administration of high doses, (100–600 mg/day) of lipoic acid (or dihydro-, , l, , Reduces the free radicals in brain that, otherwise contribute to Alzheimer’s disease, and multiple sclerosis., Lipoic acid stimulates production of, glutathione (GSH), besides helping in the, recycle of vitamins E and C., Reduces insulin resistance, and brings down, plasma low density lipoproteins., May be useful in the prevention of stroke and, myocardial infarction., , + Distinct deficiency conditions of certain B-complex vitamins are known, Thiamine — Beri-beri, , Riboflavin — Cheilosis, glossitis, , Niacin, , Pyridoxine — Peripheral neuropathy, , — Pellagra, , Folic acid — Macrocytic anemia, , Cobalamin — Pernicious anemia, , + B-complex vitamin deficiencies are usually multiple rather than individual with, overlapping symptoms., , + A combined therapy of vitamin B12 and folic acid is commonly employed to treat the, patients of megaloblastic anemias., , + Megadoses of niacin are useful in the treatment of hyperlipidemia., + Long term use of isoniazid for the treatment of tuberculosis causes B6 deficiency., + Folic acid supplementation reduces elevated plasma homocysteine level which is, associated with atherosclerosis and thrombosis., , + Sulfonamides serve as antibacterial drugs by inhibiting the incorporation of PABA to, produce folic acid., , + Aminopterin and amethopterin, the structural analogues of folic acid, are employed in, the treatment of cancers., , + Lipoic acid is therapeutically useful as an antioxidant to prevent stroke, myocardial, infarction, etc.
Page 169 :
159, , Chapter 7 : VITAMINS, , PARA AMINOBENZOIC ACID, Para aminobenzoic acid (PABA) is a structural, constituent of folic acid. PABA may be regarded, as a vitamin in another vitamin (folic acid), COOH, , NH2, PABA, , SO2NH 2, , NH2, Sulfonilamide, , The deficiency of PABA was first found to be, associated with failure of lactation and greying, of black hair in rats. The specific functions of, PABA in humans, except that it is a component, of folic acid, have not been identified., PABA is synthesized by the bacteria and is, essential for their growth. The sulfa drug, sulfonilamide (p-amino benzene sulfanilamide) is, a structural analogue of PABA. Sulfonilamide, competes with PABA and acts as a bacteriostatic, agent. Ingestion of large doses of PABA, will compete with the action of drugs and, therefore should be avoided during sulfonilamide, therapy (trade name—sulfonamides)., , TABLE 7.4 Selected list of antivitamins/vitamin, antagonists along with corresponding vitamins, , Antivitamin/vitamin antgonists, , Vitamin, , Dicumarol, Warfarin, , Vitamin K, , Thiaminase, Pyrithiamine, Oxythiamine, , Thiamine, , Galactoflavin, , Riboflavin, , Isoniazid, Deoxypyridoxine, , Niacin, , Avidin, Desthiobiotin, , Biotin, , Aminopterin, Methotrexate, Trimethoprim, , Folic acid, , Sulfonilamide, , Para-aminobenzoic, acid, , Bioflavonoids act as antioxidants and protect, ascorbic acid from being destroyed. It is, suggested that this antioxidant property may be, responsible for maintenance of capillary, permeability. Bioflavonoids have been used to, correct the vascular abnormality in humans., Bioflavonoids are found in peel and pulp of, citrus fruits, tobacco leaves and many, vegetables. The requirement of these compounds, in humans has not been established., , BIOFLAVONOIDS, Szent-Gyorgi and his associates (1936), observed that flavonoids, isolated from lemon, peel (known as citrin) were responsible for, maintenance of normal capillary permeability., The term vitamin P (P for permeability) was used, to this group of substances. However, they are, commonly known as bioflavonoids., , ANTIVITAMINS, Antivitamins are antagonistic to (oppose and, block) the action of vitamins. They usually have, structural similarities with vitamins. Administration, of antivitamins causes vitamin deficiencies. The, common antivitamins are discussed as antagonists, for each vitamin, and are given in Table 7.4.
Page 170 :
160, , BIOCHEMISTRY, , 1. Vitamins are accessory food factors required in the diet. They are classified as fat, soluble (A, D, E and K) and water soluble (B-complex and C)., 2. Vitamin A is involved in vision, proper growth, differentiation and maintenance of, epithelial cells. Its deficiency results in night blindness., 3. The active form of vitamin D is calcitriol which functions like a steroid hormone and, regulates plasma levels of calcium and phosphate. Vitamin D deficiency leads to rickets, in children and osteomalacia in adults., 4. Vitamin E is a natural antioxidant necessary for normal reproduction in many animals., 5. Vitamin K has a specific coenzyme function. It catalyses the carboxylation of glutamic, acid residues in blood clotting factors (II, VII, IX and X) and converts them to active, form., 6. Thiamine (B1), as a cocarboxylase (TPP) is involved in energy releasing reactions. Its, deficiency leads to beri-beri., 7. The coenzymes of riboflavin (FAD and FMN) and niacin (NAD+ and NADP+) take part, in a variety of oxidation-reduction reactions connected with energy generation., Riboflavin deficiency results in cheilosis and glossitis whereas niacin deficiency leads to, pellagra., 8. Pyridoxal phosphate (PLP), the coenzyme of vitamin B6, is mostly associated with, amino acid metabolism. PLP participates in transamination, decarboxylation,, deamination and condensation reactions., 9. Biotin (anti-egg white injury factor) participates as a coenzyme in carboxylation, reactions of gluconeogenesis, fatty acid synthesis etc., 10. Coenzyme A (of pantothenic acid) is involved in the metabolism of carbohydrates, lipids, and amino acids, and their integration., 11. Tetrahydrofolate (THF), the coenzyme of folic acid participates in the transfer of one, carbon units (formyl, methyl etc.) in amino acid and nucleotide metabolism., Megaloblastic anemia is caused by folic acid deficiency., 12. Vitamin B12 has two coenzymes, deoxyadenosylcobalamin and methylcobalamin. B12, deficiency results in pernicious anemia., 13. Vitamin C (ascorbic acid) is involved in the hydroxylation of proline and lysine in the, formation of collagen. Scurvy is caused by ascorbic acid deficiency. Therapeutic use of, megadoses of vitamin C, to cure everything from common cold to cancer, has become, controversial., 14. Certain vitamin like compounds (choline, inositol, PABA, lipoic acid) participate in, many biochemical reactions.
Page 171 :
161, , Chapter 7 : VITAMINS, , I. Essay questions, 1. Classify vitamins and briefly discuss their functions and deficiency disorders., 2. Describe the chemistry, biochemical functions, daily requirements, sources and deficiency, manifestations of vitamin A., 3. Write an account of folic acid involvement in one carbon metabolism., 4. Discuss the biochemical functions of vitamin C. Add a note on the therapeutic use of megadoses, of this vitamin., 5. Write briefly about the coenzymes involved in oxidation-reduction reactions., , II. Short notes, (a) Vitamin D is a hormone-justify, (b) Thiamine pyrophosphate, (c) Coenzymes of niacin,, (d) Pyridoxal phosphate in transamination, (e) Folate trap, (f) Tocopherol, (g) Vitamin K in, carboxylation, (h) Biocytin, (i) Choline, (j) Pernicious anemia., , III. Fill in the blanks, 1. The A in coenzyme A stands for_____________________., 2. The vitamin containing isoalloxazine ring_____________________., 3. The vitamin that is regarded as a vitamin in search of a disease_____________________., 4. Anti-tuberculosis drug, isonicotinic acid hydrazide (INH) leads to the deficiency of, vitamin_____________________., 5. The egg injury factor present in raw egg white_____________________., 6. The ‘burning feet syndrome’ in man is associated with the deficiency of_____________________., 7. The vitamin that is synthesized by only microorganisms_____________________., 8. The three Ds in pellagra stand for, _____________, ____________ and ____________., 9. The fat soluble vitamin required for carboxylation reaction_____________________., 10. FIGLU (formimino glutamic acid), vitamin_____________________., , is, , excreted, , in, , urine, , in, , IV. Multiple choice questions, 11. Which one of the vitamin A functions as a steroid hormone, (a) Retinal (b) Retinol (c) Provitamin A (d) E-Carotene., 12. The functionally active form of vitamin D is, (a) Cholecalciferol (b) Ergocalciferol (c) Dehydrocholesterol (d) Calcitriol., 13. The metabolite excreted in urine in thiamine deficiency, (a) Pyruvate (b) Glucose (c) Xanthurenic acid (d) FIGLU., 14. The coenzyme directly concerned with the synthesis of biogenic amines, (a) TPP (b) NADP+ (c) Biotin (d) Pyridoxal phosphate., 15. Folic acid antagonist(s) used in the treatment of cancer, (a) Methotrexate (b) Trimethoprim (c) Sulfonamide (d) All the three., , the, , deficiency, , of
Page 172 :
“This page intentionally left blank"
Page 173 :
PHYSIOLOGICAL, OGICAL BIOCHEMISTRY, PHYSIOL, 8, ■, 9, ■, 10, ■, 11, ■, , Digestion and Absorption, , 165, , Plasma Proteins, , 182, , Hemoglobin and Porphyrins, , 196, , Biological Oxidation, , 221, , Section, , IIII
Page 174 :
“This page intentionally left blank"
Page 175 :
Section 2, , Physiological Biochemistry, , Chapter, , Digestion and Absorption, , 8, , The natural foodstuffs speak :, , “Complex is the ingested food,, But digested to simpler products,, Absorbed by intestinal mucosal cells,, Assimilated and utilized by all cells.”, , F, , ood is the basic and essential requirement of, man for his very existence. The food we eat, consists of carbohydrates, proteins, lipids,, vitamins and minerals. The bulk of the food, ingested is mostly in a complex macromolecular, form which cannot, as such, be absorbed by the, body., , TABLE 8.1, Organs of gastrointestinal tract with their, major functions in digestion and absorption, Organ, , Major function(s), , Mouth, , Production of saliva containing D-amylase;, partial digestion of polysaccharides, , Digestion is a process involving the, hydrolysis of large and complex organic, molecules of foodstuffs into smaller and, preferably water-soluble molecules which can, be easily absorbed by the gastrointestinal tract, for utilization by the organism. Digestion of, macromolecules also promotes the absorption of, fat soluble vitamins and certain minerals., , Stomach, , Elaboration of gastric juice with HCl and, proteases; partial digestion of proteins, , Pancreas, , Release of NaHCO3 and many enzymes, required for intestinal digestion, , Liver, , Synthesis of bile acids, , Gall bladder, , Storage of bile, , Small intestine, , Final digestion of foodstuffs; absorption of, digested products, , Cooking of the food, and mastication (in the, mouth) significantly improve the digestibility of, foodstuffs by the enzymes., , Large intestine, , Mostly absorption of electrolytes; bacterial, utilization of certain non-digested and/or, unabsorbed foods, , Gastrointestinal tract, Digestion as well as absorption are, complicated processes that occur in the gastrointestinal tract (GIT) involving many organs. The, , diagrammatic representation of GIT is depicted, in Fig.8.1, and the essential organs with, their respective major functions are given in, Table 8.1. The digestive organs possess a large, , 165
Page 176 :
166, , BIOCHEMISTRY, , saccharides (glucose, fructose). The structures of, carbohydrates are described in Chapter 2., , Digestion, , Mouth, Oesophagus, , Liver, Stomach, Pyloric, sphincter, , Gall bladder, Duodenum, (~ 0.25 m), , Pancreas, , Jejunum, (~ 2.3 m), Small, intestine, Ileum, (~ 4.6 m), , Colon, (~ 1.8 m), , Large, intestine, , Rectum, , Fig. 8.1 : Diagrammatic representation, of gastrointestinal tract., , reserve capacity. For instance, pancreas secretes, enzymes 5-10 fold higher than required for, digestion of foods normally ingested., , The digestion of carbohydrates occurs briefly, in mouth and largely in the intestine. The, polysaccharides get hydrated during heating, which is essential for their efficient digestion., The hydrolysis of glycosidic bonds is carried out, by a group of enzymes called glycosidases, (Fig.8.2). These enzymes are specific to the, bond, structure and configuration of monosaccharide units., Digestion in the mouth : Carbohydrates are, the only nutrients for which the digestion begins, in the mouth to a significant extent. During the, process of mastication, salivary D-amylase, (ptyalin) acts on starch randomly and cleaves D1,4-glycosidic bonds. The products formed, include D-limit dextrins, (containing about 8, glucose units with one or more D-1,6-glycosidic, bonds) maltotriose and maltose., Carbohydrates not digested in the stomach :, The enzyme salivary amylase is inactivated by, high acidity (low pH) in the stomach. Consequently,, the ongoing degradation of starch is stopped., Digestion in the small intestine : The acidic, dietary contents of the stomach, on reaching, small intestine, are neutralized by bicarbonate, produced by pancreas. The pancreatic, D-amylase acts on starch and continues the, digestion process. Amylase specifically acts on, D-1,4-glycosidic bonds and not on D-1,6-bonds., , O, , The digestion and absorption of individual, foods, namely carbohydrates, proteins, lipids and, nucleic acids, is described here. The gastrointestinal, hormones are discussed under hormones, (Chapter 19)., , The principal dietary carbohydrates are, polysaccharides (starch, glycogen), disaccharides, (lactose, sucrose) and, to a minor extent, mono-, , H, , O, , O, H2 O, O, , CARBOHYDRATES, , H, , H, OH, , Glycosidase, , H, , O, , HO, , Fig. 8.2 : Hydrolysis of a glycosidic bond.
Page 177 :
167, , Chapter 8 : DIGESTION AND ABSORPTION, D�(1-4), , D�(1-6), , Amylopectin, D-Amylase, , Maltose, , Isomaltose, , Maltotriose, , Oligosaccharides, , Fig. 8.3 : Degradation of amylopectin by salivary or pancreatic D-amylase., , The resultant products are disaccharides, (maltose, isomaltose) and oligosaccharides, (Fig.8.3)., The final digestion of di- and oligosaccharides to monosaccharides (Fig.8.4), primarily occurs at the mucosal lining, of the upper jejunum. This is carried out, by oligosaccharidases (e.g. glucoamylase, acting on amylose) and disaccharidases, (e.g. maltase, sucrase, lactase). The enzyme, sucrase is capable of hydrolysing a, large quantity of table sugar (sucrose). In, , contrast, lactase (E-galactosidase) is the ratelimiting, and, consequently, the utilization of, milk sugar (lactose) is limited in humans., , Absorption of monosaccharides, The principal monosaccharides produced by, the digestion of carbohydrates are glucose,, fructose and galactose. Of these, glucose, accounts for nearly 80% of the total, monosaccharides. The absorption of sugars, mostly takes place in the duodenum and upper, jejunum of small intestine., , Mouth, Starch, Salivary, D-amylase, Dextrins, Isomaltose, Maltose, Lactose, Sucrose, , Small intestine, , Stomach, Low pH stops, amylase activity, , Pancreatic D-amylase,, D-Glucoamylase, Isomaltase, Maltase, Lactase, Sucrase, , Glucose, Glucose, Fructose, Galactose, , Cellulose, (not-digested), , Cellulose, , Excreted, , Fig. 8.4 : Overview of digestion of carbohydrates.
Page 178 :
168, , BIOCHEMISTRY, , There exists a considerable, variation in the absorption of, different monosaccharides. The, relative rates of absorption of, important monosaccharides in, comparison with glucose are given, below, Glucose, Galactose, Fructose, Mannose, Xylose, Arabinose, , —, —, —, —, —, —, , Capillaries, , Intestinal mucosal cell, Carrier, Glucose, , Glucose, , Glucose, , Na+, Na+, , 100, 110, 43, 20, 15, 9, , It is observed that hexoses are, more rapidly absorbed than, pentoses. Further, among the, monosaccharides, galactose is most, efficiently absorbed followed by, glucose and fructose. Insulin has no, effect on the absorption of sugars., , Membrane, , Intestinal, lumen, , ATP, , K+, , ADP + Pi, Na+-K+ ATPase, , Na+, , K+, , Fig. 8.5 : Transport of glucose across intestinal epithelium, (Note : Transport of amino acids also occurs by a similar, mechanism; replace glucose in figure by amino acid)., , Mechanism of absorption, Different sugars possess different mechanisms, for their absorption. Glucose is transported into, the intestinal mucosal cells by a carrier mediated, and energy requiring process (Fig.8.5)., , Glucose and Na+ share the same transport, system (symport) which is referred to as sodiumdependent glucose transporter. The concentration of Na+ is higher in the intestinal lumen, compared to mucosal cells. Na+, therefore,, moves into the cells along its concentration, gradient and simultaneously glucose is, transported into the intestinal cells. This is, mediated by the same carrier system. Thus, Na+, diffuses into the cell and it drags glucose along, with it. The intestinal Na+ gradient is the, immediate energy source for glucose transport., This energy is indirectly supplied by ATP since, the reentry of Na+ (against the concentration, gradient) into the intestinal lumen is an energyrequiring active process. The enzyme Na +-K+, ATPase is involved in the transport of Na+ in, exchange of K+ against the concentration, gradient (for details see Chapter 33)., Oral rehydration therapy (ORT) : ORT is the, most common treatment of diarrhea. The oral, , rehydration fluid contains glucose and sodium., Intestinal absorption of sodium is facilitated by, the presence of glucose., The mechanism of absorption of galactose is, similar to that of glucose. The inhibitor phlorizin, blocks the Na+ dependent transport of glucose, and galactose., Absorption of fructose : Fructose absorption, is relatively simple. It does not require energy, and is independent of Na+ transport. Fructose is, transported by facilitated diffusion mediated by a, carrier. Inside the epithelial cell, most of the, fructose is converted to glucose. The latter then, enters the circulation., , Pentoses are absorbed by a process of simple, diffusion., , Non-digestible carbohydrates, The plant foods are rich in fibrous material, which cannot be digested either by the human, enzymes or intestinal bacteria. The fibers are, chemically complex carbohydrates which, include cellulose, hemicellulose, pectins, lignin, and gums. Fiber in nutrition is of special, importance which is described under nutrition, (Chapter 23).
Page 179 :
169, , Chapter 8 : DIGESTION AND ABSORPTION, , Abnormalities of, carbohydrate digestion, In general, humans possess an efficient system, of carbohydrate digestion and absorption. Since, only the monosaccharides are absorbed, any, defect in the activities of disaccharidases results in, the passage of undigested disaccharides into, the large intestine. The disaccharides draw, water from intestinal mucosa by osmosis and, cause diarrhea. Further, bacterial action of these, undigested carbohydrates leads to flatulence., Disaccharidases are the intestinal brush, border enzymes. Any alteration in the mucosa of, the small intestine caused by severe diarrhea,, malnutrition, intestinal diseases or drug therapy, will lead to a temporary acquired deficiency of, disaccharidases. The patients with such disorders, are advised to restrict the consumption of, sucrose and lactose., Hereditary disorders with deficiency of, individual disaccharidases in infants and children, cause intolerance of specific disaccharides., , Lactose intolerance, Lactose intolerance is the most common, disorder of carbohydrate digestion in humans., This is due to a defect in the enzyme lactase, (E-galactosidase). It is estimated that more than, half of the world’s adult population is affected, by lactose intolerance. It is more commonly, found in Africans (blacks) and Asians compared, to Europeans., Continued consumption of lactose by lactose, intolerant individuals causes typical symptoms, of flatulence (described later)., Lactose intolerance may be primary, (congenital) or secondary (acquired). Acquired, lactose intolerance may occur due to a sudden, and high intake of milk-based diets. Lactase is an, inducible enzyme. Therefore in acquired, intolerance, if milk is withdrawn temporarily,, diarrhea will be limited. For lactose intolerant, people, consumption of curd is benefical,, since lactobacilli present in curd contain the, enzyme lactase. Further, yeast rich in lactase,, can also be used for treatment of lactose, intolerance., , The best treatment for lactose intolerance is, elimination of lactose from the diet (i.e. severe, restriction of milk and dairy products)., , Sucrase deficiency, The deficiency of the enzyme sucrase causes, intolerance to dietary sucrose. It is estimated that, about 10% of Eskimos of Greenland and 2%, of North Americans are affected by this, disorder. The treatment is to remove sucrose, from the diet., , The problem of flatulence, Flatulence is characterized by increased, intestinal motility, cramps and irritation. This, occurs after ingestion of certain carbohydrates, and is explained as follows., The carbohydrates (di-, oligo-, and polysaccharides) not hydrolysed by D-amylase and, other intestinal enzymes cannot be absorbed., Lactose is not hydrolysed in some individuals, due to the deficiency of lactase. The di-, and, oligosaccharides can be degraded by the, bacteria present in ileum (lower part of small, intestine) to liberate monosaccharides. The latter, can be metabolized by the bacteria., As the monosaccharides are utilized by the, intestinal bacteria, gases such as hydrogen,, methane and carbon dioxide—besides lactate, and short chain fatty acids—are released. These, compounds cause flatulence., The occurrence of flatulence after the ingestion, of leguminous seeds (bengal gram, redgram,, beans, peas, soya bean) is very common. They, contain several nondigestible oligosaccharides by, human intestinal enzymes. These compounds are, degraded and utilised by intestinal bacteria, causing, flatulence., Raffinose, containing, galactose, glucose and fructose is a predominant, oligosaccharide found in leguminous seeds., , PROTEINS, The proteins subjected to digestion and, absorption are obtained from two sources—, dietary and endogenous.
Page 180 :
170, The intake of dietary protein is in the range, of 50-100 g/day. About 30-100 g/day of, endogenous protein is derived form the digestive, enzymes and worn out cells of the digestive, tract. The digestion and absorption of proteins is, very efficient in healthy humans, hence very little, protein (about 5-10 g/day) is lost through feces., Dietary proteins are denatured on cooking and, therefore, easily digested., Proteins are degraded by a class of enzymes—, namely hydrolases—which specifically cleave, the peptide bonds, hence known as peptidases., They are divided into two groups, 1. Endopeptidases (proteases) which attack, the internal peptide bonds and release peptide, fragments, e.g. pepsin, trypsin., 2. Exopeptidases which act on the peptide, bonds of terminal amino acids. Exopeptidases, are subdivided into carboxypeptidases (act on, C-terminal amino acid) and aminopeptidases (act, on N-terminal amino acid)., The proteolytic enzymes responsible for the, digestion of proteins are produced by the, stomach, the pancreas and the small intestine., Proteins are not digested in the mouth due to the, absence of proteases in saliva., , I. Digestion of proteins, by gastric secretion, Protein digestion begins in the stomach., Gastric juice produced by stomach contains, hydrochloric acid and a protease proenzyme, namely pepsinogen., Hydrochloric acid : The pH of the stomach is, < 2 due to the presence of HCl, secreted by, parietal (oxyntic) cells of gastric gland. This acid, performs two important functions-denaturation of, proteins and killing of certain microorganisms., The denatured proteins are more susceptible to, proteases for digestion., Pepsin : Pepsin (Greek : pepsis—digestion) is, produced by the serous cells of the stomach as, pepsinogen, the inactive zymogen or proenzyme., Pepsinogen is converted to active pepsin either, by autocatalysis, brought about by other pepsin, molecules or by gastric HCl (pH < 2). Removal of, a fragment of polypeptide chain (44 amino acids, in case of pig enzyme) makes the inactive enzyme, active after attaining a proper conformation., , BIOCHEMISTRY, , Pepsin is an acid-stable endopeptidase, optimally active at a very low pH (2.0). The, active site of the enzyme contains 2 carboxyl, groups, which are maintained at low pH. Pepsin, A is the most predominant gastric protease which, preferentially cleaves peptide bonds formed by, amino groups of phenylalanine or tyrosine or, leucine., Pepsin digestion of proteins results in peptides, and a few amino acids which act as stimulants, for the release of the hormone cholecystokinin, from the duodenum., Rennin : This enzyme, also called chymosin,, is found in the stomach of infants and children., Rennin is involved in the curdling of milk. It, converts milk protein casein to calcium, paracaseinate which can be effectively digested, by pepsin. Rennin is absent in adults., , II. Digestion of proteins, by pancreatic proteases, The proteases of pancreatic juice are secreted, as zymogens (proenzymes) and then converted, to active forms. These processes are initiated, by the release of two polypeptide hormones,, namely cholecystokinin and secretin from the, intestine (Fig.8.6)., , Peptides, Amino acids, , CCK, , Intestine, CCK, secretin, Pancreas, , Trypsinogen, Enteropeptidase, Trypsin, Chymotrypsinogen, Proelastase, Procarboxypeptidases, (A and B), , Chymotrypsin, Elastase, Carboxypeptidases, (A and B), , Fig. 8.6 : Formation and activation of pancreatic, proteases (CCK-Cholecystokinin).
Page 181 :
171, , Chapter 8 : DIGESTION AND ABSORPTION, , Rn, , R1, , R, , –, , Protein…CO NH CH CO NH CH CO NH…………CO NH CH COO, , Enzyme, , Nature of Rn, , Enzyme, , Nature of R, , Pepsin A, , Tyr, Phe, Leu, , Carboxypeptidase A, , Ala, Ile, Leu, Val, , Trypsin, , Arg, Lys, , Carboxypeptidase B, , Arg, Lys, , Chymotrypsin, , Trp, Tyr, Phe,, Leu, Met, , Elastase, , Ala, Gly, Ser, , Fig. 8.7 : Digestion of proteins—Specificity of enzyme cleavage of peptide bonds. ( R1 can be from any amino acid), , Release and activation of zymogens : The key, enzyme for activation of zymogen is enteropeptidase (formerly enterokinase) produced by, intestinal (mostly duodenal) mucosal epithelial, cells. Enteropeptidase cleaves off a hexapeptide, (6 amino acid fragment) from the N-terminal end, of trypsinogen to produce trypsin, the active, enzyme. Trypsin, in turn, activates other, trypsinogen molecules (autocatalysis). Further,, trypsin is the common activator of all other, pancreatic zymogens to produce the active, proteases, namely chymotrypsin, elastase and, carboxypeptidases (A and B)., Specificity and action of pancreatic proteases :, Trypsin, chymotrypsin and elastase are, endopeptidases active at neutral pH. Gastric HCl, is neutralized by pancreatic NaHCO3 in the, intestine and this creates favourable pH for the, action of proteases., The substrate specificity of pancreatic, proteases is depicted in Fig.8.7. For instance,, trypsin cleaves the peptide bonds, the carbonyl, ( CO ) group of which is contributed by, arginine or lysine., The amino acid serine is essential at the active, centre to bring about the catalysis of all the three, pancreatic proteases, hence these enzymes are, referred to as serine proteases., Action of carboxypeptidases : The pancreatic, carboxypeptidases (A and B) are metalloenzymes, that are dependent on Zn2+ for their catalytic, activity, hence they are sometimes called, , Zn-proteases. They also possess certain degree, of substrate specificity in their action. For, example, carboxypeptidase B acts on peptide, bonds of COOH-terminal amino acid, the amino, group of which is contributed by arginine or, lysine (Fig.8.7)., The combined action of pancreatic proteases, results in the formation of free amino acids and, small peptides (2-8 amino acids)., , III. Digestion of proteins, by small intestinal enzymes, The luminal surface of intestinal epithelial, cells contains aminopeptidases and dipeptidases., Aminopeptidase is a non-specific exopeptidase, which repeatedly cleaves N-terminal amino, acids one by one to produce free amino acids, and smaller peptides. The dipeptidases act on, different dipeptides to liberate amino acids, (Fig.8.8)., , Absorption of amino, acids and dipeptides, The free amino acids, dipeptides and to some, extent tripeptides are absorbed by intestinal, epithelial cells., The di- and tripeptides, after being absorbed, are hydrolysed into free amino acids in the, cytosol of epithelial cells. The activities of, dipeptidases are high in these cells. Therefore,, after a protein meal, only the free amino acids, are found in the portal vein.
Page 182 :
172, , BIOCHEMISTRY, , Mouth, , Small Intestine, Stomach, Pepsin, , Proteins, , Polypeptides, Amino acids, , Trypsin, Chymotrypsin, Elastase, , Unchanged, , Amino acids, Oligopeptides, , Carboxypeptidases, Aminopeptidases, Dipeptidases, Amino acids, Dipeptides, , Fig. 8.8 : Overview of digestion of proteins., , The small intestine possesses an efficient, system to absorb free amino acids. L-Amino acids, are more rapidly absorbed than D-amino acids., The transport of L-amino acids occurs by an, active process (against a concentration gradient),, in contrast to D-amino acids which takes place, by a simple diffusion., , Mechanism of amino acid, absorption, Amino acids are primarily absorbed by a, similar mechanism, as described for the transport, of D-glucose. It is basically a Na+-dependent, active process linked with the transport of Na+., As the Na+ diffuses along the concentration, gradient, the amino acid also enters the intestinal, cell. Both Na+ and amino acids share a common, carrier and are transported together. The energy, is supplied indirectly by ATP (for details, see, absorption of monosaccharides and Fig.8.5)., A Na+-independent system of amino acid, transport across intestinal cells has also been, identified. The compound cytochalasin B inhibits, Na+-independent transport system., Another transport system to explain the, mechanism of amino acid transfer across, membrane in the intestine and kidney has been, put forth. This is known as J-glutamyl cycle, or Meister cycle and involves a tripeptide, namely glutathione (J-glutamylcysteinylglycine)., , Three ATP are utilized for the transport of a, single amino acid by this cycle. For this reason,, Meister cycle is not a common transport, system for amino acid. However, this cycle is, operative for rapid transport of cysteine and, glutamine., The J-glutamyl cycle appears to be important, for the metabolism of glutathione, since this, tripeptide undergoes rapid turnover in the cells., There may be more physiological significance of, J-glutamyl cycle., , Absorption of intact proteins, and polypeptides, For a short period, immediately after birth, the, small intestine of infants can absorb intact, proteins and polypeptides. The uptake of proteins, occurs by a process known as endocytosis or, pinocytosis. The macromolecules are ingested by, formation of small vesicles of plasma membrane, followed by their internalization. The direct, absorption of intact proteins is very important, for the transfer of maternal immunoglobulins, (J-globulins) to the offspring., The intact proteins and polypeptides are not, absorbed by the adult intestine. However, the, macromolecular absorption in certain individuals, appears to be responsible for antibody formation, that often causes food allergy .
Page 183 :
173, , Chapter 8 : DIGESTION AND ABSORPTION, , Abnormalities of protein digestion, and amino acid absorption, , Minor digestion of, lipids in the stomach, , Any defect in the pancreatic secretion impairs, protein and fat digestion. This causes the loss of, undigested protein in the feces along with the, abnormal appearance of lipids. Deficiency of, pancreatic secretion may be due to pancreatitis,, cystic fibrosis or surgical removal of pancreas., , The digestion of lipids is initiated in the, stomach, catalysed by acid-stable lipase. This, enzyme (also called lingual lipase) is believed to, originate from the glands at the back of tongue., Stomach contains a separate gastric lipase which, can degrade fat containing short chain fatty acids, at neutral pH. The digestion of lipids in the, stomach of an adult is almost negligible, since, lipids are not emulsified and made ready for, lipase action. Further, the low pH in the stomach, is unfavourable for the action of gastric lipase., , Hartnup’s disease, (neutral amino aciduria), Hartnup is the name of the family in whom, this disease was first discovered. It is, characterized by the inability of intestinal and, renal epithelial cells to absorb neutral amino, acids (tryptophan, alanine, serine, threonine,, valine). Tryptophan absorption is most severely, affected with a result that typical symptoms of, pellagra are observed in the patients of Hartnup’s, disease. This is related to the impairment in the, conversion of tryptophan to NAD+ and NADP+,, the coenzymes of niacin. The treatment, includes high protein diet and nicotinamide, supplementation., , LIPIDS, There is considerable variation in the daily, consumption of lipids which mostly depends on, the economic status and dietary habits. The, intake of lipids is much less (often < 60 g/day) in, poorer sections of the society, particularly in the, less developed countries. In the developed, countries, an adult ingests about 60-150 g of, lipids per day. Of this, more than 90% is fat, (triacylglycerol). The rest of the dietary lipid is, made up of phospholipids, cholesterol,, cholesteryl esters and free fatty acids., Lipids are insoluble or sparingly soluble in, aqueous solution. The digestive enzymes,, however, are present in aqueous medium. This, poses certain problems for the digestion and, absorption of lipids. Fortunately, the digestive, tract possesses specialized machinery to, 1. Increase the surface area of lipids for, digestion;, 2. Solubilize, absorption., , the, , digested, , products, , for, , In case of infants, the milk fat (with short, chain fatty acids) can be hydrolysed by gastric, lipase to some extent. This is because the, stomach pH of infants is close to neutrality, ideal, for gastric lipase action., , Emulsification of lipids, in the small intestine, Emulsification is the phenomenon of, dispersion of lipids into smaller droplets due to, reduction in the surface tension. This is, accompanied by increase in the surface area of, lipid droplets. Emulsification is essential for, effective digestion of lipids, since the enzymes, can act only on the surface of lipid droplets., More correctly, lipases act at the interfacial area, between the aqueous and lipid phase., The process of emulsification occurs by three, complementary mechanisms, 1. Detergent action of bile salts;, 2. Surfactant action of degraded lipids;, 3. Mechanical mixing due to peristalsis., 1. Bile salts : The terms bile salts and bile, acids are often used interchangeably. At, physiological pH, the bile acids are mostly, present as anions. Bile salts are the biological, detergents synthesized from cholesterol in the, liver. They are secreted with bile into the, duodenum. Bile salts possess steroid nucleus, the, side chain of which is attached to either glycine, (glycocholic acid) or taurine (taurocholic acid)., For the synthesis and other details on bile acids,, refer cholesterol metabolism (Chapter 14). Bile, salts are the most effective biological emulsifying
Page 184 :
174, , BIOCHEMISTRY, , O, O, , CH2 O C R1, , R2 C O CH, , Pancreatic lipase, , O, , CH2 O C R3, , 2 H2O, , Triacylglycerol, , O, , CH2 OH, , R2 C O CH, , +, , CH2 OH, 2-Monoacylglycerol, , R1COOH, R3COOH, Free fatty acids, , Fig. 8.9 : Enzymatic cleavage of dietary fat., , agents. They interact with lipid particles and the, aqueous duodenal contents and convert them, into smaller particles (emulsified droplets)., Further, bile salts stabilize the smaller particles, by preventing them from coalescing., 2. Surfactant action of degraded lipids : The, initial digestive products of lipids (catalysed by, lipase) namely free fatty acids, monoacylglycerols promote emulsification. These, compounds along with phospholipids are known, as surfactants. They are characterized by, possessing polar and non-polar groups., Surfactants get absorbed to the water-lipid, interfaces and increase the interfacial area of, lipid droplets. Thus the initial action of the, enzyme lipase helps in further digestion of lipids., 3. Besides the action of bile salts and surfactants, the mechanical mixing due to peristalsis, also helps in the emulsification of lipids. The, smaller lipid emulsion droplets are good, substrates for digestion., , Digestion of lipids, by pancreatic enzymes, The pancreatic enzymes are primarily, responsible for the degradation of dietary triacylglycerols, cholesteryl esters and phospholipids., , Degradation of triacylglycerols (fat), Pancreatic lipase is the major enzyme that, digests dietary fats. This enzyme preferentially, cleaves fatty acids (particularly long chain, above, 10 carbons) at position 1 and 3 of triacylglycerols. The products are 2-monoacylglycerol, (formerly 2-monoglyceride) and free fatty acids, (Fig.8.9). The activity of pancreatic lipase is, inhibited by bile acids which are present along, with the enzyme in the small intestine. This, problem is overcome by a small protein, colipase, , (mol. wt. 12,000). It is also secreted by pancreas, as procolipase and converted to active form by, trypsin. Colipase binds at the lipid-aqueous, interface and helps to anchor and stabilize lipase., , Lipid esterase is a less specific enzyme, present in pancreatic juice. It acts on, monoacylglycerols, cholesteryl esters, vitamin, esters etc. to liberate free fatty acids. The, presence of bile acids is essential for the activity, of lipid esterase., , Degradation of cholesteryl esters, A specific enzyme namely pancreatic cholesterol esterase (cholesteryl ester hydrolase), cleaves cholesteryl esters to produce cholesterol, and free fatty acids (Fig.8.10)., , Degradation of phospholipids, Phospholipases are enzymes responsible for, the hydrolysis of phospholipids. Pancreatic juice, is rich in phospholipase A2 which cleaves the, fatty acid at the 2nd position of phospholipids., The products are a free fatty acid and a, lysophospholipid. Phospholipase A2 is secreted, as a zymogen which is activated in the intestine, by the action of trypsin., An overview of the digestion of lipids is given, in Fig.8.11., , Absorption- of lipids, The former and present theories to explain, the absorption of lipids are briefly described, hereunder, 1. Lipolytic theory put forth by Verzar :, According to this, fats are completely hydrolysed, to glycerol and free fatty acids. The latter are, absorbed either as soaps or in association with, bile salts.
Page 185 :
175, , Chapter 8 : DIGESTION AND ABSORPTION, , Cholesteryl esterase, H 2O, , O, R C O, , + R COOH, , HO, , Fatty acid, , Cholesterol, , Cholesteryl ester, , Fig. 8.10 : Enzymatic cleavage of cholesteryl ester., , 2. Partition theory proposed by Frazer : This, theory states that the digestion of triacylglycerols, is partial and not complete. The partially, digested triacylglycerols, in association with bile, salts, form emulsions. The lipids are taken up by, the intestinal mucosal cells. As per this theory,, resynthesis of lipids is not necessary for their entry, into the circulation., 3. Bergstrom theory : This is a more recent, and comprehensive theory to explain lipid, absorption. It has almost replaced the earlier, theories, and is briefly described hereunder, The primary products obtained from the lipid, digestion are 2-monoacylglycerol, free fatty acids, and free cholesterol., , Role of bile salts in lipid absorption, Besides their participation in digestion, bile, salts are essential for absorption of lipids. Bile, salts form mixed micelles with lipids. These, micelles are smaller in size than the lipid, emulsion droplets (utilized for digestion,, described above). The micelles have a disk like, shape with lipids (monoacylglycerol, fatty acids,, cholesterol and phospholipids) at the interior and, bile salts at the periphery. The hydrophilic, groups of the lipids are oriented to the outside, (close to the aqueous environment) and the, hydrophobic groups to the inside. In this fashion,, the bile salt micelles exert a solubilizing effect, on the lipids., , Mouth, , Small Intestine, Stomach, , Triacylglycerols, Phospholipids, Cholesteryl esters, , Almost, unchanged, , Pancreatic lipase, Phospholipase A2, Cholesteryl esterase, , Unchanged, , Fig. 8.11 : Overview of digestion of lipids., , Monoacylglycerols, Free fatty acids, Glycerol, Cholesterol
Page 186 :
176, , BIOCHEMISTRY, , 2-Monoacylglycerol, , Free fatty acid, INTESTINAL MUCOSAL CELL, , Bile salt, micelle, , Phospholipid, , Mixed, micelle, , Cholesterol, , Fig. 8.12 : Absorption of lipids by intestinal mucosal cell., , Mechanism of lipid absorption, The mixed micelles serve as the major, vehicles for the transport of lipids from the, intestinal lumen to the membrane of the, intestinal mucosal cells, the site of lipid, absorption. The lipid components pass through, the unstirred fluid layer and are absorbed, through the plasma membrane by diffusion, (Fig.8.12). Absorption is almost complete for, monoacylglycerols and free fatty acids which are, slightly water soluble. However, for water, insoluble lipids, the absorption is incomplete., For instance, less than 40% of the dietary, cholesterol is absorbed., The micelle formation is also essential for the, absorption of fat soluble vitamins, particularly, vitamins A and K., The efficiency of lipid absorption is, dependent on the quantity of bile salts to, solubilize digested lipids in the mixed micelles., It may, however, be noted that in the absence of, bile salts, the lipid absorption occurs to a minor, extent. This is mostly due to the slightly water, soluble nature of monoacylglycerols and free, fatty acids. Further, short and medium chain fatty, acids are not dependent on micelle formation for, the absorption., , Synthesis of lipids in the, intestinal mucosal cells, The fatty acids of short and medium chain, length (< 10 carbons), after their absorption into, , the intestinal cells, do not undergo any modification. They enter the portal circulation and, are transported to the liver in a bound form to, albumin., The long chain fatty acids are activated by, thiokinase (fatty acyl CoA synthetase) in the, intestinal cells. The acyl CoA derivatives so, formed combine with 2-monoacylglycerols to, produce triacylglycerols. These reactions are, catalysed by a group of enzymes, namely, acyltransferases (Fig.8.13). Further, within, the intestinal cells, cholesterol is converted, to cholesterylester, and phospholipids are, regenerated from the absorbed lysophospholipids. The newly synthesized lipids are usually, different from those consumed in the diet., , Secretion of lipids from, the intestinal mucosal cells, The lipids that are resynthesized (described, above) in the intestinal cells are hydrophobic in, nature. They are put together as lipid droplets, and surrounded by a thin layer consisting of, mostly apolipoproteins (AI and B-48) and, phospholipids. This package of lipids enveloped, in the layer stabilizes the droplets and increases, their solubility. These particles are known as, chylomicrons., Chylomicrons migrate to the plasma, membrane of intestinal mucosal cells. They are, released into the lymphatic vessels by exocytosis.
Page 187 :
177, , Chapter 8 : DIGESTION AND ABSORPTION, , INTESTINAL MUCOSAL CELL, , Phospholipids Apolipoproteins, , Thiokinase, , Long chain, fatty acid, , Fatty acyl-CoA, , Fatty acid, ATP, CoA AMP, 2Pi, CoA, , 2-Monoacylglycerol, , CoA, Triacylglycerol, , 2-Monoacylglycerol, Acyltransferase, , Cholesterol, , Cholesterol, , Acyltransferase, Fatty, acyl CoA, , Short chain, fatty acids, , CHYLOMICRON, Cholesteryl ester, , CoA, , Short chain fatty acids, , Lymphatic system, , Portal circulation, , Blood, , Liver, , Peripheral tissues, , Fig. 8.13 : Formation and secretion of chylomicrons by intestinal mucosal cells., , The presence of chylomicrons (Greek: chylos–, juice) gives the lymph a milky appearance,, which is observed after a lipid-rich meal., Chylomicrons enter the large body veins via the, thoracic duct. Blood from here flows to the heart, and then to the peripheral tissues (muscle,, adipose tissue) and, finally, to the liver. Adipose, tissue and muscle take up a large proportion of, dietary lipids from chylomicrons for storage and, transport. It is believed that this bypass, arrangement (passage of chylomicrons through, peripheral tissues) protects the liver from a lipid, overload after a meal., , Abnormalities of lipid, digestion and absorption, The gastrointestinal tract possesses an efficient, system for digestion and absorption of lipids. It, can comfortably handle as much as 4 times the, normal daily intake of lipids., Steatorrhea : It is a condition characterized, by the loss of lipids in the feces. Steatorrhea may, be due to, 1. A defect in the secretion of bile or, pancreatic juice into the intestine;
Page 188 :
178, , BIOCHEMISTRY, , 2. Impairment in the lipid absorption by the, intestinal cells., , fat digestion, absorption, and thus obesity are in, use. Two approaches are given below, , Steatorrhea is commonly seen in disorders, associated with pancreas, biliary obstruction,, severe liver dysfunction etc., , 1. Pancreatic, lipase, degrades, dietary, triacylglycerol to fatty acids and glycerol which, are absorbed. Orlistat is a non-hydrolysable, analog of triacylglycerol, and is a powerful, inhibitor of pancreatic lipase, hence prevents fat, digestion, and absorption., , Cholesterol stones, Cholesterol stone formation in gall-bladder, (gall stones) is a frequent health complication. It, is found more frequently in females than in males, often in association with obesity. Cholesterol gall, stones are formed when liver secretes bile, (containing phospholipids, bile acids etc.),, supersaturated with respect to cholesterol., , OBESITY AND FAT ABSORPTION, Obesity is a major problem in many parts of, the world as the availability of food is generally, abundant and overeating is common. Intake of, lipids largely contributes to obesity. In recent, years, pharmacological interventions to prevent, , 2. Olestra is a synthetic lipid, produced by, esterification of natural fatty acids with sucrose, (instead of glycerol). Olestra tastes like a natural, lipid. However, it cannot be hydrolysed and, therefore, gets excreted., , NUCLEIC ACIDS, Nucleic acids (DNA and RNA), and their, bases purines and pyrimidines can be, synthesized in the body, and thus they are, dietarily non-essential., , + Cooking of food significantly improves the digestibility by enzymes., + Lactose intolerance due to a defect in the enzyme lactase (E-galactosidase) is very, +, +, , +, +, +, +, +, +, , common. The treatment advocated is severe restriction of lactose (milk and milk, products) in the diet., Flatulence, occurring after ingestion of certain non-digestible oligosaccharides, is, characterized by increased intestinal motility, cramps and irritation., Direct intestinal absorption of proteins and polypeptides is observed in the infants,, immediately after birth. This is important for the transfer of maternal immunoglobulins, (via breast-feeding) to the offspring., In some adults, macromolecular (protein) absorption by intestine is responsible for, antibody formation, often causing food allergy., Emulsification of lipids is essential for their effective digestion, since lipases can act only, on the surface of lipid droplets. Bile salts are the most efficient biological emulsifying agents., Pharmacological interventions (e.g. Orlistat, Olestra) to block fat digestion and/or, absorption so as to prevent obesity are in recent use., Steatorrhea, characterized by the loss of lipids in feces is commonly associated with, impaired pancreatic function and biliary obstruction., Gastric ulcers are mainly caused by the bacterium H. pylori. The antibiotics that, eliminate this bacterium are effective in the treatment., Acute pancreatitis is caused by autodigestion of pancreas while chronic pancreatitis is, associated with excessive consumption of alcohol.
Page 189 :
179, , Chapter 8 : DIGESTION AND ABSORPTION, , Mouth, , Small Intestine, , Nucleic acids, (DNA, RNA), , Stomach, , Ribonucleases, , Low pH, , Deoxyribonucleases, , DNA, RNA, denatured, , Oligonucleotides, Phosphodiesterases, Nucleotides, Pi, , Nucleotidases, , Nucleosides, Nucleosidases, (Deoxy) ribose, , Unchanged, , Purines, Pyrimidines, , Fig. 8.14 : Overview of digestion of nucleic acids., , The digestion of dietary nucleic acids is, carried out in the small intestine, primarily by, the enzymes of pancreatic juice. Ribonucleases, and deoxyribonucleases, respectively, hydrolyse, RNA and DNA to oligonucleotides (Fig.8.14)., The latter are degraded by phosphodiesterases to, form mononucleotides. Nucleotidases act on, nucleotides, to, liberate, phosphate, and, nucleosides. The nucleosides may be either, directly absorbed or degraded to free bases, before absorption. Some of the unabsorbed, purines are metabolized by the intestinal, bacteria., The dietary purines and pyrimidines are not of, much utility for the synthesis of tissue nucleic, acids. Further, the purines after their absorption, are mostly converted to uric acid by the intestinal, mucosal cells and excreted in the urine., , Peptic ulcers, Gastric and duodenal ulcers are collectively, known as peptic ulcers. Ulceration occurs due, to the autodigestion of mucosa by the gastric, secretions (pepsin and HCl). In the patients, of peptic ulcer, gastric HCl is always present in, the pyloric regions of stomach and the, duodenum. Gastic ulcers are mainly caused by, the bacterium Helicobacter pylori which lives in, the nutrient-rich gastric mucosa. H. pylori, induces chronic inflammation in the stomach, tissues, which gets exposed to acid damage. For, this reason, the best mode of treatment for, gastric ulcers is the use of antibiotics that, eliminate H. pylori., , Achlorhydria is a less serious disorder, involving the failure to secrete gastric HCl., , Pancreatitis, ABNORMALITIES RELATED TO, DIGESTION AND ABSORPTION, The following are the major abnormalities (of, interest to biochemists) concerned with digestion, and absorption of food in the gastrointestinal, tract., Lactose intolerance, deficiency of sucrase,, Hartnup’s disease and steatorrhea have already, been described. Peptic ulcer, pancreatitis and, celiac disease are other important disorders, associated with digestive system., , Inflammation of the pancreas is known as, pancreatitis. Acute pancreatitis is caused by the, autodigestion of pancreas due to the unusual, conversion of zymogens into the active enzymes, by trypsin. In normal circumstances, this is, prevented by trypsin inhibitor., Acute pancreatitis is a life-threatening, disorder. Measurement of serum amylase (highly, elevated) is used in the diagnosis of pancreatitis., Excessive consumption of alcohol over a long, period is blamed as the prime cause of chronic, pancreatitis.
Page 190 :
180, , BIOCHEMISTRY, , Celiac disease (celiac sprue), Celiac disease is a disease of malabsorption, caused by immune-mediated damage to, , as a result of ingestion of gluten (or gliadin, derived from gluten), a protein found in, what, barley and rye., , small intestine. This occurs in some individuals, , 1. Digestion is a process that converts complex foodstuffs into simpler ones which can be, readily absorbed by the gastrointestinal tract., 2. Stomach, duodenum and upper part of small intestine are the major sites of digestion., The small intestine is the prime site for the absorption of digested foods., 3. The digestion of carbohydrates is initiated in the mouth by salivary D-amylase and is, completed in the small intestine by pancreatic D-amylase, oligosaccharidases and, disaccharidases., 4. Monosaccharides are the final absorbable products of carbohydrate digestion. Glucose, is transported into the intestinal mucosal cells by a carrier mediated, Na+-dependent, energy requiring process., 5. Lactose intolerance due to a defect in the enzyme lactase (E-galactosidase) resulting in the, inability to hydrolyse lactose (milk sugar) is the common abnormality of carbohydrate digestion., 6. Protein digestion begins in the stomach by pepsin, which is aided by gastric HCl., Pancreatic proteases (trypsin, chymotrypsin and elastase) and intestinal aminopeptidases and dipeptidases complete the degradation of proteins to amino acids and, some dipeptides., 7. The intestinal absorption of amino acids occurs by different transport systems (at least, six known). The uptake of amino acids is primarily a Na+-dependent energy requiring, process., 8. Digestion of lipids occurs in the small intestine. Emulsification of lipids, brought about, by bile salts, is a prerequisite for their digestion. Pancreatic lipase aided by a colipase, degrades triacylglycerol to 2-monoacylglycerol and free fatty acids. Cholesterol esterase, and phospholipases, respectively, hydrolyse cholesteryl esters and phospholipids., 9. Lipid absorption occurs through mixed micelles, formed by bile salts in association with, products of lipid digestion (primarily 2-monoacylglycerol, cholesterol and free fatty, acids). In the intestinal mucosal cells, lipids are resynthesized from the absorbed, components and packed as chylomicrons which enter the lymphatic vessels and then, the blood., 10. Dietary nucleic acids (DNA and RNA) are digested in the small intestine to nucleosides, and/or bases (purines and pyrimidines) which are absorbed.
Page 191 :
Chapter 8 : DIGESTION AND ABSORPTION, , 181, , I. Essay questions, 1., 2., 3., 4., 5., , Write an account of the digestion and absorption of lipids., Describe briefly the digestion of carbohydrates and proteins., Give an account of the Na+ dependent intestinal transport of glucose and amino acids., Describe the role of intestine in the digestion of foodstuffs., Write briefly on the enzymes of gastrointestinal tract involved in the digestion of foodstuffs., , II. Short notes, (a) Mixed micelles, (b) Lactose intolerance, (c) Salivary amylase, (d) Disaccharidases, (e) JGlutamyl cycle, (f) Zymogens, (g) Specificity of proteases, (h) Bile salts, (i) Synthesis of chylomicrons, in the intestinal mucosal cells, (j) Pancreatic juice., , III. Fill in the blanks, 1. Cellulose is not digested in humans due to lack of the enzyme that hydrolyses ________________, bonds., 2. The most important carbohydrate associated with flatulence caused by ingestion of leguminous, seeds ________________., 3. Lactose intolerance is caused by the deficiency of the enzyme ________________., 4. The non-digested carbohydrates are collectively called ________________., 5. Gastric HCl is secreted by ________________., 6. Name of the peptide believed to be involved in the transport of amino acids ________________., 7. The disease characterized by impairment in the absorption of neutral amino, acids ________________., 8. Trypsin hydrolyses peptide bonds, the carbonyl group of which is contributed by the amino, acids ________________ or ________________., 9. The inhibition of the enzyme pancreatic lipase by bile salts is overcome by a protein,, namely ________________., 10. The vehicles for the transport of lipids from the intestinal lumen to the membrane of mucosal, cells ________________., , IV. Multiple choice questions, 11. Transport of glucose from the lumen to the intestinal mucosal cells is coupled with diffusion of, (a) Na+ (b) K+ (c) Cl– (d) HCO3–., 12. The key enzyme that converts trypsinogen to trypsin is, (a) Secretin (b) Chymotrypsin (c) Elastase (d) Enteropeptidase., 13. The products obtained by the action of pancreatic lipase on triacylglycerols are, (a) Glycerol and free fatty acids (b) 1-Acylglycerol and free fatty acids (c) 2-Acylglycerol and free, fatty acids (d) 3-Acylglycerol and free fatty acids., 14. The lipoproteins synthesized in the intestinal mucosal cells from the absorbed lipids are, (a) High density lipoproteins (b) Chylomicrons (c) Low density lipoproteins (d) Very low density, lipoproteins., 15. Salivary D-amylase becomes inactive in the stomach primarily due to, (a) Inactivation by low pH (b) Degradation by gastric pepsin (c) Inhibition by Cl– (d) Inhibition, by peptides.
Page 192 :
Section 2, , Physiological Biochemistry, , Chapter, , Plasma Proteins, , 9, , The official ‘spokesperson’ of, plasma proteins, albumin, speaks :, , “I am the most abundant plasma protein;, Produced exclusively by the liver;, Perform osmotic, transport and nutritive functions;, Estimated in lab to assess liver function.”, , T, , he plasma is the liquid medium of blood, (55-60%), in which the cell components—, namely erythrocytes, leukocytes, platelets—are, suspended. If blood containing anticoagulants, (e.g. heparin, potassium oxalate) is centrifuged,, the plasma separates out as a supernatant while, the cells remain at the bottom. The packed cell, volume or hematocrit is about 45%., , The term serum is applied to the liquid, medium which separates out after the blood clots, (coagulates). Serum does not contain fibrinogen, and other clotting factors. Thus, the main, difference between plasma and serum is the, presence or absence of fibrinogen., , Importance of blood, The total volume of blood in an adult is, around 4.5 to 5 liters. Blood performs several, diversified functions. These include respiration,, excretion, acid-base maintenance, water, balance, transport of metabolites, hormones and, drugs, body defense and coagulation., , Separation of plasma proteins, The total concentration of plasma proteins is, about 6-8 g/dl. The plasma is a complex mixture, of proteins, and several techniques are employed, to separate them. An age-old technique is based, on the use of varying concentrations of, ammonium sulfate or sodium sulfate. By this, method, which is known as salting out process,, the plasma proteins can be separated into, three groups—namely albumin, globulins and, fibrinogen., Electrophoresis : This is the most commonly, employed analytical technique for the separation, of plasma (serum) proteins. The basic principles, of electrophoresis are described in Chapter 43., Paper or agar gel electrophoresis with vernol, buffer (pH-8.6) separates plasma proteins into, 5 distinct bands namely albumin, D1, D2, E and, J globulins (Fig.9.1). The concentration of each, one of these fractions can be estimated by a, densitometer., , 182
Page 193 :
183, , Chapter 9 : PLASMA PROTEINS, , Start, , 2. Excretion of albumin into urine in kidney, damage., 3. Increased, production, of, globulins, associated with chronic infections, multiple, myelomas etc., , (A), , Albumin, , D1, , D2, , E, , J, , Globulins, , Components of plasma proteins, The important plasma proteins along with, their characteristics (based on electrophoretic, pattern) and major functions are given in, Table 9.1. Some selected plasma proteins are, discussed hereunder., , (B), , ALBUMIN, Albumin, , D1, , D2, E, Globulins, , J, , Fig. 9.1 : Electrophoresis of plasma proteins—, (A) Separated bands, (B) Densitometer scannning., , Abnormal electrophoretic pattern, Electrophoresis of serum proteins is, conveniently used for the diagnosis of certain, diseases, 1. Multiple myeloma : A sharp and distinct, M band appears in the J-globulin fraction., 2. Acute infections : D1- and D2- globulins, are increased., 3. Nephrotic syndrome : Decreased albumin, with sharp and prominent D2-globulin., 4. Primary immune deficiency : Diminished, J-globulin band., 5. D1-Antitrypsin deficiency : Diminished D1globulin band., Albumin/globulin (A/G) ratio : The albumin, concentration of plasma is 3.5 to 5.0 g/dl while, that of total globulins is 2.5 to 3.5 g/dl. The, normal A/G ratio is 1.2 to 1.5 : 1. The A/G ratio, is lowered either due to decrease in albumin or, increase in globulins, as found in the following, conditions, 1. Decreased synthesis of albumin by liver—, usually found in liver diseases and severe protein, malnutrition., , Albumin is the major constituent (60%) of, plasma proteins with a concentration of 3.5–5.0, g/dl. Human albumin has a molecular weight of, 69,000, and consists of a single polypeptide, chain of 585 amino acids with 17 disulfide, bonds., , Synthesis of albumin, Albumin is exclusively synthesized by the, liver. For this reason, measurement of serum, albumin concentration is conveniently used to, assess liver function (synthesis decreased in liver, diseases). Liver produces about 12 g albumin per, day which represents 25% of the total hepatic, protein synthesis. Albumin has an half-life of 20, days., , Functions of albumin, Plasma albumin performs osmotic, transport, and nutritive functions, 1. Osmotic function : Due to its high, concentration and low molecular weight,, albumin contributes to 75–80% of the total, plasma osmotic pressure (25 mm Hg). Thus,, albumin plays a predominant role in maintaining, blood volume and body fluid distribution., Decrease in plasma albumin level results in a, fall in osmotic pressure, leading to enhanced, fluid retention in tissue spaces, causing edema., The edema observed in kwashiorkor, a disorder, of protein-energy malnutrition, is attributed to a, drastic reduction in plasma albumin level.
Page 194 :
184, , BIOCHEMISTRY, , TABLE 9.1 Important plasma proteins, characteristics and major functions, , Protein, , Plasma, concentration, , Molecular, weight, , Albumin, , 3.5–5.0 g/dl, , 69,000, , Osmotic, transport, nutritive and buffering, , Prealbumin, , 25–30 mg/dl, , 61,000, , Transports thyroxine to some extent, , D1-Globulins, , 0.3–0.5 g/dl, , —, , D1-Antitrypsin, , < 0.2 g/dl, , D1-Lipoproteins (HDL), , 0.2–0.3 g/dl, , Orosomucoid, , < 0.1 g/dl, , 44,000, , Binds with progesterone, , Retinol binding protein (RBP), , 3–6 mg/dl, , 21,000, , Transports vitamin A, , Thyroxine binding globulin (TBG), , 1–2 mg/dl, , 58,000, , Transports thyroid hormones, , Transcortin or cortisol, binding protein (CBG), , 3–4 mg/dl, , 52,000, , Major transporter of steroid hormones (e.g., cortisol, corticosterone), , D2-Globulins, , 0.4–0.8 g/dl, , —, , D2-Macroglobulin, , 0.2–0.3 g/dl, , 800,000, , Haptoglobins, (Hp 1-1; Hp 2-1 and Hp 2-2), , < 0.3 g/dl, , 90,000, , Binds with plasma free hemoglobin and, prevents its excretion, , Prothrombin, , < 0.02 g/dl, , 63,000, , Participates in blood coagulation, , Ceruloplasmin, , < 0.03 g/dl, , 150,000, , E-Globulins, , 0.6–1.1 g/dl, , —, , E-Lipoproteins (LDL), , 0.2–0.5 g/dl, , —, , Transferrin, , 0.2–0.3 g/dl, , 76,000, , Transports iron, , Hemopexin, , < 0.1 g/dl, , 57,000, , Transports heme, , Plasminogen, , < 0.05 g/dl, , 140,000, , J-Globulins, , 0.8–1.8 mg/dl, , 54,000, —, , —, , Major function(s), , —, Inhibitor of trypsin, Transports cholesterol and phospholipids, , —, Antitrypsin and antiplasmin activity, , Transport of copper; oxidation of Fe2+ to Fe3+., —, Transports triacylglycerols and cholesterol, , Forms plasmin, involved in fibrinolysis, Antibody functions, , (Immunoglobulins—IgG, IgA, IgM, IgD and IgE; refer Table 9.2 for details), Fibrinogen, , 0.2–0.4 g/dl, , 2. Transport functions : Plasma albumin, binds to several biochemically important, compounds and transports them in the, circulation. These include free fatty acids,, bilirubin, steroid hormones, calcium and copper., [Note : Besides albumin, there are several, other plasma transport proteins. These include, prealbumin, retinol binding protein, thyroxine, binding protein, transcortin and others as stated, in the functions of plasma proteins in Table 9.1]., , 340,000, , Participates in blood coagulation, , 3. Nutritive functions : Albumin serves as a, source of amino acids for tissue protein synthesis, to a limited extent, particularly in nutritional, deprivation of amino acids., 4. Buffering function : Among the plasma, proteins, albumin has the maximum buffering, capacity. However, the buffering action of, albumin in plasma is not significant compared to, bicarbonate buffer system.
Page 195 :
185, , Chapter 9 : PLASMA PROTEINS, , Clinical significance of albumin, 1. Albumin, binding to certain compounds in, the plasma, prevents them from crossing the, blood-brain barrier e.g. albumin-bilirubin, complex, albumin-free fatty acid complex., 2. Hypoalbuminemia, (lowered, plasma, albumin), observed in malnutrition, nephrotic, syndrome and cirrhosis of liver is associated with, edema., The osmotic pressure of albumm significantly, contributes to maintaining plasma volume, and, fluid volume of interstitial fluids. In hypoalbuminemia (albumin <2g/dl), plasma osmotic, pressure is decreased, leading to flow of water, from plasma to interstitial compartment that, results in edema of legs and other body parts., 3. Albumin, is, excreted, into, urine, (albuminuria) in nephrotic syndrome and in, certain inflammatory conditions of urinary tract., Microalbuminuria (30-300 mg/day) is clinically, important for predicting the future risk of renal, diseases (Refer Chapter 36)., 4. Albumin is therapeutically useful for the, treatment of burns and hemorrhage., , GLOBULINS, Globulins constitute several proteins that are, separated into four distinct bands (D�, D�, E and, J-globulins) on electrophoresis (See Fig.9.1)., Globulins, in general, are bigger in size, than albumin. In Table 9.1, the important, globulins are given, some of them are discussed, hereunder., , D1-Antitrypsin, D1-Antitrypsin, more recently called as D-antiproteinase, is a glycoprotein with 394 amino acids, and a molecular weight of 54,000. It is a major, constituent of D1-globulin fraction of plasma, proteins with a normal concentration of about 200, mg/dl. D1-Antitrypsin is a serine protease inhibitor., It combines with trypsin, elastase and other, protease enzymes and inhibits their activity., , Clinical significance, of D1-antitrypsin, D1-Antitrypsin deficiency has been implicated, in two diseases, namely, emphysema and D1-AT, deficiency liver disease., , Emphysema (Greek : emphusan—to inflate) is, a term used to represent the abnormal distension, of lungs by air. At least 5% of emphysema cases, are due to the deficiency of D1-AT. This is, associated with lung infections (e.g. pneumonia), and increase in the activity of macrophages to, release elastase that damages lung tissues. In the, normal circumstances, elastase activity is, inhibited by D1-AT., Effect of smoking on D1-AT : The amino acid, methionine at position 358 of D1-AT is involved, in binding with proteases. Smoking causes, oxidation of this methionine to methionine, sulfoxide. As a result, D1-AT with methionine, sulfoxide cannot bind and inactivate proteases., Emphysema is more commonly associated with, heavy smoking and the situation becomes worse, in persons with D1-AT deficiency., D1-Antitrypsin deficiency and liver disease :, This is due to the accumulation of a mutant, D1-AT which aggregates to form polymers. These, polymers, in turn cause liver damage (hepatitis), followed by accumulation of collagen resulting, in fibrosis (cirrhosis)., , D2-Macroglobulin, D2-Macroglobulin concentration in plasma is, elevated in nephrotic syndrome. This is due to, the fact that majority of the low molecular, weight proteins are lost in urine (proteinuria) in, this disorder., , HAPTOGLOBIN, Haptoglobin (Hp), a glycoprotein, is an acute, phase protein. Its plasma concentration is, increased in several inflammatory conditions., , Functions of haptoglobin, Haptoglobin binds with the free hemoglobin, (known as extra-corpuscular hemoglobin) that, spills into the plasma due to hemolysis., The haptoglobin-hemoglobin (Hp-Hb) complex
Page 196 :
186, , Clinical significance of Hp : Plasma, concentration of Hp is decreased in hemolytic, anemia. This is explained as follows. The halflife of Hp is about 5 days while that of Hp-Hb, complex is 90 min. In hemolytic anemia, free, Hb in plasma is elevated leading to increased, formation of Hp-Hb complex. This complex in, turn, is rapidly cleared from the plasma resulting, in decreased Hp levels., , CERULOPLASMIN, Ceruloplasmin is a blue coloured, copper—, containing D2-globulin with a molecular weight, of 150,000. Its plasma concentration is about 30, mg/dl. Ceruloplasmin binds with almost 90% of, plasma copper (6 atoms of Cu bind to a, molecule). This binding is rather tight and, as a, result, copper from ceruloplasmin is not readily, released to the tissues. Albumin carrying only, 10% of plasma copper is the major supplier of, copper to the tissues. Ceruloplasmin possesses, oxidase activity, and it is associated with, Wilson’s disease which is discussed under, copper metabolism (Chapter 18)., , TRANSFERRIN, Transferrin (Tf) is a glycoprotein with a, molecular weight of 76,000. It is associated with, E-globulin fraction. Transferrin is a transporter, of iron in the circulation., , ACUTE PHASE PROTEINS, Acute phase response refers to a non-specific, response to the stimulus of infection, injury,, various inflammatory conditions (affecting, tissue/organs), cancer etc. This phase is, associated with a characteristic pattern of, changes in certain plasma proteins, collectively, referred to as acute phase proteins e.g. D1antitrypsin, ceruloplasmin, complement proteins,, C-reactive protein. During the acute phase,, synthesis of certain plasma proteins decreases,, and they are regarded as negative acute phase, reactants e.g. albumin, transferrin., , 200, Serum CRP (mg/l), , (mol. wt. 155,000) cannot pass through glomeruli, of kidney while free Hb (mol. wt. 65,000) can., Haptoglobin, therefore, prevents the loss of free, Hb into urine., , BIOCHEMISTRY, , 150, Infection, , Treatment, , 100, Normal, , 50, , 0, , 1, , 2, , 3, , 4, , 5 6, Days, , 7, , 8, , 9 10 11 12, , Fig. 9.2 : The response of C-reactive protein (CRP) in, response to surgery (The normal acute phase is depicted, by blue line, the development of infection by red line and, the response after treatment by green line)., , C-reactive protein (CRP), CRP is a major component of acute phase, proteins. It is produced in the liver and is present, in the circulation in minute concentration, (< 1 mg/dl). C-reactive protein (C strands for, carbohydrate to which it binds on the capsule of, pneumococi) is involved in the promotion of, immune system through the activation of, complement cascade., Estimation of CRP in serum is important for, the evaluation of acute phase response. The, response of CRP to surgery is depicted in, Fig.9.2. In a normal surgery, serum CRP, increases and returns to normal level within, 7-10 days. If the recovery is complicated by any, infection, it will be reflected by the continuous, elevation of CRP which requires further, treatment., Increased levels of high sensitive CRP, (hs-CRP) in th circulation (reference range, 100–300 Pg/dl) are useful for predicting the risk, of coronary heart disease., , IMMUNOGLOBULINS, The higher vertebrates, including man, have, evolved a defense system to protect themselves, against the invasion of foreign substances—a, virus, a bacterium or a protein. The defense
Page 197 :
187, , Chapter 9 : PLASMA PROTEINS, Interchain, disulfide bonds, , +, , H3N, , +, , H3N, , S, , VL, , S, , S, , +, , NH3, , S, +, , L-c, ha, in, , NH3, , CL, S, , S, , H- S S, ch, ain, , S, , S, S, , S, , S, S, , S, , S, S, , Hinge region, , VH, , S, , S, S, , Fab, , S, , S, CH1, , S, S, , CHO, , Papain, , CHO, S, , S, , S, , S, , S, , S, , S, , S, , CH2, Fc, , Intrachain, disulfide bonds, , COO–, , CH3, , COO–, , Fig. 9.3 : Diagrammatic representation of human IgG molecule (V—variable region, C—constant region;, CHO—carbohydrate; Each heavy chain is composed of four units—VH , CH1 , CH2 , CH3, while light chain consists of two units—VL, CL)., , strategies of the body are collectively referred to, as immunity, and are briefly described under, immunology (Chapter 42). Immunoglobulins (or, antibodies) are described here., , Immunoglobulins—basic concepts, Immunoglobulins, a specialised group of, proteins are mostly associated with J-globulin, fraction (on electrophoresis) of plasma proteins., Some immunoglobulins however, separate along, with E and D-globulins. Therefore, it should be, noted that J-globulin and immunoglobulin, are not synonymous. Immunoglobulin is a, functional term while J-globulin is a physical, term., , Structure of immunoglobulins, All the immunoglobulin (Ig) molecules, basically consist of two identical heavy (H), , chains (mol. wt. 53,000 to 75,000 each) and two, identical light (L) chains (mol. wt. 23,000 each), held together by disulfide linkages and noncovalent interactions (Fig.9.3). Thus, immunoglobulin is a Y-shaped tetramer (H2L2). Each, heavy chain contains approximately 450 amino, acids while each light chain has 212 amino, acids. The heavy chains of Ig are linked to, carbohydrates, hence immunoglobulins are, glycoproteins., Constant and variable regions : Each chain, (L or H) of Ig has two regions (domains), namely, the constant and the variable. The amino, terminal half of the light chain is the variable, region (VL) while the carboxy terminal half is the, constant region (CL). As regards heavy chain,, approximately one-quarter of the amino terminal, region is variable (VH) while the remaining threequarters is constant (CH , CH2, CH3). The amino, 1
Page 198 :
188, , BIOCHEMISTRY, , TABLE 9.2 Characteristics of human immunoglobulins, , Type, , H-Chain, , L-Chains, , Molecular, formula, , Molecular, weight, , Percentage, carbohydrate, , Serum conc., mg/dl, , IgG, , J, , N or O, , J2N2 or J2O2, , ~150,000, , 3, , 800–1,500, , IgA, , D, , N or O, , (D2N2)1–3 or, (D2O2)1–3, , ~(160,000)1–3, , 8, , 150–400, , IgM, , P, , N or O, , (P2N2)5 or, (P2O2)5, , ~ 900,000, , 12, , 50–200, , IgD, , G, , N or O, , (G2N2 or G2O2), , ~180,000, , 13, , 1–10, , IgE, , H, , N or O, , H2N2 or H2O2, , ~190,000, , 12, , 0.02–0.05, , acid sequence (with its tertiary structure) of, variable regions of light and heavy chains is, responsible for the specific binding of, immunoglobulin (antibody) with antigen., Proteolytic cleavage of Ig : An immunoglobulin can be split by the enzyme papain to, their fragments. These are two identical antigen, binding fragments (Fab) and one crystallizable, fragment (Fc). Papain cleaves the immunoglobin, molecule at the site between CH1 and CH2, regions which is referred to as hinge region., , CLASSES OF IMMUNOGLOBULINS, Humans have five classes of immunoglobulins—namely IgG, IgA, IgM, IgD and, IgE—containing the heavy chains J, D, P, G and, H, respectively. The type of heavy chain, ultimately determines the class and the function, of a given Ig., Two types of light chains—namely kappa (N), and lambda O —are found in immunoglobulins., They differ in their structure in CL regions. An, immunoglobulin (of any class) contains two N or, two O light chains and never a mixture. The, occurrence of N chains is more common in, human immunoglobulins than O chains., The characteristics of the 5 classes of human, immunoglobulins are given in Table 9.2., , Major function(s), Mostly responsible for, humoral immunity, Protects the body, surfaces, Humoral immunity,, serves as first line of, defense, B-cell receptor ?, Humoral sensitivity, and histamine release., , Immunoglobulin G (IgG), IgG is the most abundant (75–80%) class of, immunoglobulins. IgG is composed of a single, Y-shaped unit (monomer). It can traverse blood, vessels readily. IgG is the only immunoglobulin, that can cross the placenta and transfer the, mother’s immunity to the developing fetus. IgG, triggers foreign cell destruction mediated by, complement system., , Immunoglobulin A (IgA), IgA occurs as a single (monomer) or double, unit (dimer) held together by J chain. It is mostly, found in the body secretions such as saliva, tears,, sweat, milk and the walls of intestine. IgA is the, most predominant antibody in the colostrum, the, initial secretion from the mother’s breast after a, baby is born. The IgA molecules bind with, bacterial antigens present on the body (outer, epithelial) surfaces and remove them. In this, way, IgA prevents the foreign substances from, entering the body cells., , Immunoglobulin M (IgM), IgM is the largest immunoglobulin composed, of 5 Y-shaped units (IgG type) held together by, a J polypeptide chain. Thus IgM is a pentamer., Due to its large size, IgM cannot traverse blood, vessels, hence it is restricted to the blood stream.
Page 199 :
Chapter 9 : PLASMA PROTEINS, , IgM is the first antibody to be produced in, response to an antigen and is the most effective, against invading microorganisms. It may be, noted that IgM can simultaneously combine with, 5 antigenic sites due to its pentameric structure., , Immunoglobulin D (IgD), IgD is composed of a single Y-shaped unit, and is present in a low concentration in the, circulation. IgD molecules are present on the, surface of B cells. Their function, however, is not, known for certain. Some workers believe that, IgD may function as B-cell receptor., , Immunoglobulin E (IgE), IgE is a single Y-shaped monomer. It is normally, present in minute concentration in blood. IgE levels, are elevated in individuals with allergies as it is, associated with the body’s allergic responses. The, IgE molecules tightly bind with mast cells which, release histamine and cause allergy., , Production of immunoglobulins, by multiple genes, As already discussed, immunoglobulins are, composed of light and heavy chains. Each light, chain is produced by 3 separate genes, namely, a variable region (VL) gene, a constant region, (CL) gene and a joining region (J) gene. Each, heavy chain is produced by at least 4 different, genes—a variable region (VH) gene, a constant, region (CH) gene, a joining region (J) gene and, diversity region (D) gene. Thus multiple genes, are responsible for the synthesis of any one of, the immunoglobulins., Antibody diversity : A person is capable of, generating antibodies to almost an unlimited, range of antigens (more than one billion!). It, should, however, be remembered that humans, do not contain millions of genes to separately, code for individual immunoglobulin molecules., The antibody diversity is achieved by two special, processes, namely combination of various, structural genes and somatic mutations., , MULTIPLE MYELOMA, Multiple myeloma, a plasma cell cancer,, constitutes about 1% of all cancers affecting the, , 189, population. Females are more susceptible than, males for this disorder and it usually occurs in, the age group 45-60 years., Abnormal Ig production : Multiple myeloma is, due to the malignancy of a single clone of plasma, cells in the bone marrow. This results in the, overproduction of abnormal immunoglobulins,, mostly (75%) IgG and in some cases (25%) IgA, or IgM. IgD type multiple myeloma found in, younger adults is less common (<2%) but more, severe. In patients of multiple myeloma, the, synthesis of normal immunoglobulins is, diminished causing depressed immunity. Hence, recurrent infections are common in these patients., Electrophoretic pattern : The plasma of, multiple myeloma patients shows a characteristic, pattern of electrophoresis. There is a sharp and, distinct band (M band, for myeloma globulin), between E-and J-globulins. Further, this M band, almost replaces the J-globulin band due to the, diminished synthesis of normal J-globulins., Bence Jones proteins : Henry Bence Jones first, described them in 1847. These are the light, chains (N or O) of immunoglobulins that are, synthesized in excess. Bence Jones proteins have, a molecular weight of 20,000 or 40,000 (for, dimer). In about 20% of the patients of multiple, myeloma, Bence Jones proteins are excreted in, urine which often damages the renal tubules., , Amyloidosis is characterized by the deposits, of light chain fragments in the tissue (liver,, kidney, intestine) of multiple myeloma patients., The presence of Bence Jones proteins in urine, can be detected by specific tests., 1. Electrophoresis of a concentrated urine is, the best test to detect Bence Jones proteins in, urine., 2. The classical heat test involves the, precipitation of Bence Jones proteins when, slightly acidified urine is heated to 40-50°C. This, precipitate redissolves on further heating of urine, to boiling point. It reappears again on cooling, urine to about 70°C., 3. Bradshaw’s test involves layering of urine, on concentrated HCl that forms a white ring of, precipitate, if Bence Jones proteins are present.
Page 200 :
190, , BIOCHEMISTRY, , Intrinsic, pathway, , Extrinsic, pathway, , Factor Xa, , Factor X, , Prothrombin (II), , Factor X, , Thrombin (IIa), , Fibrinogen (I), , Fibrin, (blood clot), , Fig. 9.4 : Overview of blood clotting with the final common pathway., , BLOOD CLOTTING, The term hemostasis is applied to the, sequence of physiological responses to stop, bleeding (loss of blood after an injury). This is, carried out by blood clotting., Blood clotting or coagulation is the body’s, major defense mechanism against blood loss. A, blood clot is formed as a result of a series of, reactions involving nearly 20 different, substances, most of them being glycoproteins,, synthesized by the liver., Blood clotting process, independent pathways, , involves, , two, , 1. The extrinsic pathway is the initial process, in clotting and involves the factors that are not, present in the blood (hence the name)., 2. The intrinsic pathway involves a series of, reactions participated by the factors present in, the blood., Strictly speaking, the extrinsic and intrinsic, pathways are not independent, since they are, coupled together. Further, the final reactions are, identical for both pathways that ultimately lead, to the activation of prothrombin to thrombin and, the conversion of fibrinogen to fibrin clot, (Fig.9.4)., The blood coagulation factors in human, plasma along with their common names and, molecular weights are listed in Table 9.3. All but, , two of these factors are designated by a Roman, numeral. It should, however, be noted that the, numbers represent the order of their discovery, and not the order of their action. The cascade of, blood clotting process is depicted in Fig.9.5 and, the salient features are discussed below. The, active form of a factor is designated by a, subscript a. The active clotting factors (with, exception of fibrin) are serine proteases., , Conversion of fibrinogen to fibrin, Fibrinogen (factor I) is a soluble glycoprotein, that constitutes 2-3% of plasma proteins (plasma, concentration 0.3 g/dl). Fibrinogen consists of 6, polypeptide chains-two A D, two B E and two J, making the structure (A D)2 (B E)2 J2., Fibrinogen undergoes proteolytic cleavage, catalysed by thrombin to release small, fibrinopeptides (A and B). This results in the, formation of fibrin monomers which can stick, together to form hard clots (Fig.9.6). Clot, formation is further stabilized by covalent crosslinking between glutamine and lysine residues., This reaction cross-links fibrin clots and is, catalysed by fibrin stabilizing factor (XIII). The, red colour of the clot is due to the presence of, red cells entangled in the fibrin cross-links., , Conversion of prothrombin, to thrombin, Prothrombin (II) is the inactive zymogen form, of thrombin (IIa). The activation of prothrombin
Page 201 :
191, , Chapter 9 : PLASMA PROTEINS, , occurs on the platelets and requires the presence, of factors Va and Xa, besides phospholipids and, Ca2+., , The extrinsic pathway, The extrinsic pathway is very rapid and, occurs in response to tissue injury. This pathway, essentially, involves, the, conversion, of, proconvertin (VII) to its active form (VIIa) and the, generation factor Xa. The tissue factor (III),, found to be necessary to accelerate the action, VIIa on a factor X, is present in lung and brain., , The intrinsic pathway, The intrinsic pathway is rather slow. It, involves the participation of a contact system, (wounded surface) and a series of factors to, generate factor Xa., The Hageman factor (XII) is activated (XIIa) on, exposure to activating wound surface containing, collagen or platelet membranes. The formation, of XIIa is accelerated by kallikrein and HMK., The activated Hageman factor (XIIa) activates, factor XI. The XIa activate the Christmas factor, , (IX). The Christmas factor is also activated by, active proconvertin (VIIa)., In the next step, the Staurt factor (X) is, activated by Christmas factor (IXa) and this, reaction requires the presence of antihemophilic, factor (VIIIa), Ca2+ and phospholipids., The extrinsic and intrinsic pathways lead to, the formation of factor Xa which then, participates in the final common pathway to, ultimately result in the formation of fibrin clot., , Anticoagulants, Several substances, known as anticoagulants,, are in use to inhibit the blood clotting. Calcium, is essentially required for certain reactions of, blood coagulation. The substances which bind, with Ca2+ are very effective as anticoagulants., These include oxalate, fluoride, EDTA and, citrate., , Heparin is an anticoagulant used to maintain, normal hemostasis. It is a heteropolysaccharide, found in many tissues including mast cells in the, endothelium of blood vessels. Heparin combines, with antithrombin III which in turn, inhibits the, , TABLE 9.3 Blood coagulation factors in humans, , Factor number, I, II, III, IV, V, VII, VIII, IX, X, XI, XII, XIII, —, —, , Common name(s), Fibrinogen, Prothrombin, Tissue factor, thromboplastin, Calcium (Ca2+), Proaccelerin, labile factor, Proconvertin, serum prothrombin conversion accelerator (SPCA), Antihemophilic factor A, antihemophilic globulin (AHG), Christmas factor, antihemophilic factor B,, Plasma thromboplastin component (PTC), Staurt-Prower factor, Plasma thromboplastin antecedent (PTA), Hageman factor, Fibrin-stabilizing factor (FSF), fibrinoligase, Liki Lorand factor, Prekallikrein, High molecular weight kininogen (HMK), , Subunit molecular weight, 340,000, 720,000, 370,000, —, 330,000, 50,000, 330,000, 56,000, 56,000, 160,000, 80,000, 320,000, 88,000, 150,000, , Note : The numbers represent the order of their discovery and not the order of their action. Factor Va was once referred to as factor VI,, hence there is no factor VI.
Page 202 :
192, , BIOCHEMISTRY, , Intrinsic, pathway, , Kallikrein, Wounded, surface, , Prekallikrein, XIIa, , Factor XII, , Extrinsic, pathway, , XIa, , Factor XI, , Factor VIII, , Factor VII, , VIIa, , IXa, , Factor IX, , Factor III, , VIIIa, Xa, , Factor X, Factor V, Final, common, pathway, , Factor X, , Va, Prothrombin (II), , Thrombin (IIa), , Fibrinogen (I), Factor XIII, , Fibrin, (monomer), , XIIIa, Fibrin, (hard clot), , Fig. 9.5 : The blood clotting cascade in humans, (the active forms of the factors are represented in red with subscript ‘a’)., , clotting factors II, IX, X, XI, XII and kallikrein., Heparin can be administered to patients during, and after surgery to retard blood clotting., The blood contains another anticoagulant—, namely protein C—which is activated by, thrombin. Active protein C hydrolyses and, inactivates clotting factors V and VIII., , Warfarin, a vitamin K antagonist may be, considered as an oral anticoagulant. This acts by, reducing the synthesis of certain clotting factors, (II, VII, IX and X)., , Streptokinase is a therapeutic fibrinolytic, agent which activates plasminogen., , Fibrinogen, , Thrombin, , Fibrinopeptides A and B, (, ), , Fibrin monomer, , Fibrinolysis, The term fibrinolysis refers to the dissolution, or lysis of blood clots. Plasmin is mostly, responsible for the dissolution of fibrin clots., Plasminogen, synthesized in the kidney, is the, inactive, precursor, of, plasmin., Tissue, plasminogen activator (TPA) and urokinase, convert plasminogen to plasmin., , Fibrin clot, , Fig. 9.6 : Diagrammatic representation of, fibrin clot formation from fibrinogen.
Page 203 :
193, , Chapter 9 : PLASMA PROTEINS, , Abnormalities in blood clotting, Several abnormalities associated with blood, clotting are known. These are due to defects in, clotting factors which may be inherited or, acquired. Hemophilia, Von Willebrand’s disease, etc., are examples of inherited disorder while, afibrinogenemia is an acquired disease., Hemophilia A (classical hemophilia) : This is, a sex-linked disorder transmitted by females, affecting males. Hemophilia A is the most, common clotting abnormality and is due to the, deficiency of antihemophilic factor (VIII). The, affected individuals have prolonged clotting time, , and suffer from internal bleeding (particularly in, joints and gastrointestinal tract). Hemophilia A, has gained importance due to the fact that the, Royal families of Britain are among the affected, individuals., Hemophilia B (Christmas disease) : This is, due to the deficiency of Christmas factor (IX)., The clinical symptoms are almost similar to that, found in hemophilia A., Von Willebrand’s disease : This disorder is, characterized by failure of platelets to aggregate, and is due to a defect in the platelet adherence, factor., , + Albumin, the most abundant plasma protein, is involved in osmotic function,, transport of several compounds (fatty acids, steroid hormones), besides the buffering, action., , + Hypoalbuminemia and albuminuria are observed in nephrotic syndrome., + D1-Antitrypsin deficiency has been implicated in emphysema (abnormal distension of, lungs by air) which is more commonly associated with heavy smoking., , + Haptoglobin prevents the possible loss of free hemoglobin from the plasma through the, kidneys by forming haptoglobin-hemoglobin complex., , + Immunoglobulins (antibodies), a specialized group of plasma globular proteins, are, actively involved in immunity. IgG and IgM are primarily concerned with humoral, immunity while IgE is associated with allergic reactions., , + Multiple myeloma, a plasma cell cancer disease of bone marrow, is characterized by, overproduction of abnormal immunoglobulins (mostly IgG). Laboratory diagnosis of, multiple myeloma can be made by the presence of a distinct M band on plasma/serum, electrophoresis., , + Blood clotting or coagulation is the body’s major defense mechanism against, blood loss. Defects in clotting factors cause coagulation abnormalities such, as hemophilia A (deficiency of factor VIII) and Christmas disease (deficiency of, factor IX)., , + Anticoagulants inhibit blood clotting. These include heparin, oxalate, fluoride, EDTA, and citrate.
Page 204 :
194, , BIOCHEMISTRY, , 1. The total concentration of plasma proteins is about 6-8 g/dl. Electrophoresis separates, plasma proteins into 5 distinct bands, namely albumin, D1, D2, E and J globulins., 2. Albumin is the major constituent (60%) of plasma proteins with a concentration 3.5 to, 5.0 g/dl. It is exclusively synthesized by the liver. Albumin performs osmotic, transport, and nutritive functions., 3. D1-Antitrypsin is a major constituent of D1 globulin fraction. D1-Antitrypsin deficiency, has been implicated in emphysema and a specific liver disease., 4. Haptoglobin (Hp) binds with free hemoglobin (Hb) that spills into the plasma due to, hemolysis. The Hp-Hb complex cannot pass through the glomeruli, hence haptoglobin, prevents the loss of free hemoglobin into urine., 5. Alterations in the acute phase proteins (e.g. D1-antitrypsin, ceruloplasmin, C-reactive, protein) are observed as a result of non-specific response to the stimulus of infection,, injury, inflammation etc. Estimation of serum C-reactive protein is used for the, evaluation of acute phase response., 6. Immunoglobulins are specialized proteins to defend the body against the foreign, substances. They are mostly associated with J-globulin fraction of plasma proteins. The, immunoglobulins essentially consist of two identical heavy chains and two identical, light chains, held together by disulfide linkages., 7. Five classes of immunoglobulins—namely IgG, IgA, IgM, IgD and IgE—are found in, humans. IgG is most abundant and is mainly responsible for humoral immunity. IgA, protects body surfaces. IgM serves as a first line of defense for humoral immunity while, IgE is associated with allergic reactions., 8. Multiple myeloma is due to the malignancy of a single clone of plasma cells in the bone, marrow. This causes the overproduction of abnormal IgG. The plasma of multiple, myeloma patients on electrophoresis shows a distinct M-band., 9. Blood clotting is the body’s major defense mechanism against blood loss. The extrinsic, and intrinsic pathways lead to the formation of factor Xa which then participates in the, final common pathway to activate prothrombin to thrombin. Fibrinogen is then, converted to fibrin clot., 10. Plasmin is mostly responsible for the dissolution of fibrin clots. Plasminogen,, synthesized by the kidney, is the inactive precursor of plasmin. Tissue plasminogen, activator (TPA) and urokinase convert plasminogen to plasmin.
Page 205 :
Chapter 9 : PLASMA PROTEINS, , 195, , I. Essay questions, 1. Describe the characteristics and major functions of plasma proteins., 2. Give an account of different types of immunoglobulins along with their functions., 3. Discuss the cascade of blood clotting process., 4. Describe the structure of different immunogloublins., 5. Discuss the role of acute phase proteins in health and disease., , II. Short notes, (a) Electrophoresis of plasma proteins, (b) Functions of albumin, (c) D1-Antitrypsin, (d) Haptoglobin,, (e) Immunoglobulin G, (f) Multiple myeloma, (g) Bence-Jones proteins, (h) Fibrinogen,, (i) Anticoagulants, (j) Hemophilia., , III. Fill in the blanks, 1. The difference between plasma and serum is the presence or absence of ______________., 2. The most commonly employed technique for separation of plasma proteins ______________., 3. Haptoglobin binds and prevents the excretion of the compound ______________., 4. The cells responsible for the production of immunoglobulins ______________., 5. The immunoglobulin that can cross the placenta and transfer the mother’s immunity to the, developing fetus ______________., 6. The immunoglobulins that can bind with mast cells and release histamine ______________., 7. Bence-Jones proteins are precipitated when urine is heated to ______________., 8. The major component of acute phase proteins used for the evaluation of acute phase response, ______________., 9. The extrinsic and intrinsic pathways result in the formation of a common activated, factor ______________., 10. The factor mostly responsible for the lysis of blood clot ______________., , IV. Multiple choice questions, 11. Hemophilia A is due to the deficiency of clotting factor, (a) X (b) V (c) VIII (d) II., 12. Plasma albumin performs the following functions, (a) Osmotic (b) Transport (c) Nutritive (d) All of them., 13. The immunoglobulin present in most abundant quantity, (a) IgG (b) IgA (c) IgM (d) IgE., 14. Name the immunoglobulin involved in body allergic reactions, (a) IgA (b) IgE (c) IgD (d) IgM., 15. The following anticoagulant binds with Ca2+ and prevents blood clotting, (a) Heparin (b) Oxalate (c) Protein C (d) All of them.
Page 206 :
Section 2, , Physiological Biochemistry, , Chapter, , Hemoglobin and Porphyrins, , 10, , The hemoglobin speaks :, , “I am the red of blood, responsible for respiration;, Deliver O2 to tissues and return CO2 to lungs;, Influenced by factors pH, BPG and Cl – in my functions;, Disturbed in my duties by structural abnormalities.”, , T, , he structure, functions and abnormalities of, hemoglobin, the synthesis and degradation, of heme, the porphyrin containing compounds, are discussed in this chapter., , E�, , D�, , HEMOGLOBIN, Hemoglobin (Hb) is the red blood pigment,, exclusively found in erythrocytes (Greek:, erythrose—red; kytos—a hollow vessel). The, normal concentration of Hb in blood in males, is 14–16 g/dl, and in females 13–15 g/dl., Hemoglobin performs two important biological, functions concerned with respiration, 1. Delivery of O2 from the lungs to the, tissues., 2. Transport of CO2 and protons from tissues, to lungs for excretion., , Structure of hemoglobin, Hemoglobin (mol. wt. 64,450) is a conjugated, protein, containing globin—the apoprotein, , E�, , D�, , Fig. 10.1 : Diagrammatic representation of hemoglobin, with 2D and 2E chains (Red blocks–Heme)., , part—and the heme—the non-protein part, (prosthetic group). Hemoglobin is a tetrameric, allosteric protein (Fig.10.1)., Structure of globin : Globin consists of four, polypeptide chains of two different primary, structures (monomeric units). The common form, of adult hemoglobin (HbA1) is made up of two, D-chains and two E-chains (D2E2). Some authors, consider hemoglobin consisting of two identical, dimers—(DE)1 and (DE)2. Each D-chain contains, 141 amino acids while E-chain contains 146, amino acids. Thus HbA1 has a total of 574, , 196
Page 207 :
197, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , M, , V, , O O, , A, N, M, , M, D N, , Fe, , 2+, , N B, V, , P, N, , Hereditary persistence of fetal hemoglobin, (HPFH) is a condition in which fetal hemoglobin, synthesis is not terminated at birth but, continues, into, adulthood., Glycosylated, hemoglobin (HbA1c), formed by covalent, binding of glucose is also found in low, concentration. It is increased in diabetes mellitus, which is successfully utilized for the prognosis of, these patients (Refer Chapter 36)., , C, , Myoglobin, N, , P, , M, , Histidine of, globin, , HN, , CH2, , Fig. 10.2 : Structure of heme [M-Methyl ( CH3); V-Vinyl, ( CH2 CH2 ); P-Propionyl ( CH2 CH2 CH2)., , amino acid residues. The four subunits of, hemoglobin are held together by non-covalent, interactions primarily hydrophobic, ionic and, hydrogen bonds. Each subunit contains a heme, group., Structure of heme : The characteristic red, colour of hemoglobin (ultimately blood) is due, to heme. Heme contains a porphyrin molecule, namely protoporphyrin IX, with iron at its, center. Protoporphyrin IX consists of four pyrrole, rings to which four methyl, two propionyl and, two vinyl groups are attached (Fig.10.2)., Heme is common prosthetic group present in, cytochromes, in certain enzymes such as, catalase, tryptophan pyrolase, and chlorophyll, (Mg2+). In case of cytochromes, oxidation and, reduction of iron (Fe2+, electron transport chain., , Fe3+) is essential for, , Other forms of hemoglobin, Besides the adult hemoglobin (HbA1), described above, other minor hemoglobins are, also found in humans (Table 10.1). In adults a, small fraction (< 5%) of hemoglobin, known as, HbA2 is present. HbA2 is composed of two D, and two G (delta) chains. Fetal hemoglobin (HbF), is synthesized during the fetal development and, a little of it may be present even in adults., , Myoglobin (Mb) is monomeric oxygen, binding hemoprotein found in heart and skeletal, muscle. It has a single polypeptide (153 amino, acids) chain with heme moiety. Myoglobin (mol., wt. 17,000) structurally resembles the individual, subunits of hemoglobin molecule. For this, reason, the more complex properties of, hemoglobin have been conveniently elucidated, through the study of myoglobin., Myoglobin functions as a reservoir for, oxygen. It further serves as oxygen carrier that, promotes the transport of oxygen to the rapidly, respiring muscle cells., , Functions of hemoglobin, Hemoglobin is largely responsible for the, transport of O2 from lungs to tissues. It also helps, to transport CO2 from the tissues to the lungs., , Binding of O2 to hemoglobin, One molecule of hemoglobin (with four, hemes) can bind with four molecules of O2. This, is in contrast to myoglobin (with one heme), which can bind with only one molecule of, oxygen. In other words, each heme moiety can, bind with one O2., TABLE 10.1 Normal major types of hemoglobins, , Type, , Composition and, symbol, , Percentage of, total hemoglobin, , HbA1, , D2E2, , 90%, , HbA2, , D2G2, , < 5%, , HbF, , D 2 J2, , < 2%, , HbA1c, , D2E2-glucose, , < 5%
Page 208 :
198, , BIOCHEMISTRY, , 100, , Myoglobin, , % Saturation with O2, , pO2 in lungs, , Hemoglobin, , 50, , pO2 in tissues, , 0, 50, pO2 (mm Hg), , 100, , Fig. 10.3 : Oxygen dissociation curves of hemoglobin, and myoglobin (pO2 Partial pressure of oxygen)., , Oxygen dissociation curve : The binding, ability of hemoglobin with O2 at different partial, pressures of oxygen (pO2) can be measured by a, graphic representation known as O2 dissociation, curve. The curves obtained for hemoglobin and, myoglobin are depicted in Fig.10.3., It is evident from the graph that myoglobin, has much higher affinity for O2 than, hemoglobin. Hence O2 is bound more tightly, with myoglobin than with hemoglobin. Further,, pO2 needed for half saturation (50% binding) of, myoglobin is about 1 mm Hg compared to about, 26 mm Hg for hemoglobin., , Cooperative binding, of O2 to hemoglobin, The oxygen dissociation curve for hemoglobin, is sigmoidal in shape (Fig.10.3). This indicates, that the binding of oxygen to one heme increases, , O2, O2, , O2, O2, , the binding of oxygen to other hemes. Thus the, affinity of Hb for the last O2 is about 100 times, greater than the binding of the first O2 to Hb., This phenomenon is referred to as cooperative, binding of O2 to Hb or simply heme-heme, interaction (Fig.10.4). On the other hand, release, of O2 from one heme facilitates the release of, O2 from others. In short, there is a, communication among heme groups in the, hemoglobin function., , Transport of O2 to the tissues, In the lungs, where the concentration of O2 is, high (hence high pO2), the hemoglobin gets fully, saturated (loaded) with O2. Conversely, at the, tissue level, where the O2 concentration is low, (hence low pO2), the oxyhemoglobin releases, (unloads) its O2 for cellular respiration. This is, often mediated by binding O2 to myoglobin, which serves as the immediate reservoir and, supplier of O2 to the tissues (Fig.10.5)., , T and R forms of hemoglobin, The four subunits (D2E2) of hemoglobin are, held together by weak forces. The relative, position of these subunits is different in, oxyhemoglobin compared to deoxyhemoglobin., T-form of Hb : The deoxy form of hemoglobin, exists in a T or taut (tense) form. The hydrogen, and ionic bonds limit the movement of, monomers. Therefore, the T-form of Hb has low, oxygen affinity., R-form of Hb : The binding of O2 destabilizes, some of the hydrogen and ionic bonds, particularly between DE dimers. This results in a, relaxed form or R-form of Hb wherein the, , O2, , O2, , O2, , O2, , O2, , O2, , O2, , O2, , O2, , O2, Increasing affinity for O2, , Fig. 10.4 : Cooperative binding of O2 to hemoglobin (, , : T or taut form;, , : R or relaxed form ).
Page 209 :
199, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , subunits move a little freely. Therefore,, the R-form has high oxygen affinity., , LUNGS, , The existence of hemoglobin in two, forms (T and R) suitably explains the, allosteric behaviour of hemoglobin, (Fig.10.4)., , CO2, , O2, , Transport of CO2, by hemoglobin, In aerobic metabolism, for every, molecule of O2 utilized, one molecule of, CO2 is liberated. Hemoglobin actively, participates in the transport of CO2 from, the tissues to the lungs. About 15% of, CO2 carried in blood directly binds with, Hb. The rest of the tissue CO2 is, –, transported as bicarbonate (HCO3)., , –, , NHCOO, , O2, , Fe, , Fe Fe, , CarbamylHb, , Fe, , Carbon dioxide molecules are bound, to the uncharged D-amino acids of, hemoglobin, to, form, carbamyl, hemoglobin as shown below, Hb – NH2 + CO2, , Fe, , O2, , OxyHb, , Fe Fe, , Fe, O2, , –, , NHCOO, , O2, , TISSUES, Myoglobin, , –, , Hb – NH – COO + H+, , The oxyHb can bind 0.15 moles CO2/, mole heme, whereas deoxyHb can bind, 0.40 moles CO2/mole heme. The binding, of CO2 stabilizes the T (taut) form of, hemoglobin structure, resulting in, decreased O2 affinity for Hb., Hemoglobin also helps in the, transport of CO2 as bicarbonate, as, explained below (Fig.10.6)., As the CO2 enters the blood from tissues, the, enzyme carbonic anhydrase present in, erythrocytes catalyses the formation of carbonic, acid (H2CO3). Bicarbonate (HCO3–) and proton, (H+) are released on dissociation of carbonic, acid. Hemoglobin acts as a buffer and, immediately binds with protons. It is estimated, that for every 2 protons bound to Hb, 4 oxygen, molecules are released to the tissues. In the, lungs, binding O2 to Hb results in the release of, protons. The bicarbonate and protons combine, to form carbonic acid. The latter is acted upon, by carbonic anhydrase to release CO2, which is, exhaled., , O2, Myoglobin O2, CO2, O2, Cellular, respiration, , Fig. 10.5 : Diagrammatic representation of, transport of O2 and CO2 by hemoglobin., , BOHR EFFECT, The binding of oxygen to hemoglobin, decreases with increasing H+ concentration, (lower pH) or when the hemoglobin is exposed, to increased partial pressure of CO2 (pCO2). This, phenomenon is known as Bohr effect. It is due to, a change in the binding affinity of oxygen to, hemoglobin. Bohr effect causes a shift in, the oxygen dissociation curve to the right, (Fig.10.7)., Bohr effect is primarily responsible for the, release of O2 from the oxyhemoglobin to the, tissue. This is because of increased pCO2 and, decreased pH in the actively metabolizing cells.
Page 210 :
200, , BIOCHEMISTRY, , 2CO2 + 2H2O, , Exhaled, , Carbonic, anhydrase, , 2CO2 + 2H2O, , 2H2CO3, 2H2CO3, Hb.4O2, –, , 2H+, , 2HCO3, , –, , 2H+ + 2HCO3, , 4O2, , 4O2, Hb.2H+, PERIPHERAL, TISSUES, , LUNGS, , Fig. 10.6 : Transport of CO2 through the mediation of hemoglobin., , The Bohr effect may be simplified as follows, HbO2 + H+ o Hb H+ + O2, Any increase in protons and/or lower pO2, shifts the equilibrium to the right to produce, deoxyhemoglobin as happens in the tissues. On, the other hand, any increase in pO2 and / or a, decrease in H+ shifts the equilibrium to the left,, which occurs in lungs., When CO2 binds to hemoglobin, carbamyl, hemoglobin is produced (details described under, transport of CO2). This causes the removal of, protons from the terminal NH2 group and, stabilizes the structure of Hb in the T form, (deoxyhemoglobin). Therefore, the binding of, CO2 promotes the release of oxygen (in tissues)., On the other hand, when hemoglobin is, oxygenated in lungs, CO2 is released as it binds, loosely with R-form of Hb., , Role of Cl– in oxygen transport, Chloride (Cl–) is bound more tightly to deoxyhemoglobin than to oxyhemoglobin. This, facilitates the release of O2 which is explained, as follows, (HCO–3), , is freely permeable, Bicarbonate, across the erythrocyte membrane. Once, produced in the erythrocytes, HCO3– freely, moves out and equilibrates with the surrounding, , The, four, substances, namely 2,3-bisphosphoglycerate, (described below), CO2, H+ and, Cl– are collectively called as, allosteric, effectors., They, interact with the hemoglobin, molecule and facilitate the, release of O2 from oxyhemoglobin., , EFFECT OF 2,3-BISPHOSPHOGLYCERATE ON O2 AFFINITY, OF Hb, 2,3-Bisphosphoglycerate (2,3-BPG; formerly,, 2,3-diphosphoglycerate) is the most abundant, organic phosphate in the erythrocytes. Its molar, concentration is approximately equivalent to that, of hemoglobin. 2,3-BPG is produced in, the erythrocytes from an intermediate (1,3bisphosphoglycerate) of glycolysis. This short, pathway, referred to as Rapaport-Leubering, cycle, is described in carbohydrate metabolism, (Chapter 13)., , 100, pH-7.6, , % Saturation with O2, , Mechanism of Bohr effect, , plasma. In order to maintain, neutrality, Cl– enters the, erythrocytes and binds with, deoxyhemoglobin. The concentration of Cl– is greater in, venous blood than in arterial, blood., , pH-7.2, 50, , 0, 50, pO2 (mm Hg), , 100, , Fig. 10.7 : Effect of pH (Bohr effect) on oxygen, dissociation curve (pO2-Partial pressure of O2 ).
Page 211 :
201, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , Stripped Hb, (no 2, 3-BPG), , On oxygenation of hemoglobin, 2,3-BPG is, expelled from the pocket and the oxyhemoglobin, attains the R-form of structure., , % Saturation with O2, , 100, , Normal blood, (with 2,3-BPG), Blood of anemic patient, (2,3-BPGn), , 50, , 0, 50, pO2 (mm Hg), , 100, , Fig. 10.8 : Effect of pH (Bohr effect) on oxygen, dissociation curve (pO2-Partial pressure of O2)., , Binding of 2,3-BPG, to deoxyhemoglobin, 2,3-BPG regulates the binding of O2 to, hemoglobin. It specifically binds to deoxyhemoglobin (and not to oxyhemoglobin) and, decreases the O2 affinity to Hb. The effect of, 2,3-BPG on Hb may be summarized as follows, HbO2 + 2,3-BPG o Hb-2,3-BPG + O2, OxyHb, , DeoxyHb bound, to 2,3-BPG, , The reduced affinity of O2 to Hb facilitates, the release of O2 at the partial pressure found in, the tissues. This 2,3-BPG shifts the oxygen, dissociation curve to the right (Fig.10.8)., , Clinical significance of 2,3-BPG, Since the binding of 2,3-BPG with, hemoglobin is primarily associated with the, release of O2 to the tissues, this small molecule, assumes a lot of biomedical significance. The, erythrocyte levels of 2,3-BPG are related to tissue, demands of oxygen supply., 1. In hypoxia : The concentration of 2,3-BPG, in erythrocytes is elevated in chronic hypoxic, conditions associated with difficulty in O2, supply. These include adaptation to high, altitude, obstructive pulmonary emphysema, (airflow in the bronchioles blocked) etc., 2. In anemia : 2,3-BPG, levels, are, increased in severe anemia in order to cope up, with the oxygen demands of the body. This is an, adaptation to supply as much O2 as possible to, the tissue, despite the low hemoglobin levels., 3. In blood transfusion : Storage of blood in, acid citrate-dextrose medium results in the, decreased concentration of 2,3-BPG. Such, blood when transfused fails to supply O2 to the, tissues immediately., Addition of inosine (hypoxanthine-ribose) to, the stored blood prevents the decrease of 2,3BPG. The ribose moiety of inosine gets, phosphorylated and enters the hexose, monophosphate pathway and finally gets, converted to 2,3-BPG., , Mechanism of action of 2,3-BPG, One molecule of 2,3-BPG binds with one, molecule (tetramer) of deoxyhemoglobin in the, central cavity of the four subunits. This central, pocket has positively charged (e.g. histidine,, lysine) two E-globin chains. Ionic bonds (salt, bridges) are formed between the positively, charged amino acids (of E globins) with the, negatively charged phosphate groups of 2,3-BPG, (Fig.10.9). The binding of 2,3-BPG stabilizes the, deoxygenated hemoglobin (T-form) by crosslinking the E-chains., , O–, , O, E�, , E�, ++, +++, , ++, +++, , –, , H C O P O, H C H O, O, , D�, (A), , O, –, , 2, 3-BPG, , D�, , C, , O P O, , –, , –, , O, , (B), , Fig. 10.9 : (A) Diagrammatic representation of binding of, 2,3-BPG to deoxyhemoglobin; (B) Structure of 2,3-BPG.
Page 212 :
202, , BIOCHEMISTRY, , 4. Fetal hemoglobin (HbF) : The binding of, 2,3-BPG to fetal hemoglobin is very weak., Therefore, HbF has higher affinity for O2, compared to adult hemoglobin (HbA). This may, be needed for the transfer of oxygen from the, maternal blood to the fetus., , HEMOGLOBIN DERIVATIVES, Hemoglobin (specifically heme) combines, with different ligands and forms hemoglobin, derivatives. The normal blood contains oxyHb, and deoxyHb. Besides these, methemoglobin, (metHb) and carboxyhemoglobin are the other, important Hb derivatives. The Hb derivatives, have characteristic colour and they can be, detected by absorption spectra., , Methemoglobin, For the biological function of hemoglobin—to, carry oxygen—the iron should remain in the, ferrous (Fe2+) state. Hemoglobin (Fe2+) can be, oxidized to methemoglobin (Fe3+). In normal, circumstances, however, molecular oxygen does, not oxidize Hb, it only loosely binds to form, oxyhemoglobin., The, oxidation, of, hemoglobin, to, methemoglobin (metHb) may be caused in the, living system by H2O2, free radicals and drugs., The methemoglobin (with Fe3+) is unable to bind, to O2. Instead, a water molecule occupies the, oxygen site in the heme of metHb., In normal circumstances, the occasional, oxidation of hemoglobin is corrected by the, enzyme methemoglobin reductase present in, erythrocytes (Fig.10.10)., , Carboxyhemoglobin (COHb), Carbon monoxide (CO) is a toxic compound, (an industrial pollutant) that can bind with Hb in, the same manner as O2 binds. However, CO has, about 200 times more affinity than O2 for, binding with Hb., Clinical manifestations of CO toxicity are, observed when the COHb concentration exceeds, 20%. The symptoms include headache, nausea,, , H2O2, free radicals, drugs, , Hemoglobin, , Methemoglobin, , (Fe 2+), , (Fe3+), Methemoglobin, reductase, NAD+, , NADH + H+, , Fig. 10.10 : Conversion of hemoglobin to, methemoglobin and vice versa., , breathlessness, vomiting and irritability. Administration of O2 through oxygen masks will help to, reverse the manifestations of CO toxicity., , ABNORMAL HEMOGLOBINS, Abnormal hemoglobins are the resultant of, mutations in the genes that code for D or E, chains of globin. As many as 400 mutant, hemoglobins are known. About 95% of them are, due to alteration in a single amino acid of globin., , Basic concepts of globin synthesis, For a better understanding of abnormal, hemoglobins, it is worthwhile to have a basic, knowledge of globin synthesis. The globin genes, are organised into two gene families or clusters, (Fig.10.11)., 1. D-Gene family : There are two genes, coding for D-globin chain present on each one, of chromosome 16. The ]-gene, other member, of D-gene cluster is also found on chromosome, 16 and is active during the embryonic, development., 2. E-Gene family : The synthesis of E-globin, occurs from a single gene located on each one, of chromosome 11., This chromosome also contains four other, genes., One H-gene expressed in the early stages of, embryonic development.
Page 213 :
203, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, D2, , ], , D1, D-Globin-like genes, (chromosome 16), D, , ], , Globin chains, , ]2H2, , D2J2, , D2G2, , D2E2, , Hemoglobins, , H, , J, , G, , E, , Globin chains, , E-Globin-like genes, (chromosome 11), H, , GJ, , AJ, , G, , E, , Fig. 10.11 : Diagrammatic representation of globin genes with the synthesis of globin chains, and hemoglobins (]2H2-Hb Gower 1; D2J2-HbF; D2G2-HbA2; D2E2-HbA1 )., , Two J-genes (GJ and AJ) synthesize J-globin, chains of fetal hemoglobin (HbF)., One G-gene producing G-globin chain found, in adults to a minor extent (HbA2)., , Hemoglobinopathies, It is a term used to describe the disorders, caused by the synthesis of abnormal hemoglobin, molecule or the production of insufficient, quantities of normal hemoglobin or rarely both., , Sickle-cell anemia (HbS) and hemoglobin C, disease (HbC) are the classical examples of, abnormal hemoglobins. Thalassemias, on the, other hand, are caused by decreased synthesis of, normal hemoglobin., , SICKLE-CELL ANEMIA OR, SICKLE-CELL HEMOGLOBIN, , the black population. It is estimated that 1 in, 500 newborn black infants in the USA are, affected by sickle-cell anemia., , Molecular basis of HbS, The structure of hemoglobin (as described, already) contains two D-and two E-globin chains., In case of sickle-cell anemia, the hemoglobin, (HbS) has two normal D-globin chains and two, abnormal (mutant) E-globin chains. This is due, to a difference in a single amino acid. In HbS,, glutamate at sixth position of E-chain is, replaced by valine (Glu E6 o Val)., Sickle-cell anemia is due to a change, (missense mutation) in the single nucleotide, (thymine o adenine) of E-globin gene. This error, causes the formation of altered codon (GUG in, , Sickle-cell anemia (HbS) is the most common, form of abnormal hemoglobins. It is so named, because the erythrocytes of these patients adopt, a sickle shape (crescent like) at low oxygen, concentration (Fig.10.12)., , Occurrence of the disease, Sickle-cell anemia is largely confined to, tropical areas of the world. It primarily occurs in, , (A), , (B), , Fig. 10.12 : Erythrocytes : (A) From a normal person;, (B) From a patient of sickel-cell anemia.
Page 214 :
204, , BIOCHEMISTRY, , CTC, , CAC, , DNA, , GAG, , GUG, , RNA (codon), , HN CH CO, , Amino acid, , HN CH CO, , CH2, H3C, , CH2, , CH, CH3, , –, , (, , COO, Glu, , ), , (, , Hemoglobin A, , Val, , ), , E-Chain 6th position, , Hemoglobin S, , Fig. 10.13 : Formation of E-chain of hemoglobin in normal and sickle cell anemia (Note : Single base mutation in, DNA (T o A ) causes replacement of glutamate by valine at 6th position of E-chain)., , place of GAG) which leads to the incorporation, of valine instead of glutamate at the sixth, position in E-chain (Fig.10.13)., Homozygous and heterozygous HbS : Sicklecell anemia is said to be homozygous, if caused, by inheritance of two mutant genes (one from, each parent) that code for E-chains. In case of, heterozygous HbS, only one gene (of E-chain), is affected while the other is normal. The, erythrocytes of heterozygotes contain both HbS, and HbA and the disease is referred to as sicklecell trait which is more common in blacks, (almost 1 in 10 are affected). The individuals, of sickle-cell trait lead a normal life, and do, not usually show clinical symptoms. This, is in contrast to homozygous sickle-cell, anemia., , Abnormalities associated with HbS, Sickle-cell anemia is characterized by the, following abnormalities, 1. Life-long hemolytic anemia : The sickled, erythrocytes are fragile and their continuous, breakdown leads to life-long anemia., 2. Tissue damage and pain : The sickled cells, block the capillaries resulting in poor blood, supply to tissues. This leads to extensive damage, and inflammation of certain tissues causing pain., 3. Increased susceptibility to infection :, Hemolysis and tissue damage are accompanied, by increased susceptibility to infection and, diseases., , 4. Premature death : Homozygous individuals, of sickle-cell anemia die before they reach, adulthood (< 20 years)., , Mechanism of sickling, in sickle-cell anemia, Glutamate is a polar amino acid and it is, replaced by a non-polar valine in sickle-cell, hemoglobin. This causes a marked decrease in, the solubility of HbS in deoxygenated form, (T-form). However, solubility of oxygenated HbS, is unaffected., , Sticky patches and formation of, deoxyhemoglobin fibres, The substitution of valine for glutamate results, in a sticky patch on the outer surface of E-chains., It is present on oxy- and deoxyhemoglobin S but, absent on HbA. There is a site or receptor, complementary to sticky patch on deoxyHbS., The sticky patch of one deoxyHbS binds with, the receptor of another deoxyHbS and this, process continuous resulting in the formation of, long aggregate molecules of deoxyHbS, (Fig.10.14). Thus, the polymerization of deoxyHbS molecules leads to long fibrous precipitates, (Fig.10.15). These stiff fibres distort the, erythrocytes into a sickle or crescent shape, (Fig.10.12). The sickled erythrocytes are highly, vulnerable to lysis., In case of oxyHbS, the complementary, receptor is masked, although the sticky patch is
Page 215 :
205, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , OxyHbA, E, , D, , D, , E, , DeoxyHbA, , OxyHbS, , DeoxyHbS, , Fig. 10.14 : Diagrammatic representation of sticky patch (Blue) and sticky patch receptor ( > ), in the formation of long aggregates of deoxyhemoglobins., , present (Fig.10.14). Hence, the molecules of, oxyHbS cannot bind among themselves or with, the molecules of deoxyHbS., Normal deoxyHbA lacks sticky patches but, contains receptors. Absence of sticky patches, does not allow the deoxyHbA to participate in, the formation of aggregates., As explained above, sickling is due to, polymerization of deoxyHbS. Therefore, if HbS, is maintained in the oxygenated form (or with, minimum deoxyHbS), sickling can be prevented., , Sickle-cell trait provides, resistance to malaria, , 2. More recent studies indicate that malarial, parasite increases the acidity of erythrocytes (pH, down by 0.4). The lowered pH increases the, sickling of erythrocytes to about 40% from the, normally occurring 2%. Therefore, the entry of, malarial parasite promotes sickling leading to, lysis, of, erythrocytes., Furthermore,, the, concentration of K+ is low in sickled cells which, is unfavourable for the parasite to survive., Sickle-cell trait appears to be an adaptation, for the survival of the individuals in malariainfested regions. Unfortunately, homozygous, individuals, the patients of sickle-cell anemia, (much less frequent than the trait), cannot live, beyond 20 years., , The incidence of sickle-cell disease coincides, with the high incidence of malaria in tropical, areas of the world (particularly among the black, Africans)., , Sickle-cell trait (heterozygous state with about, 40% HbS) provides resistance to malaria which, is a major cause of death in tropical areas. This, is explained as follows, 1. Malaria is a parasitic disease caused by, Plasmodium falciparum in Africa. The malarial, parasite spends a part of its life cycle in, erythrocytes. Increased lysis of sickled cells, (shorter life span of erythrocytes) interrupts the, parasite cycle., , Fig. 10.15 : Diagrammatic representation, a fibre of aggregated deoxyhemoglobin.
Page 216 :
206, , BIOCHEMISTRY, , Diagnosis of sickle-cell anemia, 1. Sickling test : This is a simple microscopic, examination of blood smear prepared by adding, reducing agents such as sodium dithionite., Sickled erythrocytes can be detected under the, microscope., 2. Electrophoresis : When subjected to, electrophoresis in alkaline medium (pH 8.6),, sickle-cell hemoglobin (HbS) moves slowly, towards anode (positive electrode) than, does adult hemoglobin (HbA). The slow mobility, of HbS is due to less negative charge, caused, by the absence of glutamate residues that, carry negative charge. In case of sickle-cell, trait, the fast moving HbA and slow moving, HbS are observed. The electrophoresis of, hemoglobin obtained from lysed erythrocytes, can be routinely used for the diagnosis of sicklecell anemia and sickle-cell trait (Fig.10.16)., , Management of sickle-cell disease, Administration of sodium cyanate inhibits, sickling of erythrocytes, Cyanate increases the, affinity of O2 to HbS and lowers the formation of, deoxyHbS. However, it causes certain sideeffects like peripheral nerve damage., In patients with severe anemia, repeated, blood transfusion is required. This may result in, iron overload and cirrhosis of liver., Replacement of HbS with other forms of, hemoglobins has been tried. Fetal hemoglobin, (HbF) reduces sickling. Sickle-cell disease awaits, gene-replacement therapy!, , Hemoglobin C disease, Cooley’s hemoglobinemia (HbC) is characterized by substitution of glutamate by lysine in, the sixth position of E-chain. Due to the presence, of lysine, HbC moves more slowly on, electrophoresis compared to HbA and HbS. HbC, disease occurs only in blacks. Both homozygous, and heterozygous individuals of HbC disease are, known. This disease is characterized by mild, hemolytic anemia. No specific therapy is, recommended., , Normal Sickle-cell Sickle-cell, trait, anemia, HbA, HbS, Origin, , Fig. 10.16 : Electrophoresis of hemoglobins, at pH 8.6 (HbA–Normal adult hemoglobin;, HbS–Sickle cell hemoglobin)., , Hemoglobin D, This is caused by the substitution of glutamine, in place of glutamate in the 121st positioin of, E-chain. Several variants of HbD are identified, from different places indicated by the suffix., For instance, HbD (Punjab), HbD (Los Angeles)., HbD, on electrophoresis moves along with, HbS., , Hemoglobin E, This is the most common abnormal, hemoglobin after HbS. It is estimated that about, 10% of the population in South-East Asia, (Bangladesh,, Thailand,, Myanmar), suffer, from HbE disease. In India, it is prevalent in, West Bengal. HbE is characterized by, replacement of glutamate by lysine at 26th, position of E-chain. The individuals of HbE, (either homozygous or heterozygous) have no, clinical manifestations., , THALASSEMIAS, Thalassemias are a group of hereditary, hemolytic disorders characterized by impairment/, imbalance in the synthesis of globin chains, of Hb., Thalassemias (Greek: thalassa-sea) mostly, occur in the regions surrounding the, Mediterranean sea, hence the name. These, diseases, however, are also prevalent in Central, Africa, India and the Far East.
Page 217 :
207, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , Molecular basis of thalassemias, The basic concepts in the synthesis of globin, chains have been described (See Fig.10.14)., Hemoglobin contains 2D and 2E globin chains., The synthesis of individual chains is so, coordinated that each D-chain has a, E-chain partner and they combine to finally, give hemoglobin (D2E2). Thalassemias are, characterized by a defect in the production of, D-or E-globin chain. There is however, no, abnormality in the amino acids of the individual, chains., Thalassemias occur due to a variety of, molecular defects, 1. Gene deletion or substitution,, 2. Underproduction or instability of mRNA,, 3. Defect in the initiation of chain synthesis,, 4. Premature chain termination., , D-Thalassemias, D-Thalassemias are caused by a decreased, synthesis or total absence of D-globin chain of, Hb. There are four copies of D-globin gene, two, on each one of the chromosome 16. Four types, of D-thalassemias occur which depend on the, , number of missing D-globin genes. The salient, features of different D-thalassemias are given in, Table 10.2., 1. Silent carrier state is due to loss of one of, the four D-globin genes with no physical, manifestations., 2. D-Thalassemia trait caused by loss of two, genes (both from the same gene pair or one from, each gene pair). Minor anemia is observed., 3. Hemoglobin H disease, due to missing of, three genes, is associated with moderate anemia., 4. Hydrops fetalis is the most severe form of, D-thalassemias due to lack of all the four genes., The fetus usually survives until birth and then, dies., , E-Thalassemias, Decreased synthesis or total lack of the, formation of E-globin chain causes Ethalassemias. The production of D-globin chain, continues to be normal, leading to the formation, of a globin tetramer (D4) that precipitate. This, causes premature death of erythrocytes. There, are mainly two types of E-thalassemias, (Fig.10.17), , TABLE 10.2 Summary of different types of D-thalassemias, , Type of, thalassemia, , Number of, missing genes, , Normal, Silent carrier, , 1, , D-Thalassemia trait, (heterozygous form), , 2, , Hemoglobin H disease, , 3, , Hydrops fetalis, (, , Nil, , : represent functional genes;, , 4, , Schematic representation, of genes on chromosome 16, ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , ��D1, , ��D2, , : represent missing genes), , Clinical symptoms, Nil, No symptoms, Minor anemia, Mild to moderate anemia, may lead normal life., Fetal death usually, occurs at birth.
Page 218 :
208, , BIOCHEMISTRY, , E, , E, , E, , E, , E, E, , Genes for, E-globin chain, , No-E chain, 2 E Chain, Normal, , 1E Chain, E Thalassemia, minor, , E Thalassemia, major, , Fig. 10.17: Diagrammatic representation of gene deletions in E-thalassemias, (each one of the chromosome pair of 11 has one gene for E-globin)., , 1. E-Thalassemia minor : This is an heterozygous state with a defect in only one of the two, E-globin gene pairs on chromosome 11. This, disorder, also known as E-thalassemia trait, is, usually asymptomatic, since the individuals can, make some amount of E-globin from the, affected gene., 2. E-Thalassemia major : This is a, homozygous state with a defect in both the genes, responsible for E-globin synthesis. The infants, born with E-thalassemia major are healthy at, birth since E-globin is not synthesized during the, fetal development. They become severely, anemic and die within 1-2 years. Frequent blood, transfusion is required for these children. This is, associated with iron overload which in turn may, lead to death within 15-20 years., , PORPHYRINS, Porphyrins are cyclic compounds composed, of 4 pyrrole rings held together by methenyl, ( CH ) bridges (Fig.10.18). Metal ions can bind, with nitrogen atoms of pyrrole rings to form, complexes. Heme is an iron-containing, porphyrin (See Fig.10.2) while chlorophyll is a, magnesium-containing porphyrin. Thus heme, and chlorophyll are the classical examples of, metalloporphyrins., , Presentation and nomenclature, of porphyrins, The structure of porphyrins (C20H14N4), has four pyrrole rings namely I, II, III and IV., , Naturally, occurring, porphyrins, contain, substituent groups replacing the 8 hydrogen, atoms of the porphyrin nucleus., Hans Fischer, the father of porphyrin, chemistry, proposed a shorthand model for, presentation of porphyrin structures. Accordingly,, each pyrrole ring is represented as a bracket., Thus porphyrin has 4 closed brackets with the, 8 substituent positions numbered as shown in, Fig.10.18., Type I porphyrins : When the substituent, groups on the 8 positions are symmetrically, arranged they are known as type I porphyrins,, e.g. uroporphyrin I., Type III porphyrins : They contain asymmetric, groups at the 8 positions and are more common, in the biological system. Originally, Fischer, placed them as IX series hence they are more, popularly known as type IX porphyrins. It may be, observed that the structure of uroporphyrin is, asymmetric since on ring IV, the order of, substituent groups is reversed (P, A instead of, A, P)., The Fischer’s shorthand models of important, porphyrins (uroporphyrin I and III; coproporphyrin, I and III; protoporphyrin IX and heme) are, depicted in Fig.10.19., , Porphyrins in cancer therapy, The photodynamic properties of porphyrins, can be used in the treatment of certain cancers., This is carried out by a technique called cancer, phototherapy. Tumors are capable of taking up
Page 219 :
209, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , HC, , A, , CH, , HC, , CH, , N, H, , N, H, , P, , P, , A, , A, , P, , Pyrrole, , H, C, G, , HC, , I, , C, , HC, , N, , C, HN, N, C, C, III, C, C, H, H, , HC, , CH, , E, , CH, , A, P, , P A, Uroporphyrin III, , CH, M P, , Porphyrin, 1, , A, P, , II, C, , J, , 2, , P, , M, , M, , P, , P M, Coproporphyrin l, , I, N, 8, , M P, , 3, , HN, , IV NH, , P, , CH, , C, C, , A, , D, , C, , IV NH, HC, , P A, Uroporphyrin I, , H, C, , II, 4, , 7, , M, , M, , P, , P, , N, P M, Coproporphyrin III, , III, 5, , 6, , Porphyrin, 1, , M V, , 2, , I, 8, , IV, , II, III, 6, , M, , P, , V, , 3, 4, , 7, , M, , P M, Protoporphyrin IX (III), , 5, , Fischer’s model, , Fig. 10.18 : Structures of pyrrole and porphyrin, [I-IV are pyrrole rings; 1-8 are substituent positions;, D, E, J, G are methylene ( CH ) bridges.], , more porphyrins than normal tissues. The cancer, phototherapy is carried out by administering, hematoporphyrin (or other related compounds), to the cancer patient. When the tumor is exposed, to an argon laser, the porphyrins get excited and, produce cytotoxic effects on tumor cells., , M V, M, P, , Fe2+, , M, V, , P M, Heme, , Fig. 10.19 : Fischer’s shorthand models of, physiologically important porphyrins [A–Acetate, ( CH3COO–); P–Propionyl ( CH2CH2COO–);, M–Methyl ( CH3); V–Vinyl ( CH CH2)].
Page 220 :
210, , BIOCHEMISTRY, , BIOSYNTHESIS OF HEME, Heme is the most important porphyrin, containing compound. It is primarily synthesized, in the liver and the erythrocyte-producing cells, of bone marrow (erythroid cells). Heme synthesis, also occurs to some extent in other tissues., However, mature erythrocytes lacking mitochondria are a notable exception. Biosynthesis, of heme occurs in the following stages, (Fig.10.20)., 1. Formation of G-aminolevulinate : Glycine,, a non-essential amino acid and succinyl CoA, an, intermediate in the citric acid cycle, are the, starting materials for porphyrin synthesis., Glycine combines with succinyl CoA to form, G-aminolevulinate (ALA). This reaction catalysed, by a pyridoxal phosphate dependent G-aminolevulinate synthase occurs in the mitochondria., It is a rate-controlling step in porphyrin synthesis., 2. Synthesis of porphobilinogen : Two molecules of G-aminolevulinate condense to form, porphobilinogen (PBG) in the cytosol. This, reaction is catalysed by a Zn-containing enzyme, ALA dehydratase. It is sensitive to inhibition by, heavy metals such as lead., 3. Formation of porphyrin ring : Porphyrin synthesis occurs by condensation of, four molecules of porphobilinogen. The four, pyrrole rings in porphyrin are interconnected, by methylene ( CH2) bridges derived from, D-carbon of glycine., The interaction of two enzymes—namely, uroporphyrinogen I synthase and uroporphyrinogen III cosynthase—results in condensation, of porphobilinogen followed by ring closure and, isomerization to produce uroporphyrinogen III., 4. Conversion of uroporphyrinogen III to, protoporphyrin IX : This is catalysed by a series, of reactions, (a) Uroporphyrinogen decarboxylase decarboxylates all the four acetate (A) side chains, to form methyl groups (M), to produce, coproporphyrinogen., (b) Coproporphyrinogen oxidase converts, (oxidative decarboxylation) two of the, , propionate side chains (P) to vinyl groups, (V) and results in the formation of protoporphyrinogen., (c) Protoporphyrinogen oxidase oxidizes, methylene, groups, ( CH2 ), interconnecting pyrrole rings to methenyl, groups ( CH ). This leads to the, synthesis of protoporphyrin IX., 5. Synthesis of heme from protoporphyrin, IX : The incorporation of ferrous iron (Fe2+) into, protoporphyrin IX is catalysed by the enzyme, ferrochelatase or heme synthetase. This enzyme, can be inhibited by lead. It is found that the, induction of Fe2+ into protoporphyrin IX can, occur spontaneously but at a slow rate., , Regulation of heme synthesis, Heme production in the liver is required for, the formation of hemoproteins (e.g. cytochrome, P450 involved in detoxification) while in the, erythroid cells, it is necessary for the synthesis of, hemoglobin. Two different mechanisms exist for, the regulation of heme biosynthesis in the liver, and the erythroid cells., Regulation in the liver : The first committed, step in heme biosynthesis, catalysed by G-aminolevulinate (ALA) synthase, is regulatory. Heme, or its oxidized product hemin (Fe3+) controls this, enzyme activity by three mechanisms, 1. Feedback inhibition, 2. Repression of ALA synthatase, 3. Inhibition of transport of ALA synthase, from cytosol to mitochondria (the site of action)., Effect of drugs on ALA synthase activity : The, activity of ALA synthase is markedly increased, by the administration of a large number of drugs, e.g. phenobarbital, insecticides, carcinogens etc., This is expected since these compounds are, mostly metabolized by a heme containing, protein, cytochrome P450. On administration of, drugs, cellular levels of heme are depleted due, to its increased incorporation into cytochrome, P450. The reduced heme concentration increases, the synthesis (derepression) of ALA synthase to, meet the cellular demands.
Page 223 :
213, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , TABLE 10.3 A general summary of porphyrias, , Type of porphyria, , Enzyme defect, , Characteristics, , Hepatic, Acute intermittent porphyria, , Uroporphyrinogen I synthase, , Abdominal pain, neuropsychiatric, symptoms, , Porphyria cutanea tarda, , Uroporphyrinogen decarboxylase, , Photosensitivity, , Hereditary coproporphyria, , Corpoporphyrinogen oxidase, , Abdominal pain, photosensitivity,, neuropsychiatric symptoms, , Variegate porphyria, , Protoporphyrinogen oxidase, , Abdominal pain, photosensitivity,, neuropsychiatric symptoms, , Congenital erythropoietic porphyria, , Uroporphyrinogen III cosynthase, , Photosensitivity, increased hemolysis, , Protoporphyria, , Ferrochelatase, , Photosensitivity, , Erythropoietic, , (caused by depleted heme levels), resulting, in the accumulation of tryptophan and, 5-hydroxytryptamine., l, , l, , The symptoms are more severe after, administration of drugs (e.g. barbiturates) that, induce the synthesis of cytochrome P450. This, is due to the increased activity of ALA synthase, causing accumulation of PBG and ALA., These patients are not photosensitive since the, enzyme defect occurs prior to the formation of, uroporphyrinogen., , Acute intermittent porphyria is treated by, administration of hematin which inhibits the, enzyme ALA synthase and the accumulation of, porphobilinogen., [The disease—acute intermittent porphyria—, has historical importance. King George III (17601820) ruled England during the period of, American revolution. He was a victim of this, disease and possessed the characteristic, manifestations (such as red colour urine) and was, considered mad. The decisions taken by the, deranged King due to acute intermittent, porphyria had led to a war followed by, American Independence. It is widely believed, that American history would have been different,, had George III not inherited this metabolic, disorder!], , II. Congenital erythropoietic, porphyria, This disorder is due to a defect in the enzyme, uroporphyrinogen III cosynthase. Some workers,, however, believe that congenital erythropoeitic, porphyria is caused by an imbalance between, the activities of uroporphyrinogen I synthase and, uroporphyrinogen III cosynthase. This disease, has certain characteristic features, l, , l, , l, , l, , It is a rare congenital disorder caused by, autosomal recessive mode of inheritance,, mostly confined to erythropoietic tissues., The individuals excrete uroporphyrinogen I, and coproporphyrinogen I which oxidize, respectively to uroporphyrin I and coproporphyrin I (red pigments)., The patients are photosensitive (itching and, burning of skin when exposed to visible light), due to the abnormal prophyrins that, accumulate., Increased hemolysis is also observed in the, individuals affected by this disorder., , III. Porphyria cutanea tarda, This is also known as cutaneous hepatic, porphyria and is the most common porphyria,, usually associated with liver damage caused by
Page 224 :
214, alcohol overconsumption or iron overload. The, partial, deficiency, of, the, enzyme, uroporphyrinogen decarboxylase appears to be, responsible for the occurrence of porphyria, cutanea tarda. The other characteristic features, include, l, , l, , l, , Increased excretion of uroporphyrins (I and III), and rarely porphobilinogen., Cutaneous photosensitivity is the most, important clinical manifestation of these, patients., Liver exhibits fluorescence due to high, concentration of accumulated porphyrins., , IV. Hereditary coproporphyria, This disorder is due to a defect in the enzyme, coproporphyrinogen oxidase. As a result of this,, coproporphyrinogen III and other intermediates, (ALA and PBG) of heme synthesis prior to the, blockade are excreted in urine and feces. The, victims of hereditary coproporphyria are, photosensitive. They exhibit the clinical, manifestations observed in the patients of acute, intermittent porphyria., Infusion of hematin is used to control this, disorder. Hematin inhibits ALA synthase and, thus reduces the accumulation of various intermediates., , V. Variegate porphyria, The enzyme protoporphyrinogen oxidase, is defective in this disorder. Due to this, blockade, protoporphyrin IX required for the, ultimate synthesis of heme is not produced., Almost all the intermediates (porphobilinogen,, coproporphyrin, uroporphyrin, protoporphyrin, etc.) of heme synthesis accumulate in the body, and are excreted in urine and feces. The urine of, these patients is coloured and they exhibit, photosensitivity., , VI. Protoporphyria, This disorder, also known as erythropoietic, protoporphyria, is caused by a deficiency of the, enzyme ferrochelatase. Protoporphyrin IX accumulates in the tissues and is excreted into urine, , BIOCHEMISTRY, , and feces. Reticulocytes (young RBC) and skin, biopsy exhibit red flourescence., , Acquired (toxic) porphyrias, The porphyrias, though not inherited, may be, acquired due to the toxicity of several, compounds. Exposure of the body to heavy, metals (e.g. lead), toxic compounds (e.g., hexachlorobenzene) and drugs (e.g. griseofulvin), inhibits many enzymes in heme synthesis., These include ALA dehydratase, uroporphyrin I, synthase and ferrochelatase., , DEGRADATION OF HEME, TO BILE PIGMENTS, Erythrocytes have a life span of 120 days. At, the end of this period, they are removed from, the circulation. Erythrocytes are taken up and, degraded by the macrophages of the, reticuloendothelial (RE) system in the spleen and, liver. The hemoglobin is cleaved to the protein, part globin and non-protein heme. About 6 g of, hemoglobin per day is broken down, and, resynthesized in an adult man (70 kg)., Fate of globin : The globin may be reutilized, as such for the formation of hemoglobin or, degraded to the individual amino acids. The, latter undergo their own metabolism, including, participation in fresh globin synthesis., Sources of heme : It is estimated that about, 80% of the heme that is subjected for, degradation comes from the erythrocytes and the, rest (20%) comes from immature RBC,, myoglobin and cytochromes., Heme oxygenase : A complex microsomal, enzyme namely heme oxygenase utilizes, NADPH and O2 and cleaves the methenyl, bridges between the two pyrrole rings (A and B), to form biliverdin. Simultaneously, ferrous iron, (Fe2+) is oxidized to ferric form (Fe3+) and, released. The products of heme oxygenase, reaction are biliverdin (a green pigment), Fe3+, and carbon monoxide (CO). Heme promotes the, activity of this enzyme.
Page 225 :
215, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , Aged erythrocytes, MACROPHAGE, , O, , E, , E, D, , D, , E, , Apoproteins, , 2+, , Amino, acids, , H, , H, , O, , Bilirubin, Bilirubin, glucuronyltransferase, , Glucuronic, acid, , V, , Glucuronic, acid, , O C, , V, , M, , Heme oxygenase, , O, , 3+, , Fe , CO, , +, , NADP, , M, , B, , C, , N, H, , N, H, , C O, , CH2, , CH2, , CH2, , CH2, , C, H2, , M, , V, , M, , D, , A, , N, H, , N, H, , O, , Bilirubin diglucuronide (to bile), , P, , M, , P, , C, , D, , A, , N, , N, H, , N, , N, , Biliverdin, , INTESTINE/KIDNEY, , V, , M, , B, , H, , H, , 2 UDPglucuronate, , +, , O, , N, H, , H, , LIVER, , M, P, Heme, , M, , A, , N B, , N, C, , V, , D, N, , 2 UDP, , P, , M, , C, N, , M, , Fe, , O2, NADPH + H, , B, N, H, , Reutilized or, degraded, , A, N, D N, , V, , M, , Bilirubin-albumin, complex, , E, D, , V, , M, , M, , P, , BLOOD, , D, M, , P, , M, , Bilirubin, , Hemoglobin, , Other heme, proteins, , V, , M, , O, , H, , Microbial, enzymes, (intestine), , Urobilinogen, , Microbial, enzymes, , Kidney, , +, , NADPH + H, +, , Biliverdin, reductase, , Urobilin, , Stercobilin, , To urine, , To feces, , NADP, , Fig. 10.22 contd. next column, , Fig. 10.22 : Degradation of heme to bile pigments, (Note : Colours used in structures represent change in the specific reaction only)., , Biliverdin is excreted in birds and amphibia, while in mammals it is further degraded., Biliverdin reductase : Biliverdin’s methenyl, bridges (between the pyrrole rings C and D) are, reduced to methylene group to form bilirubin, (yellow pigment). This reaction is catalysed by, an NADPH dependent soluble enzyme,, biliverdin reductase (Fig.10.22). One gram of, hemoglobin on degradation finally yields about, 35 mg bilirubin. Approximately 250-350 mg of, , bilirubin is daily produced in human adults. The, term bile pigments is used to collectively, represent bilirubin and its derivatives., Transport of bilirubin to liver : Bilirubin is, lipophilic and therefore insoluble in aqueous, solution. Bilirubin is transported in the plasma in, a bound (non-covalently) form to albumin., Albumin has two binding sites for bilirubin—a, high affinity site and a low affinity site., Approximately 25 mg of bilirubin can bind
Page 226 :
216, tightly to albumin (at high affinity sites) per 100, ml of plasma. The rest of the bilirubin binds, loosely (at the low affinity sites) which can be, easily detached from albumin to enter the, tissues. Certain drugs and antibiotics (e.g, sulfonamides, salicylates) can displace bilirubin, from albumin. Due to this, bilirubin can enter, the central nervous system and cause damage to, neurons., As the albumin-bilirubin complex enters the, liver, bilirubin dissociates and is taken up by, sinusoidal surface of the hepatocytes by a carrier, mediated active transport. The transport system, has a very high capacity and therefore is not a, limitation for further metabolism of bilirubin., Inside the hepatocytes, bilirubin binds to a, specific intracellular protein namely ligandin., , Conjugation of bilirubin, In the liver, bilirubin is conjugated with two, molecules of glucuronate supplied by UDPglucuronate. This reaction, catalysed by bilirubin, glucuronyltransferase (of smooth endoplasmic, reticulum) results in the formation of a water, soluble bilirubin diglucuronide (Figs.10.22 and, 10.23). When bilirubin is in excess, bilirubin, monoglucuronides also accumulate in the body., The enzyme bilirubin glucuronyltransferase can, be induced by a number of drugs (e.g., phenobarbital),, , Excretion of bilirubin into bile, Conjugated bilirubin is excreted into the bile, canaliculi against a concentration gradient which, then enters the bile. The transport of bilirubin, diglucuronide is an active, energy-dependent, and rate limiting process. This step is easily, susceptible to any impairment in liver function., Normally, there is a good coordination between, the bilirubin conjugation and its excretion into, bile. Thus almost all the bilirubin (> 98%) that, enters bile is in the conjugated form., , Fate of bilirubin, Bilirubin glucuronides are hydrolysed in the, intestine by specific bacterial enzymes namely, E-glucuronidases to liberate bilirubin. The latter, is then converted to urobilinogen (colourless, , BIOCHEMISTRY, , compound), a small part of which may be, reabsorbed into the circulation. Urobilinogen, can be converted to urobilin (an yellow colour, compound) in the kidney and excreted. The, characteristic colour of urine is due to urobilin., A major part of urobilinogen is converted by, bacteria to stercobilin which is excreted along, with feces. The characteristic brown colour of, feces is due to stercobilin., , JAUNDICE, The normal serum total bilirubin concentration is in the range of 0.2 to 1.0 mg/dl. Of, this, about 0.2-0.6 mg/dl is unconjugated while, 0.2 to 0.4 mg/dl is conjugated bilirubin., Jaundice (French : Jaune-yellow) is a clinical, condition characterized by yellow colour of the, white of the eyes (sclerae) and skin. It is caused, by the deposition of bilirubin due to its elevated, levels in the serum. The term hyperbilirubinemia, is often used to represent the increased, concentration of serum bilirubin. (Note : For, some more details on jaundice, refer Chapter 20), , Classification of jaundice, Jaundice (also known as icterus) may be more, appropriately considered as a symptom rather, than a disease. It is rather difficult to classify, jaundice, since it is frequently caused due to, multiple factors. For the sake of convenience to, understand, jaundice is classified into three, major types—hemolytic, hepatic and obstructive., 1. Hemolytic jaundice : This condition is associated with increased hemolysis of erythrocytes, (e.g. incompatible blood transfusion, malaria,, sickle-cell anemia). This results in the overproduction of bilirubin beyond the ability of the, liver to conjugate and excrete the same. It should,, however be noted that liver possesses a large, capacity to conjugate about 3.0 g of bilirubin per, day against the normal bilirubin production of, 0.3 g/day., In hemolytic jaundice, more bilirubin is, excreted into the bile leading to the increased
Page 227 :
217, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , l, , Blood, Bilirubin—albumin, , I. Uptake, , l, , l, , Bilirubin, 2UDP-GlcUA, , Liver, , III. Secretion, , l, , Bilirubin-diglucuronide, Bile duct, , l, , Fig. 10.23 : Summary of bilirubin metabolism, (UDP-GlcUA—UDP-glucuronic acid)., l, , formation of urobilinogen and stercobilinogen., Hemolytic jaundice is characterized by, l, , Elevation in the serum unconjugated bilirubin., , l, , Increased excretion of urobilinogen in urine., Dark brown colour of feces due to high, content of stercobilinogen., , 2. Hepatic (hepatocellular) jaundice : This, type of jaundice is caused by dysfunction of the, liver due to damage to the parenchymal cells., This may be attributed to viral infection (viral, hepatitis), poisons and toxins (chloroform,, carbon tetrachloride, phosphorus etc.) cirrhosis, of liver, cardiac failure etc. Among these, viral, hepatitis is the most common., Damage to the liver adversely affects the, bilirubin uptake and its conjugation by liver, cells. Hepatic jaundice is characterized by, , l, , The affected individuals experience nausea, and anorexia (loss of appetite)., , Due to the blockage in bile duct, the, conjugated bilirubin from the liver enters the, circulation. Obstructive jaundice is characterized, by, , II. Conjugation, , l, , The patients pass pale, clay coloured stools, due to the absence of stercobilinogen., , 3. Obstructive (regurgitation) jaundice : This, is due to an obstruction in the bile duct that, prevents the passage of bile into the intestine., The obstruction may be caused by gall stones,, tumors etc., , UDP-glucuronyltransferase, , Bilirubindiglucuronide, , l, , Increased activities of alanine transaminase, (SGPT) and aspartate transaminase (SGOT), released into circulation due to damage to, hepatocytes., , Increased, levels, of, conjugated, unconjugated bilirubin in the serum., , and, , Dark coloured urine due to the excessive, excretion of bilirubin and urobilinogen., , l, , l, , Increased concentration, bilirubin in serum., , of, , conjugated, , Serum alkaline phosphatase is elevated as it is, released from the cells of the damaged bile, duct., Dark coloured urine due to elevated excretion, of bilirubin and clay coloured feces due to, absence of stercobilinogen., Feces contain excess fat indicating impairment in fat digestion and absorption in the, absence of bile (specifically bile salts)., The patients experience nausea and gastrointestinal pain., , JAUNDICE DUE TO, GENETIC DEFECTS, There are certain hereditary abnormalities that, cause jaundice., , Neonatal-physiologic jaundice, Physiological jaundice is not truly a genetic, defect. It is caused by increased hemolysis, coupled with immature hepatic system for the, uptake, conjugation and secretion of bilirubin., The activity of the enzyme UDP-glucuronyltransferase is low in the newborn. Further, there, is a limitation in the availability of the substrate, UDP-glucuronic acid for conjugation. The, net effect is that in some infants the serum
Page 228 :
218, , BIOCHEMISTRY, , uncojugated bilirubin is highly elevated (may go, beyond 25mg/dl), which can cross the bloodbrain barrier. This results in hyperbilirubinemic, toxic encephalopathy or kernicterus that causes, mental retardation. The drug phenobarbital, is used in the treatment of neonatal jaundice,, as it can induce bilirubin metabolising, enzymes in liver. In some neonates, blood, transfusion may be necessary to prevent brain, damage., , bilirubin gets converted into a non-toxic isomer, namely lumirubin. Lumirubin can be easily, excreted by the kidneys in the unconjugated, form (in contrast to bilirubin which cannot be, excreted). Serum bilirubin is monitored every, 12–24 hours, and phototherapy is continuously, carried out till the serum bilirubin becomes, normal (< 1 mg/dl)., , Phototherapy : Bilirubin can absorb blue light, (420–470 nm) maximally. Phototherapy deals, with the exposure of the jaundiced neonates to, blue light. By a process called photoisomerization, the toxic native unconjugated, , This is also known as congenital nonhemolytic jaundice. It is a rare disorder and is, due to a defect in the hepatic enzyme UDPglucuronyltransferase. Generally, the children, die within first two years of life., , Crigler-Najjar syndrome type I, , + Hemoglobin is primarily responsible for the delivery of O2 from lungs to tissue and the, transport of CO2 from tissues to lungs., , + Increased erythrocyte 2,3-BPG levels in anemia and chronic hypoxia facilitate the, release of more O2 from the oxyhemoglobin to the tissues., + Storage of blood causes a decrease in the concentration of 2,3-BPG. This can be, prevented by the addition of ionosine., , + Hemoglobin (Fe2+) on oxidation by H2O2, free radicals or drugs, forms methemoglobin, , (Fe3+) which cannot transport O2., + Carboxyhemoglobin is produced when carbon monoxide, an industrial pollutant, binds, to hemoglobin. The clinical manifestations of CO toxicity (> 20% COHb) include, headache, nausea, breathlessness and vomiting., , + Sickle cell hemoglobin (HbS) causes hemolytic anemia, increased susceptibility to, infection and premature death. However, HbS offers protection against malaria., , + Thalassemias are hemolytic disorders caused by impairment / imbalance in the synthesis, of globin chains of Hb. These include D-thalassemia trait, hydrops fetalis and, E-thalassemias., , + Administration of porphyrins can be used in the treatment certain cancers by, phototherapy., , + Abnormalities in heme synthesis cause porphyrias which may be erythropoietic (enzyme, defect in RBC) or hepatic (enzyme defect in liver). Porphyrias are associated with, elevated excretion of porphyrins, neuropsychiatric disturbances and cardiovascular, abnormalities., , + Jaundice is caused by elevated serum bilirubin (normal < 0.8 mg/dl) levels and is, characterized by yellow coloration of white of the eyes, and skin., , + Phototherapy (by exposure to blue light) is used in to control severe cases of neonatal, physiologic jaundice.
Page 229 :
219, , Chapter 10 : HEMOGLOBIN AND PORPHYRINS, , Crigler-Najjar syndrome type II, This is again a rare hereditary disorder and is, due to a less severe defect in the bilirubin, conjugation. It is believed that hepatic UDPglucuronyltransferase that catalyses the addition, of second glucuronyl group is defective. The, serum bilirubin concentration is usually less than, 20 mg/dl and this is less dangerous than type I., , Gilbert’s disease : This is not a single disease, but a combination of disorders. These include, 1. A defect in the uptake of bilirubin by liver, cells., 2. An impairment in conjugation due to, reduced activity of UDP-glucuronyltransferase., 3. Decreased hepatic clearance of bilirubin., , 1. Hemoglobin (HbA1, mol. wt. 64,450) is a conjugated protein containing globin, the, apoprotein and the heme, the nonprotein moiety (prosthetic group). It is a tetrameric,, allosteric protein with 2D and 2E polypeptide chains held by non-covalent interactions., Each subunit contains a heme with iron in the ferrous state., 2. Hemoglobin is responsible for the transport of O2 from lungs to the tissues. Each heme, (of Hb) can bind with one molecule of O2 and this is facilitated by cooperative hemeheme interaction., 3. Hemoglobin actively participates in the transport of CO2 from tissues to lungs., Increased partial pressure of CO2 (pCO2) accompanied by elevated H+ decreases the, binding of O2 to Hb, a phenomenon known as Bohr effect., 4. The four compounds namely 2,3-bisphosphoglycerate, CO2, H+ and Cl– are collectively, known as allosteric effectors. They interact with hemoglobin and facilitate the release, of O2 from oxyHb., 5. Sickle-cell anemia (HbS) is a classical example of abnormal hemoglobins. It is caused, when glutamate at 6th position of E-chain is replaced by valine. HbS is characterized, by hemolytic anemia, tissue damage, increased susceptibility to infection and premature, death. Sickle-cell anemia, however offers resistance to malaria., 6. Thalassemias are a group of hereditary hemolytic disorders characterized by impairment/, imbalance in the synthesis of globin (D or E) chain of Hb. Hydrops fetalis, the most, severe form of D-thalassemia is characterized by the death of infant at birth. EThalassemia major is another serious disorder with severe anemia and death of child, within 1-2 years., 7. Heme is the most important porphyrin compound, primarily synthesized in the liver, from the precursors-glycine and succinyl CoA. Heme productioin is regulated by, G-aminolevulinate synthase., 8. Porphyrias are the metabolic disorders of heme synthesis, characterized by the increased, excretion of porphyrins or their precursors. Acute intermittent porphyria occurs due to, the deficiency of the enzyme uroporphyrinogen I synthase and is characterized by, increased excretion of porphobilinogen and G-aminolevulinate. The clinical symptoms, include neuropsychiatric disturbances and cardiovascular abnormalities., 9. Heme is degraded mainly to bilirubin, an yellow colour bile pigment. In the liver, it is, conjugated to bilirubin diglucuronide, a more easily excretable form into bile., 10. Jaundice is a clinical condition caused by elevated serum bilirubin concentration, (normal <1.0 mg/dl). Jaundice is of three types-hemolytic (due to increased hemolysis),, hepatic (due to impaired conjugation) and obstructive (due to obstruction in the bile duct).
Page 230 :
220, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the structure of hemoglobin and discuss oxygen transport., 2. Write an account of hemoglobinopathies with special reference to sickle-cell anemia., 3. Discuss the biosynthesis of heme. Add a note on the regulation of heme synthesis., 4. What are porphyrias? Describe any three porphyrias in detail., 5. Write an account of the degradation of heme to bile pigments. Add a note on jaundice., , II. Short notes, (a) Methemoglobin, (b) Heme—heme interaction, (c) Bohr effect, (d) 2,3—BPG, (e) Sickle cell, anemia and malaria, (f) Thalassemias, (g) Acute intermittent porphyria, (h) Heme oxygenase,, (i) Bilirubin diglucuronide, (j) Carboxyhemoglobin., , III. Fill in the blanks, 1. The total number of amino acids present in adult hemoglobin ________________., 2. The oxidation of ferrous (Fe2+) iron to ferric (Fe3+) iron in hemoglobin results in the formation, of a compound namely ________________., 3. The enzyme that catalyses the formation of carbonic acid ________________., 4. Name the compound that is increased in RBC of anemic patients to facilitate the supply of O2, to the tissues ________________., 5. Sickling of RBC in sickle-cell anemia is due to polymerization of ________________., 6. The disorders characterized by decreased synthesis or total absence of globin chains of, hemoglobin are collectively known as ________________., 7. The intermediate of citric acid cycle that is involved in heme synthesis ________________., 8. The enzyme defect in acute intermittent porphyria ________________., 9. The enzyme that is regulated by feedback inhibition in heme synthesis is ________________., 10. The product formed when heme oxygenase cleaves heme ________________., , IV. Multiple choice questions, 11. The characteristic red colour of hemoglobin is due to, (a) Heme (b) D-Globin (c) E-Globin (d) All of them., 12. The number of heme groups present in myoglobin, (a) 1 (b) 2 (c) 3 (d) 4., 13. The patients of sickle-cell anemia are resistant to, (a) Filaria (b) Malaria (c) Diabetes (d) Trypanosomiasis., 14. The compound that facilitates the release of O2 from oxyhemoglobin, (a) 2, 3-BPG (b) H+ (c) C1– (d) All of them., 15. Name the amino acid that directly participates in the synthesis of heme, (a) Methionine (b) Aspartate (c) Glycine (d) Tryptophan.
Page 231 :
Section 2, , Physiological Biochemistry, , Chapter, , Biological Oxidation, , 11, , The high energy, compound, ATP speaks :, , NH2, N, N, N Adenine, , N, O, , O, , O, O, , O– P ~ O P ~ O P O CH2, O–, , O–, , O–, , Triphosphate, , H, , H, , H, , OH, , OH, , Ribose, , H, , “I am the energy currency of the cell !, Continuous consumption and regeneration is my thrill;, Without me, all biochemical functions come to a standstill;, Existence of life is unimaginable without my will.”, , F, , or a better understanding of biological, oxidation, it is worthwhile to have a basic, knowledge of bioenergetics and the role of highenergy compounds in biological processes., , BIOENERGETICS, Bioenergetics or biochemical thermodynamics, deals with the study of energy changes (transfer, and utilization) in biochemical reactions. The, reactions are broadly classified as exergonic, (energy releasing) and endergonic (energy, consuming). Bioenergetics is concerned with the, initial and final states of energy component of the, reactants and not the mechanism of chemical, reactions., , Free energy, The energy actually available to do work, (utilizable) is known as free energy. Changes in, the free energy ('G) are valuable in predicting, the feasibility of chemical reactions. The, reactions can occur spontaneously if they are, accompanied by decrease in free energy., , During a chemical reaction, heat may be, released or absorbed. Enthalpy ('H) is a measure, of the change in heat content of the reactants,, compared to products., , Entropy ('S) represents a change in the, randomness or disorder of reactants and, products. Entropy attains a maximum as the, reaction approaches equilibrium. The reactions, of biological systems involve a temporary, decrease in entropy., The relation between the changes of free, energy ('G), enthalpy ('H) and entropy ('S) is, expressed as, 'G = 'H – T'S, T represents the absolute temperature in Kelvin, (K = 273 + °C)., The term standard free energy represented by, 'G° (note the superscript°) is often used. It, indicates the free energy change when the, reactants or products are at a concentration of 1, mol/l at pH 7.0., , 221
Page 232 :
222, , BIOCHEMISTRY, , Negative and positive 'G, If free energy change ('G) is represented by a, negative sign, there is a loss of free energy. The, reaction is said to be exergonic, and proceeds, spontaneously. On the other hand, a positive 'G, indicates that energy must be supplied to the, reactants. The reaction cannot proceed spontaneously and is endergonic in character., The hydrolysis of ATP is a classical example, of exergonic reaction, ATP + H2O o ADP + Pi ('G° = –7.3 Cal/mol), The reversal of the reaction (ADP + Pi o ATP), is endergonic and occurs only when there is a, supply of energy of at least 7.3 Cal/mol ('G° is, positive)., The free energy change becomes zero ('G = 0), when a reaction is at equilibrium., At a constant temperature and pressure, 'G is, dependent on the actual concentration of, reactants and products. For the conversion of, reactant A to product B (A o B), the following, mathematical relation can be derived, 'G = 'G° + RT In [B], [A], where 'G° = Standard free energy change, R = Gas constant (1.987 Cal/mol), T = Absolute temperature (273 + °C), In = Natural logarithm, [B] = Concentration of product, [A] = Concentration of reactant., , 'G° is related to, equilibrium constant (Keq), When a reaction A, B is at equilibrium, (eq), the free energy change is zero. The above, equation may be written as, [B] eq., 'G = 0 = 'G° + RT In, [A] eq., , Hence 'G° = – RT In Keq., , 'G is an additive value for pathways, Biochemical pathways often involve a series, of reactions. For such reactions, free energy, , change is an additive value. The sum of 'G is, crucial in determining whether a particular, pathway will proceed or not. As long as the sum, of 'Gs of individual reactions is negative, the, pathway can operate. This happens despite the, fact that some of the individual reactions may, have positive 'G., , HIGH-ENERGY COMPOUNDS, Certain compounds are encountered in the, biological system which, on hydrolysis, yield, energy. The term high-energy compounds or, energy rich compounds is usually applied to, substances which possess sufficient free, energy to liberate at least 7 Cal/mol at pH 7.0, (Table 11.1). Certain other compounds which, liberate less than 7.0 Cal/mol (lower than ATP, hydrolysis to ADP + Pi) are referred to as lowenergy compounds., , TABLE 11.1 Standard free energy of, hydrolysis of some important compounds, , Compounds, , 'G° (Cal/mol), , High-energy phosphates, Phosphoenol pyruvate, Carbamoyl phosphate, Cyclic AMP, 1,3-Bisphosphoglycerate, Phosphocreatine, Acetyl phosphate, S-Adenosylmethionine*, Pyrophosphate, Acetyl CoA**, ATP o ADP + Pi, , –14.8, –12.3, –12.0, –11.8, –10.3, –10.3, –10.0, –8.0, –7.7, –7.3, , Low-energy phosphates, ADP o AMP + Pi, , –6.6, , Glucose 1-phosphate, , –5.0, , Fructose 6-phosphate, , –3.8, , Glucose 6-phosphate, , –3.3, , Glycerol 3-phosphate, , –2.2, , * Sulfonium compound, ** Thioester
Page 233 :
223, , Chapter 11 : BIOLOGICAL OXIDATION, , TABLE 11.2 High-energy compounds, , Class, Pyrophosphates, , Bond, C, , P, , Example(s), P, , ATP, pyrophosphate, , O, Acyl phosphates, , C O~ P, , 1,3-Bisphosphoglycerate,, carbamoyl phosphate,, acetyl phosphate, , CH, Enol phosphates, , C O~ P, , Phosphoenol pyruvate, , C, Thiol esters, (thioesters), Guanidio phosphates, (Phosphagens), , C O~ S, , N~P, , Acetyl CoA, acyl CoA, , Phosphocreatine,, phosphoarginine, , All the high-energy compounds—when, hydrolysed—liberate more energy than that of, ATP. These include phosphoenol pyruvate, 1,3bisphosphoglycerate, phosphocreatine etc., Most of the high-energy compounds contain, phosphate group (exception acetyl CoA), hence they are called high-energy phosphate, compounds., , energy bonds, since the free energy is liberated, when these bonds are hydrolysed. Lipmann, suggested use of the symbol ~ to represent highenergy bond. For instance, ATP is written as, AMP~P~P., , ATP – the most important, high-energy compound, Adenosine triphosphate (ATP) is a unique and, the most important high-energy molecule in the, living cells. It consists of an adenine, a ribose, and a triphosphate moiety (Fig.11.1). ATP is a, high-energy compound due to the presence of, two phosphoanhydride bonds in the triphosphate, unit. ATP serves as the energy currency of the, cell as is evident from the ATP-ADP cycle., , ATP-ADP Cycle, The hydrolysis of ATP is associated with the, release of large amount of energy., ATP + H2O o ADP + Pi + 7.3 Cal., , Classification of, high-energy compounds, , The energy liberated is utilized for various, processes like muscle contraction, active, transport etc. ATP can also act as a donor of, high-energy, phosphate, to, low-energy, compounds, to make them energy rich. On the, other hand, ADP can accept high-energy, phosphate from the compounds possessing, higher free energy content to form ATP., , There are at least 5 groups of high-energy, compounds., , ATP serves as an immediately available, energy currency of the cell which is constantly, , 1. Pyrophosphates e.g. ATP., 2. Acyl phosphates e.g. 1,3-bisphosphoglycerate., , NH2, , 3. Enol phosphates e.g. phosphoenolpyruvate., , N, , N, , 4. Thioesters e.g. acetyl CoA., 5. Phosphagens e.g. phosphocreatine., , Table 11.2 gives some more details on the, high-energy compounds, including the highenergy bonds present in each category., High-energy bonds : The high-energy compounds possess acid anhydride bonds (mostly, phosphoanhydride bonds) which are formed by, the condensation of two acidic groups or related, compounds. These bonds are referred to as high-, , N Adenine, , N, O, , O, , O, , O– P~O P~O P O CH2, –, , O, , –, , O, , O, , –, , O, , Triphosphate, , H, , H, , H, , OH, , OH, , Fig. 11.1 : Structure of ATP., , Ribose, , H
Page 234 :
224, , BIOCHEMISTRY, , Oxidative, phosphorylation, , ADP, , ~P, Substrate level, phosphorylation, , Muscle contraction, Active transport, ~P, Biosyntheses, Phosphorylations, , compound. In invertebrates, phosphoarginine, (arginine phosphate) replaces phosphocreatine., , ATP, , BIOLOGICAL OXIDATION, , ~P, , Oxidation is defined as the loss of electrons, and reduction as the gain of electrons. This may, be illustrated by the interconversion of ferrous, ion (Fe2+) to ferric ion (Fe3+)., , Creatine, , Creatine ~P, , e–, ~P, , Fig. 11.2 : ATP-ADP cycle along with sources and, utilization of ATP (Note that ~P does not exist in free, form, but is only transferred)., , being utilized and regenerated. This is, represented by ATP-ADP cycle, the fundamental, basis of energy exchange reactions in living, system (Fig.11.2). The turnover of ATP is very, high., ATP acts as an energy link between the, catabolism (degradation of molecules) and, anabolism (synthesis) in the biological system., , Fe2+, , Oxidation, , Acceptor, (reduced), , Fe3+, , Reduction, , e–, , The electron lost in the oxidation is accepted, by an acceptor which is said to be reduced. Thus, the oxidation-reduction is a tightly coupled, process., The general principle of oxidation-reduction, is applicable to biological systems also. The, oxidation of NADH to NAD+ coupled with the, reduction of FMN to FMNH2 is illustrated, NADH + H+, , FMN, , 2H+ + 2e–, , Synthesis of ATP, ATP can be synthesized in two ways, 1. Oxidative phosphorylation : This is the, major source of ATP in aerobic organisms. It is, linked with the mitochondrial electron transport, chain (details described later)., 2. Substrate level phosphorylation : ATP, may be directly synthesized during substrate, oxidation in the metabolism. The high-energy, compounds such as phosphoenolpyruvate, and 1,3-bisphosphoglycerate (intermediates of, glycolysis) and succinyl CoA (of citric acid cycle), can transfer high-energy phosphate to ultimately, produce ATP., , Storage forms of, high-energy phosphates, Phosphocreatine (creatine phosphate) stored, in vertebrate muscle and brain is an energy-rich, , NAD+, , FMNH2, , In the above illustration, there are two redox, pairs NADH/NAD+ and FMN/FMNH2. The redox, pairs differ in their tendency to lose or gain, electrons., , Redox potential (E0), The oxidation-reduction potential or, simply,, redox potential, is a quantitative measure of the, tendency of a redox pair to lose or gain, electrons. The redox pairs are assigned specific, standard redox potential (E0 volts) at pH 7.0 and, 25°C., The redox potentials of some biologically, important redox systems are given in Table 11.3., The more negative redox potential represents a, greater tendency (of reductant) to lose electrons.
Page 235 :
225, , Chapter 11 : BIOLOGICAL OXIDATION, , Carbohydrates, Fatty acids, Amino acids, , Table 11.3 Standard redox potential (E0), of some oxidation-reduction systems, , Redox pair, , E0 Volts, , Succinate/D-ketoglutarate, , – 0.67, , 2H+/H2, , – 0.42, , NAD+/NADH, , – 0.32, , NADP+/NADPH, , – 0.32, , FMN/FMNH2 (enzyme bound), , – 0.30, , Lipoate (ox/red), , – 0.29, , FAD/FADH2, , – 0.22, , Pyruvate/lactate, , – 0.19, , Fumarate/succinate, , + 0.03, , Cytochrome b (Fe3+/Fe2+), , + 0.07, , Coenzyme Q (ox/red), , + 0.10, , Cytochrome c1 (Fe3+/Fe2+), , + 0.23, , Cytochrome c (Fe3+/Fe2+), , + 0.25, , Cytochrome a (Fe3+/Fe2+), , + 0.29, , 1, 2, , NAD+, FAD, , O2/H2O, , CO2, H2O, , 1O, 2 2, , ADP + Pi, , and FADH2. The latter two reduced coenzymes, pass through the electron transport chain (ETC), or respiratory chain and, finally, reduce oxygen, to water. The passage of electrons through the, ETC is associated with the loss of free energy. A, part of this free energy is utilized to generate, ATP from ADP and Pi (Fig.11.3)., An overview of the ETC is depicted in, Fig.11.4., , Mitochondria – the power houses, of cell, , The redox potential (E0) is directly related to, the change in the free energy ('G°)., , The mitochondria are the centres for, metabolic oxidative reactions to generate, reduced coenzymes (NADH and FADH2) which,, in turn, are utilized in ETC to liberate energy in, the form of ATP. For this reason, mitochondrion, is appropriately regarded as the power house of, the cell., , Mitochondrial organization, The mitochondrion consists of five distinct, parts. These are the outer membrane, the inner, membrane, the intermembrane space, the cristae, and the matrix (Fig.11.5)., , NADH, , Cyts (2Fe3+), , FpH2, , NAD+, , H+, , NADH + H+, FADH2, , Fig. 11.3 : Overview of biological oxidation, (ETC-Electron transport chain)., , On the other hand, a more positive redox, potential indicates a greater tendency (of, oxidant) to accept electrons. The electrons flow, from a redox pair with more negative E0 to, another redox pair with more positive E0., , The energy-rich carbohydrates, (particularly glucose), fatty acids AH, 2, and amino acids undergo a series, of metabolic reactions and,, finally, get oxidized to CO2 and, H2O. The reducing equivlents, A, from, various, metabolic, intermediates are transferred to, coenzymes NAD+ and FAD to, produce, respectively, NADH, , ATP, ETC, , + 0.82, , ELECTRON TRANSPORT CHAIN, , H2O, , H+, , Fp, , 2H+, , Cyts (2Fe2+), , Fig. 11.4 : Overview of electron transport chain, (A–Substrate; Fp–Flavoprotein; Cyts–Cytochromes)., , H2O, , 2H, , 1, 2, , O2
Page 236 :
226, , BIOCHEMISTRY, , Phosphorylating, subunits, , Outer, membrane, , Matrix, , F1, , Cristae, , NAD +, , Intermembrane, space, , FMN, , CoQ, , b, , c, , a a3, , Fo, ATP synthase, , Inner, membrane, ETC assembly, , Fig. 11.5 : Structure of mitochondrion depicting electron transport chain (ETC) (F0, F1—Protein subunits)., , Inner mitochondrial membrane : The electron, transport chain and ATP synthesizing system are, located on the inner mitochondrial membrane, which is a specialized structure, rich in proteins., It is impermeable to ions (H+, K+, Na+) and small, molecules (ADP, ATP). This membrane is highly, folded to form cristae. The surface area of inner, mitochondrial membrane is greatly increased, due to cristae. The inner surface of the inner, mitochondrial membrane possesses specialized, particles (that look like lollipops), the phosphorylating subunits which are the centres for ATP, production., , there are certain mobile electron carriers in the, respiratory chain. These include NADH,, coenzyme Q, cytochrome C and oxygen., , Mitochondrial matrix : The interior ground, substance forms the matrix of mitochondria. It is, rich in the enzymes responsible for the citric, acid cycle, E-oxidation of fatty acids and, oxidation of amino acids., , There are five distinct carriers that participate, in the electron transport chain (ETC). These, carriers are sequentially arranged (Fig.11.7) and, are responsible for the transfer of electrons from, a given substrate to ultimately combine with, proton and oxygen to form water., , Structural organization, of respiratory chain, The inner mitochondrial membrane can be, disrupted into five distinct respiratory or enzyme, complexes, denoted as complex I, II, III, IV and, V (Fig.11.6). The complexes I-IV are carriers of, electrons while complex V is responsible for ATP, synthesis. Besides these enzyme complexes,, , The enzyme complexes (I-IV) and the mobile, carriers are collectively involved in the transport, of electrons which, ultimately, combine with, oxygen to produce water. The largest proportion, of the oxygen supplied to the body is utilized by, the mitochondria for the operation of electron, transport chain., , Components and reactions, of the electron transport chain, , I. Nicotinamide nucleotides, Of the two coenzymes NAD+ and NADP+, derived from the vitamin niacin, NAD+ is more, actively involved in the ETC. NAD+ is reduced, to NADH + H+ by dehydrogenases with, the removal of two hydrogen atoms from, the substrate (AH2). The substrates include
Page 238 :
228, , BIOCHEMISTRY, , IV. Coenzyme Q, Coenzyme Q is also known as ubiquinone, since it is ubiquitous in living system. It is a, quinone derivative with a variable isoprenoid, side chain. The mammalian tissues possess a, quinone with 10 isoprenoid units which is, known as coenzyme Q10 (CoQ10)., O, H3CO, , CH3, CH3, (CH2 CH C CH2)n H, , H3CO, O, , Ubiquinone (oxidized form), , Coenzyme Q is a lipophilic electron carrier. It, can accept electrons from FMNH2 produced in, the ETC by NADH dehydrogenase or FADH2, produced, outside, ETC, (e.g., succinate, dehydrogenase, acyl CoA dehydrogenase)., Coenzyme Q is not found in mycobacteria., Vitamin K performs similar function as coenzyme, Q in these organisms. Coenzyme Q has no, known vitamin precursor in animals. It is directly, synthesized in the body. (Refer cholesterol, biosynthesis, Chapter 14), , V. Cytochromes, The cytochromes are conjugated proteins, containing heme group. The latter consists of a, porphyrin ring with iron atom. The heme group, of cytochromes differ from that found in the, structure of hemoglobin and myoglobin. The iron, of heme in cytochromes is alternately oxidized, (Fe3+) and reduced (Fe2+), which is essential for, the transport of electrons in the ETC. This is in, contrast to the heme iron of hemoglobin and, myoglobin which remains in the ferrous (Fe2+), state., Three cytochromes were initially discovered, from the mammalian mitochondria. They were, designated as cytochrome a, b and c depending, on the type of heme present and the respective, absorption spectrum. Additional cytochromes, such as c1, b1, b2, a3 etc. were discovered later., The electrons are transported from coenzyme, Q to cytochromes (in the order) b, c1, c, a and, a3. The property of reversible oxidation-, , Fe3+ present in, reduction of heme iron Fe2+, cytochromes allows them to function as effective, carriers of electrons in ETC., , Cytochrome c (mol. wt. 13,000) is a small, protein containing 104 amino acids and a heme, group. It is a central member of ETC with an, intermediate redox potential. It is rather loosely, bound to inner mitochondrial membrane and, can be easily extracted., Cytochrome a and a3 : The term cytochrome, oxidase is frequently used to collectively, represent cytochrome a and a3 which is the, terminal component of ETC. Cytochrome oxidase, is the only electron carrier, the heme iron of, which can directly react with molecular oxygen., Besides heme (with iron), this oxidase also, contains copper that undergoes oxidationreduction (Cu2+, Cu+) during the transport, of electrons., In the final stage of ETC, the transported, electrons, the free protons and the molecular, oxygen combine to produce water., , OXIDATIVE PHOSPHORYLATION, The transport of electrons through the ETC is, linked with the release of free energy. The process, of synthesizing ATP from ADP and Pi coupled, with the electron transport chain is known, as oxidative phosphorylation. The complex V, (See Fig.11.6) of the inner mitochondrial, membrane is the site of oxidative phosphorylation., , P : O Ratio, The P : O ratio refers to the number of, inorganic phosphate molecules utilized for ATP, generation for every atom of oxygen consumed., More appropriately, P : O ratio represents the, number of molecules of ATP synthesized per pair, of electrons carried through ETC., The mitochondrial oxidation of NADH with a, classical P : O ratio of 3 can be represented by, the following equation :, NADH + H+ +, , 1, 2, , O2 + 3ADP + 3Pi o, NAD+ + 3ATP + 4H2O
Page 239 :
229, , Chapter 11 : BIOLOGICAL OXIDATION, , Further, a P : O ratio of 2 has been assigned, to the oxidation of FADH2. There is a strong, evidence now to suggest a P:O ratio of 2.5 for, NADH, and 1.5 for FADH2, since ten protons, (for NADH) and six protons (for FADH2) are, pumped across mitochondrial membrane., Synthesis of one ATP requires four protons., , Sites of oxidative, phosphorylation in ETC, , 1. Oxidation of FMNH2 by coenzyme Q., 2. Oxidation of cytochrome b by cytochrome c1., 3. Cytochrome oxidase reaction., Each one of the above reactions represents a, coupling site for ATP production. There are only, two coupling sites for the oxidation of FADH2, (P : O ratio 2), since the first site is bypassed., , Energetics of oxidative, phosphorylation, , This hypothesis was put forth by Edward, Slater (1953). According to chemical coupling, hypothesis, during the course of electron transfer, in respiratory chain, a series of phosphorylated, high-energy intermediates are first produced, which are utilized for the synthesis of ATP. These, reactions are believed to be analogous to the, substrate level phosphorylation that occurs in, glycolysis or citric acid cycle. However, this, hypothesis lacks experimental evidence, since, all attempts, so far, to isolate any one of, the high-energy intermediates have not been, successful., , Chemiosmotic hypothesis, , The transport of electrons from redox pair, NAD+/NADH (E0 = – 0.32) to finally the redox, pair 1 O2/H2O (E0 = + 0.82) may be simplified, 2, , and represented in the following equation, O2 + NADH + H+ o H2O + NAD+, , The redox potential difference between these, two redox pairs is 1.14 V, which is equivalent to, an energy 52 Cal/mol., Three ATP are synthesized in the ETC when, NADH is oxidized which equals to 21.9 Cal, (each ATP = 7.3 Cal)., The efficiency of energy conservation is, calculated as, 219 u 100, 52, , Several hypotheses have been put forth to, explain the process of oxidative phosphorylation., The most important among them—namely,, chemical coupling, and chemiosmotic—are, discussed below., , Chemical coupling hypothesis, , There are three sites in the ETC that are, exergonic to result in the synthesis of 3 ATP, molecules (See Fig.11.7)., , 1, 2, , MECHANISM OF OXIDATIVE, PHOSPHORYLATION, , 42%., , Therefore, when NADH is oxidized, about, 42% of energy is trapped in the form of 3 ATP, and the remaining is lost as heat. The heat, liberation is not a wasteful process, since it, allows ETC to go on continuously to generate, ATP. Further, this heat is necessary to maintain, body temperature., , This mechanism, originally proposed by Peter, Mitchell (1961), is now widely accepted. It, explains how the transport of electrons through, the respiratory chain is effectively utilized to, produce ATP from ADP + Pi. The concept of, chemiosmotic hypothesis is comparable with, energy stored in a battery separated by positive, and negative charges., Proton gradient : The inner mitochondrial, membrane, as such, is impermeable to protons, (H+) and hydroxyl ions (OH–). The transport of, electrons through ETC is coupled with the, translocation of protons (H+) across the inner, mitochondrial membrane (coupling membrane), from the matrix to the intermembrane space. The, pumping of protons results in an electrochemical, or proton gradient. This is due to the, accumulation of more H+ ions (low pH) on the, outer side of the inner mitochondrial membrane, than the inner side (Fig.11.8). The proton, gradient developed due to the electron flow in, the respiratory chain is sufficient to result in the, synthesis of ATP from ADP and Pi.
Page 240 :
230, , BIOCHEMISTRY, , High H+, ATP, synthesis, Low H+, , Outer mitochondrial, membrane, Matrix, Intermembrane, space, Inner mitochondrial, membrane, , Fig. 11.8 : Outline of chemiosmotic hypothesis for oxidative phosphorylation., , Enzyme system for ATP synthesis : ATP, synthase, present in the complex V, utilizes the, proton gradient for the synthesis of ATP. This, enzyme is also known as ATPase since it can, hydrolyse ATP to ADP and Pi. ATP synthase is a, complex enzyme and consists of two functional, subunits, namely F1 and F0 (Fig.11.9). Its, structure is comparable with ‘lollipops’., , The protons that accumulate on the, intermembrane space re-enter the mitochondrial, matrix leading to the synthesis of ATP., , Rotary motor model for ATP, generation, Paul Boyer in 1964 proposed (Nobel Prize,, 1997) that a conformational change in the, , +, , 2H+, , Cy, , tc, , +, , 2H, , III, , Q, , 2H+, , I, , NAD+, , 2H, , Co, , IV, 2H+, , NADH + H+, H+ n, (acidic), , 3ADP + 3Pi, 6H+, , OH– n, (alkaline), , 3ATP, , V, F1, , F0, , 6H+, , pH gradient, Mitochondrial, matrix, Inner mitochondrial, membrane, Intermembrane, space, Outer mitochondrial membrane, , Fig. 11.9 : Diagrammatic representation of chemiosmotic hypothesis for oxidative phosphorylation, (I, III, IV and V–Respiratory chain complexes; F0, F1–Protein subunits for phosphorylation).
Page 241 :
231, , Chapter 11 : BIOLOGICAL OXIDATION, , E, ATP, , ADP + Pi, F1, J, , H+, , Inside, Inner, mitochondrial, membrane, , C-units, , F0, , Outside, H+, , Fig. 11.10 : Structure of mitochondrial ATP synthase, (F0F1) complex (C units-channel protein subunits;, D, E, and J are the subunits of F1-ATP synthase)., , mitochondrial membrane proteins leads to the, synthesis of ATP. The original Boyer hypothesis,, now considered as rotary motor/engine driving, model or binding change model, is widely, accepted for the generation ATP., , The enzyme ATP synthase acts as a protondriving motor, and is an example of rotary, catalysis. Thus, ATP synthase is the world’s, smallest molecular motor., , Inherited disorders of, oxidative phosphorylation, It is estimated that about 100 polypeptides, are required for oxidative phosphorylation. Of, these, 13 are coded by mitochondrial DNA, (mtDNA) and synthesized in the mitochondria,, while the rest are produced in the cytosol (coded, by nuclear DNA) and transported. mtDNA is, maternally inherited since mitochondria from, the sperm do not enter the fertilized ovum., , In response to the proton flux, the J subunit, physically rotates. This induces conformational, changes in the E3 subunits that finally lead to the, release of ATP., , T, , O, , ATP, , AT, P, , ATP, T, , J, , O, , L, ADP + Pi, , ATP, O, , J, T, ATP, , Fig. 11.11 : The binding change model, (rotary motor/engine driving model), for ATP synthesis by F1-ATP synthase., , L, , Pi, , By an unknown mechanism, protons induce, the rotation of J subunit, which in turn induces, conformation changes in E subunits. The, substrates ADP and Pi bind to E subunit in, , J, , P+, AD, , According to the binding change mechanism,, the three E subunits of F1-ATP synthase adopt, different conformations. One subunit has, open (O) conformation, the second has loose (L), conformation while the third one has tight (T), conformation (Fig.11.11)., , L, , P, AT, , The enzyme ATP synthase is F0F1 complex (of, complex V). The F0 subcomplex is composed of, channel protein ‘C’ subunits to which F1-ATP, synthase is attached (Fig.11.10). F1-ATP synthase, consists of a central J subunit surrounded by, alternating D and E subunits (D3 and E3)., , It may be noted that the ATP release in O, conformation is energy dependent (and not ATP, synthesis) and very crucial in rotary motor model, for ATP generation., , i, , D, , +P, , E, , D, , L-conformation. The L site changes to T, conformation, and this leads to the synthesis of, ATP. The O site changes to L conformation, which binds to ADP and Pi. The T site changes, to O conformation, and releases ATP. This cycle, of conformation changes of E subunits is, repeated. And three ATP are generated for each, revolution (Fig.11.11)., , AD, P, , D, , E, ATP, J
Page 242 :
232, , BIOCHEMISTRY, , Mitochondrial DNA is about 10 times more, susceptible to mutations than nuclear DNA., mtDNA mutations are more commonly seen, in tissues with high rate of oxidative, phosphorylation (e.g. central nervous system,, skeletal and heart muscle, liver)., , Leber’s hereditary optic neuropathy is an, example for mutations in mtDNA. This disorder, is characterized by loss of bilateral vision due to, neuroretinal degeneration., , Inhibitors of electron, transport chain, Many site-specific inhibitors of ETC have, contributed to the present knowledge of, mitochondrial respiration. Selected examples, of these inhibitors haven been given in, Fig.11.7. The inhibitors bind to one of the, components of ETC and block the transport of, electrons. This causes the accumulation of, reduced components before the inhibitor, , blockade step and oxidized components after, that step., The synthesis of ATP (phosphorylation) is, dependent on electron transport. Hence, all the, site-specific inhibitors of ETC also inhibit ATP, formation. Three possible sites of action for the, inhibitors of ETC are identified, 1. NADH and coenzyme Q : Fish poison, rotenone, barbituate drug amytal and antibiotic, piercidin A inhibit this site., 2. Between cytochrome b & c1 : Antimycin A, — an antibiotic, British antilewisite (BAL)—, an antidote used against war-gas—are the, two important inhibitors of the site between, cytochrome b and c1., 3. Inhibitors of cytochrome oxidase : Carbon, monoxide, cyanide, hydrogen sulphide and, azide effectively inhibit cytochrome oxidase., Carbon monoxide reacts with reduced form of, the cytochrome while cyanide and azide react, with oxidized form., , + The most important function of food is to supply energy to the living cells. This is, finally achieved through biological oxidation., , + The supply of O2 is very essential for the survival of life (exception—anaerobic bacteria)., + ATP, the energy currency of the cell, acts as a link between the catabolism and, anabolism in the living system. The major production of body’s ATP occurs in the, mitochondria through oxidative phosphorylation coupled with respiration., , + Respiratory chain or electron transport chain (ETC) is blocked by site specific inhibitors, such as rotenone, amytal, antimycin A, BAL, carbon monoxide and cyanide., , + Uncoupling of respiration from oxidative phosphorylation under natural conditions, assumes biological significance. The brown adipose tissue, rich in electron carriers,, brings about oxidation uncoupled from phosphorylation. The presence of active brown, adipose tissue in some individuals is believed to protect them from becoming obese., This is because the excess calories consumed by these people are burnt and liberated, as heat instead of being stored as fat., , + Inherited disorders of oxidative phosphorylation caused by the mutations in, mitochondrial DNA have been identified e.g. Leber’s hereditary optic neuropathy.
Page 243 :
233, , Chapter 11 : BIOLOGICAL OXIDATION, , Cyanide poisoning : Cyanide is probably the, most potent inhibitor of ETC. It binds to Fe3+ of, cytochrome oxidase blocking mitochondrial, respiration leading to cell death. Cyanide, poisoning causes death due to tissue asphyxia, (mostly of central nervous system)., , INHIBITORS OF OXIDATIVE, PHOSPHORYLATION, Uncouplers, The mitochondiral transport of electrons is, tightly coupled with oxidative phosporylation, (ATP synthesis). In other words, oxidation and, phosphorylation proceed simultaneously. There, are certain compounds that can uncouple (or, delink) the electron transport from oxidative, phosphorylation. Such compounds, known as, uncouplers, increase the permeability of inner, mitochondrial membrane to protons (H+). The, result is that ATP synthesis does not occur. The, energy linked with the transport of electrons is, dissipated as heat. The uncouplers allow (often, at accelerated rate) oxidation of substrates (via, NADH or FADH2) without ATP formation., The uncoupler, 2,4-dinitrophenol (DNP), has, been extensively studied. It is a small lipophilic, molecule. DNP is a proton-carrier and can easily, diffuse through the inner mitochondrial, membrane. In the people seeking to lose weight,, DNP was used as a drug. However, this is now, discontinued, as it produces hyperthermia and, other side effects. In fact, Food and Drug, Administration (USA) has banned the use of, DNP., The other uncouplers include dinitrocresol,, pentachlorophenol,, trifluorocarbonylcyanide, phenylhydrazone (FCCP). The last compound, (FCCP) is said to be 100 times more effective as, an uncoupler than dinitrophenol. When, administered in high doses, the drug aspirin acts, as an uncoupler., Physiological uncouplers : Certain physiological substances which act as uncouplers at, higher concentration have been identified. These, include thermogenin, thyroxine and long chain, free fatty acids. The unconjugated bilirubin is, , also believed to act as an uncoupler. This is,, however, yet to be proved beyond doubt., , Significance of uncoupling, Uncoupling of respiration from oxidative, phosphorylation under natural conditions, assumes, biological, significance., The, maintenance of body temperature is particularly, important in hairless animals, hibernating, animals and the animals adapted to cold. These, animals possess a specialized tissue called, brown adipose tissue in the upper back and neck, portions. The mitochondria of brown adipose, tissue are rich in electron carriers and are, specialized to carry out an oxidation uncoupled, from phosphorylation. This causes liberation of, heat when fat is oxidized in the brown adipose, tissue. Brown adipose tissue may be considered, as a site of non-shivering thermogenesis. The, presence of active brown adipose tissue in, certain individuals is believed to protect them, from becoming obese. The excess calories, consumed by these people are burnt and, liberated as heat, instead of being stored as fat., , Thermogenin (or uncoupling protein, UCPI) is, a physiological uncoupler, located in the inner, mitochondrial membrane of brown adipose, tissue. It blocks the formation of ATP, and, generates heat. This assumes significant in the, newborn, and during hibernation in animals., Ionophores : The term ‘ionophores’ is used to, collectively represent the lipophilic substances, that promote the transport of ions across, biological membranes., All the uncouplers (described above) are, in, fact, proton ionophores., The antibiotics valinomycin, gramicidin A and, nigercin act as ionophores for K+ ions. Both these, compounds are also capable of dissipating proton, gradient across the inner mitochondrial, membrane and inhibit oxidative phosphorylation., , Other inhibitors of, oxidative phosphorylation, Oligomycin : This antibiotic prevents the, mitochondrial, oxidation, as, well, as, phosphorylation. It binds with the enzyme ATP
Page 244 :
234, , BIOCHEMISTRY, , NADH + H, , +, , +, , NAD, , CH2OH, , CH2OH, , C O, CH2O, , Cytosolic glycerol, 3-phosphate dehydrogenase, , P, , HO C H, CH2O, , P, , Glycerol 3-phosphate, , Dihydroxyacetone, phosphate, CYTOSOL, , MITOCHONDRIAL MATRIX, , CH2OH, , Mitochondrial glycerol, 3-phosphate dehydrogenase, , C O, CH2O, , CH2OH, HO C H, , P, , FADH2, Dihydroxyacetone, phosphate, ETC, 2ATP, , CH2O, FAD, , P, , Glycerol 3-phosphate, , H2O, , Fig. 11.12 : Glycerol-phosphate shuttle (reducing equivalents transported are shown in Blue)., , synthase and blocks the proton (H+) channels. It, thus prevents the translocation (re-entry) of, protons into the mitochondrial matrix. Due to, this, protons get accumulated at higher, concentration in the intermembrane space., Electron transport (respiration) ultimately stops,, since protons cannot be pumped out against, steep proton gradients., Atractyloside : This is a plant toxin and, inhibits oxidative phosphorylation by an indirect, mechanism. Adenine nucleotide carrier system, facilitates the transport of ATP and ADP., Atractyloside inhibits adenine nucleotide carrier, and, thus, blocks the adequate supply of ADP,, thereby preventing phosphorylation., , TRANSPORT OF REDUCING, EQUIVALENTS—SHUTTLE, PATHWAYS, The inner mitochondrial membrane is, impermeable to NADH. Therefore, the NADH, produced in the cytosol cannot directly enter the, mitochondria. Two pathways—namely glycerol-, , phosphate shuttle and malate-aspartate shuttle—, are operative to do this job. They transport the, reducing, equivalents, from, cytosol, to, mitochondria and not vice versa., , I. Glycerol-phosphate shuttle, Cytosolic glycerol 3-phosphate dehydrogenase, oxidizes NADH to NAD+. The reducing, equivalents are transported through glycerol, 3-phosphate into the mitochondria. Glycerol, 3-phosphate dehydrogenase—present on outer, surface of inner mitochondrial membrane—, reduces FAD to FADH2. Dihydroxyacetone, phosphate escapes into the cytosol and, the shuttling continues as depicted in Fig.11.12., FADH2 gets oxidized via ETC to generate, 2 ATP., , II. Malate-aspartate shuttle, In the cytosol, oxaloacetate accepts the, reducing equivalents (NADH) and becomes, malate. Malate then enters mitochondria where, it is oxidized by mitochondrial malate dehydro-
Page 246 :
236, , BIOCHEMISTRY, , substrate molecules by dehydrogenases) to the, final acceptor, oxygen., Some flavoproteins containing FAD or, FMN also belong to the category of oxidases., e.g., L-amino acid oxidase (FMN), xanthine, oxidase (FAD)., 2. Dehydrogenases : As the name indicates,, these enzymes cannot utilize oxygen as, hydrogen acceptor. They catalyse the reversible, transfer of hydrogen from one substrate to, another and, thus, bring about oxidationreduction reactions. There are a large number of, enzymes belonging to this group, l, , l, , l, , l, , l, , NAD+ dependent dehydrogenases, e.g., alcohol dehydrogenase, glycerol 3-phosphate, dehydrogenase., NADP+ dependent dehydrogenases, e.g. HMG, CoA reductase, enoyl reductase., FMN dependent dehydrogenases, e.g. NADH, dehydrogenase., FAD dependent dehydrogenases, e.g. succinate dehydrogenase, acyl CoA dehydrogenase., The cytochromes : All the cytochromes of, electron transport chain (b, c1 and c) except, the terminal cytochrome oxidase (a+a3) belong, to this group., , 3. Hydroperoxidases : Hydrogen peroxide is, the substrate for these enzymes. There is a, constant production of H2O2 in the reactions, catalysed by the aerobic dehydrogenases. The, harmful effects of H2O2 are prevented by, hydroperoxidases, e.g. peroxidase and catalase., 2H2O2 o 2H2O + O2, (Note : The reader must refer Chapter 34 for, details on free radicals and antioxidants), , 4. Oxygenases : This group of enzymes, catalyses the direct incorporation of oxygen into, the substrate molecules., l, , l, , Dioxygenases (true oxygenases) : They are, responsible for the incorporation of both the, atoms of oxygen (O2) into the substrate, e.g., homogentisate oxidase, L-tryptophan pyrrolase., Monooxygenases (mixed function oxidases) : They catalyse the incorporation of one, atom of oxygen ( 1 O2) while the other oxygen, 2, atom is reduced to H2O. NADPH usually, provides, the reducing equivalents, e.g., cytochrome P450 monooxygenase system of, microsomes is responsible for the metabolism, of many drugs (amino pyrine, morphine,, aniline etc.) and biosynthesis of steroid, hormones (from cholesterol). The action of Cyt, P450 is depicted here., , O2, , H2O, , RH, NADPH + H+, , ROH, NADP+, , Electron transport in prokaryotes, In contrast to eukaryotes, the prokaryotes lack, mitochondria. However, prokaryotes possess a, separate system for biological oxidation. A set of, electron carriers (different from that found in, mitochondria) and enzymes of oxidative, phosphorylation are bound to the inner cell, membrane in prokaryotes. This arrangement of, oxidative machinery is one of the reasons to, believe that mitochondria of higher organisms, have descended from prokaryotic cells.
Page 247 :
Chapter 11 : BIOLOGICAL OXIDATION, , 1. Bioenergetics deals with the study of energy changes in biochemical reactions. Change, in free energy ('G) is valuable in predicting the feasibility of a reaction. A negative and, a positive 'G, respectively, represent an exergonic (energy-releasing) and endergonic, (energy-consuming) reactions., 2. High-energy compounds ('G > –7.0 Cal/mol) play a crucial role in the energy transfer, of biochemical reactions (e.g. ATP, phosphocreatine, phosphoenolpyruvate)., 3. ATP is the energy currency of the cell. ATP-ADP cycle acts as a connecting energy link, between catabolic and anabolic reactions., 4. Respiratory chain or electron transport chain (ETC) located in the inner mitochondrial, membrane represents the final stage of oxidizing the reducing equivalents (NADH and, FADH2) derived from the metabolic intermediates to water., 5. ETC is organized into five distinct complexes. The complexes I to IV are electron, carriers while complex V is responsible for ATP production. The components of ETC, are arranged in the sequence, NAD+ o FMN o CoQ o� Cyt b o Cyt c1 o Cyt c o Cyt a + a3 o O2, , 6. The process of synthesizing ATP from ADP and Pi coupled with ETC is known as, oxidative phosphorylation. NADH oxidation with a P : O ratio 3 indicates that 3 ATP, are synthesized while FADH2 oxidation (P : O ratio 2) results in the production of 2, ATP., 7. Among the hypotheses put forth to explain the mechanism of oxidative, phosphorylation, the chemiosmotic hypothesis (of Mitchell) is widely accepted. The, rotary motor model (of Boyer) involving the conformation changes in the E-subunits of, ATP synthase explains the ATP generation., 8. NADH produced in the cytosol cannot directly enter mitochondria. Glycerol-phosphate, shuttle (generates 2 ATP) and malate-asparate shuttle (generates 3 ATP) operate to, overcome the difficulty., 9. There are many inhibitors of electron transport chain (rotenone, amytal, antimycin,, CO,-CN, H2S etc.) and oxidative phosphorylation (oligomycin, atractyloside)., Uncouplers (e.g. dinitrophenol) are the substances that delink ETC from oxidative, phosphorylation., 10. The enzymes participating in biological oxidation belong to the class oxidoreductases., There are five groups, namely oxidases, aerobic dehydrogenases, anaerobic dehydrogenases, hydroperoxidases and oxygenases., , 237
Page 248 :
238, , BIOCHEMISTRY, , I. Essay questions, 1. Write an account of the high-energy compounds in metabolism, 2. Describe the components of electron transport chain and discuss the oxidation of NADH., 3. Define oxidative phosphorylation. Discuss chemiosmotic hypothesis in detail., 4. Give an account of the enzymes involved in biological oxidation., 5. Discuss about the inhibitors of ETC and oxidative phosphorylation., , II. Short notes, (a) High-energy bonds, (b) Uncouplers, (c) P : O ratio, (d) Redox loops, (e) ATP synthase,, (f) Cytochromes, (g) Sites of oxidative phosphorylation, (h) Coenzyme Q, (I) Redox potential, (j) ATP, as energy currency., , III. Fill in the blanks, 1. The relation between the change of free energy ('G), enthalpy ('H) and entropy ('S) is, expressed by the equation ______________., 2. A negative sign of free energy indicates that the reaction is ______________., 3. The bonds responsible for a majority of high-energy compounds are ______________., 4. The storage form of high-energy compound in invertebrates is ______________., 5. A more negative redox potential represents a greater tendency to lose ______________., 6. The electron transport chain is located in ______________., 7. The prosthetic group present in cytochromes ______________., 8. The component of electron transport chain which can directly react with molecular, oxygen ______________., 9. The site of ETC inhibited by cyanide ______________., 10. Superoxide is converted to H2O2 by the enzyme ______________., , IV. Multiple choice questions, 11. Name the compound with the greatest standard free energy., (a) ATP (b) Phosphocreatine (c) Cyclic AMP (d) Phosphoenolpyruvate., 12. One of the following components of ETC possesses isoprenoid units, (a) Coenzyme Q (b) Cytochrome (c) Cytochrome b (d) Non-heme iron., 13. The P : O ratio for the oxidation of FADH2 is, (a) 1 (b) 2 (c) 3 (d) 4., 14. Inner mitochondrial membrane is impermeable to, (a) H+ (b) K+ (c) OH– (d) All of them., 15. ATP synthase activity is associated with the mitochondrial enzyme complex, (a) V (b) III (c) IV (d) I.
Page 249 :
ME, METABOLISMS, MET, TABOLISMS, ABOLISMS, 12, ■, 13, ■, 14, ■, 15, ■, 16, ■, 17, ■, 18, ■, , Introduction to Metabolism, , 241, , Metabolism of Carbohydrates 244, Metabolism of Lipids, , 285, , Metabolism of Amino Acids, , 330, , Integration of Metabolism, , 380, , Metabolism of Nucleotides, , 387, , Mineral Metabolism, , 403, , Section, , IIIIII
Page 250 :
“This page intentionally left blank"
Page 251 :
Section 3, , Metabolisms, , Chapter, , Introduction to Metabolism, , 12, , Energy rich, complex molecules, ADP + Pi, NADP+, , CATABOLISM, , ANABOLISM, , ATP, NADPH, Energy poor, products, , The metabolism introduces itself :, , “I represent the chemical reactions of life;, Composed of catabolism and anabolism;, Catabolism is degradative to generate energy;, Anabolism is synthetic that consumes energy.”, , H, , undreds of reactions simultaneously take, place in a living cell, in a well-organized, and integrated manner. The entire spectrum of, chemical reactions, occurring in the living, system, are collectively referred to as, metabolism., , Energy rich, complex molecules, ADP + Pi, NADP+, , CATABOLISM, , A metabolic pathway (or metabolic map), constitutes a series of enzymatic reactions to, produce specific products. The term metabolite, is applied to a substrate or an intermediate or a, product in the metabolic reactions., , ANABOLISM, , ATP, NADPH, Energy poor, products, , Metabolism is broadly divided into two, categories (Fig.12.1)., , Fig. 12.1 : An outline of catabolism and anabolism., , 1. Catabolism : The degradative processes, concerned with the breakdown of complex, molecules to simpler ones, with a concomitant, release of energy., , several intermediates common to both the, processes. The term amphibolism is also in use, for reactions which are both catabolic and, anabolic in nature., , 2. Anabolism : The biosynthetic reactions, involving the formation of complex molecules, from simple precursors., , Catabolism, , A clear demarcation between catabolism and, anabolism is rather difficult, since there are, , The very purpose of catabolism is to trap the, energy of the biomolecules in the form of ATP, and to generate the substances (precursors), , 241
Page 252 :
242, , BIOCHEMISTRY, , Polysaccharides, , Lipids, , Proteins, , Monosaccharides, , Fatty acids, and glycerol, , Amino acids, , Stage 1, , Stage 2, , Acetyl CoA, , Citric acid, cycle, NADH, FADH2, Stage 3, , 1, 2 O2, , H 2O, , ETC, , CO2, ADP, ATP, , Fig. 12.2 : The three stages of catabolism (ETC–Electron transport chain)., , required for the synthesis of complex molecules., Catabolism occurs in three stages (Fig.12.2)., 1. Conversion of complex molecules into, their building blocks : Polysaccharides are, broken down to monosaccharides, lipids to free, fatty acids and glycerol, proteins to amino acids., 2. Formation of simple intermediates : The, building blocks produced in stage (1) are, degraded to simple intermediates such as, pyruvate and acetyl CoA. These intermediates, are not readily identifiable as carbohydrates,, lipids or proteins. A small quantity of energy (as, ATP) is captured in stage 2., 3. Final oxidation of acetyl CoA : Acetyl CoA, is completely oxidized to CO2, liberating NADH, and FADH2 that finally get oxidized to release, large quantity of energy (as ATP). Krebs cycle (or, citric acid cycle) is the common metabolic, pathway involved in the final oxidation of all, energy-rich molecules. This pathway accepts the, carbon compounds (pyruvate, succinate etc.), derived from carbohydrates, lipids or proteins., , Anabolism, For the synthesis of a large variety of complex, molecules, the starting materials are relatively, , few. These include pyruvate, acetyl CoA and the, intermediates of citric acid cycle. Besides the, availability of precursors, the anabolic reactions, are dependent on the supply of energy (as ATP, or GTP) and reducing equivalents (as NADPH +, H+)., The anabolic and catabolic pathways are not, reversible and operate independently. As such,, the metabolic pathways occur in specific cellular, locations (mitochondria, microsomes etc.) and, are controlled by different regulatory signals., The terms—intermediary metabolism and, energy, metabolism—are, also, in, use., Intermediary metabolism refers to the entire, range of catabolic and anabolic reactions, not, involving nucleic acids. Energy metabolism, deals with the metabolic pathways concerned, with the storage and liberation of energy., , Types of metabolic reactions, The biochemical reactions are mainly of four, types, 1. Oxidation-reduction., 2. Group transfer., 3. Rearrangement and isomerization.
Page 253 :
243, , Chapter 12 : INTRODUCTION TO METABOLISM, , 4. Make and break of carbon-carbon bonds., These reactions are catalysed by specific, enzymes—more than 2,000 known so far., , Methods employed, to study metabolism, The metabolic reactions do not occur in, isolation. They are interdependent and integrated, into specific series that constitute metabolic, pathways. It is, therefore, not an easy task to, study metabolisms. Fortunately, the basic, metabolic pathways in most organisms are, essentially identical. For this reason, many, organisms can be used to understand, metabolisms., Several methods are employed to elucidate, biochemical reactions and the metabolic, pathways. These experimental approaches may, be broadly divided into 3 categories, 1. Use of whole organisms or its components., 2. Utility of metabolic probes., 3. Application of isotopes., The actual methods employed may be either, in vivo (in the living system) or in vitro (in the, test tube) or, more frequently, both., 1. Use of whole organism or its components :, (a) Whole organisms : The ultimate aim of, a biochemist is to know the, metabolism in the organism as a, whole. Glucose tolerance test (GTT),, , employed to measure the response of, man (or other animals) towards, carbohydrate metabolism is a good, example of the use of whole organism., (b) Isolated organs, tissue slices, whole, cells, subcellular organelles, cell-free, systems, and, recently, purified, components are frequently used to, elucidate biochemical reactions and, metabolic pathways., 2. Utility of metabolic probes : Two types of, metabolic probes are commonly used to trace, out biochemical pathways. These are metabolic, inhibitors and mutations. In both the cases, there, is a specific blockade in a metabolic reaction, which helps to understand the pathway., Inhibitors of electron transport chain have been, largely responsible to elucidate the sequence of, electron carriers (Chapter 11). The inborn errors, of metabolism in higher organisms and the, genetic manipulations in the microorganisms, have also contributed a lot to the understanding, of metabolisms., 3. Application of isotopes : Isotopes are the, atoms with the same number of protons but, different neutrons. By use of isotopes, the, molecules of the living system can be labelled, without altering their chemical properties., Application of isotopes in biochemistry has, revolutionized the study of metabolisms. More, details on the utility of isotopes in biochemistry, are given elsewhere (Chapter 41)., , 1. The wide range of chemical reactions occurring in the living system are collectively, known as metabolism. Catabolism is concerned with the degradation of complex, molecules to simpler ones coupled with the liberation of energy (ATP). On the other, hand, anabolism deals with the synthetic reactions converting simple precursors to, complex molecules, coupled with the consumption of energy (ATP). A metabolic, pathway constitutes a series of enzymatic reactions to produce specific products., 2. Several methods are employed to study metabolism. These include the use of the whole, organism or its components (organ, tissue, cells, organelles etc.), utility of metabolic, probes (inhibitors and mutations) and application of isotopes.
Page 254 :
Section 3, , Metabolisms, , Chapter, , Metabolism of Carbohydrates, , 13, , HO C H, H C OH, HO C H, H C OH, H C, CH2 OH, Glucose, , The official spokesperson of carbohydrate metabolism, ‘glucose’, speaks :, , “I burn myself to provide fuel to life!, Generated through gluconeogenesis by my friends;, Engaged in the synthesis of lipids, amino acids;, Deranged in my duties due to diabetes mellitus.”, , C, , arbohydrates are the major source of energy, for the living cells. As such, carbohydrates, are the first cellular constituents, synthesized by, green plants during photosynthesis from carbon, dioxide and water, on absorption of light. Thus,, light is the ultimate source of energy for all, biological processes., , The monosaccharide glucose is the central, molecule in carbohydrate metabolism since all, the major pathways of carbohydrate metabolism, are connected with it (Fig.13.1). Glucose is, utilized as a source of energy, it is synthesized, from non-carbohydrate precursors and stored as, glycogen to release glucose as and when the, need arises. The other monosaccharides, important in carbohydrate metabolism are, fructose, galactose and mannose., The fasting blood glucose level in normal, individuals is 70-100 mg/dl (4.5-5.5 mmol/l) and, it is very efficiently maintained at this level (for, details refer Chapter 36). Liver plays a key role, in monitoring and stabilizing blood glucose, levels. Thus liver may be appropriately, considered as glucostat monitor., , Major pathways, of carbohydrate metabolism, The important pathways of carbohydrate, metabolism are listed, 1. Glycolysis (Embden-Meyerhof pathway) :, The oxidation of glucose to pyruvate and lactate., 2. Citric acid cycle (Krebs cycle or, tricarboxylic acid cycle) : The oxidation of acetyl, CoA to CO2. Krebs cycle is the final common, oxidative pathway for carbohydrates, fats or, amino acids, through acetyl CoA., 3. Gluconeogenesis : The synthesis of, glucose from non-carbohydrate precursors (e.g., amino acids, glycerol etc.)., 4. Glycogenesis : The formation of glycogen, from glucose., 5. Glycogenolysis, glycogen to glucose., , :, , The, , breakdown, , of, , 6. Hexose monophosphate shunt (pentose, phosphate pathway or direct oxidative pathway) :, This pathway is an alternative to glycolysis and, , 244
Page 255 :
245, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , OXIDATIVE PATHWAYS, , SYNTHETIC PATHWAYS, , Glycolysis, , Hexose monophosphate shunt, , Uronic acid, pathway, , G, L, U, C, O, S, E, , Other carbohydrates, (galactose, fructose), Glycogenesis, , Lipogenesis, (synthesis of fat), Non-essential, amino acids, , Fig. 13.1 : Overview of glucose metabolism., (Note : For majority of the pathways, glucose, participates as glucose 6-phosphate)., , TCA cycle for the oxidation of glucose (directly to, carbon dioxide and water)., 7. Uronic acid pathway : Glucose is, converted to glucuronic acid, pentoses and, in, some animals, to ascorbic acid (not in man). This, pathway is also an alternative oxidative pathway, for glucose., 8. Galactose metabolism : The pathways, concerned with the conversion of galactose to, glucose and the synthesis of lactose., 9. Fructose metabolism : The oxidation of, fructose to pyruvate and the relation between, fructose and glucose metabolism., , 2. Insulin-dependent transport system : This, occurs in muscle and adipose tissue., Glucose transporters : In recent years, at least, six glucose transporters (GLUT-1 to GLUT-5 and, GLUT-7) in the cell membranes have been, identified. They exhibit tissue specificity. For, instance, GLUT-1 is abundant in erythrocytes, whereas GLUT-4 is abundant in skeletal muscle, and adipose tissue., Insulin increases the number and promotes, the activity of GLUT-4 in skeletal muscle and, adipose tissue. In type 2 diabetes mellitus,, insulin resistance is observed in these tissues., This is due to the reduction in the quantity of, GLUT-4 in insulin deficiency., , GLYCOLYSIS, Glycolysis is derived from the Greek words, (glycose—sweet or sugar; lysis—dissolution). It is, a universal pathway in the living cells. The, complete pathway of glycolysis was elucidated, in 1940. This pathway is often referred to as, Embden-Meyerhof pathway (E.M. pathway) in, honour of the two biochemists who made a, major contribution to the knowledge of, glycolysis., , 10. Amino sugar and mucopolysaccharide, metabolism : The synthesis of amino sugars and, other sugars for the formation of mucopolysaccharides and glycoproteins., , Glycolysis is defined as the sequence of, reactions converting glucose (or glycogen) to, pyruvate or lactate, with the production of ATP., , Entry of glucose into cells, , 1. Glycolysis takes place in all cells of the, body. The enzymes of this pathway are present, in the cytosomal fraction of the cell., , Glucose concentration is very low in the cells, compared to plasma (for humans < 100 mg/dl)., However, glucose does not enter the cells by, simple diffusion. Two specific transport systems, are recognized for the entry of glucose into the, cells, 1. Insulin-independent transport system of, glucose : This is a carrier mediated uptake of, glucose which is not dependent on the hormone, insulin. This is operative in hepatocytes, erythrocytes and brain., , Salient features, , 2. Glycolysis occurs in the absence of oxygen, (anaerobic) or in the presence of oxygen, (aerobic). Lactate is the end product under, anaerobic condition. In the aerobic condition,, pyruvate is formed, which is then oxidized to, CO2 and H2O., 3. Glycolysis is a major pathway for ATP, synthesis in tissues lacking mitochondria, e.g., erythrocytes, cornea, lens etc.
Page 256 :
246, , BIOCHEMISTRY, , 4. Glycolysis is very essential for brain which, is dependent on glucose for energy. The glucose, in brain has to undergo glycolysis before it is, oxidized to CO2 and H2O., 5. Glycolysis (anaerobic) may be summarized, by the net reaction, Glucose + 2ADP + 2Pi o 2Lactate + 2ATP, 6. Glycolysis is a central metabolic pathway, with many of its intermediates providing branch, point to other pathways. Thus, the intermediates, of glycolysis are useful for the synthesis of amino, acids and fat., 7. Reversal of glycolysis along with the, alternate arrangements at the irreversible, steps, will result in the synthesis of glucose, (gluconeogenesis)., , Reactions of glycolysis, The sequence of reactions of glycolysis is, given in Fig.13.2. The pathway can be divided, into three distinct phases, A. Energy investment phase or priming stage, B. Splitting phase, C. Energy generation phase., The sequence of reactions are discussed, below., A. Energy investment phase, 1. Glucose is phosphorylated to glucose, hexokinase, or, 6-phosphate, by, glucokinase (both are isoenzymes)., This is an irreversible reaction,, dependent on ATP and Mg2+. The, enzyme hexokinase is present in almost, all the tissues. It catalyses the, phosphorylation of various hexoses, (fructose, mannose etc.), has low Km, for substrates (about 0.1 mM) and is, inhibited by glucose 6-phosphate., Glucokinase present in liver, catalyses, the phosphorylation of only glucose,, has high Km for glucose (10 mM) and is, not inhibited by glucose 6-phosphate., Due to high affinity (low Km), glucose, is utilized by hexokinase even at low, concentration, whereas glucokinase, , acts only at higher levels of glucose, i.e., after a meal when blood glucose, concentration is above 100 mg/dl., , Glucose 6-phosphate is impermeable, to the cell membrane. It is a central, molecule with a variety of metabolic, fates—glycolysis, glycogenesis, gluconeogenesis and pentose phosphate, pathway., 2. Glucose 6-phosphate undergoes isomerization to give fructose 6-phosphate in, the presence of the enzyme phosphohexose isomerase and Mg2+., 3. Fructose 6-phosphate is phosphorylated, to fructose 1,6-bisphosphate by phosphofructokinase (PFK). This is an irreversible, and a regulatory step in glycolysis., B. Splitting phase, 4. The, six, carbon, fructose, 1,6bisphosphate is split (hence the name, glycolysis), to, two, three-carbon, compounds, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, by the enzyme aldolase (fructose 1,6bisphosphate aldolase)., 5. The enzyme phosphotriose isomerase, catalyses the reversible interconversion, of glyceraldehyde 3-phosphate and, dihydroxyacetone phosphate. Thus,, two molecules of glyceraldehyde, 3-phosphate are obtained from one, molecule of glucose., C. Energy generation phase, 6. Glyceraldehyde 3-phosphate dehydrogenase, converts, glyceraldehyde, 3-phosphate to 1,3-bisphosphoglycerate., This step is important as it is involved in, the formation of NADH + H+ and a high, energy compound 1,3-bisphosphoglycerate. Iodoacetate and arsenate, inhibit the enzyme glyceraldehyde, 3-phosphate dehydrogenase. In aerobic, condition, NADH passes through the, electron transport chain and 6 ATP, (2 u 3 ATP) are synthesized by oxidative, phosphorylation.
Page 258 :
248, , BIOCHEMISTRY, , COO–, C OH, CH2, Pyruvate (enol), Spontaneous, COO–, C O, CH3, Pyruvate (keto), NADH + H, , +, , NAD, , +, , Lactate, dehydrogenase, COO–, , H C OH, CH3, L-Lactate, , Fig. 13.2 : The reactions in the pathway of, glycolysis (The three steps catalysed by hexokinase,, phosphofructokinase and pyruvate kinase,, shown in thick lines are irreversible)., , 7. The, enzyme, phosphoglycerate, kinase acts on 1,3-bisphosphoglycerate, resulting in the synthesis of ATP and, formation of 3-phosphoglycerate. This, step is a good example of substrate, level phosphorylation, since ATP, is synthesized from the substrate, without the involvement of electron, transport chain. Phosphoglycerate, kinase reaction is reversible, a rare, example among the kinase reactions., 8. 3-Phosphoglycerate is converted to, 2-phosphoglycerate by phosphoglycerate, mutase. This is an isomerization reaction., 9. The high energy compound phosphoenol pyruvate is generated from, 2-phosphoglycerate by the enzyme, enolase. This enzyme requires Mg2+ or, Mn2+ and is inhibited by fluoride. For, blood glucose estimation in the, laboratory, fluoride is added to the blood, to prevent glycolysis by the cells, so that, blood glucose is correctly estimated., (Fluoride combines with Mg2+ and, , phosphate to form a complex that binds, with active site of enolase and blocks, access of substrate. Thus, fluoride is an, unusual competitive inhibitor)., 10. The enzyme pyruvate kinase catalyses, the transfer of high energy phosphate, from phosphoenol pyruvate to ADP,, leading to the formation of ATP., This step also is a substrate level, phosphorylation. This reaction is, irreversible., , Conversion of pyruvate to, lactate—significance, Under anaerobic conditions (lack of O2),, pyruvate is reduced by NADH to lactate in, presence of the enzyme lactate dehydrogenase, (competitive inhibitor—oxamate). The NADH, utilized in this step is obtained from the reaction, catalysed by glyceraldehyde 3-phosphate, dehydrogenase. The formation of lactate allows, the regeneration of NAD+ which can be reused, by glyceraldehyde 3-phosphate dehydrogenase, so that glycolysis proceeds even in the absence, of oxygen to supply ATP., The occurrence of uninterrupted glycolysis is, very essential in skeletal muscle during strenous, exercise where oxygen supply is very limited., Glycolysis in the erythrocytes leads to lactate, production, since mitochondria—the centres for, aerobic oxidation—are absent. Brain, retina,, skin, renal medulla and gastrointestinal tract, derive most of their energy from glycolysis., , Lactic acidosis, Lactic acid is a three carbon hydroxy acid., Elevation of lactic acid in the circulation (normal, plasma 4–15 mg/dl) may occur due to its, increased production or decreased utilization., Mild forms of lactic acidosis (not life-threatening), are associated with strenuous exercise, shock,, respiratory diseases, cancers, low pyruvate, dehydrogenase activity, von Gierke’s disease etc., Severe forms of lactic acidosis are observed, due to impairment/collapse of circulatory system, which is often encountered in myocardial, infarction, pulmonary embolism, uncontrolled, hemorrhage and severe shock. This type of lactic
Page 259 :
249, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , acidosis is due to inadequate supply of O2 to the, tissues with a drastic reduction in ATP synthesis, (since the cells have to survive in anaerobic, conditions) which may even lead to death. The, term oxygen debt refers to the excess amount of, O2 required to recover. In clinical practice,, measurement of plasma lactic acid is useful to, know about the oxygen debt, and monitor the, patient’s recovery, and save the patient from, morbidity and mortality., , Production of ATP in glycolysis, The details of ATP generation in glycolysis, (from glucose) are given in Table 13.1. Under, anaerobic conditions, 2 ATP are synthesized, while, under aerobic conditions, 8 or 6 ATP are, synthesized—depending on the shuttle pathway, that operates., When the glycolysis occurs from glycogen,, one more ATP is generated. This is because no, ATP is consumed for the activation of glucose, (glycogen directly produces glucose 1-phosphate, , which forms glucose 6-phosphate). Thus, in, anaerobic glycolysis, 3 ATP are produced from, glycogen., , Glycolysis and shuttle pathways, In the presence of mitochondria and oxygen,, the NADH produced in glycolysis can participate, in the shuttle pathways (Refer Chapter 11) for, the synthesis of ATP. If the cytosolic NADH uses, malate-aspartate shuttle, 3 ATP are generated, from each molecule of NADH. This is in contrast, to glycerolphosphate shuttle that produces, only 2 ATP., , Cancer and glycolysis, Cancer cells display increased uptake of, glucose, and glycolysis. As the tumors grow, rapidly, the blood vessels are unable to supply, adequate oxygen, and thus a condition, of hypoxia exists. Due to this, anaerobic, glycolysis predominantly occurs to supply, energy. The cancer cells get adapted to hypoxic, , TABLE 13.1 Generation of ATP in glucose metabolism, , Pathway, Glycolysis, , Enzyme (method of ATP synthesis), , Number of, ATP synthesized, , Glyceraldehyde 3-phosphate dehydrogenase, (2 NADH, ETC, oxidative phosphorylation), , 6(5), , Phosphoglycerate kinase (substrate level phosphorylation), , 2, , Pyruvate kinase (substrate level phosphorylation), Two ATP are consumed in the reactions catalysed by hexokinase and, , 2, –2, , phosphofructokinase, , Citric acid cycle, , Net ATP synthesis in glycolysis in aerobic condition, , 8(7), , Pyruvate dehydrogenase (2 NADH, ETC, oxidative phosphorylation), , 6(5), , Isocitrate dehydrogenase (2 NADH, ETC, oxidative phosphorylation), , 6(5), , D-Ketoglutarate dehydrogenase, , 6(5), , Succinate thiokinase (substrate level phosphorylation), , 2, , Succinate dehydrogenase (2 FADH2, ETC, oxidative phosphorylation), , 4(3), , Malate dehydrogenase (2 NADH, ETC, oxidative phosphorylation), , 6(5), , Total ATP per mole of glucose under aerobic condition, Total ATP per mole of glucose under anaerobic condition, , 38(32), 2, , Note : Values in brackets in red colour represent ATP synthesized as per the new P:O ratios of 2.5 for NADH and 1.5 for FADH 2.
Page 260 :
250, glycolysis through the involvement of a, transcription factor namely hypoxia-inducible, transcription factor (HIF). HIF increases the, synthesis of glycolytic enzymes and the glucose, transporters. However, the cancer cells cannot, grow and survive without proper vascularization., One of the modalities of cancer treatment is to, use drugs that can inhibit vascularization of, tumors., , Irreversible steps in glycolysis, Most of the reactions of glycolysis are, reversible. However, the three steps catalysed by, the enzymes hexokinase (or glucokinase),, phosphofructokinase and pyruvate kinase, are, irreversible. These three stages mainly regulate, glycolysis. The reversal of glycolysis, with, alternate arrangements made at the three, irreversible stages, leads to the synthesis of, glucose from pyruvate (gluconeogenesis)., , Regulation of glycolysis, The three enzymes namely hexokinase (and, glucokinase), phosphofructokinase and pyruvate, kinase, catalysing the irreversible reactions, regulate glycolysis., , Hexokinase is inhibited by glucose, 6-phosphate. This enzyme prevents the, accumulation of glucose 6-phosphate due to, product, inhibition., Glucokinase,, which, specifically phosphorylates glucose, is an, inducible enzyme. The substrate glucose,, probably through the involvement of insulin,, induces glucokinase., Phosphofructokinase (PFK) is the most, important regulatory enzyme in glycolysis. This, enzyme catalyses the rate limiting committed, step. PFK is an allosteric enzyme regulated by, allosteric effectors. ATP, citrate and H+ ions (low, pH) are the most important allosteric inhibitors,, whereas, fructose 2,6-bisphosphate, ADP, AMP, and Pi are the allosteric activators., , Role of fructose 2,6-bisphosphate, in glycolysis, Fructose 2,6-bisphosphate (F2,6-BP) is considered to be the most important regulatory factor, , BIOCHEMISTRY, , cAMP, Phosphofructokinase -2, ATP, , ADP, , Fructose, 6-phosphate, , Fructose 2,6bisphosphate, Pi, Fructose 2,6bisphosphatase, , cAMP, , Fig. 13.3 : Regulation of fructose 2,6-bisphosphatase., , (activator) for controlling PFK and, ultimately,, glycolysis in the liver. F2,6-BP is synthesized, from fructose 6-phosphate by the enzyme, phosphofructokinase called PFK-2 (PFK-1 is the, glycolytic enzyme). F2,6-BP is hydrolysed by, fructose 2,6-bisphosphatase. The function of, synthesis and degradation of F2,6-BP is brought, out by a single enzyme (same polypeptide with, two active sites) which is referred to as, bifunctional enzyme (Fig.13.3). In fact, the, combined name of phosphofructokinase-2/, fructose 2,6-bisphosphatase is used to refer to the, enzyme that synthesizes and degrades F2,6-BP., The activity of PFK-2 and fructose 2,6bisphosphatase is controlled by covalent, modification which, in turn, is regulated by, cyclic AMP (cAMP is the second messenger for, certain hormones). Cyclic AMP brings about, dephosphorylation of the bifunctional enzyme,, resulting in inactivation of active site responsible, for the synthesis of F2,6-BP but activation of the, active site responsible for the hydrolysis of, F2,6-BP., , Pyruvate kinase also regulates glycolysis. This, enzyme is inhibited by ATP and activated by, F1,6-BP. Pyruvate kinase is active (a) in, dephosphorylated state and inactive (b) in, phosphorylated state. Inactivation of pyruvate, kinase by phosphorylation is brought about by, cAMP-dependent protein kinase. The hormone—, glucagon inhibits hepatic glycolysis by this, mechanism (Fig.13.4).
Page 261 :
251, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Glucagon, , cAMP, , Protein kinase, ATP, , P, , ADP, , Pyruvate kinase a, (active enzyme), , Pyruvate kinase b, (inactive enzyme), Phosphatase, , Pi, , Fig. 13.4 : Regulation of pyruvate kinase., , Pasteur effect, The inhibition of glycolysis by oxygen, (aerobic condition) is known as Pasteur effect. It, was discovered by Louis Pasteur, more than a, century ago, while studying fermentation by, yeast. He observed that when anaerobic yeast, cultures were exposed to air, the utiliziation of, glucose decreased by nearly seven fold., In the aerobic condition, the levels of, glycolytic intermediates from fructose 1,6bisphosphate onwards decrease while the earlier, intermediates accumulate. This clearly indicates, that Pasteur effect is due to the inhibition of the, enzyme phosphofructokinase. The inhibitory, effect of citrate and ATP (produced in the, presence of oxygen) on phosphofructokinase, explains the Pasteur effect., , Crabtree effect, The phenomenon of inhibition of oxygen, consumption by the addition of glucose to tissues, having high aerobic glycolysis is known as, Crabtree effect. Basically, this is opposite to that, of Pasteur effect. Crabtree effect is due to, increased competition of glycolysis for inorganic, phosphate (Pi) and NAD+ which limits their, availability for phosphorylation and oxidation., , Glycolysis and dental caries, Dental caries refers to the destruction or, decalcification of hard teeth due to organic, acids released by bacterial infections. The, , anaerobic bacteria (e.g. Streptococcus mutans,, Lactobacillus sps) that colonize the oral cavily, contribute to the development of dental caries., These bacteria grow optimally on refined and, fermentable sugars (e.g. sucrose of chocolates,, candies) by utilizing anaerobic glycolysis. Lactic, acid and other acids (produced by bacteria), erode tooth enamel and dentin, and dissolve, hydroxyapatite matrix of teeth that results in, cavity formation. Low levels of fluoride, from, tooth pastes or when applied topically can, inhibit the enzyme enolase and reduce glycolysis, and thus tooth decay. Further, fluoride integrates, into tooth surface to form fluoroapatite which, offers resistance to demineralization., , RAPAPORT-LEUBERING CYCLE, This is a supplementary pathway to glycolysis, which is operative in the erythrocytes of, man and other mammals. Rapaport-Leubering, cycle is mainly concerned with the synthesis of, 2,3-bisphosphoglycerate (2,3-BPG) in the RBC., 1,3-Bisphosphoglycerate (1,3-BPG) produced, in glycolysis is converted to 2,3-BPG by, the enzyme 2,3-bisphosphoglycerate mutase, (Fig.13.5). 2,3-BPG is hydrolysed to 3-phosphoglycerate by bisphosphoglycerate phosphatase. It, is now believed that bisphosphoglycerate mutase, is a bifunctional enzyme with mutase and, phosphatase activities catalysed by two different, sites present on the same enzyme., About 15-25% of the glucose that gets, converted to lactate in erythrocytes goes via 2,3BPG synthesis., , Significance of 2,3-BPG, 1. Production of 2,3-BPG allows the, glycolysis to proceed without the synthesis of, ATP. Rapaport-Leubering cycle, therefore is a, shunt pathway of glycolysis to dissipate or waste, the energy not needed by erythrocytes., 2. 2,3-BPG, is not a waste molecule, It combines with hemoglobin (Hb) and, Hb affinity with oxygen. Therefore,, presence of 2,3-BPG, oxyhemoglobin, more oxygen to the tissues., , in RBC., reduces, in the, unloads, , Increase in erythrocyte 2,3-BPG is observed, in hypoxic condition, high altitude, fetal tissues,
Page 262 :
252, , BIOCHEMISTRY, , CONVERSION OF, PYRUVATE TO ACETYL CoA, , Glucose, , Pyruvate is converted to acetyl CoA by, oxidative decarboxylation. This is an irreversible, reaction, catalysed by a multienzyme complex,, known as pyruvate dehydrogenase complex, (PDH), which is found only in the mitochondria., High activities of PDH are found in cardiac, muscle and kidney. The enzyme PDH requires, five cofactors (coenzymes), namely—TPP,, lipoamide, FAD, coenzyme A and NAD+, (lipoamide contains lipoic acid linked to H-amino, group of lysine). The overall reaction of PDH is, , H C O, H C OH, CH2 O, , P, , Glyceraldehyde, 3-phosphate, Pi, Glyceraldehyde, 3-phosphate, dehydrogenase, , +, , NAD, , +, , NADH + H, , O, C O, , Pyruvate + NAD+ + CoA, , P, , 2,3-Bisphosphoglycerate, mutase, , H C OH, CH2 O, , P, , COO–, 2+, , Mg, , Phosphoglycerate, kinase, , H C O, CH2O, , ATP, , P, P, , 2,3-Bisphosphoglycerate, , COO–, H C OH, CH2 O, , P, , 3-Phosphoglycerate, , Pi, , Acetyl CoA +, CO2 + NADH + H+, , Reactions of PDH complex, , 1,3-Bisphosphoglycerate, ADP, , PDH, , 2,3-Bisphosphoglycerate, phosphatase, , Pyruvate, , Fig. 13.5 : Rapaport-Leubering cycle for the synthesis, of 2,3-bisphosphoglycerate (2,3-BPG)., , anemic conditions etc. In all these cases, 2,3BPG will enhance supply of oxygen to tissues., 3. Glycolysis in the erythrocytes is linked with, 2,3-BPG production and oxygen transport. In the, deficiency of the enzyme hexokinase, glucose is, not phosphorylated, hence the synthesis and, concentration of 2,3-BPG are low in RBC., The hemoglobin exhibits high oxygen affinity in, hexokinase-defective patients. On the other, hand, in the patients with pyruvate kinase, deficiency, the level of 2,3-BPG in erythrocytes, is high, resulting in low oxygen affinity. (For a, more detailed discussion on the functions of 2,3BPG, refer Chapter 10)., , The sequence of reactions brought about by, different enzymes of PDH complex in, association with the coenzymes is depicted in, Fig.13.6. Pyruvate is decarboxylated to, give hydroxyethyl TPP, catalysed by PDH, (decarboxylase activity). Dihydrolipoyl transacetylase brings about the formation of acetyl, lipoamide (from hydroxethyl-TPP) and then, catalyses the transfer of acetyl group to, coenzyme A to produce acetyl CoA. The cycle is, complete when reduced lipoamide is converted, to oxidized lipoamide by dihydrolipoyl dehydrogenase, transferring the reducing equivalents to, FAD. FADH2, in turn, transfers the reducing, equivalents to NAD+ to give NADH + H+, which, can pass through the respiratory chain to give, 3 ATP (6 ATP from 2 moles of pyruvate formed, from glucose) by oxidative phosphorylation., The intermediates of PDH catalysed reaction, are not free but bound with enzyme complex., In mammals, the PDH complex has an, approximate molecular weight of 9 u 106. It, contains 60 molecules of dihydrolipoyltransacetylase and about 20–30 molecules, each of the other two enzymes (pyruvate, dehydrogenase & dihydrolipoyl dehydrogenase)., A comparable enzyme with PDH is, D-ketoglutarate dehydrogenase complex of citric, acid cycle which catalyses oxidative decarboxylation of D-ketoglutarate to succinyl CoA.
Page 263 :
253, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , O, CH3 C, , O, COO–, , TPP, , CH3 C S Lip SH, , Pyruvate, , Acetyl-lipoamide, , CoA-SH, O, CH3 C ~ S CoA, , Pyruvate, dehydrogenase, , Dihydrolipoyl, transacetylase, , Lip, , OH, CH3 CH TPP, , CO2, , Hydroxyethyl-TPP, , Lip, , SH, SH, FAD, , S, S, , Oxidized, lipoamide, , Dihydrolipoyl, dehydrogenase, , NADH + H+, ETC, , FADH2, , NAD+, , 3 ATP, , Fig. 13.6 : The mechanism of action of pyruvate dehydrogenase complex. (Note : The reaction involving the, conversion of pyruvate to acetyl CoA requires five coenzymes—TPP, lipoamide, CoASH, FAD and NAD+)., , Arsenic poisoning : The enzymes PDH and, D-ketoglutarate dehydrogenase are inhibited by, arsenite. Arsenite binds to thiol ( SH) groups of, lipoic acid and makes it unavailable to serve as, cofactor., , Regulation of PDH, Pyruvate dehydrogenase is a good example, for end product (acetyl CoA, NADH) inhibition., Besides this, PDH is also regulated by, phosphorylation and dephosphorylation (Fig.13.7), PDH is active as a dephosphoenzyme while it is, inactive as a phosphoenzyme. PDH phosphatase, activity is promoted by Ca2+, Mg+ and, insulin (in adipose tissue). It is of interest to note, that calcium released during muscle contraction, stimulates PDH (by increasing phosphatase, activity) for energy production., PDH kinase (responsible to form inactive, PDH) is promoted by ATP, NADH and acetyl, CoA, while it is inhibited by NAD+, CoA and, pyruvate. The net result is that in the presence of, high energy signals (ATP, NADH), the PDH is, turned off., , Biochemical importance of PDH, 1. Lack of TPP (due to deficiency of thiamine), inhibits PDH activity resulting in the, accumulation of pyruvate., , 2. In the thiamine deficient alcoholics,, pyruvate is rapidly converted to lactate, resulting, in lactic acidosis., 3. In patients with inherited deficiency of, PDH, lactic acidosis (usually after glucose load), is observed., 4. PDH activity can be inhibited by arsenic, and mercuric ions. This is brought about by, binding of these ions with SH groups of lipoic, acid., , Metabolic importance of pyruvate, Pyruvate is a key metabolite. Besides its, conversion to acetyl CoA (utilized in a wide, range of metabolic reactions-citric acid cycle,, fatty acid synthesis etc.), pyruvate is a good, substrate for gluconeogenesis., P, , ADP, NAD+, CoA, Pyruvate, , PDH complex-b, inactive phosphoenzyme, Ca2+, Mg2+, , PDH kinase, ATP, NADH, Acetyl CoA, , ATP, , PDH phosphatase, , Pi, PDH complex, active dephosphoenzyme, , Insulin, (adipose, tissue), , Fig. 13.7 : Regulation of pyruvate dihydrogenase, (PDH) complex.
Page 264 :
254, , BIOCHEMISTRY, , CITRIC ACID CYCLE, The citric acid cycle (Krebs cycle or, tricarboxylic acid—TCA cycle) is the most, important metabolic pathway for the energy, supply to the body. About 65-70% of the ATP is, synthesized in Krebs cycle. Citric acid cycle, essentially involves the oxidation of acetyl CoA, to CO2 and H2O. This cycle utilizes about twothirds of total oxygen consumed by the body., The name TCA cycle is used, since, at the outset, of the cycle, tricarboxylic acids (citrate, cisaconitate and isocitrate) participate., , Acetyl CoA, CoA, (2C), , Citrate, (6C), , Oxaloacetate, (4C), Succinyl CoA, (4C), , CO2, D-Ketoglutarate, (5C), CO2, , Fig. 13.8 : An overview of Krebs cycle., , TCA cycle—an overview, TCA cycle—the central, metabolic pathway, The citric acid cycle is the final common, oxidative pathway for carbohydrates, fats and, amino acids. This cycle not only supplies energy, but also provides many intermediates required, for the synthesis of amino acids, glucose, heme, etc. Krebs cycle is the most important, central pathway connecting almost all the, individual metabolic pathways (either directly or, indirectly)., , Brief history, The citric acid cycle was proposed by Hans, Adolf Krebs in 1937, based on the studies of, oxygen consumption in pigeon breast muscle., The cycle is named in his honour (Nobel Prize, for Physiology and Medicine in 1953.), [Note : It is of interest to note that the original, manuscript on TCA cycle submitted by Krebs to, the journal ‘Nature’ was not accepted. He, published it in another journal Enzymoligia., Krebs used to carry the rejection letter (of Nature), with him, and advise the researches never to be, discouraged by research paper rejection]., , Location of TCA cycle, The enzymes of TCA cycle are located in, mitochondrial matrix, in close proximity to the, electron transport chain. This enables the, synthesis of ATP by oxidative phosphorylation, without any hindrance., , Krebs, cycle, basically, involves, the, combination of a two carbon acetyl CoA with a, four carbon oxaloacetate to produce a six carbon, tricarboxylic acid, citrate. In the reactions that, follow, the two carbons are oxidized to CO2 and, oxaloacetate is regenerated and recycled., Oxaloacetate is considered to play a catalytic, role in citric acid cycle. An overview of Krebs, cycle is depicted in Fig.13.8., , TCA cycle—an open cycle, Krebs cycle is a cyclic process. However, it, should not be viewed as a closed circle, since, many compounds enter the cycle and leave. TCA, cycle is comparable to a heavy traffic circle in a, national highway with many connecting roads., Each intermediate of the cycle connecting, another pathway is a road!, , Reactions of citric acid cycle, Oxidative decarboxylation of pyruvate to, acetyl CoA by pyruvate dehydrogenase complex, is discussed above. This step is a connecting link, between glycolysis and TCA cycle. A few, authors, however, describe the conversion of, pyruvate to acetyl CoA along with citric acid, cycle. The events of TCA cycle are described, hereunder (Fig.13.9)., 1. Formation of citrate : Krebs cycle proper, starts with the condensation of acetyl CoA and, oxaloacetate, catalysed by the enzyme citrate, synthase.
Page 266 :
256, 2. and 3. Citrate is isomerized to isocitrate, by the enzyme aconitase. This is achieved in a, two stage reaction of dehydration followed by, hydration through the formation of an, intermediate—cis-aconitate., 4. and 5. Formation of D-ketoglutarate :, The enzyme isocitrate dehydrogenase (ICD), catalyses the conversion (oxidative decarboxylation) of isocitrate to oxalosuccinate and then to, D-ketoglutarate. The formation of NADH and the, liberation of CO2 occur at this stage., 6. Conversion of D-ketoglutarate to succinyl, CoA occurs through oxidative decarboxylation,, catalysed by D-ketoglutarate dehydrogenase, complex. This enzyme is dependent on five, cofactors—TPP, lipoamide, NAD+, FAD and, CoA. The mechanism of the reaction is, analogous to the conversion of pyruvate to acetyl, CoA (See Fig.13.6)., 7. Formation of succinate : Succinyl CoA is, converted to succinate by succinate thiokinase., This, reaction, is, coupled, with, the, phosphorylation of GDP to GTP. This is a, substrate level phosphorylation. GTP is, converted to ATP by the enzyme nucleoside, diphosphate kinase., GTP + ADP l ATP + GDP, 8. Conversion of succinate to fumarate :, Succinate is oxidized by succinate dehydrogenase to fumarate. This reaction results in the, production of FADH2 and not NADH., 9. Formation of malate : The enzyme, fumarase catalyses the conversion of fumarate to, malate with the addition of H2O., 10. Conversion of malate to oxaloacetate :, Malate is then oxidized to oxaloacetate by, malate dehydrogenase. The third and final, synthesis of NADH occurs at this stage. The, oxaloacetate is regenerated which can combine, with another molecule of acetyl CoA, and, continue the cycle., , Summary of TCA cycle, The events of Krebs cycle may be summarized, as given in the next column, Acetyl CoA + 3 NAD+ + FAD + GDP + Pi +, 2H2O o 2CO2 + 3NADH + 3H+ + FADH2 +, GTP + CoA, , BIOCHEMISTRY, , Requirement of O2 by TCA cycle, There is no direct participation of oxygen in, Krebs cycle. However, the cycle operates only, under aerobic conditions. This is due to the fact, that NAD+ and FAD (from NADH and FADH2,, respectively) required for the operation of the, cycle can be regenerated in the ETC only in the, presence of O2. Therefore, citric acid cycle is, strictly aerobic in contrast to glycolysis which, operates in both aerobic and anaerobic, conditions., , Energetics of citric acid cycle, During the process of oxidation of acetyl CoA, via citric acid cycle, 4 reducing equivalents (3 as, NADH and one as FADH2) are produced., Oxidation of 3 NADH by electron transport, chain coupled with oxidative phosphorylation, results in the synthesis of 9 ATP, whereas FADH2, leads to the formation of 2 ATP. Besides, there is, one substrate level phosphorylation. Thus, a total, of twelve ATP (10 as per recent evidence) are, produced from one acetyl CoA., , Role of vitamins in TCA cycle, Four B-complex vitamins are essential for, Krebs cycle, and thus energy generation, 1. Thiamine (as TPP) as a coenzyme for, D-ketoglutarate dehydrogenase., 2. Riboflavin (as FAD) as a coenzyme for, succinate dehydrogenase., 3. Niacin (as NAD+) as electron acceptor, for isocitrate dehydrogenase, D-ketoglutarate, dehydrogenase and malate dehydrogenase., 4. Pantothenic acid (as coenzyme A) attached, to active carboxylic acid residues i.e. acetyl CoA,, succinyl CoA., , Inhibitors of Krebs cycle, The important enzymes of TCA cycle, inhibited by the respective inhibitors are listed, Enzyme, , Aconitase, D-Ketoglutarate, dehydrogenase, Succinate, dehydrogenase, , Inhibitor, , Fluoroacetate, (non-competitive), Arsenite, (non-competitive), Malonate, (competitive)
Page 267 :
257, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Fluoroacetate – a suicide substrate : The, inhibitor fluoroacetate is first activated to, fluoroacetyl CoA which then condenses with, oxaloacetate to form fluorocitrate. TCA cycle, (enzyme-aconitase) is inhibited by fluorocitrate., The compound fluoroacetate, as such, is a, harmless substrate. But it is converted to a toxic, compound (fluorocitrate) by cellular metabolism., This is a suicide reaction committed by the cell,, and thus fluoroacetate is regarded as a suicide, substrate, , Regulation of citric acid cycle, The cellular demands of ATP are crucial in, controlling the rate of citric acid cycle. The, regulation is brought about either by enzymes or, the levels of ADP. Three enzymes—namely, citrate synthase, isocitrate dehydrogenase and, D-ketoglutarate dehydrogenase—regulate citric, acid cycle., 1. Citrate synthase is inhibited by ATP,, NADH, acetyl CoA and succinyl CoA., 2. Isocitrate dehydrogenase is activated by, ADP, and inhibited by ATP and NADH., 3. D-Ketoglutarate dehydrogenase is inhibited, by succinyl CoA and NADH., 4. Availability of ADP is very important for, the citric acid cycle to proceed. This is due to, the fact that unless sufficient levels of ADP are, available, oxidation (coupled with phosphorylation of ADP to ATP) of NADH and FADH2, through electron transport chain stops. The, accumulation of NADH and FADH2 will lead to, inhibition of the enzymes (as stated above) and, also limits the supply of NAD+ and FAD which, are essential for TCA cycle to proceed., , The most important synthetic (anabolic) reactions, connected with TCA cycle are given (Fig.13.10), 1. Oxaloacetate and D-ketoglutarate, respectively, serve as precursors for the synthesis of, aspartate and glutamate which, in turn, are, required for the synthesis of other non-essential, amino acids, purines and pyrimidines., 2. Succinyl CoA is used for the synthesis of, porphyrins and heme., 3. Mitochondrial citrate is transported to the, cytosol, where it is cleaved to provide acetyl, CoA for the biosynthesis of fatty acids, sterols, etc., , Anaplerosis or anaplerotic reactions, The synthetic reactions described above, deplete the intermediates of citric acid cycle. The, cycle will cease to operate unless the, intermediates drawn out are replenished., The reactions concerned to replenish or to fill, up the intermediates of citric acid cycle are, called anaplerotic reactions or anaplerosis, (Greek : fill up). In Fig.13.10, the important, synthetic pathways that draw the intermediates, of TCA cycle and the anaplerotic reactions to fill, them up are given., The salient features of important anaplerotic, reactions are described, 1. Pyruvate carboxylase catalyses the, conversion of pyruvate to oxaloacetate. This is, an ATP dependent carboxylation reaction., Pyruvate + CO2 + ATP o, Oxaloacetate + ADP + Pi, The details of the above reaction are, described under gluconeogenesis., , Amphibolic nature, of the citric acid cycle, , 2. Pyruvate is converted to malate by NADP +, dependent malate dehydrogenase (malic enzyme)., , The citric acid cycle provides various, intermediates for the synthesis of many, compounds needed by the body. Krebs cycle is, both catabolic and anabolic in nature, hence, regarded as amphibolic., , Pyruvate + CO2 + NADPH + H+, , TCA cycle is actively involved in gluconeogenesis, transamination and deamination., , Malate + NADP+ + H2O, , 3. Transamination is a process wherein an, amino acid transfers its amino group to a keto, acid and itself gets converted to a keto acid. The, formation of D-ketoglutarate and oxaloacetate, occurs by this mechanism.
Page 268 :
258, , BIOCHEMISTRY, , Non-essential, amino acids,, purines,, pyrimidines, , Aspartate, Transamination, , Acetyl CoA, , Pyruvate, carboxylase, Pyruvate, , Oxaloacetate, , Citrate, , Fatty acids, sterols, , Citric acid, cycle, Malic enzyme, Pyruvate, , D-Ketoglutarate, , Malate, , n, , io, at, in, , m, sa, an, Tr, , Succinyl CoA, , Heme, Glutamate, , Non-essential, amino acids, purines, , Fig. 13.10 : Major synthetic and anaplerotic pathways of the intermediates of citric acid cycle., , 4. D-Ketoglutarate can also be synthesized, from glutamate by glutamate dehydrogenase, action., Glutamate + NAD(P)+ + H2O l, D-Ketoglutarate + NAD(P)H + H+ + NH4+, , living system, energy is trapped leading to the, synthesis of 38 ATP which is equivalent to 1,159, KJ (1 ATP has high energy bond equivalent to, 30.5 KJ). That is, about 48% of the energy in, glucose combustion is actually captured for ATP, generation., , Energetics of glucose oxidation, When a molecule of glucose (6 carbon), undergoes glycolysis, 2 molecules of pyruvate or, lactate (3 carbon) are produced. Pyruvate is, oxidatively decarboxylated to acetyl CoA (2, carbon) which enters the citric acid cycle and, gets completely oxidized to CO2 and H2O. The, overall process of glucose being completely, oxidized to CO2 and H2O via glycolysis and, citric acid cycle is as follows, C6H12O6 + 6O2 + 38ADP + 38Pi o, 6CO2 + 6H2O + 38ATP, The enzymes of glucose metabolism, responsible for generating ATP are given in, Table 13.1., When a molecule of glucose is burnt in a, calorimeter, 2,780 KJ of heat is liberated. In the, , GLUCONEOGENESIS, The synthesis of glucose from noncarbohydrate compounds is known as gluconeogenesis. The major substrates/precursors, for gluconeogenesis are lactate, pyruvate,, glucogenic amino acids, propionate and glycerol., , Location of gluconeogenesis, Gluconeogenesis occurs mainly in the, cytosol, although some precursors are produced, in the mitochondria. Gluconeogenesis mostly, takes place in liver (about 1 kg glucose, synthesized everyday) and, to some extent, in, kidney matrix (about one-tenth of liver capacity).
Page 269 :
259, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Importance of gluconeogenesis, Glucose occupies a key position in the, metabolism and its continuous supply is, absolutely essential to the body for a variety of, functions, 1. Brain and central nervous system,, erythrocytes, testes and kidney medulla are, dependent on glucose for continuous supply of, energy. Human brain alone requires about 120 g, of glucose per day, out of about 160 g needed, by the entire body., 2. Glucose is the only source that supplies, energy to the skeletal muscle, under anaerobic, conditions., 3. In fasting even more than a day,, gluconeogenesis must occur to meet the basal, requirements of the body for glucose and to, maintain the intermediates of citric acid cycle., This is essential for the survival of humans and, other animals., , 4. Certain metabolites produced in the tissues, accumulate in the blood, e.g. lactate, glycerol,, propionate etc. Gluconeogenesis effectively, clears them from the blood., , Reactions of gluconeogenesis, Gluconeogenesis closely resembles the, reversed pathway of glycolysis, although it is not, the complete reversal of glycolysis. Essentially, 3, (out of 10) reactions of glycolysis are irreversible., The seven reactions are common for both, glycolysis and gluconeogenesis (Fig.13.11). The, three irreversible steps of glycolysis are, catalysed by the enzymes, namely hexokinase,, phosphofructokinase and pyruvate kinase. These, three stages—bypassed by alternate enzymes, specific to gluconeogenesis—are discussed, 1. Conversion of pyruvate to phosphoenolpyruvate : This takes place in two steps, (Fig.13.12). Pyruvate carboxylase is a biotin—, dependent mitochondrial enzyme that converts, pyruvate to oxaloacetate in presence of ATP and, , + Glycolysis is an important source of energy supply for brain, retina, skin and renal medulla., + The crucial significance of glycolysis is its ability to generate ATP in the absence of oxygen., + Skeletal muscle, during strenous exercise, requires the occurrence of uninterrupted, glycolysis. This is due to the limited supply of oxygen., , + The cardiac muscle cannot survive for long in the absence of oxygen since it is not well, adapted for glycolysis under anaerobic conditions., , + Glycolysis in erythrocytes is associated with 2, 3-bisphosphoglycerate (2,3-BPG) production. In the presence of 2, 3-BPG, oxyhemoglobin unloads more oxygen to the tissues., , + The occurrence of glycolysis is very much elevated in rapidly growing cancer cells., + Lactic acidosis is also observed in patients with deficiency of the enzyme pyruvate, dehydrogenase. It could also be due to collapse of circulatory system encountered in, myocardial infarction and pulmonary embolism., , + Citric acid cycle is the final common oxidative pathway for carbohydrates, fats and, amino acids. It utilizes (indirectly) about 2/3 of the total oxygen consumed by the body, and generates about 2/3 of the total energy (ATP)., , + Unlike the other metabolic pathways/cycles, very few genetic abnormalities of Krebs, cycle are known. This may be due to the vital importance of this metabolic cycle for, the survival of life.
Page 271 :
261, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Gluconeogenesis from amino acids, , O, COO–, , CH3 C, , Pyruvate, ATP, Pyruvate, carboxylase, , CO2, , The carbon skeleton of glucogenic amino, acids (all except leucine and lysine) results in the, formation of pyruvate or the intermediates of, citric acid cycle (Fig.13.11) which, ultimately,, result in the synthesis of glucose., , ADP + Pi, , O, –OOC, , CH2 C COO–, Oxaloacetate, GTP, Phosphoenolpyruvate, carboxykinase, , GDP, CO2, , P, O, CH2 C COO–, Phosphoenolpyruvate, , Fig. 13.12 : Conversion of pyruvate to, phosphoenolpyruvate., , 2. Conversion of fructose 1,6-bisphosphate, to fructose 6-phosphate : Phosphoenolpyruvate, undergoes the reversal of glycolysis until fructose, 1,6-bisphosphate is produced. The enzyme, fructose 1,6-bisphosphatase converts fructose, 1,6-bisphosphate to fructose 6-phosphate. This, enzyme requires Mg2+ ions. Fructose 1,6bisphosphatase is absent in smooth muscle and, heart muscle. This enzyme is also regulatory in, gluconeogenesis., 3. Conversion of glucose 6-phosphate to, glucose : Glucose 6-phosphatase catalyses the, conversion of glucose 6-phosphate to glucose., The presence or absence of this enzyme in a, tissue determines whether the tissue is capable, of contributing glucose to the blood or not. It is, mostly present in liver and kidney but absent in, muscle, brain and adipose tissue., The overall summary of gluconeogenesis for, the conversion of pyruvate to glucose is, shown below, 2 Pyruvate + 4ATP + 2GTP + 2NADH + 2H+, + 6H2O� o Glucose + 2NAD+ + 4ADP +, 2GDP + 6Pi + 6H+, , Gluconeogenesis from glycerol, Glycerol is liberated mostly in the adipose, tissue by the hydrolysis of fats (triacylglycerols)., The enzyme glycerokinase (found in liver and, kidney, absent in adipose tissue) activates, glycerol to glycerol 3-phosphate. The latter, is converted to dihydroxyacetone phosphate, by glycerol 3-phosphate dehydrogenase., Dihydroxyacetone phosphate is an intermediate, in glycolysis which can be conveniently used for, glucose production., , Gluconeogenesis from propionate, Oxidation of odd chain fatty acids and the, breakdown of some amino acids (methionine,, isoleucine) yields a three carbon propionyl CoA., Propionyl CoA carboxylase acts on this in, presence of ATP and biotin and converts to, methyl malonyl CoA which is then converted to, succinyl CoA in presence of B12 coenzyme, (Refer Fig.7.38). Succinyl CoA formed from, propionyl CoA enters gluconeogenesis via citric, acid cycle., , Gluconeogenesis, from lactate (Cori cycle), Lactate produced by active skeletal muscle is, a major precursor for gluconeogenesis. Under, anaerobic conditions, pyruvate is reduced to, lactate by lactate dehydrogenase (LDH), Pyruvate + NADH + H+, , LDH, , Lactate + NAD+, , Lactate is a dead end in glycolysis, since it, must be reconverted to pyruvate for its further, metabolism. The very purpose of lactate, production is to regenerate NADH so that, glycolysis proceeds uninterrupted in skeletal, muscle. Lactate or pyruvate produced in the, muscle cannot be utilized for the synthesis of
Page 272 :
262, , BIOCHEMISTRY, , MUSCLE, , BLOOD, , Glucose, , Glucose, , Glucose, LIVER, , Glycogen, , Glucose 6-phosphate, , Glucose 6-phosphate, , Glycogen, , Pyruvate, Pyruvate, , Transamination, , Lactate, , Lactate, , Alanine, , Lactate, , Transamination, , Alanine, , Fig. 13.13 : The Cori cycle (blue) and glucose-alanine (red) cycle (other reactions common for both cycles)., , glucose due to the absence of the key enzymes, of gluconeogenesis (glucose 6-phosphatase and, fructose 1,6-bisphosphatase)., The plasma membrane is freely permeable to, lactate. Lactate is carried from the skeletal, muscle through blood and handed over to liver,, where it is oxidized to pyruvate. Pyruvate, so, produced, is converted to glucose by, gluconeogenesis, which is then transported to, the skeletal muscle., The cycle involving the synthesis of glucose, in liver from the skeletal muscle lactate and the, reuse of glucose thus synthesized by the muscle, for energy purpose is known as Cori cycle, (Fig.13.13)., , Glucose-alanine cycle, There is a continuous transport of amino acids, from muscle to liver, which predominantly, occurs during starvation. Alanine dominates, among the transported amino acids. It is, postulated that pyruvate in skeletal muscle, undergoes transamination to produce alanine., Alanine is transported to liver and used for, gluconeogenesis. This cycle is referred to as, glucose-alanine cycle (Fig.13.13)., , Regulation of gluconeogenesis, The hormone glucagon and the availability of, substrates mainly regulate gluconeogenesis, as, discussed hereunder., Influence of glucagon : This is a hormone,, secreted by D-cells of the pancreatic islets., Glucagon stimulates gluconeogenesis by two, mechanisms, 1. Active form of pyruvate kinase is, converted to inactive form through the mediation, of cyclic AMP, brought about by glucagon., Decreased pyruvate kinase results in the reduced, conversion of phosphoenol pyruvate to pyruvate, and the former is diverted for the synthesis of, glucose., 2. Glucagon reduces the concentration of, fructose 2,6-bisphosphate. This compound allosterically inhibits phosphofructokinase and, activates fructose 1,6-bisphosphatase, both, favour increased gluconeogenesis., Availability of substrates : Among the various, substrates, glucogenic amino acids have, stimulating influence on gluconeogenesis. This is, particularly important in a condition like, diabetes mellitus (decreased insulin level) where
Page 273 :
263, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , amino acids are mobilized from muscle protein, for the purpose of gluconeogenesis., Acetyl CoA promotes gluconeogenesis :, During starvation—due to excessive lipolysis in, adipose tissue—acetyl CoA accumulates in the, liver. Acetyl CoA allosterically activates pyruvate, carboxylase resulting in enhanced glucose, production., , Alcohol inhibits gluconeogenesis, Ethanol oxidation in the liver to acetaldehyde, by the enzyme alcohol dehydrogenase utilizes, NAD+. The excess NADH produced in the liver, interferes with gluconeogenesis as illustrated, below., Ethanol + NAD+ o Acetaldehyde + NADH + H+, Pyruvate + NADH + H+ l Lactate + NAD+, Oxaloacetate + NADH + H+ l Malate + NAD+, It is evident from the above reactions that, pyruvate and oxaloacetate, the predominant, substrates for gluconeogenesis, are made, unavailable by alcohol intoxication. This, happens due to overconsumption of NAD+ and, excessive production of NADH by alcohol., Alcohol consumption increases the risk of, hypoglycemia (reduced plasma glucose) due to, reduced gluconeogenesis. Hypoglycemia is, frequently observed in diabetic patients, (particularly on insulin treatment), and undernourished persons consuming alcohol., , Gluconeogenesis from fat?, , GLYCOGEN METABOLISM, Glycogen is the storage form of glucose in, animals, as is starch in plants. It is stored mostly, in liver (6-8%) and muscle (1-2%). Due to more, muscle mass, the quantity of glycogen in muscle, (250 g) is about three times higher than that in, the liver (75 g). Glycogen is stored as granules in, the cytosol, where most of the enzymes of, glycogen synthesis and breakdown are present., , Functions of glycogen, The prime function of liver glycogen is to, maintain the blood glucose levels, particularly, between meals. Liver glycogen stores increase in, a well-fed state which are depleted during, fasting. Muscle glycogen serves as a fuel reserve, for the supply of ATP during muscle contraction., , Why store glycogen, as a fuel reserve?, As such, fat is the fuel reserve of the body., However, fat is not preferred, instead glycogen is, chosen for a routine, and day to day use of, energy for the following reasons, l, , l, , l, , Glycogen can be rapidly mobilized, Glycogen can generate energy in the absence, of oxygen, Brain depends on continuous glucose supply, (which mostly comes from glycogen.), , On the other hand, fat mobilization is slow,, needs O2 for energy production and cannot, produce glucose (to a significant extent). Thus,, fat may be considered as a fixed deposit while, glycogen is in the current/saving account in a, bank!, , It is often stated that glucose cannot be, synthesized from fat. In a sense, it is certainly, true, since the fatty acids (most of them being, even chain), on oxidation, produce acetyl CoA, which cannot be converted to pyruvate. Further,, the two carbons of acetyl CoA disappear as 2, moles of CO2 in TCA cycle. Therefore, even, chain fatty acids cannot serve as precursors for, glucose formation. The prime reason why, animals cannot convert fat to glucose is the, absence of glyoxylate cycle (described later)., , The synthesis of glycogen from glucose is, glycogenesis (Fig.13.14). Glycogenesis takes, place in the cytosol and requires ATP and UTP,, besides glucose., , However, the glycerol released from lipolysis, and the propionate obtained from the oxidation, of odd chain fatty acids are good substrates for, gluconeogenesis, as discussed above., , 1. Synthesis of UDP-glucose : The enzymes, hexokinase (in muscle) and glucokinase (in liver), convert glucose to glucose 6-phosphate., Phosphoglucomutase catalyses the conversion of, , GLYCOGENESIS
Page 274 :
264, , BIOCHEMISTRY, , glucose 6-phosphate to glucose 1-phosphate., Uridine diphosphate glucose (UDPG) is, synthesized from glucose 1-phosphate and UTP, by UDP-glucose pyrophosphorylase., 2. Requirement of primer to initiate glycogenesis : A small fragment of pre-existing, glycogen must act as a ‘primer’ to initiate, glycogen synthesis. It is recently found that in, the absence of glycogen primer, a specific, protein—namely, ‘glycogenin’—can, accept, glucose from UDPG. The hydroxyl group of the, amino acid tyrosine of glycogenin is the site at, which the initial glucose unit is attached. The, enzyme glycogen initiator synthase transfers the, first molecule of glucose to glycogenin. Then, glycogenin itself takes up a few glucose residues, to form a fragment of primer which serves as an, acceptor for the rest of the glucose molecules., 3. Glycogen synthesis by glycogen synthase :, Glycogen synthase is responsible for the, formation of 1,4-glycosidic linkages. This, enzyme transfers the glucose from UDP-glucose, to the non-reducing end of glycogen to form D1,4 linkages., 4. Formation of branches in glycogen :, Glycogen synthase can catalyse the synthesis of, a linear unbranched molecule with 1,4 Dglycosidic linkages. Glycogen, however, is a, branched tree-like structure. The formation of, branches is brought about by the action of a, branching enzyme, namely glucosyl D-4-6, transferase. (amylo D 1,4 o 1,6 transglucosidase). This enzyme transfers a small, fragment of five to eight glucose residues from, the non-reducing end of glycogen chain (by, breaking D-1,4 linkages) to another glucose, residue where it is linked by D-1,6 bond. This, leads to the formation of a new non-reducing, end, besides the existing one. Glycogen is further, elongated and branched, respectively, by the, enzymes glycogen synthase and glucosyl 4-6, transferase., The overall reaction of the glycogen synthesis, for the addition of each glucose residue is, (Glucose)n + Glucose + 2ATP o, (Glucose)n+1 + 2 ADP + Pi, , Glucose, ATP, Glucokinase, ADP, Glucose 6-phosphate, Phosphoglucomutase, Glucose 1-phosphate, UTP, , UDP-glucose, pyrophosphorylase, , PPi, UDP-glucose, (UDP, ), , OH, Glycogen initiator, synthase, , Glycogenin, , UDP, 1, , 2, , 3, , O, Glycogen primer, 13 (UDP, , ), Glycogen synthase, , 13 UDP, 1, , 10, , 16, , Glucosyl (D�4-6), transferase, 1, , 7, 11, , 10, , D�1-6-Bond, , 16, , Elongation by, glycogen synthase, (forming D 1,4-bonds), , Branching by, glucosyl 4-6 transferase, (D 1,6-bonds), GLYCOGEN, , Fig. 13.14 : Glycogen synthesis from glucose, (glycogenesis).
Page 275 :
265, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Of the two ATP utilized, one is required for, the phosphorylation of glucose while the other is, needed for conversion of UDP to UTP., , GLYCOGENOLYSIS, The degradation of stored glycogen in liver, and muscle constitutes glycogenolysis. The, pathways for the synthesis and degradation of, glycogen are not reversible. An independent set, of enzymes present in the cytosol carry out, glycogenolysis. Glycogen is degraded by, breaking D-1,4- and D-1,6-glycosidic bonds, (Fig.13.15)., 1. Action of glycogen phosphorylase : The D1,4-glycosidic bonds (from the non-reducing, ends) are cleaved sequentially by the enzyme, glycogen phosphorylase, to yield glucose, 1-phosphate. This process—called phosphorolysis—continues until four glucose residues, remain on either side of branching point (D-1,6glycosidic link). The glycogen so formed is, known as limit dextrin which cannot be further, degraded, by, phosphorylase., Glycogen, phosphorylase possesses a molecule of pyridoxal, phosphate, covalently bound to the enzyme., 2. Action of debranching enzyme : The, branches of glycogen are cleaved by two, enzyme activities present on a single polypeptide, called debranching enzyme, hence it is a, bifunctional enzyme., , D 1,6-bond, Glycogen, Pi (–), , Glucose 1phosphate, , D 1,6-bond, Limit dextrin, Debranching enzyme, (transferase activity), , Glucose, (free), , Glycosyl 4 : 4 transferase (oligo D-1,4 o 1,4, glucan transferase) activity removes a fragment, of three or four glucose residues attached at a, branch and transfers them to another chain., Here, one D-1,4-bond is cleaved and the same, D-1,4 bond is made, but the places are different., Amylo D-1,6-glucosidase breaks the D-1,6, bond at the branch with a single glucose residue, and releases a free glucose., The remaining molecule of glycogen is again, available for the action of phosphorylase and, debranching enzyme to repeat the reactions, stated in 1 and 2., 3. Formation of glucose 6-phosphate and, glucose : Through the combined action of, glycogen phosphorylase and debranching, , Glycogen, phosphorylase, , Debranching enzyme, (D 1o6 glucosidase activity), , Further action of, phosphorylase, Glucose 1-phosphate, Phosphoglucomutase, , Glycolysis, , Glucose 6-phosphate, Glucose 6-phosphatase, (in liver), GLUCOSE, , Fig. 13.15 : Glycogen degradation to glucoseglycogenolysis. (The ratio of glucose, 1-phosphate to glucose is 8 : 1).
Page 276 :
266, , BIOCHEMISTRY, , enzyme, glucose 1-phosphate and free glucose, in a ratio of 8 : 1 are produced. Glucose, 1-phosphate is converted to glucose 6-phosphate, by the enzyme phosphoglucomutase., The fate of glucose 6-phosphate depends on, the tissue. The liver, kidney and intestine contain, the enzyme glucose 6-phosphatase that cleaves, glucose 6-phosphate to glucose. This enzyme is, absent in muscle and brain, hence free glucose, cannot be produced from glucose 6-phosphate, in these tissues. Therefore, liver is the major, glycogen storage organ to provide glucose into, the circulation to be utilised by various tissues., In the peripheral tissues, glucose 6-phosphate, produced by glycogenolysis will be used for, glycolysis. It may be noted that though glucose, 6-phosphatase is absent in muscle, some amount, of free glucose (8-10% of glycogen) is produced, in glycogenolysis due to the action of, debranching enzyme (D-1,6-glucosidase activity)., , Degradation of glycogen, by lysosomal acid maltase, Acid maltase or D-1,4-glucosidase is a, lysosomal enzyme. This enzyme continuously, degrades a small quantity of glycogen. The, significance of this pathway is not very clear., However, it has been observed that the, deficiency of lysosomal enzyme D-1,4, glucosidase results in glycogen accumulation,, causing a serious glycogen storage disease type, II (i.e. Pompe’s disease)., , Regulation of glycogenesis, and glycogenolysis, A good coordination and regulation of, glycogen synthesis and its degradation are, essential to maintain the blood glucose, levels. Glycogenesis and glycogenolysis are,, respectively, controlled by the enzymes, glycogen synthase and glycogen phosphorylase., Regulation of these enzymes is accomplished by, three mechanisms, 1. Allosteric regulation, 2. Hormonal regulation, 3. Influence of calcium., , LIVER, , Glucose, Glucose 6phosphate, , MUSCLE, , ATP, , Ca, , 2+, , AMP, , Glycogen phosphorylase, , Glycogen, , Glucose, 1-phosphate, Glycogen synthase, , Glucose 6phosphate, , Fig. 13.16 : Allosteric regulation of glycogenolysis and, glycogenesis (s : Inhibition; r : Activation)., , 1. Allosteric regulation of glycogen metabolism : There are certain metabolites that, allosterically regulate the activities of glycogen, synthase and glycogen phosphorylase. The, control is carried out in such a way that glycogen, synthesis is increased when substrate availability, and energy levels are high. On the other hand,, glycogen breakdown is enhanced when glucose, concentration and energy levels are low. The, allosteric regulation of glycogen metabolism is, depicted in Fig.13.16. In a well-fed state, the, availability of glucose 6-phosphate is high which, allosterically activates glycogen synthase for, more glycogen synthesis. On the other hand,, glucose 6-phosphate and ATP allosterically, inhibit glycogen phosphorylase. Free glucose in, liver also acts as an allosteric inhibitor of, glycogen phosphorylase., 2. Hormonal regulation of glycogen metabolism : The hormones, through a complex series, of reactions, bring about covalent modification,, namely phosphorylation and dephosphorylation, of enzyme proteins which, ultimately control, glycogen synthesis or its degradation., cAMP as second messenger for hormones :, Hormones like epinephrine and norepinephrine,, and glucagon (in liver) activate adenylate cyclase
Page 277 :
267, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , to increase the production of cAMP. The, enzyme phosphodiesterase breaks down cAMP., The, hormone, insulin, increases, the, phosphodiesterase activity in liver and lowers the, cAMP levels., , In the Fig.13.17, the inhibition of glycogen, synthesis brought by epinephrine (also, norepinephrine) and glucagon through cAMP by, converting active glycogen synthase ‘a’ to, inactive synthase ‘b’, is given., , Regulation of glycogen synthesis by cAMP :, The glycogenesis is regulated by glycogen, synthase. This enzyme exists in two forms—, glycogen, synthase, ‘a’—which, is, not, phosphorylated and most active, and secondly,, glycogen synthase ‘b’ as phosphorylated inactive, form. Glycogen synthase ‘a’ can be converted to, ‘b’ form (inactive) by phsophorylation. The, degree of phosphorylation is proportional to the, inactive state of enzyme. The process of, phosphorylation is catalysed by a cAMPdependent protein kinase. The protein kinase, phosphorylates, and, inactivates, glycogen, synthase by converting ‘a’ form to ‘b’ form. The, glycogen synthase ‘b’ can be converted back to, synthase ‘a’ by protein phosphatase I., , Regulation of glycogen degradation by, cAMP : The hormones like epinephrine and, glucagon bring about glycogenolysis by their, action on glycogen phosphorylase through, cAMP as illustrated in Fig.13.18. Glycogen, phosphorylase exists in two forms, an active ‘a’, form and inactive form ‘b’., , Glucagon, (liver), , The cAMP—formed due to hormonal, stimulus—activates cAMP dependent protein, kinase. This active protein kinase phosphorylates, inactive form of glycogen phsophorylase, kinase to active form. (The enzyme protein, phosphatase removes phosphate and inactivates, phosphorylase kinase). The active phosphorylase kinase phosphorylates inactive glycogen, phosphorylase ‘b’ to active glycogen phospho-, , Epinephrine, (liver, muscle), , Adenylate, cyclase, (inactive), , Adenylate, cyclase, (active), , ATP, , cAMP dependent, protein kinase, (inactive), , PLASMA MEMBRANE, , cAMP, , Phosphodiesterase, , 5c AMP, , cAMP dependent, protein kinase, (active), ATP, , Glycogen, synthase a, (active), , Pi, , ADP, , Glycogen, synthase b, (inactive), , Protein H2O, phosphatase I, , Fig. 13.17 : Hormonal regulation of glycogen synthesis (glycogenesis)., , Glycogenesis, Inhibited
Page 278 :
268, , BIOCHEMISTRY, , Glucagon Epinephrine, (liver), (liver, muscle), , Adenylate, cyclase, (active), , Adenylate, cyclase, (inactive), , PLASMA MEMBRANE, , Phosphodiesterase, ATP, , 5c AMP, , cAMP, , cAMP-dependent, protein kinase, (inactive), , cAMP dependent, protein kinase, (active), , Glycogen, , Glucose 1phosphate, , ADP, , ATP, , Glycogen, phosphorylase a, (active), , Calmodulin, phosphorylase, kinase, Phosphorylase kinase, (inactive), , Pi, , 2+, , Ca, – Ca2+, , Phosphorylase kinase, (active), , H2O, , Protein, phosphatase I, , Protein, phosphatase I, , Glycogen, phosphorylase b, (inactive), , Fig. 13.18 : Hormonal regulation of glycogen degradation (glycogenolysis)., , rylase ‘a’ which degrades glycogen. The enzyme, protein phosphatase I can dephosphorylate and, convert active glycogen phosphorylase ‘a’ to, inactive ‘b’ form., 3. Effect of Ca2+ ions on glycogenolysis :, When the muscle contracts, Ca2+ ions are, released from the sarcoplasmic reticulum. Ca2+, binds to calmodulin-calcium modulating protein, and directly activates phosphorylase kinase, without the involvement of cAMP-dependent, protein kinase., The overall effect of hormones on glycogen, metabolism is that an elevated glucagon, or epinephrine level increases glycogen, degradation whereas an elevated insulin results, in increased glycogen synthesis., , FUTILE CYCLES, The synthesis and degradative pathways of, metabolism (particularly reactions involving, phosphorylation and dephosphorylation utilizing, ATP) are well regulated and subjected to fine, tuning to meet the body demands, with minimal, wastage of energy and metabolites. Thus,, glycolysis and gluconeogenesis (breakdown of, glucose to pyruvate, and conversion of pyruvate, to glucose), glycogenolysis and glycogenesis, operate in a selective fashion to suit the cellular, demands. If on the other hand, the synthesis and, degradative metabolic pathways of a particular, substance (say gluconeogenesis and glycolysis, related to glucose) operate to the same extent
Page 279 :
269, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , simultaneously, this would result in futile cycles., However, futile cycles, consuming energy (ATP), are wasteful metabolic exercises. They are, minimally operative due to a well coordinated, metabolic machinery., , glycogen metabolism along with the defective, enzymes in the glycogen storage disorders is, depicted in Fig.13.19. The biochemical lesions, and the characteristic features of the disorders, are given in Table 13.2., , Recent studies suggest that futile cycles may, have some physiological (operative in arousal of, hibernating animals) and pathological (malignant, hyperthermia due to loss of control)significance., , von Gierke’s disease (type I), , GLYCOGEN STORAGE DISEASES, The metabolic defects concerned with the, glycogen synthesis and degradation are, collectively referred to as glycogen storage, diseases. These disorders are characterized by, deposition of normal or abnormal type of, glycogen in one or more tissues. A summary of, , The incidence of type I glycogen storage, disease is 1 per 200,000 persons. It is transmitted, by autosomal recessive trait. This disorder results, in various biochemical manifestations., 1. Fasting hypoglycemia : Due to the defect, in the enzyme glucose 6-phosphatase, enough, free glucose is not released from the liver., 2. Lactic acidemia : Glucose is not, synthesized from lactate produced in muscle and, liver. Lactate level in blood increases and the pH, is lowered (acidosis)., , TABLE 13.2 Glycogen storage diseases – biochemical lesions and characteristic features, Type, , Name, , Enzyme defect, , Organ(s) involved, , Characteristic features, , I, , von Gierke’s disease, (type I glycogenosis), , Glucose 6-phosphatase, , Liver, kidney and, intestine, , Glycogen accumulates in hepatocytes and renal cells,, enlarged liver and kidney, fasting hypoglycemia, lactic, acidemia; hyperlipidemia; ketosis; gouty arthritis., , II, , Pompe’s disease, , Lysosomal D-1,4 glucosidase (acid maltase), , All organs, , Glycogen accumulates in lysosomes in almost all the, tissues; heart is mostly involved; enlarged liver and, heart, nervous system is also affected; death occurs at, an early age due to heart failure., , III, , Cori’s disease, (limit dextrinosis,, Forbe’s disease), , Amylo D-1,6-glucosidase, (debranching enzyme), , Liver, muscle,, heart, leucocytes, , Branched chain glycogen accumulates; liver enlarged;, clinical manifestations are similar but milder compared to, von Gierke’s disease., , IV, , Anderson’s disease, (amylopectinosis), , Glucosyl 4-6 transferase, (branching enzyme), , Most tissues, , A rare disease, glycogen with only few branches, accumulate; cirrhosis of liver, impairment in liver function., , V, , McArdle’s disease, (type V glycogenosis), , Muscle glycogen, phosphorylase, , Skeletal muscle, , Muscle glycogen stores very high, not available during, exercise; subjects cannot perform strenous exercise;, suffer from muscle cramps; blood lactate and pyruvate, do not increase after exercise; muscles may get, damaged due to inadequate energy supply., , VI, , Her’s disease, , Liver glycogen, phosphorylase, , Liver, , Liver enlarged; liver glycogen cannot form glucose; mild, hypoglycemia and ketosis seen., , VII, , Tarui’s disease, , Phosphofructokinase, , Skeletal muscle,, erythrocytes, , Muscle cramps due to exercise; blood lactate not, elevated; hemolysis occurs., , Rare glycogen disorders VIII, IX, X and XI have been identified. They are due to defects in the enzymes concerned with activating and, deactivating liver phosphorylase.
Page 280 :
270, , BIOCHEMISTRY, , Glucose, Hexokinase, I, , Glucose 6phosphatase, 6-Phosphofructokinase, , Glucose 6-phosphate, VII, UTP, , Lactate, , Glucose 1-phosphate, , Glucose, , Fructose 1,6bisphosphate, , Debranching, enzyme, , PPi, , III, Limit dextrin, , UDP-glucose, , Glucose 1phosphate, , Primer, , Glycogen, phosphorylase, , Glycogen, synthase, , V (muscle), UDP, , VI (liver), Glycogen, (D 1,4 and 1,6-bonds), , IV, , Glycogen unbranched, (D 1,4-bonds), Glucosyl (4-6), transferase, , Lysosomal, D-glucosidase, II, Glucose +, oligosaccharides, , Fig. 13.19 : Summary of glycogen metabolism with glycogen storage diseases (Red blocks, indicate storage disease, I–von Gierke’s disease; II–Pompe’s disease; III–Cori’s disease;, IV–Anderson’s disease; V–Mc Ardle’s disease; VI–Her’s disease; VII–Tarui’s disease)., , 3. Hyperlipidemia : There is a blockade in, gluconeogenesis. Hence more fat is mobilized to, meet energy requirements of the body. This, results in increased plasma free fatty acids and, ketone bodies., 4. Hyperuricemia : Glucose 6-phosphate that, accumulates is diverted to pentose phosphate, pathway, leading to increased synthesis of ribose, phosphates which increase the cellular levels of, phosphoribosyl pyrophosphate and enhance the, metabolism of purine nucleotides to uric acid., Elevated, plasma, levels, of, uric, acid, (hyperuricemia) are often associated with gouty, arthritis (painful joints)., , The important features of the glycogen storage, diseases are given in Table 13.2., , HEXOSE MONOPHOSPHATE SHUNT, Hexose monophosphate pathway or HMP, shunt is also called pentose phosphate pathway, or phosphogluconate pathway. This is an, alternative pathway to glycolysis and TCA cycle, for the oxidation of glucose. However, HMP, shunt is more anabolic in nature, since it is, concerned with the biosynthesis of NADPH and, pentoses.
Page 281 :
271, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , HMP shunt—a unique, multifunctional pathway, , HO C H, , The pathway starts with glucose 6-phosphate., As such, no ATP is directly utilized or produced, in HMP pathway. It is a unique multifunctional, pathway, since there are several interconvertible, substances produced which may proceed in, different directions in the metabolic reactions., , HO C H, , H C OH, , H C, CH2O, , The enzymes of HMP shunt are located in the, cytosol. The tissues such as liver, adipose tissue,, adrenal gland, erythrocytes, testes and lactating, mammary gland, are highly active in HMP shunt., Most of these tissues are involved in the, biosynthesis of fatty acids and steroids which are, dependent on the supply of NADPH., , Reactions of the pathway, , P, , Glucose 6-phosphate, NADP, , Location of the pathway, , O, , H C OH, , Mg, , +, , Glucose 6-phosphate, dehydrogenase, , 2+, , +, , NADPH + H, , O, C, H C OH, HO C H, , O, , H C OH, H C, CH2O, , The sequence of reactions of HMP shunt, (Fig.13.20) is divided into two phases—oxidative, and non-oxidative., 1. Oxidative phase : Glucose 6-phosphate, dehydrogenase (G6PD) is an NADP-dependent, enzyme that converts glucose 6-phosphate to, 6-phosphogluconolactone. The latter is then, hydrolysed by the gluconolactone hydrolase to, 6-phosphogluconate. The next reaction involving, the synthesis of NADPH is catalysed by 6-phosphogluconate dehydrogenase to produce 3 keto, 6-phosphogluconate which then undergoes, decarboxylation to give ribulose 5-phosphate., G6PD regulates HMP shunt : The first, reaction catalysed by G6PD is most regulatory in, HMP shunt. This enzyme catalyses an, irreversible reaction. NADPH competitively, inhibits G6PD. It is the ratio of NADPH/NAD+, that ultimately determines the flux of this cycle., 2. Non-oxidative phase : The non-oxidative, reactions are concerned with the interconversion, of three, four, five and seven carbon monosaccharides. Ribulose 5-phosphate is acted upon by, an epimerase to produce xylulose 5-phosphate, while ribose 5-phosphate ketoisomerase converts, ribulose 5-phosphate to ribose 5-phosphate., , P, , 6-Phosphogluconolactone, H 2O, Gluconolactone, hydrolase, COO–, H C OH, HO C H, H C OH, H C OH, CH2O, , P, , 6-Phosphogluconate, NADP+, NADPH + H, , +, , Phosphogluconate, dehydrogenase, Mg2+, , CO2, CH2OH, C O, H C OH, H C OH, CH2O, , P, , Ribulose 5-phosphate, , Fig. 13.20 contd. next page
Page 284 :
274, 6-phosphate to 6CO2, we have to start with 6, molecules of glucose 6-phosphate. Of these 6, 5, moles are regenerated with the production of 12, NADPH., The overall reaction may be represented as, 6 Glucose 6-phosphate + 12 NADP+ + 6H2O, o 6CO2 + 12 NADPH + 12H+ + 5 Glucose, 6-phosphate., , BIOCHEMISTRY, , 2 GSH, (reduced), , H 2O2, Glutathione, peroxidase, 2 H2O, , +, , NADP, , Glutathione, reductase, G-S-S-G, (oxidized), , NADPH + H+, , Significance of HMP shunt, , Glutathione (reduced, GSH) detoxifies H2O2,, peroxidase catalyses this reaction. NADPH is, responsible for the regeneration of reduced, glutathione from the oxidized one., , HMP shunt is unique in generating two, important products—pentoses and NADPH—, needed for the biosynthetic reactions and other, functions., , 4. Microsomal cytochrome P450 system (in, liver) brings about the detoxification of drugs, and foreign compounds by hydroxylation, reactions involving NADPH., , Importance of pentoses, , 5. Phagocytosis is the engulfment of foreign, particles, including microorganisms, carried out, by white blood cells. The process requires the, supply of NADPH., , In the HMP shunt, hexoses are converted into, pentoses, the most important being ribose, 5-phosphate. This pentose or its derivatives are, useful for the synthesis of nucleic acids (RNA, and DNA) and many nucleotides such as ATP,, NAD+, FAD and CoA., Skeletal muscle is capable of synthesizing, pentoses, although only the first few enzymes of, HMP shunt are active. It, therefore, appears that, the complete pathway of HMP shunt may not be, required for the synthesis of pentoses., , Importance of NADPH, 1. NADPH is required for the reductive, biosynthesis of fatty acids and steroids, hence, HMP shunt is more active in the tissues, concerned with lipogenesis, e.g. adipose tissue,, liver etc., 2. NADPH is used in the synthesis of certain, amino acids involving the enzyme glutamate, dehydrogenase., 3. There is a continuous production of H2O2, in the living cells which can chemically damage, unsaturated lipids, proteins and DNA. This is,, however, prevented to a large extent through, antioxidant (free radical scavenging) reactions, involving NADPH. Gluta-thione mediated, reduction of H2O2 is given in the next column., , 6. Special functions of NADPH in RBC :, NADPH produced in erythrocytes has special, functions to perform. It maintains the, concentration of reduced glutathione (reaction, explained in 3) which is essentially required to, preserve the integrity of RBC membrane., NADPH is also necessary to keep the ferrous, iron (Fe2+) of hemoglobin in the reduced state so, that accumulation of methemoglobin (Fe3+) is, prevented., 7. High concentration of NADPH in lens of, eyes is necessary to preserve the transparency of, the lenses., , Glucose 6-phosphate, dehydrogenase deficiency, G6PD deficiency is an inherited sex-linked, trait. Although the deficiency occurs in all the, cells of the affected individuals, it is more severe, in RBC., HMP shunt is the only means of providing, NADPH in the erythrocytes. Decreased activity, of G6PD impairs the synthesis of NADPH in, RBC. This results in the accumulation of, methemoglobin and peroxides in erythrocytes, leading to hemolysis., Clinical manifestations in G6PD deficiency :, Most of the patients with G6PD deficiency do not
Page 285 :
275, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , usually exhibit clinical symptoms. Some of them,, however, develop hemolytic anemia if they, are administered oxidant drugs or exposed to a, severe infection. The drugs such as primaquine, (antimalarial),, acetanilide, (antipyretic),, sulfamethoxazole (antibiotic) or ingestion of fava, beans (favism) produce hemolytic jaundice in, these patients. Severe infection results in the, generation of free radicals (in macrophages), which can enter RBC and cause hemolysis (due, to decreased NADPH and reduced GSH)., G6PD deficiency and resistance to malaria :, It is interesting to note that G6PD deficiency is, associated with resistance to malaria (caused by, Plasmodium falciparum). This is explained from, the fact that the parasites that cause malaria are, dependent on HMP shunt and reduced, glutathione for their optimum growth in RBC., Therefore, G6PD deficiency—which is seen, frequently in Africans—protects them from, malaria, a common disease in this region. It is, regarded as an adaptability of the people living, in malaria-infected regions of the world., Biochemical diagnosis can be done by, detecting reduced activity of G6PD in RBC. The, management of G6PD deficiency includes, avoiding oxidative stress and symptomatic, treatment of hemolysis., , Wernicke-Korsakoff syndrome, This is a genetic disorder associated with, HMP shunt. An alteration in transketolase, activity that reduces its affinity (by tenfold or so), with thiamine pyrophosphate is the biochemical, lesion. The symptoms of Wernicke-Korsakoff, syndrome include mental disorder, loss of, memory and partial paralysis. The symptoms are, manifested in vitamin-deficient alcoholics., , URONIC ACID PATHWAY, This is an alternative oxidative pathway for, glucose and is also known as glucuronic acid, pathway (Fig.13.22). It is concerned with the, synthesis of glucuronic acid, pentoses and, vitamin, ascorbic acid (except in primates and, guinea pigs). Dietary xylulose enters uronic acid, pathway through which it can participate in, , other metabolisms. In most of the pathways of, carbohydrate metabolism, phosphate esters, participate, whereas, in uronic acid pathway, the, free sugars or sugar acids are involved., 1. Formation and importance of UDPglucuronate : Glucose 6-phosphate is first, converted to glucose 1-phosphate. UDP-glucose, is then synthesized by the enzyme UDP-glucose, pyrophosphorylase. Till this step, the reactions, are the same as described in glycogenesis, (Fig.13.14)., UDP-glucose, dehydrogenase, oxidizes UDP-glucose to UDP-glucuronate., UDP-glucuronate is the metabolically active, form of glucuronate which is utilized for, conjugation with many substances like bilirubin,, steroid hormones and certain drugs. Several, insoluble compounds are converted to soluble, ones through conjugation and, further, the drugs, are detoxified. UDP-glucuronate is also required, for the synthesis of glycosaminoglycans and, proteoglycans., 2. Conversion of UDP-glucuronate to, L-gulonate : UDP-glucuronate loses its UDP, moiety in a hydrolytic reaction and releases Dglucuronate which is reduced to L-gulonate by, an NADPH-dependent reaction., 3. Synthesis of ascorbic acid in some, animals : L-Gulonate is the precursor for the, synthesis of ascorbic acid (vitamin C) in many, animals. The enzyme L-gulonolactone oxidase—, which converts gulonate to ascorbic acid—is, absent in man, other primates and guinea pigs., Therefore, vitamin C has to be supplemented in, the diet for these animals., 4. Oxidation of L-gulonate : L-Gulonate is, oxidized, to, 3-ketogulonate, and, then, decarboxylated to a pentose, L-xylulose., L-Xylulose is converted to D-xylulose via xylitol, by a reduction (NADPH-dependent) followed by, an oxidation (NAD+-dependent) reaction. This is, necessary since the D-xylulose (and not, L-form)—after getting phosphorylated—can enter, the hexose monophosphate shunt, for further, metabolism., , Effect of drugs, on uronic acid pathway, Administration of drugs (barbital, chlorobutanol etc.) significantly increases the uronic
Page 288 :
278, , BIOCHEMISTRY, , Glycogen, +, , NADP, , NADPH + H, , +, , Hexokinase, , Sorbitol, NAD+, NADH + H+, , Glucose, ATP, Glucose 6-phosphate, Aldose, reductase, Glucose 6-phosphatase, Phosphohexose, Sorbitol, isomerase, dehydrogenase, , Hexokinase (minor), , Fructose, , Fructose 6-phosphate, , ATP, Fructokinase, , Phosphofructokinase, , ATP, (1), , ATP, Fructose 1, 6bisphosphate, , Fructose 1-phosphate, , Fructose 1, 6bisphosphatase, Aldolase A, , Aldolase B, (2), Dihydroxyacetone, phosphate (DHAP), Triosephosphate, isomerase, , Glyceraldehyde, Triokinase, NADH + H +, +, , ATP, , Alcohol, dehydrogenase, , Triose phosphate, isomerase, , NAD, , Glycerol, , Glyceraldehyde, 3-phosphate, , Glycerol kinase, ATP, , Glycerol, 3-phosphate, , Glycerol 3-phosphate, dehydrogenase, , Glycolysis, (pyruvate), , DHAP, , Triacylglycerols Phospholipids, , Fig. 13.24 : Metabolism of fructose (Metabolic defects 1–Fructosuria; 2–Fructose intolerance)., , phosphorylation of galactose, will also result in, galactosemia and galactosuria. Here again, galactose is shunted to the formation of, galactitol. Generally, galactokinase-deficient, individuals do not develop hepatic and renal, complications. Development of cataract occurs, at a very early age, sometimes within an year, after birth. The treatment is the removal of, galactose and lactose from the diet., , METABOLISM OF FRUCTOSE, The major dietary source of fructose is the, disaccharide sucrose (cane sugar), and highfructose corn syrups (HFCS) used in the, manufactured foods and beverages. It is also, , found in free form in honey and many fruits. In, the body, entry of fructose into the cells is not, controlled by the hormone insulin. This is in, contrast to glucose which is regulated for its, entry into majority of the tissues., Fructose is mostly phosphorylated by fructokinase to fructose 1-phosphate. Fructokinase has, been identified in liver, kidney and intestine., Hexokinase, which phosphorylates various, monosaccharides, can also act on fructose to, produce fructose 6-phosphate. However,, hexokinase has low affinity (high Km) for, fructose, hence this is a minor pathway., Fructose 1-phosphate is cleaved to glyceraldehyde and dihydroxyacetone phosphate, (DHAP) by aldolase B (Fig.13.24). This is in
Page 289 :
279, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , contrast to fructose 6-phosphate which is, converted to fructose 1, 6-bisphosphate and split, by aldolase A (details in glycolysis—See, Fig.13.2). Glyceraldehyde is phosphorylated by, the enzyme triokinase to glyceraldehyde 3phosphate which, along with DHAP, enters, glycolysis or gluconeogenesis., The fructose is more rapidly metabolized (via, glycolysis) by the liver than glucose. This is due, to the fact that the rate limiting reaction in, glycolysis catalysed by phosphofructokinase is, bypassed. Increased dietary intake of fructose, significantly elevates the production of acetyl, CoA and lipogenesis (fatty acid, triacylglycerol, and very low density lipoprotein synthesis)., , Ingestion of large quantities of fructose or, sucrose, is, linked, with, many, health, complications., , Sorbitol / Polyol pathway, Polyol pathway (so termed since sorbitol is a, polyhydroxy sugar) basically involves the, conversion of glucose to fructose via sorbitol, (Fig.13.24). This pathway is absent in liver., Sorbitol pathway is directly related to glucose, concentration, and is higher in uncontrolled, diabetes., The enzyme aldose reductase reduces glucose, to sorbitol (glucitol) in the presence of NADPH., , + A continuous presence of glucose—supplied through diet or synthesized in the body, (gluconeogenesis)—is essential for the survival of the organism. Alcohol intoxication, reduces gluconeogenesis, particularly in diabetic patients and undernourished persons., , + Human brain consumes about 120 g of glucose per day out of the 160 g needed by, the body. Insufficient supply of glucose to brain may lead to coma and death., , + Liver glycogen serves as an immediate source for maintaining blood glucose levels,, particularly between the meals. The glycogen stores in the liver get depleted after 1218 hours of fasting., , + Muscle glycogen is primarily concerned with the supply of hexoses that undergo, glycolysis to provide energy during muscle contraction., , + Glycogen storage diseases—characterized by deposition of normal or abnormal type of, glycogen in one or more tissues—result in muscular weakness, or even death., , + The occurrence of HMP shunt (NADPH production) in the RBC is necessary to maintain, the integrity of erythrocyte membrane and to prevent the accumulation of, methemoglobin., , + Deficiency of glucose 6-phosphate dehydrogenase results in hemolysis of RBC, causing, hemolytic anemia. The subjects of G6PD deficiency are, however, resistant to malaria., , + Uronic acid pathway is concerned with the production of glucuronic acid (involved in, detoxification), pentoses and vitamin C. Man is incapable of synthesizing vitamin C due, to the absence of a single enzyme—L-gulonolactone oxidase., , + The conversion of glucose to fructose is impaired in diabetes mellitus, causing, accumulation of sorbitol. This compound has been implicated in the development of, cataract, nephropathy, peripheral neuropathy etc., , + Severe cases of galactosemia are associated with the development of cataract, believed, to be due to the accumulation of galactitol.
Page 290 :
280, Sorbitol is then oxidized to fructose by sorbitol, dehydrogenase and NAD+. Aldose reductase is, absent in liver but found in many tissues like, lens and retina of the eye, kidney, placenta,, Schwann cells of peripheral nerves, erythrocytes, and seminal vesicles. The enzyme sorbitol, dehydrogenase is present in seminal vesicle,, spleen and ovaries. Fructose is a preferred, carbohydrate for energy needs of sperm cells due, to the presence of sorbitol pathway., , Sorbitol pathway in, diabetes mellitus, In uncontrolled diabetes (hyperglycemia),, large amounts of glucose enter the cells which, are not dependent on insulin. Significantly, the, cells with increased intracellular glucose levels, in diabetes (lens, retina, nerve cells, kidney etc.), possess high activity of aldose reductase and, sufficient supply of NADPH. This results in a, rapid and efficient conversion of glucose to, sorbitol. The enzyme sorbitol dehydrogenase,, however, is either low in activity or absent in, these cells, hence sorbitol is not converted to, fructose. Sorbitol cannot freely pass through the, cell membrane, and accumulate in the cells, where it is produced. Sorbitol—due to its, hydrophilic nature—causes strong osmotic, effects leading to swelling of the cells. Some of, the pathological changes associated with, diabetes (like cataract formation, peripheral, neuropathy, nephropathy etc.) are believed to, be due to the accumulation of sorbitol, as, explained above., It is clearly known that in diabetic animals, sorbitol content of lens, nerve, and glomerulus is, elevated. This causes damage to tissues. It thus, appears that majority of the complications, associated with diabetes share a common, pathogenesis as a consequence of polyol, pathway. Certain inhibitors of aldose reductase, can prevent the accumulation of sorbitol, and, thus the associated complications. However, this, approach is still at the experimental stage., , Defects in fructose metabolism, 1. Essential fructosuria : Due to the, deficiency of the enzyme hepatic fructokinase,, , BIOCHEMISTRY, , fructose is not converted to fructose 1-phosphate., This is an asymptomatic condition with excretion, of fructose in urine. Treatment involves the, restriction of dietary fructose., 2. Hereditary fructose intolerance : This is, due to the absence of the enzyme aldolase B., Hereditary, fructose, intolerance, causes, intracellular accumulation of fructose 1-phosphate, severe hypoglycemia, vomiting, hepatic, failure and jaundice. Fructose 1-phosphate, allosterically inhibits liver phosphorylase and, blocks glycogenolysis leading to hypoglycemia., Early detection and intake of diet free from, fructose and sucrose, are advised to overcome, fructose intolerance., 3. Consumption of high fructose : Fructose is, rapidly converted to fructose 1-phosphate by, fructokinase. The activity of the enzyme aldolase, B is relatively less, and, due to this, fructose, 1-phosphate accumulates in the cell. This leads, to the depletion of intracellular inorganic, phosphate (Pi) levels. The phenomenon of, binding of Pi to the organic molecules (like, fructose here)—that leads to the less availability, of Pi for the essential metabolic functions—is, known as sequestering of phosphate. Due to the, decreased availability of Pi, which happens in, overconsumption of fructose, the liver, metabolism is adversely affected. This includes, the lowered synthesis of ATP from ADP and Pi., High consumption of fructose over a long period, is associated with increased uric acid in blood, leading to gout. This is due to the excessive, breakdown of ADP and AMP (accumulated due, to lack of Pi) to uric acid., , High fructose consumption and the, risk of atheroslerosis, Athesosclerosis is characterized by thickening, of arteries due to accumulation of lipids, (Refer Chapter 14)., Fructose rapidly enters tissues and increases, glycolysis, that ultimately results in lipogenesis., It is clearly established that in liver fructose, increases fatty acid and triacylglycerol synthesis,, and VLDL secretion. All these metabolic, processes finally lead to elevated triacylglycerol
Page 291 :
281, , Chapter 13 : METABOLISM OF CARBOHYDRATES, , Glucosamine, Glucose, Glutamine, Glucose, 6-phosphate, , UTP, , Glutamate, , Fructose, 6-phosphate Aminotransferase, , Glucosamine, 6-phosphate, , PPi, , Glucosamine, 1-phosphate, , UDPglucosamine, , Acetyl CoA, , N-Acetylglucosamine, 6-phosphate, , N-Acetylglucosamine, 1-phosphate, , Glycosaminoglycans, (e.g. heparin), , UTP, Epimerase, PPi, N-Acetylmannosamine, 6-phosphate, , Sialic acid, Gangliosides, Glycoproteins, , UDP-Nacetylglucosamine, , Phosphoenolpyruvate, PPi, , CTP, , CMP-NANA, , N-Acetylneuraminic, acid 9-phosphate, , Epimerase, , Glycoproteins,, Glycosaminoglycans, (e.g. chondroitins), , UDP-Nacetylgalactosamine, , Fig. 13.25 : Summary of the synthesis of amino sugars., , and LDL-cholesterol in circulation, thereby, increasing the risk of atherosclerosis., , METABOLISM OF AMINO SUGARS, When a hydroxyl group of a sugar is replaced, by an amino group, the resultant compound is, an amino sugar., The important amino sugars are glucosamine,, galactosamine, mannosamine, sialic acid etc., They are essential components of glycosaminoglycans, glycolipids (gangliosides) and, glycoproteins. They are also found in some, oligosaccharides and certain antibiotics. It is, estimated that about 20% of the glucose is, utilized for the synthesis of amino sugars, which, mostly occurs in the connective tissue., The outline of the pathway for the synthesis, of amino sugars is given in Fig.13.25., Fructose 6-phosphate is the major precursor, for glucosamine, N-acetylgalactosamine and, N-acetylneuraminic acid (NANA). The utilization, , of the amino sugars for the formation of, glycosaminolgycans,, glycoproteins, and, gangliosides is also indicated in this figure., , Mucopolysaccharidoses, The lysosomal storage diseases caused, by enzyme defects in the degradation, of glycosaminoglycans (GAGs) are known, as, mucopolysaccharidoses, (Table, 13.3)., Mucopolysaccharidoses are characterized by the, accumulation of GAGs in various tissues that may, result in skeletal deformities, and mental, retardation. Mucopolysaccharidoses are important, for elucidating the role of lysosomes in health and, disease., , GLYOXYLATE CYCLE, The animals, including man, cannot carry out, the net synthesis of carbohydrate from fat., However, the plants and many microorganisms, are equipped with the metabolic machinery—
Page 293 :
Chapter 13 : METABOLISM OF CARBOHYDRATES, , 1. Carbohydrates are the major source of energy for the living cells. Glucose (normal, fasting blood level 70-100 mg/dl) is the central molecule in carbohydrate metabolism,, actively participating in a number of metabolic pathways—glycolysis, gluconeogenesis, glycogenesis, glycogenolysis, hexose monophosphate shunt, uronic acid, pathway etc., 2. Glucose is oxidized in glycolysis, either in anaerobic (2 ATP formed) or aerobic (8 ATP, formed) conditions, resulting in the formation of 2 moles of lactate or pyruvate,, respectively., 3. Acetyl CoA is produced from pyruvate which is completely oxidized in citric acid cycle,, the final common oxidative pathway for all foodstuffs. The complete oxidation of one, mole of glucose generates 38 ATP., 4. Gluconeogenesis is the synthesis of glucose from noncarbohydrate precursors like, amino acids (except leucine and lysine), lactate, glycerol, propionate etc. The reversal, of glycolysis with alternate arrangements made at three irreversible reactions of, glycolysis constitutes gluconeogenesis., 5. Glycogen is the storage form of glucose. The degradation of glycogen (glycogenolysis) in, muscle meets the immediate fuel requirements, whereas the liver glycogen maintains the, blood glucose level. Enzyme defects in synthesis or degradation of glycogen lead to storage, disorders. von Gierke’s disease (Type I) is due to the defect in the enzyme glucose, 6-phosphatase., 6. Hexose monophosphate shunt (HMP shunt) is the direct oxidative pathway of glucose., HMP shunt assumes significance since it generates NADPH and pentoses, respectively, required for the synthesis of lipids and nucleic acids., 7. Glucuronate—involved in the conjugation of bilirubin, steroid hormones and, detoxification of drugs—is synthesized in uronic acid pathway. Due to a single enzyme, defect (gulonolactone oxidase) in this pathway, man cannot synthesize ascorbic acid, (vitamin C) whereas some animals can., 8. Galactosemia is mostly due to the defect in the enzyme galactose 1-phosphate, uridyltransferase. This results in the diversion of galactose to produce galactitol which has, been implicated in the development of cataract., 9. Glucose can be converted to fructose via sorbitol pathway. In prolonged hyperglycemia, (uncontrolled diabetes), sorbitol accumulates in the tissues, resulting in cataract,, nephropathy, peripheral neuropathy etc., 10. Amino sugars (glucosamine, galactosamine, mannosamine etc.), synthesized from, fructose 6-phosphate are essential components of glycosaminoglycans, glycolipids and, glycoproteins., , 283
Page 294 :
284, , BIOCHEMISTRY, , I. Essay questions, 1. Describe briefly the metabolism of glucose 6-phosphate., 2. Give an account of glycogen metabolism., 3. Justify that citric acid cycle is the final common metabolic pathway for the oxidation of, foodstuffs., 4. Discuss the synthesis of glucose from non-carbohydrate sources., 5. Describe the hexose monophosphate shunt and add a note on its significance., , II. Short notes, (a) Glycogenolysis, (b) UDPG, (c) Galactosemia, (d) Cori cycle, (e) 2, 3- BPG, (f) Glycogen storage, diseases, (g) Essential fructosuria, (h) Conversion of pyruvate to acetyl CoA, (i) Energetics of TCA, cycle, (j) TPP in carbohydrate metabolism., , III. Fill in the blanks, 1. Name the five vitamins required by pyruvate dehydrogenase or D-ketoglutarate dehydrogenase, complex ______________., 2. Muscle glycogen does not directly contribute to blood glucose due to absence of the enyme, ______________., 3. Ascorbic acid is not synthesized in man due to lack of the enzyme ______________., 4. The compound implicated in the development of cataract in diabetic patients is ______________., 5. Galactosemia is mostly due to the deficiency of the enzyme ______________., 6. The two amino acids that are never glucogenic are ______________ and ______________., 7. Substrate level phosphorylation in citric acid cycle is catalysed by the enzyme ______________., 8. The metabolic pathway concerned with the conversion of L-xylulose to D-xylulose is, ______________., 9. The name of the protein that has been identified to serve as a primer for glycogen synthesis is, ______________., 10. The metabolite among the citric acid cycle intermediates performing a catalytic role, ______________., , IV. Multiple choice questions, 11. One of the following enzymes in glycolysis catalyses an irreversible reaction., (a) Hexokinase (b) Phosphofructokinase (c) Pyruvate kinase (d) All of them., 12. Synthesis of 2, 3-bisphosphoglycerate occurs in the tissue namely., (a) Liver (b) Kidney (c) Erythrocytes (d) Brain., 13. The hormone that lowers cAMP concentration in liver cells is, (a) Glucagon (b) Insulin (c) Epinephrine (d) Thyroxine., 14. The number of ATP produced when a molecule of acetyl CoA is oxidized through citric acid, cycle, (a) 12 (b) 24 (c) 38 (d) 15., 15. The connecting link between HMP shunt and lipid synthesis is, (a) Ribose (b) NADPH (c) Sedoheptulose 7-phosphate (d) NADH.
Page 295 :
Section 3, , Metabolisms, , Chapter, , Metabolism of Lipids, , 14, , Cholesterol, the most, feared among lipids, speaks :, , HO, , Cholesterol (27C), , “Consumed through diet and produced in the body;, Participate in innumerable cellular functions;, Implicated in several health complications;, And blamed I am, for no fault of mine!”, , L, , ipids are indispensable for cell structure and, function. Due to their hydrophobic and nonpolar nature, lipids differ from rest of the body, compounds and are unique in their action., , Triacylglycerols, —the body fuel reserve, Lipids constitute about 15-20% of the body, weight in humans. Triacylglycerols (formerly, triglycerides) are the most abundant lipids, comprising 85-90% of body lipids. Most of the, triacylglycerols (TG; also called neutral fat or, depot fat) are stored in the adipose tissue and, serve as energy reserve of the body. This is in, contrast to carbohydrates and proteins which, cannot be stored to a significant extent for energy, purposes. Fat also acts as an insulating material, for maintaining the body temperature of animals., , Why should fat be the fuel, reserve of the body?, Triacylglycerols are the most predominant, storage form of energy. There are two main, reasons for fat being the fuel reserve of the body, , 1. Triacylglycerols (TG) are highly concentrated form of energy, yielding 9 Cal/g, in, contrast to carbohydrates and proteins that, produce only 4 Cal/g. This is because fatty acids, found in TG are in the reduced form., 2. The triacylglycerols are non-polar and, hydrophobic in nature, hence stored in pure, form without any association with water, (anhydrous form). On the other hand, glycogen, and proteins are polar. One gram of glycogen, combines with 2 g of water for storage., For the two reasons stated above, one gram of, fat stored in the body yields nearly six times as, much energy as one gram of (hydrated), glycogen. In a healthy adult individual (weighing, 70 kg), about 10-11 kg of fat is stored (mostly in, adipose tissue) which corresponds to a, fuel reserve of 100,000 Cals. If this much of, energy were to be stored as glycogen (instead of, fat), then the weight of the person would, increase by at least 55 kg! This explains why fat, has been chosen as a fuel reserve during, evolution., , 285
Page 296 :
286, Long chain fatty acids (of fat) are the ideal, storage fuel reserves of the body. Fats can, support the body’s energy needs for long periods, of food deprivation. In extreme cases, humans, can fast and survive for 60–90 days, and the, obese persons can survive even longer (6 months, to one year!) without food., Hibernating animals provide good example, for utilizing fat reserve as fuel. For instance,, bears go on hibernation for about 7 months and,, during this entire period, the energy is derived, from the degradation of fat stores. The rubythroated humming birds fly non-stop between, New England and West Indies (2,400 km!) at a, speed of 40 km/hr for 60 hours! This is possible, only due to the stored fat., , Other important body lipids, Phospholipids, glycolipids and cholesterol are, major components of cell membranes., Cholesterol is also a precursor for bile acids and, steroid hormones. Arachidonic acid—an, unsaturated fatty acid—is the substrate for the, synthesis of certain intercellular regulators—, prostaglandins, thromboxanes, prostacyclins etc., , Transport of lipids, The insoluble lipids are solubilized in association with proteins to form lipoproteins in which, form lipids are transported in the blood stream., Free lipids are undetectable in blood., Chylomicrons, very low density lipoproteins, (VLDL), low density lipoproteins (LDL), high, density lipoproteins (HDL) and albumin-free fatty, acids are the different lipoprotein complexes that, transport lipids in the blood stream. Details of, plasma lipoproteins and their metabolism are, discussed later., , Plasma lipids, The various fractions of lipids in the plasma, can be estimated by different methods after, extracting them with lipid solvents. The plasma, levels of lipids (Table 14.1) are often useful for, assessing the health of the individuals., , Dynamic state of body lipids, It was earlier thought that the lipids are inert, storage compounds and are less significant, , BIOCHEMISTRY, , TABLE 14.1 The plasma concentration of lipids, (lipid profile) in humans, , Lipid fraction, , Reference values (mg/dl), , Total lipid, , 400-600, , Total cholesterol, , 150–200, , LDL-cholesterol, , 80–150, , HDL-cholesterol, , 30–60, , VLDL-cholesterol, , 20–40, , Triglycerides, , 75–150, , Phospholipids, , 150–200, , Free fatty acids, , 5–15, , metabolically. However, later experiments with, isotope studies have proved that the body lipids, are, continuously, being, degraded, and, resynthesized. As already stated, fat stored in the, adipose tissue is the fuel reserve of the body., This is in a dynamic state., The triacylglycerols transported from intestine, (as chylomicrons) and liver (as VLDL) are stored, in the adipose tissue. Besides, they are also, utilized by muscle, liver, heart etc., as per the, needs of the body. An overview of fat, metabolism is depicted in Fig.14.1., , Synthesis of, lipoproteins, (VLDL), , Synthesis of, chylomicrons from, dietary lipids, Small intestine, , Liver, , Chylomicrons, with, TG transported, , VLDL with TG, transported, Stored, triacylglycerol, Adipose, tissue, Fatty, acids, , Triacylglycerols, and fatty acids utilized, , ENERGY, , Muscle, liver, heart etc., , Fig. 14.1 : Overview of fat metabolism.
Page 297 :
287, , Chapter 14 : METABOLISM OF LIPIDS, , O, O, , CH2 C O R1, , R2 C O CH, CH2 C O R3, , Lipolysis, + 3H2O, , R1COOH, , CH2 OH, HO CH, , +, , R2COOH, , CH2OH, , R3COOH, , O, Triacylglycerol, , Glycerol, , Free fatty acids (3), , Fig. 14.2 : Complete hydrolysis (lipolysis) of triacylglycerol., , Mobilization of fat, from adipose tissue, Triacylglycerol (TG) is the stored fat in the, adipose tissue. The enzyme, namely hormonesensitive triacylglycerol lipase, removes the, fatty acid either from carbon 1 or 3 of the, triacylglycerol to form diacylglycerol. The other, two fatty acids of TG are cleaved by additional, lipases, specific, for, diacylglycerol, and, monoacylglycerol. The complete degradation of, triacylglycerol to glycerol and free acids is, known as lipolysis (Fig.14.2)., , Regulation of hormone-sensitive, TG-lipase, Hormone-sensitive TG-lipase is so named, because its activity is mostly controlled by, hormones. Lipase is present in an inactive form, ‘b’ and is activated (phosphorylated) by a cAMP, dependent protein kinase to lipase ‘a’. Several, hormones—such as epinephrine (most effective),, norepinephrine, glucagon, thyroxine, ACTH, etc.— enhance the activity of adenylate cyclase, and, thus, increase lipolysis. On the other hand,, insulin decreases cAMP levels and thereby, inactivates lipase. Caffeine promotes lipolysis by, increasing cAMP levels through its inhibition on, phosphodiesterase activity. The control of cAMP, mediated lipolysis is illustrated in Fig.14.3., Fate of glycerol : The adipose tissue lacks the, enzyme glycerol kinase, hence glycerol, produced in lipolysis cannot be phosporylated, here. It is transported to liver where it is, activated to glycerol 3-phosphate. The latter may, be used for the synthesis of triacylglycerols and, phospholipids. Glycerol 3-phosphate may also, enter glycolysis by getting converted to, dihydroxyacetone phosphate (Fig.14.4)., , Fate of free fatty acids : The fatty acids, released in the adipocytes enter the circulation, and are transported in a bound form to albumin., The free fatty acids enter various tissues and are, utilized for the energy. About 95% of the energy, obtained from fat comes from the oxidation of, fatty acids. Certain tissues, however, cannot, oxidize fatty acids, e.g. brain, erythrocytes., , Triacylglycerol/fatty acid cycle, During starvation, TG stored in adipose tissue, is hydrolysed to free fatty acids (for oxidation) to, provide energy to skeletal and cardiac muscle., However, about 65% of these FFA are converted, to TG, and sent back to adipose tissue for, deposition. This process of lipolysis of TG and, resterification of FFA to TG is termed as, triacylglycerol/fatty acid cycle., , FATTY ACID OXIDATION, The fatty acids in the body are mostly, oxidized by E-oxidation. E-Oxidation may be, defined as the oxidation of fatty acids on the, E-carbon atom. This results in the sequential, removal of a two carbon fragment, acetyl CoA., , Fatty acid oxidation, —stages and tissues, The E-oxidation of fatty acids involves three, stages, I. Activation of fatty acids occurring in the, cytosol, II. Transport of fatty acids into mitochondria, III. E-Oxidation proper in the mitochondrial, matrix.
Page 298 :
288, , BIOCHEMISTRY, , ATP, Epinephrine, Norepinephrine, Glucagon, Thyroxine, Glucocorticoids, TSH, ACTH, GH, , Adenylate cyclase, , Insulin, niacin, PGE1, , Hormone sensitive, TG lipase b, (inactive), ATP, , cAMP, Insulin, Caffeine, , Phosphodiesterase, 5c AMP, , Pi, , Protein, kinase, , Phosphatase, , ADP, Hormone sensitive, TG lipase a, (active), Triacylglycerol, , Diacylglycerol, Free fatty acid, , FFA, , Monoacylglycerol, FFA, Glycerol, Fig. 14.3 : Control of lipolysis in adipose tissue through cyclic AMP ( –Promoting and, –Inhibiting effect;, TSH–Thyroid stimulating hormone; ACTH–Adrenocorticotrophic hormone; GH–Growth hormone;, PGE1–Prostaglandin E1; TG–Triacylglycerol; FFA–Free fatty acid)., , Fatty acids are oxidized by most of the tissues, in the body. However, brain, erythrocytes and, adrenal medulla cannot utilize fatty acids for, energy requirement., , I. Fatty acid activation, Fatty acids are activated to acyl CoA by, thiokinases or acyl CoA synthetases. The, reaction occurs in two steps and requires ATP,, coenzyme A and Mg2+. Fatty acid reacts with, ATP to form acyladenylate which then combines, with coenzyme A to produce acyl CoA, (Fig.14.5). In the activation, two high energy, phosphates are utilized, since ATP is converted, to pyrophosphate (PPi). The enzyme inorganic, pyrophosphatase hydrolyses PPi to phosphate, (Pi). The immediate elimination of PPi makes this, reaction totally irreversible., Three different thiokinases, to activate long, chain (10-20 carbon), medium chain (4-12, carbon) and short chain (< 4 carbon) fatty acids, have been identified., , II. Transport of acyl CoA, into mitochondria, The inner mitochondrial membrane is, impermeable to fatty acids. A specialized, carnitine carrier system (carnitine shuttle), operates to transport activated fatty acids from, cytosol to the mitochondria. This occurs in four, steps (Fig.14.6)., 1. Acyl group of acyl CoA is transferred to, carnitine (E-hydroxy J-trimethyl aminobutyrate),, catalysed by carnitine acyltransferase I (present, on the outer surface of inner mitochondrial, membrane)., 2. The acyl-carnitine is transported across the, membrane to mitochondrial matrix by a specific, carrier protein., 3. Carnitine acyl transferase II (found on the, inner surface of inner mitochondrial membrane), converts acyl-carnitine to acyl CoA., 4. The carnitine released returns to cytosol, for reuse.
Page 299 :
289, , Chapter 14 : METABOLISM OF LIPIDS, , III. E-Oxidation proper, , CH2 OH, HO CH, , Each cycle of E-oxidation, liberating a two, carbon unit-acetyl CoA, occurs in a sequence of, four reactions (Fig.14.7)., , CH2 OH, Glycerol, , 1. Oxidation : Acyl CoA undergoes, dehydrogenation, by, an, FAD-dependent, flavoenzyme, acyl CoA dehydrogenase. A, double bond is formed between D and E carbons, (i.e., 2 and 3 carbons)., , ATP, Glycerokinase, ADP, , CH2 OH, , 2. Hydration : Enoyl CoA hydratase brings, about the hydration of the double bond to form, E-hydroxyacyl CoA., , HO CH, CH2 O, , P, , Glycerol 3-phosphate, , 3. Oxidation : E-Hydroxyacyl CoA dehydrogenase catalyses the second oxidation and generates NADH. The product formed is E-ketoacyl, CoA., , G, , ly, c, de ero, hy l 3, dr -p, og ho, +, NAD, en sp, as ha, +, e te, NADH + H, , 4. Cleavage : The final reaction in, E-oxidation is the liberation of a 2 carbon, fragment, acetyl CoA from acyl CoA. This occurs, by a thiolytic cleavage catalysed by E-ketoacyl, CoA thiolase (or simply thiolase)., , CH2 OH, C O, Synthesis of, triacylglycerols,, phospholipids, , CH2 O, , P, , Dihydroxyacetone, phosphate, , Glycolysis, , The new acyl CoA, containing two carbons, less than the original, reenters the E-oxidation, cycle. The process continues till the fatty acid is, completely oxidized., , R CH2 CH2 COO–, Fatty acid, , Fig. 14.4 : Fate of glycerol., , ATP, Thiokinase, , It should be noted that the coenzyme A used, for activation is different from the one that finally, combines with fatty acid in the mitochondria, to form acyl CoA. Thus, the cell has two, separate pools (cytosolic and mitochondrial) of, coenzyme A., Inhibitor of carnitine shuttle : Carnitine acyl, transferase I is inhibited by malonyl CoA, a key, metabolite involved in fatty acid synthesis that, occurs in cytosol (details given later). In other, words, while the fatty acid synthesis is in, progress (reflected by high concentration of, malonyl CoA), their oxidation does not occur,, since carnitine shuttle is impaired., , Pyrophosphatase, PPi, , O, R CH2 CH2 C AMP, Acyladenylate, , CoASH, AMP, , O, R CH2 CH2 C~CoA, Acyl CoA, , Fig. 14.5 : Activation of fatty acid to, acyl CoA by the enzyme thiokinase., , 2Pi
Page 300 :
290, , BIOCHEMISTRY, , Inner, mitochondrial, membrane, , Cytosol, , Mitochondrial, matrix, , O, , O, , R C SCoA, , Carnitine, , Carnitine, , R C SCoA, , Acyl CoA, , Acyl CoA, , Carrier, protein, , Carnitine acyltransferase I, , O, CoASH, , Carnitine acyltransferase II, , O, , R C Carnitine, , R C Carnitine, , Acyl-carnitine, , Acyl-carnitine, , CoASH, , Fig. 14.6 : Carnitine shuttle for transport of activated fatty acid (acyl CoA) into mitochondria., , The overall reaction for each cycle of, `-oxidation, , The standard free energy of palmitate = 2,340, Cal., , Cn Acyl CoA + FAD + NAD+ + H2O +, CoASH ±A C(n–2) Acyl CoA + Acetyl CoA +, FADH2 + NADH + H+., , The energy yield by its oxidation—129 ATP, (129 × 7.3 Cal) = 940 Cal., , The scheme of fatty acid oxidation discussed, above corresponds to saturated (no double bond), and even carbon fatty acids. This occurs most, predominantly in biological system., , Oxidation of palmitoyl CoA, The summary of `-oxidation of palmitoyl CoA, is shown below, Palmitoyl CoA + 7 CoASH + 7 FAD + 7, NAD+ + 7H2O ±A 8 Acetyl CoA + 7 FADH2 +, 7 NADH + 7H+, , The efficiency of energy conservation by fatty, acid oxidation =, , 940, 2, 340, , TABLE 14.2 Energetics of palmitic acid oxidation, , Mechanism, , ATP yield, , I. `-Oxidation 7 cycles, 7 FADH2 [oxidized by electron transport, chain (ETC), each FADH2 gives 2 ATP], , 14(10.5), , 7 NADH (oxidized by ETC, each NADH, liberates 3 ATP), , 21(17.5), , Palmitoyl CoA undergoes 7 cycles of, `-oxidation to yield 8 acetyl CoA. Acetyl CoA, can enter citric acid cycle and get completely, oxidized to CO2 and H2O., , II. From 8 acetyl CoA, , Energetics of `-oxidation, , Energy utilized for activation, (formation of palmitoyl CoA), , The ultimate aim of fatty acid oxidation is to, generate energy. The energy obtained from the, complete oxidation of palmitic acid (16 carbon), is given in Table 14.2 and Fig.14.8., , × 100 = 40%., , Oxidized by citric acid cycle, each acetyl, CoA provides 12 ATP, , 96(80), , Total energy from one mole of palmitoyl CoA 131(108), , Net yield for one molecule of palmitate, , –2, 129(106), , Note : Values in brackets in red colour represent ATP synthesized, as per P:O ratios of 2.5 for NADH and 1.5 for FADH2.
Page 301 :
291, , Chapter 14 : METABOLISM OF LIPIDS, , O, I., , R CH2 CH2 CH2 C, , Palmitoyl CoA (16 C), , O–, , Fatty acid, , ATP, , CoASH, , Mg 2+, , Thiokinase, , AMP + PPi, , 7NAD+, , O, , 7FAD, , R CH2 CH2 CH2 C SCoA, Acyl CoA, , 7NADH + H+, , CYTOSOL, , 7FADH2, , Carnitine transport system, , II., , 35 ATP (5 u 7), , MITOCHONDRION, , III., E, , O, , D, , 8 Acetyl CoA (2C), , R CH2 CH2 CH2 C SCoA, , TCA, cycle, , Acyl CoA, , FAD, , 2ATP, ETC, , 96 ATP (8 u 12), , Fig. 14.8 : An overview of oxidation of palmitic acid., , Acyl CoA, (1) dehydrogenase, , FADH2, , O, , SIDS—a disorder due, to blockade in E--oxidation, , R CH2 CH CH C SCoA, '2 trans-enoyl CoA, H2O, (2), , The sudden infant death syndrome (SIDS) is, an unexpected death of healthy infants, usually, overnight. The real cause of SIDS is not known., It is now estimated that at least 10% of SIDS is, due to deficiency of medium chain acyl CoA, dehydrogenase. The enzyme defect has a, frequency of 1 in 10,000 births and is, in fact,, more prevalent than phenylketonuria. The, occurrence of SIDS is explained as follows, , Enoyl CoA, hydratase, , OH, , O, , R CH2 CH CH2 C SCoA, E-Hydroxyacyl CoA, +, , 3ATP, , NAD, , ETC, , +, , NADH + H, , (3) E-Hydroxyacyl CoA, dehydrogenase, , O, , O, , R CH2 C CH2 C SCoA, E-Ketoacyl CoA, , CoASH, (4), , Krebs, cycle, , Thiolase, , O, , 2CO2, , O, , R CH2 C SCoA + CH3 C SCoA, Acyl CoA (–2C), , Acetyl CoA, , Fig. 14.7 : E-Oxidation of fatty acids : Palmitoyl CoA, (16 carbon) undergoes seven cycles to yield 8 acetyl, CoA [I–Activation; II–Transport; III–E Oxidation proper—, (1) Oxidation, (2) Hydration, (3) Oxidation and, (4) Cleavage]., , Glucose is the principal source of energy,, soon after eating or feeding babies. After a few, hours, the glucose level and its utilization, decrease and the rate of fatty acid oxidation must, simultaneously increase to meet the energy, needs. The sudden death in infants is due to a, blockade in E-oxidation caused by a deficiency in medium chain acyl CoA dehydrogenase, (MCAD)., , Jamaican vomiting sickness, This disease is characterized by severe, hypoglycemia, vomiting, convulsions, coma and, death. It is caused by eating unripe ackee fruit, which contains an unusual toxic amino acid,, hypoglycin A. This inhibits the enzyme acyl
Page 302 :
292, CoA dehydrogenase and thus E-oxidation, of fatty acids is blocked, leading to various, complications., , Oxidation of odd carbon, chain fatty acids, The E-oxidation of saturated fatty acids, containing odd number of carbon atoms, proceeds in the same manner, as described, above for even carbon fatty acids. The only, difference is that in the last and final, E-oxidation cycle, a three-carbon fragment, is left behind (in place of 2 carbon unit for, saturated fatty acids). This compound is, propionyl CoA which is converted to, succinyl CoA as follows (Fig.14.9), , BIOCHEMISTRY, , CO2, , Propionyl CoA, carboxylase, , CH3, CH2, CO S CoA, , CH3, H C COO–, , Biotin, ATP, ADP + Pi, , Propionyl CoA, , CO S CoA, D-Methylmalonyl CoA, , Methylmalonyl CoA, racemase, , COO–, , CH2, , Methylmalonyl CoA, mutase, B12, , CH2, , CH3, –OOC C H, , CO S CoA, , CO S CoA, , Succinyl CoA, , L-Methylmalonyl CoA, , B12 deficiency, TCA cycle, , 1. Propionyl CoA is carboxylated in the, presence of ATP, CO2 and vitamin biotin, to D-methylmalonyl CoA., , Methylmalonic acid, , Fig. 14.9 : Conversion of propionyl CoA to succinyl CoA., , 2. Methylmalonyl CoA racemase converts the, methylmalonyl CoA to L-form. This reaction, (D o L) is essential for the entry of this compound, into the metabolic reactions of the body., , same extent as saturated fatty acids. Therefore,, oxidation of unsaturated fatty acids, in general,, provides less energy than that of saturated fatty, acids., , 3. The next enzyme, methylmalonyl CoA, mutase, is dependent on vitamin B12 (deoxyadenosyl cobalamin). It catalyses the conversion, of methylmalonyl CoA (a branched compound), to succinyl CoA (a straight chain compound),, which can enter citric acid cycle., , Most of the reactions involved in the, oxidation of unsaturated fatty acids are the same, as found in the E-oxidation of saturated fatty, acids. However, the presence of double bonds, poses problem for E-oxidation to proceed. This, is overcome by two additional enzymes—an, isomerase and an epimerase., , Methylmalonic acidemia, Two types of methylmalonic acidemias are, known, 1. Due to deficiency of vitamin B12;, , E-Oxidation of fatty acids, in peroxisomes, , In either case, there is an accumulation of, methylmalonic acid in body, followed by its, increased excretion in urine. This causes severe, metabolic acidosis, damages the central nervous, system and retards the growth. It is often fatal in, the early years of life., , Peroxisomes are organelles present in most, eukaryotic cells. The E-oxidation occurs in a, modified form in peroxisomes. Acyl CoA, dehydrogenase (a flavoenzyme) leads to the, formation of FADH2, as in E-oxidation. The, reducing equivalents from FADH2 are not, transferred to the electron transport chain, but, handed over directly to O2. This results in the, formation of H2O2, which is cleaved by catalase., , Oxidation of unsaturated fatty acids, , E-FADH2 + O2 o E-FAD + H2O2, , Due to the presence of double bonds, the, unsaturated fatty acids are not reduced to the, , H2O2 o H2O +, , 2. Due to defect in the enzyme methylmalonyl, CoA mutase., , Catalase, , 1, 2, , O2
Page 303 :
293, , Chapter 14 : METABOLISM OF LIPIDS, , There is no ATP synthesized in peroxisomal, E-oxidation of fatty acids, since the reducing, equivalents do not pass through ETC. However,, heat is liberated., It is now believed that the peroxisomes carry, out the initial oxidation of long chain (C20, C22, etc.) fatty acids which is followed by, mitochondrial oxidation., , Peroxisomal oxidation is induced by high fat, diet and administration of hypolipidemic drugs, (e.g. clofibrate)., Zellweger syndrome : This is a rare disorder, characterized by the absence of peroxisomes in, almost all the tissues. As a result, the long chain, fatty acids (C26 C38) are not oxidized. They, accumulate in tissues, particularly in brain, liver, and kidney. Hence the disorder is also known as, cerebrohepatorenal syndrome., , D-Oxidation of fatty acids, E-Oxidation is the most predominant pathway, for fatty acid degradation. However, the removal, of one carbon unit at a time by the oxidation of, D-carbon atom of fatty acid is known., D-Oxidation does not involve the binding of fatty, acid to coenzyme A and no energy is produced., , Refsum’s disease is a rare but severe, neurological disorder characterized by cerebral, ataxia and peripheral neuropathy. The patients, of this disease accumulate large quantities of an, unusual fatty acid, phytanic acid. It is derived, from phytol, a constituent of chlorophyll. Hence, it is found mostly in plant foods. However, it is, also present in milk lipids and animal fats., Phytanic acid cannot undergo E-oxidation due to, the presence of a methyl group on carbon-3. This, fatty acid undergoes initial D-oxidation (to, remove D-carbon as carbon dioxide) and this is, followed by E-oxidation., Refsum’s disease is caused by a defect in the, D-oxidation due to the deficiency of the enzyme, phytanic acid D-oxidase. The result is that, phytanic acid cannot be converted to a, compound that can be degraded by E-oxidation., The patients should not consume diets containing, chlorophyll (i.e., green leafy vegetables)., , Z-Oxidation of fatty acids, This is a minor pathway. It involves, hydroxylation followed by oxidation of Z-carbon, present as a methyl group at the other end (at, one end carboxyl group is present) of fatty acid., This reaction requires cytochrome P450, NADPH, and O2, besides the enzymes. The overall, reaction may be represented as follows., CH3 (CH2)n COO–, HO H2C (CH2)n COO–, –OOC, , (CH2)n COO–, , Oxidation of fatty acids, and metabolic water, Fatty acid oxidation (even other forms of, aerobic respiration) is accompanied by the, production of water, referred to metabolic water., For instance, when one molecule of palmitic, acid is oxidized, it releases 16 molecules of, water. This metabolic water has great, significance in some animals. Camel can store, lipids in its hump which is good source of, water, besides energy supply. For this reason,, camel, can, travel, in, deserts, for, long periods even without food and water, supply. Kangaroo rat is a small animal that, is believed to live indefinitely without water., It consumes only oil rich seeds, and the, metabolic water produced is adequate to, meet its water needs. It may however, be, noted that the use of metabolic water is an, adaptation, and is accompanied by reduced, output of urine., , KETONE BODIES, The compounds namely acetone, acetoacetate and E-hydroxybutyrate (or 3-hydroxybutyrate) are known as ketone bodies (Fig.14.10)., Only the first two are true ketones while Ehydroxybutyrate does not possess a keto (C O), group. Ketone bodies are water-soluble and, energy yielding. Acetone, however, is an, exception, since it cannot be metabolized.
Page 305 :
295, , Chapter 14 : METABOLISM OF LIPIDS, , During prolonged starvation, ketone bodies, are the major fuel source for the brain and other, parts of central nervous system. It should be, noted that the ability of the brain to utilize fatty, acids for energy is very limited. The ketone, bodies can meet 50-70% of the brain’s energy, needs. This is an adaptation for the survival of, the organism during the periods of food, deprivation., , intermediate in citric acid cycle. Thiophorase is, absent in liver, hence ketone bodies are not, utilized by the liver. Thiolase cleaves acetoacetyl, CoA to two moles of acetyl CoA (Fig.14.12)., , Reactions of ketone bodies : E-Hydroxybutyrate is first converted to acetoacetate, (reversal of synthesis) and metabolized., Acetoacetate is activated to acetoacetyl CoA, by a mitochondrial enzyme thiophorase, (succinyl CoA acetoacetate CoA transferase). The, coenzyme A is donated by succinyl CoA, an, , In normal individuals, there is a constant, production of ketone bodies by liver and their, utilization by extrahepatic tissues. The concentration of ketone bodies in blood is maintained, around 1 mg/dl. Their excretion in urine is very, low and undetectable by routine tests (Rothera’s, test)., , The summary of ketone body synthesis,, utilization and excretion is depicted in, Fig.14.13., , Overproduction of ketone bodies, , + An adult human body contains about 10–11 kg of fat reserve corresponding to about, 100,000 Cal. This can meet the energy requirements for several weeks of food, deprivation in man., , + The sudden infant death syndrome (SIDS)—an unexpected overnight death of healthy, infants—is attributed to a blockade in E-oxidation of fatty acids, caused by a deficiency, of medium chain acyl CoA dehydrogenase (MCAD)., , + Jamaican vomiting sickness is due to consumption of unripe ackee fruit containing, hypoglycin A which blocks E-oxidation., , + Methylmalonic acidemia occurs either due to a deficiency of the vitamin B12 or a defect, in an enzyme methyl malonyl CoA mutase. This disorder retards growth and damages, central nervous system., , + Zellweger syndrome is caused by the absence of peroxisomes in tissues; as a result, the, long chain fatty acids cannot be oxidized., , + Refsum’s disease is due to a defect in D-oxidation of fatty acids. The patients are, advised not to consume diets containing chlorophyll., , + Ketosis is commonly associated with uncontrolled diabetes mellitus and starvation., Diabetes ketoacidosis is dangerous—may result in coma or even death. Starvation,, however, is not accompanied by ketoacidosis., , + Insulin promotes fatty acid synthesis by stimulating the conversion of pyruvate to acetyl, CoA., , + The lack of the ability of the organisms to introduce double bonds in fatty acids beyond, C9 and C10 makes linoleic and linolenic acids essential to mammals.
Page 306 :
296, , BIOCHEMISTRY, , OH, COO–, , CH3 CH CH2, , E-Hydroxybutyrate, +, , NAD, +, , E-Hydroxybutyrate, dehydrogenase, , NADH + H, , O, CH3 C CH2 COO–, Acetoacetate, , CH2 COO–, CH2 CO SCoA, Succinyl CoA, , Thiophorase, , CH2 COO–, CH2 COO–, Succinate, , O, , O, , CH3 C CH2 C SCoA, Acetoacetyl CoA, , CoASH, , O, , Thiolase, , O, , CH3 C SCoA + CH3 C SCoA, Acetyl CoA, , Acetyl CoA, , Fig. 14.12 : Metabolism (utilization) of, ketone bodies to acetyl CoA., , When the rate of synthesis of ketone bodies, exceeds the rate of utilization, their, concentration in blood increases, this is known, as ketonemia. Ketonemia is predominantly due, to incresed production of ketone bodies rather, than the deficiency in their utilization. The term, ketonuria represents the excretion of ketone, bodies in urine. The overall picture of ketonemia, and ketonuria is commonly referred to as ketosis., Smell of acetone in breath is a common feature, in ketosis. Ketosis is most commonly associated, with starvation and severe uncontrolled diabetes, mellitus., Starvation : Starvation is accompanied by, increased degradation of fatty acids (from the, fuel reserve triacylglycerol) to meet the energy, needs of the body. This causes an over-, , production of acetyl CoA which cannot be fully, handled by citric acid cycle. Furthermore, TCA, cycle is impaired due to deficiency of oxaloacetate, since most of it is diverted for glucose, synthesis to meet the essential requirements, (often unsuccessful) for tissues like brain. The, result is an accumulation of acetyl CoA and its, diversion for overproduction of ketone bodies., Ketonuria and weight loss programs : The, appearance of ketone bodies in urine is an, indication of active fat metabolism. Some, programs designed for body weight loss, encourage reduction in carbohydrate and total, calorie intake until ketone bodies appear in, urine., Diabetes mellitus : Diabetes mellitus is, associated with insulin deficiency. This results in, impaired, carbohydrate, metabolism, and, increased lipolysis, both of them ultimately, leading to the accumulation of acetyl CoA and, its conversion to ketone bodies. In severe, diabetes, the ketone body concentration in blood, plasma may reach 100 mg/dl and the urinary, excretion may be as high as 500 mg/day., , Regulation of ketogenesis, The ketone body formation (particularly, overproduction) occurs primarily due to nonavailability of carbohydrates to the tissues. This, is an outcome of excessive utilization of fatty, acids to meet the energy requirements of the, cells. The hormone glucagon stimulates, ketogenesis whereas insulin inhibits. The, increased ratio of glucagon/insulin in diabetes, mellitus promotes ketone body formation. This is, due to disturbances caused in carbohydrate and, lipid metabolisms in diabetes, as discussed, elsewhere (Chapter 36)., , Ketogenic and antiketogenic, substances, The, ketogenic, substances, (promote, ketogenesis) include fatty acids and certain, amino acids (leucine, lysine, tyrosine etc.). The, antiketogenic substances (inhibit ketogenesis) are, glucose, glycerol and glucogenic amino acids, (e.g. glycine, alanine, serine, glutamate etc.)
Page 307 :
297, , Chapter 14 : METABOLISM OF LIPIDS, , LIVER, , EXTRAHEPATIC TISSUES, (e.g. muscle), , BLOOD, Fatty acids, , Ketone bodies, , Kidneys, Fatty acids, Glucose, , Lungs, Fatty acids, Amino, Glucose, acids, , Amino, acids, , Acetyl CoA, Ketone, bodies, TCA, cycle, , 2CO2, , Ketone, bodies, , Ketone bodies, (in urine), , Acetyl CoA, , TCA, cycle, , Acetone, (exhaled), , 2CO2, , Fig. 14.13 : Summary of ketone body synthesis, utilization and excretion., , Ketoacidosis, Both acetoacetate and E-hydroxybutyrate are, strong acids. Increase in their concentration in, blood would cause acidosis. The carboxyl group, has a pKa around 4. Therefore, the ketone bodies, in the blood dissociate and release H+ ions, which lower the pH. Diabetic ketoacidosis is, dangerous—may result in coma, and even death,, if not treated. Ketosis due to starvation is not, usually accompanied by ketoacidosis., Treatment of ketoacidosis : Rapid treatment, of diabetic ketoacidosis is required to correct the, metabolic abnormalities and the associated, water and electrolyte imbalance. Administration, of insulin is necessary to stimulate uptake of, glucose by tissues and inhibition of ketogenesis., , BIOSYNTHESIS OF FATTY ACIDS, The dietary carbohydrates and amino acids,, when consumed in excess, can be converted to, fatty acids and stored as triacylglycerols. De, novo (new) synthesis of fatty acids occurs, predominantly in liver, kidney, adipose tissue, and lactating mammary glands. The enzyme, , machinery for fatty acid production is located in, the cytosomal fraction of the cell. Acetyl CoA is, the source of carbon atoms while NADPH, provides the reducing equivalents and ATP, supplies energy for fatty acid formation. The fatty, acid synthesis may be learnt in 3 stages, I. Production of acetyl CoA and NADPH, II. Conversion of acetyl CoA to malonyl CoA, III. Reactions of fatty acid synthase complex., , I. Production, and NADPH, , of, , acetyl, , CoA, , Acetyl CoA and NADPH are the prerequisites, for fatty acid synthesis. Acetyl CoA is produced, in the mitochondria by the oxidation of pyruvate, and fatty acids, degradation of carbon skeleton, of certain amino acids, and from ketone bodies., Mitochondria, however, are not permeable to, acetyl CoA. An alternate or a bypass, arrangement is made for the transfer of acetyl, CoA to cytosol. Acetyl CoA condenses with, oxaloacetate in mitochondria to form citrate., Citrate is freely transported to cytosol where it is, cleaved by citrate lyase to liberate acetyl CoA, and oxaloacetate. Oxaloacetate in the cytosol is, converted to malate (Fig.14.14).
Page 310 :
300, 1. The two carbon fragment of acetyl CoA is, transferred to ACP of fatty acid synthase,, catalysed by the enzyme, acetyl CoA-ACP, transacylase. The acetyl unit is then transferred, from ACP to cysteine residue of the enzyme., Thus ACP site falls vacant., , malonyl, CoA-ACP, 2. The, enzyme, transacylase transfers malonate from malonyl, CoA to bind to ACP., 3. The acetyl unit attached to cysteine is, transferred to malonyl group (bound to ACP)., The malonyl moiety loses CO2 which was added, by acetyl CoA carboxylase. Thus, CO2 is never, incorporated into fatty acid carbon chain. The, decarboxylation is accompanied by loss of free, energy which allows the reaction to proceed, forward. This reaction is catalyzed by E-ketoacyl, ACP synthase., 4. E-Ketoacyl, ACP, reductase, reduces, ketoacyl group to hydroxyacyl group. The, reducing equivalents are supplied by NADPH., 5. E-Hydroxyacyl ACP undergoes dehydration., A molecule of water is eliminated and a double, bond is introduced between D and E carbons., 6. A second NADPH-dependent reduction,, catalysed by enoyl-ACP reductase occurs to, produce acyl-ACP. The four-carbon unit attached, to ACP is butyryl group., The carbon chain attached to ACP is, transferred to cysteine residue and the reactions, 2-6 are repeated 6 more times. Each time, the, fatty acid chain is lengthened by a two-carbon, unit (obtained from malonyl CoA). At the end of, 7 cycles, the fatty acid synthesis is complete and, a 16-carbon fully saturated fatty acid—namely, palmitate—bound to ACP is produced., 7. The enzyme palmitoyl thioesterase, separates palmitate from fatty acid synthase. This, completes the synthesis of palmitate., , Summary of palmitate synthesis, Of the 16 carbons present in palmitate, only, two come from acetyl CoA directly. The, remaining 14 are from malonyl CoA which, in, turn, is produced by acetyl CoA. The overall, reaction of palmitate synthesis is summarized, , BIOCHEMISTRY, , 8 Acetyl CoA + 7 ATP + 14 NADPH + 14 H+, o Palmitate + 8 CoA + 7 ADP + 7 Pi + 6H2O, , Fatty acid synthase complex, The diagrammatic representation of the model, for fatty acid synthase (FAS) multienzyme, complex is depicted in Fig.14.17. This model is, tentative and is largely based on the work of, Wakil., Fatty acid synthase is a dimer composed of, two identical subunits (monomers), each with a, molecular weight of 240,000. Each subunit, contains the activities of 7 enzymes of FAS and, an ACP with 4c-phosphopantetheine SH group., The two subunits lie in antiparallel (head-to-tail), orientation. The, SH group of phosphopantetheine of one subunit is in close proximity, to the SH of cysteine residue (of the enzyme, ketoacyl synthase) of the other subunit., Each monomer of FAS contains all the, enzyme activities of fatty acid synthesis. But only, the dimer is functionally active. This is because, the functional unit consists of half of each, subunit interacting with the complementary half, of the other. Thus, the FAS structure has both, functional division and subunit division, (Fig.14.17). The two functional subunits of FAS, independently operate and synthesize two fatty, acids simultaneously., , Functional significance, of FAS complex, The organization of different enzymes of a, metabolic pathway into a single multienzyme, functional unit has distinct advantages for, cellular function, 1. The FAS complex offers great efficiency, that is free from interference of other cellular, reactions for the synthesis of fatty acids., 2. Since the entire process of the metabolic, pathway is confined to the complex, there are, no permeability barriers for the various, intermediates., 3. The multienzyme polypeptide complex is, coded by a single gene. Thus, there is a good, coordination in the synthesis of all enzymes of, the FAS complex.
Page 312 :
302, , BIOCHEMISTRY, , TABLE 14.3 Comparison of fatty acid synthesis and oxidation, , Fatty acid synthesis, , E-Oxidation, , 1., , Major tissues, , Liver, adipose tissue, , Muscle, liver, , 2., , Subcellular site, , Cytosol, , Mitochondria, , 3., , Precursor/substrate, , Acetyl CoA, , Acyl CoA, , 4., , End product, , Palmitate, , Acetyl CoA, , 5., , Intermediates are bound to, , Acyl carrier protein, , Coenzyme A, , 6., , Coenzyme requirement, , NADPH (supplying reducing, equivalents), , FAD and NAD+ (get reduced), , 7., , Carbon units added/degraded, , Malonyl CoA, , Acetyl CoA, , 8., , Transport system, , Citrate (mitochondria o� cytosol), , Carnitine (cytosol o� mitochondria), , 9., , Inhibitor, , Long chain acyl CoA (inhibits, acetyl CoA carboxylase), , Malonyl CoA (inhibits, carnitine acyltransferase I), , 10., , The pathway increased, , After rich carbohydrate diet, , In starvation, , 11., , Hormonal status that promotes, , High ratio of insulin/glucagon, , Low ratio of insulin/glucagon, , 12., , Status of enzyme(s), , Multifunctional enzyme complex, , Individual enzymes, , acid and palmitoleic acid—are, respectively,, synthesized from stearate and palmitate., Mammals lack the ability to introduce double, bonds in fatty acids between carbon 10 and, methyl terminal (Z) end. Hence, linoleic acid, (18 : 2; 9, 12) and linolenic acid (18 : 3; 9, 12,, 15) are essential for man in the diet. However,, arachidonic acid (20 : 4; 5, 8, 11, 14) can be, synthesized from linoleic acid by desaturation, and chain elongation. Arachidonic acid is the, precursor for eicosanoids (prostaglan-dins and, thromboxanes), a group of compounds with, diversified functions, discussed elsewhere, (Chapter 32)., , SYNTHESIS OF LONG CHAIN, FATTY ACIDS FROM PALMITATE, Palmitate is the end product of the reactions of, fatty acid synthase system that occurs in cytosol., Further, chain elongation can take place either, in mitochondria or in endoplasmic reticulum, (microsomes), by separate mechanisms. The, microsomal, chain, elongation, is, more, predominant and involves successive additions of, malonyl CoA with the participation of NADPH., , These reactions are similar to that catalysed by, fatty acid synthase. A specific group of enzymes,, namely elongases, bring about fatty acid chain, elongation., The mitochondrial chain elongation is almost, a reversal of E-oxidation of fatty acids. Acetyl, CoA molecules are successively added to fatty, acid to lengthen the chain. The reducing, equivalents are derived from NADPH., , Comparison between fatty acid, synthesis and oxidation, The synthesis of fatty acids and their oxidation, are two distinct and independent pathways. A, comparison between these two metabolic, pathways in given in Table 14.3., , SYNTHESIS OF TRIACYLGLYCEROLS, Triacylglycerol (TG) synthesis mostly occurs in, liver and adipose tissue, and to a lesser extent in, other tissues. Fatty acids and glycerol must be, activated prior to the synthesis of triacylglycerols. Conversion of fatty acids to acyl CoA, by thiokinase is already described (See Fig.14.5).
Page 313 :
303, , Chapter 14 : METABOLISM OF LIPIDS, , Synthesis of glycerol 3-phosphate, Two mechanisms are involved, synthesis of glycerol 3-phosphate, , for, , the, , 1. In the liver, glycerol is activated by glycerol, kinase. This enzyme is absent in adipose tissue., 2. In both liver and adipose tissue, glucose, serves as a precursor for glycerol 3-phosphate., Dihydroxyacetone phosphate (DHAP) produced, in glycolysis is reduced by glycerol 3-phosphate, dehydrogenase to glycerol 3-phosphate., , Addition of acyl groups to form TG, Glycerol 3-phosphate acyltransferase catalyses, the transfer of an acyl group to produce, lysophosphatidic acid. DHAP can also accept, acyl group, ultimately resulting in the formation, of lysophosphatidic acid. Another acyl group is, added to lysophosphatidic acid to form, phosphatidic acid (1,2-diacylglycerol phosphate)., The enzyme phosphatase cleaves off phosphate, of phosphatidic acid to produce diacylglycerol., Incorporation of another acyl group finally results, in synthesis of triacylglycerol (Fig.14.18)., The three fatty acids found in triacylglycerol, are not of the same type. A saturated fatty acid, is usually present on carbon 1, an unsaturated, fatty acid is found on carbon 2, and carbon 3, may have either., The intermediates of TG synthesis phosphatidic, acid and diacylglycerol are also utilized for, phospholipid synthesis (described later)., , METABOLISM OF PHOSPHOLIPIDS, Phospholipids are a specialized group of, lipids performing a variety of functions. These, include the membrane structure and functions,, involvement in blood clotting, and supply of, arachidonic acid for the synthesis of, prostaglandins (for details Refer Chapter 32)., , Synthesis of phospholipids, Phospholipids, are, synthesized, from, phosphatidic acid and 1,2-diacylglycerol, intermediates in the production of triacylglycerols, (Fig.14.18). Phospholipid synthesis occurs in the, smooth endoplasmic reticulum., , 1. Formation of lecithin and cephalin :, Choline and ethanolamine first get phosphorylated, and then combine with CTP to form, respectively,, CDP-choline and CDP-ethanolamine ( Fig.14.19)., Phosphatidylcholine (lecithin) is synthesized, when CDP-choline combines with 1,2-diacylglycerol. Phosphatidyl ethanolamine (cephalin) is, produced when CDP-ethanolamine reacts with, 1,2-diacylglycerol. Phosphatidyl ethanolamine, can be converted to phosphatidyl choline on, methylation., Choline and ethanolamine, used for, phospholipid synthesis, are mostly derived from, the preexisting phospholipids. Thus, the, phospholipid synthesis starting with choline or, ethanolamine is regarded as salvage pathway., 2. Synthesis of phosphatidylserine : Phosphatidyl, ethanolamine, can, exchange, its, ethanolamine group with free serine to produce, phosphatidylserine. The latter, on decarboxylation, gives the former., 3. Formation of phosphatidylinositol : CDPdiacylglycerol produced from phosphatidic acid, combines with inositol to form phosphatidyl, inositol (PI). This phospholipid contains arachidonic acid on carbon 2 of glycerol which serves, as a substrate for prostaglandin synthesis., Further, PI is important for signal transmission, across membranes., 4. Synthesis of phosphatidyl glycerol and, cardiolipin : CDP-diacylglycerol combines with, glycerol 3-phosphate to form phosphatidyl, glycerol 3-phosphate, which then forms, phosphatidylglycerol. The latter combines with, another molecule of phosphatidylglycerol to, finally, produce, cardiolipin, (Fig.14.19)., Cardiolipin is the only phospholipid possessing, antigenic properties., 5. Formation of plasmalogens : These are, phospholipids with fatty acid at carbon 1 bound, by an ether linkage instead of ester linkage. An, important plasmalogen, 1-alkenyl 2-acetyl, glycerol 3-phosphocholine, causes blood platelet, aggregation and is referred to as plateletactivating factor (PAF). The outline of the, pathway for the synthesis of plasmalogens is, depicted in Fig.14.20.
Page 318 :
308, , BIOCHEMISTRY, , Galactocerebroside, (Gal-Cer), , Krabbe’s, disease, , Gaucher’s, disease, Glucocerebroside, (Glc-Cer), , `-Galactosidase, Galactose, Farber’s, disease, , Glucose, , Fatty, acid, Sphingosine, , Ceramide, Ceramidase, , `-Glucosidase, , Choline-P, Niemann-Pick, disease, , Sphingomyelinase, , Sphingomyelin, (choline-P-Cer), , Fig. 14.25 : Degradation of cerebrosides and sphingomyelins with metabolic disorders., , Metabolic disorders of cerebrosides, The degradation of cerebrosides along with, the associated inborn errors is depicted in, Fig.14.25., Gaucher’s disease : This is due to a defect in, the enzyme `-glucosidase. As a result, tissue, glucocerebroside levels increase. This disorder is, commonly associated with enlargement of liver, and spleen, osteoporosis, pigmentation of skin,, anemia, mental retardation etc. Sometimes,, Gaucher’s disease is fatal., Krabbe’s disease : Defect in the enzyme, `-galactosidase results in the accumulation of, galactocerebrosides. A total absence of myelin, in the nervous tissue is a common feature. Severe, mental retardation, convulsions, blindness,, deafness etc. are seen. Krabbe’s disease is fatal, in early life., , Niemann-Pick disease and Farber’s disease, connected with sphingomylein metabolism are, already described. They are also depicted in, Fig.14.25., Gangliosides are complex glycosphingolipids, mostly found in ganglion cells. They contain one, or more molecules of N-acetylneuraminic acid, (NANA) bound ceramide oligosaccharides., , Defect in the degradation of gangliosides causes, gangliosidosis, Tay-Sach’s disease etc., , Sphinogolipidoses, Lipid storage diseases, representing lysosomal, storage defects, are inherited disorders. They are, characterized by the accumulation of complex, lipids., The term sphingolipidoses is often used to, collectively refer to the genetic disorders that, lead to the accumulation of any one of the, sphingolipids (glycosphingolipids and sphingomyelins). Some examples of sphiogolipidoses, (lipid storage diseases) with important features, are summarized in Table 14.4., , METABOLISM OF CHOLESTEROL, Cholesterol is found exclusively in animals,, hence it is often called as animal sterol., The total body content of cholesterol in an adult, man weighing 70 kg is about 140 g i.e.,, around 2 g/kg body weight. Cholesterol is, amphipathic in nature, since it possesses both, hydrophilic and hydrophobic regions in the, structure.
Page 319 :
309, , Chapter 14 : METABOLISM OF LIPIDS, , TABLE 14.4 Some examples of sphingolipidoses (lipid storage diseases) with their characteristics, , Disease, , Missing/defective, enzyme, , Major storage, compound, , Symptoms, , Niemann-Pick disease, , Sphingomyelinase, , Sphingomyelins, , Enlargement of liver, spleen, mental, retardation., , Farber’s disease, , Ceramidase, , Ceramide, , Painful and deformed joints., , Gaucher’s disease, , E-Glucosidase, , Glucocerebroside, , Enlargement of liver and spleen,, osteoporosis, mental retardation., , Krabbe’s disease, , E-Galactosidase, , Galactocerebrosides, , Absence of myelin formation, liver, and spleen enlargement, mental, retardation., , Tay-Sachs disease, , Hexosaminidase A, , Ganglioside GM2, , Blindness, mental retardation, death, within 2-3 years., , Fabry’s disease, , D-Galactosidase, , Ceramide trihexoside, , Renal failure, skin rash, pain in, lower extremities., , Functions of cholesterol, Cholesterol is essential to life, as it performs a, number of important functions, 1. It is a structural component of cell, membrane., , The reducing equivalents are supplied by, NADPH while ATP provides energy. For the, production of one mole of cholesterol, 18 moles, of acetyl CoA, 36 moles of ATP and 16 moles of, NADPH are required., , 3. It is an essential ingredient in the structure, of lipoproteins in which form the lipids in the, body are transported., , By administering acetate with 14C isotope, label either on the methyl ( CH3) group or, carboxyl ( COO) group, the origin of carbon, atoms in the entire molecule of cholesterol has, been established. The sources of carbon atoms, and the key intermediates of cholesterol, formation are depicted in Fig.14.26, and the, detailed reactions are given in Fig.14.27., , 4. Fatty acids are transported to liver as, cholesteryl esters for oxidation., , The synthesis of cholesterol may be learnt in, 5 stages, , 2. Cholesterol is the precursor for the, synthesis of all other steroids in the body. These, include steroid hormones, vitamin D and bile, acids., , 1. Synthesis of HMG CoA, , CHOLESTEROL BIOSYNTHESIS, About 1 g of cholesterol is synthesized per, day in adults. Almost all the tissues of the body, participate in cholesterol biosynthesis. The, largest contribution is made by liver (50%),, intestine (15%), skin, adrenal cortex, reproductive tissue etc., The enzymes involved in cholesterol synthesis, are found in the cytosol and microsomal, fractions of the cell. Acetate of acetyl CoA, provides all the carbon atoms in cholesterol., , 2. Formation of mevalonate (6C), 3. Production of isoprenoid units (5C), 4. Synthesis of squalene (30C), 5. Conversion of squalene to cholesterol, (27C)., 1. Synthesis of E-hydroxy E-methylglutaryl, CoA (HMG CoA) : Two moles of acetyl CoA, condense to form acetoacetyl CoA. Another, molecule of acetyl CoA is then added to produce, HMG CoA. These reactions are similar to that of
Page 322 :
312, , BIOCHEMISTRY, , 2 Farnesyl pyrophosphate (15C), +, , NADPH + H, 2+, , 2+, , Mg , Mn, , Squalene synthase, , +, , NADP, PPi, , CH2, CH2, Squalene (30C), , Squalene, (structure rewritten), , NADPH + H +, NADP +, O2, , Epoxidase, Hydroxylase, Cyclase, , H2O, , Lanosterol (30C), , HO, HCOOH, 2CO 2, NADPH, O2, , A series of, reactions (about 19), , NADP +, , HO, , Cholesterol (27C), , Fig. 14.27 : Biosynthesis of cholesterol., , Regulation of cholesterol synthesis, Cholesterol biosynthesis is controlled by the, rate limiting enzyme HMG CoA reductase, at, the beginning of the pathway (Fig.14.28). HMG, CoA reductase is found in association with, endoplasmic reticulum, and is subjected to, different metabolic controls., 1. Feedback control : The end product, cholesterol controls its own synthesis by a, feedback mechanism. Increase in the cellular, concentration of cholesterol reduces the, synthesis of the enzyme HMG CoA reductase., This is achieved by decreasing the transcription, of the gene responsible for the production of, HMG CoA reductase. Feedback regulation has, been investigated with regard to LDL-cholesterol, taken up by the cells, and the same mechanism, is believed to operate whenever cellular, cholesterol level is elevated., 2. Hormonal regulation : The enzyme HMG, CoA reductase exists in two interconvertible, forms. The dephosphorylated form of HMG, CoA reductase is more active while the, phosphorylated form is less active. The hormones, exert their influence through cAMP by a series of, reactions which are comparable with the control, of the enzyme glycogen synthase. The net effect, is that glucagon and glucocorticoids favour the, formation of inactive HMG CoA reductase, (phosphorylated form) and, thus, decrease, cholesterol synthesis. On the other hand, insulin, and thyroxine increase cholesterol production by, enhancing the formation of active HMG CoA, reductase (dephosphorylated form)., 3. Inhibition by drugs : The drugs compactin, and lovastatin (mevinolin) are fungal products., They are used to decrease the serum cholesterol, level in patients with hypercholesterolemia., Compactin and lovastatin are competitive, inhibitors of the enzyme HMG CoA reductase, and, therefore, reduce cholesterol synthesis., About 50 to 60% decrease in serum cholesterol, level has been reported by a combined use of, these two drugs., 4. HMG CoA reductase activity is inhibited, by bile acids. Fasting also reduces the activity of, this enzyme.
Page 323 :
313, , Chapter 14 : METABOLISM OF LIPIDS, , Compactin, lovastatin, (competitive inhibitors), , HMG CoA, Insulin, thyroxine, (enzyme dephosphorylated), , HMG CoA reductase, , Glucagon, glucocorticoids, (enzyme phosphorylated), , Mevalonate, Translation, , mRNA, Cholesterol, , Transcription, DNA, , Fig. 14.28 : Regulation of cholesterol biosynthesis by HMG CoA reductase (, , DEGRADATION OF CHOLESTEROL, The steroid nucleus (ring structure) of the, cholesterol cannot be metabolized in humans., Cholesterol (50%) is converted to bile acids,, excreted in feces, serves as a precursor for the, synthesis of steroid hormones, vitamin D,, coprostanol and cholestanol. The latter two are, the fecal sterols, besides cholesterol., , –Promoting effect;, , function as surfactants. In the bile, the, conjugated bile acids exist as sodium and, potassium salts which are known as bile salts., Cholesterol, +, , NADPH + H, +O 2, NADP, , I. Synthesis of bile acids, The bile acids possess 24 carbon atoms, 2 or, 3 hydroxyl groups in the steroid nucleus and a, side chain ending in carboxyl group. The bile, acids are amphipathic in nature since they, possess both polar and non-polar groups. They, serve as emulsifying agents in the intestine and, actively participate in the digestion and, absorption of lipids., The synthesis of primary bile acids takes place, in the liver and involves a series of reactions, (Fig.14.29). The step catalysed by 7 D-hydroxylase is inhibited by bile acids and this is the rate, limiting reaction. Cholic acid and chenodeoxycholic acid are the primary bile acids and the, former is found in the largest amount in bile. On, conjugation with glycine or taurine, conjugated, bile acids (glycocholic acid, taurocholic acid, etc.) are formed which are more efficient in their, , –Inhibitory effect)., , 7-Hydroxycholesterol, Se, ps, ve, ral, ste, l, ste, era, , v, , Se, , 7-D-Hydroxylase, , +, , ps, , Cholic acid, Glycine, Taurine, , Chenodeoxycholic, acid, , GlycoTaurocholic acid* cholic acid*, , Taurine, or, glycine, , Intestinal, bacteria, Deoxycholic acid**, , Tauro- or, glycochenodeoxycholic*, acid, Intestinal, bacteria, Lithocholic acid**, , *, , Fig. 14.29 : Outline of bile acid synthesis ( –Primary, bile acids,, –Secondary bile acids)., , **
Page 324 :
314, , BIOCHEMISTRY, , In the intestine, a portion of primary bile acids, undergoes deconjugation and dehydroxylation to, form secondary bile acids (deoxycholic acid and, lithocholic acid). These reactions are catalysed, by bacterial enzymes in the intestine., Enterohepatic circulation : The conjugated, bile salts synthesized in the liver accumulate in, gall bladder. From there they are secreted into, the small intestine where they serve as, emulsifying agents for the digestion and, absorption of fats and fat soluble vitamins. A, large portion of the bile salts (primary and, secondary) are reabsorbed and returned to the, liver through portal vein. Thus the bile salts are, recycled and reused several times in a day. This, is known as enterohepatic circulation. About 1530 g of bile salts are secreted into the intestine, each day and reabsorbed. However, a small, portion of about 0.5 g/day is lost in the feces. An, equal amount (0.5 g/day) is synthesized in liver, to replace the lost bile salts. The fecal excretion, of bile salts is the only route for the removal of, cholesterol from the body., Cholelithiasis : Bile salts and phospholipids, are responsible for keeping the cholesterol in bile, in a soluble state. Due to their deficiency, (particularly bile salts), cholesterol crystals, precipitate in the gall bladder often resulting in, cholelithiasis—cholesterol gall stone disease., Cholelithiasis may be due to defective absorption, of bile salts from the intestine, impairment in, liver function, obstruction of biliary tract etc., The patients of cholelithiasis respond to the, administration of bile acid chenodeoxy cholic, acid, commonly known as chenodiol. It is, believed that a slow but gradual dissolution of, gall stones occurs due to chenodiol. For severe, cases of cholelithiasis, surgical removal of gall, bladder is the only remedy., , II. Synthesis of steroid, hormones from cholesterol, Cholesterol is the precursor for the synthesis, of all the five classes of steroid hormones, (a) Glucocorticoids (e.g. cortisol), (b) Mineralocorticoids (e.g. aldosterone), (c) Progestins (e.g. progesterone), , Cholesterol (27C), , Pregnenolone (21C), , Progesterone (21C), , Cortisol (21C), , Aldosterone (21C), , Estradiol (18C), , Fig. 14.30 : Outline of steroid hormone synthesis, from cholesterol (Numbers in the brackets, represent the number of carbon atoms)., , (d) Androgens (e.g. testosterone), (e) Estrogens (e.g. estradiol)., A brief outline of steroid hormonal synthesis, is given in Fig.14.30 and more details are, discussed under ‘Hormones’ (Chapter 19)., , III. Synthesis of vitamin D, 7-Dehydrocholesterol, an intermediate in the, synthesis of cholesterol, is converted to cholecalciferol (vitamin D3) by ultraviolet rays in the, skin., A brief summary of prominent sources and, the major pathways for utilization of cholesterol, with the liver as the central metabolic organ is, depicted in Fig.14.31., , Transport of cholesterol, Cholesterol is present, lipoproteins in two forms, , in, , the, , plasma, , 1. About 70-75% of it is in an esterified form, with long chain fatty acids., 2. About 25-30% as free cholesterol. This, form of cholesterol readily exchanges between, different lipoproteins and also with the cell, membranes., Role of LCAT : High density lipoproteins, (HDL) and the enzyme lecithin-cholesterol, acyltransferase (LCAT) are responsible for the, transport and elimination of cholesterol from the
Page 325 :
315, , Chapter 14 : METABOLISM OF LIPIDS, , body. LCAT is a plasma enzyme,, synthesized by the liver. It catalyses, the transfer of fatty acid from the, second position of phosphatidyl, choline (lecithin) to the hydroxyl, group of cholesterol (Fig.14.32)., HDL-cholesterol is the real substrate, for LCAT and this reaction is freely, reversible., LCAT, activity, is, associated with apo-A1 of HDL., , Dietary cholesterol, (500 mg/day), LIVER, , Cholesterol, synthesis in, liver (500 mg/day), , CHOLESTEROL, POOL, (1000 mg), Lipoproteins, (variable), , Bile salts and, bile acids, (250 mg/day), Cholesterol lost, in bile, (500 mg/day), , Cholesterol from, extrahepatic, tissues (variable), , The cholesterol (cholesteryl) ester, Major sources of liver, forms an integral part of HDL. In this, Major routes of cholesterol, cholesterol, utilization, manner, the cholesterol from the, peripheral tissues is trapped in HDL,, Fig. 14.31 Summary of major sources of liver cholesterol and its, by a reaction catalysed by LCAT and, utilization (values given in brackets are variable)., then transported to liver for, degradation and excretion. This, mechanism is commonly known as reverse Carr and Dructor method and, more recently,, cholesterol oxidase method. HDL- cholesterol, cholesterol transport., can be determined after precipitating LDL and, Plasma cholesterol—, VLDL by polyethylene glycol (PEG). VLDL, cholesterol is equivalent to 1/5thof plasma, biomedical importance, triacylglycerol (TG) in a fasting state. LDLIn healthy individuals, the total plasma, cholesterol can be calculated from Friedewald, cholesterol is in the range of 150-200 mg/dl. In, formula given below., the new born, it is less than 100 mg/dl and rises, LDL-cholesterol = Total cholesterol – (HDLto about 150 mg/dl within an year. The, women have relatively lower plasma cholesterol cholesterol + TG/5)., which is attributed to the hormones-estrogens., The above formula is not valid if TG, Cholesterol level increases with increasing age, concentration is above 400 mg/dl., (in women particularly after menopause), and, In adults, the normal LDL-cholesterol is about, also in pregnancy., 80-150 mg/dl while HDL-cholesterol is around, Plasma cholesterol is associated with different, 30-60 mg/dl. Elevation in plasma HDLlipoprotein fractions (LDL, VLDL and HDL)., cholesterol is beneficial to the body, since it, Total cholesterol can be estimated by many protects the body from atherosclerosis and, methods such as Libermann-Burchard reaction, coronary heart diseases (CHD). On the other, hand, increase in LDL-cholesterol is harmful to, the body as it may lead to various complications,, Phosphatidylcholine, Cholesterol, including CHD., , HYPERCHOLESTEROLEMIA, Lecithin cholesterol, acyltransferase (LCAT), , Lysophosphatidylcholine, , Cholesterol ester, , Fig. 14.32 : Reaction catalysed by LCAT., , Increase in plasma cholesterol (> 200 mg/dl), concentration is known as hypercholesterolemia, and is observed in many disorders, 1. Diabetes mellitus : Due to increased, cholesterol synthesis since the availability of, acetyl CoA is increased.
Page 326 :
316, 2. Hypothyroidism (myxoedema) : This is, believed to be due to decrease in the HDL, receptors on hepatocytes., 3. Obstructive jaundice : Due to an, obstruction in the excretion of cholesterol, through bile., 4. Nephrotic syndrome : Increase in plasma, globulin concentration is the characteristic, feature of nephrotic syndrome. Cholesterol, elevation is due to increase in plasma lipoprotein, fractions in this disorder., Hypercholesterolemia is associated with, atherosclerosis and coronary heart disease, (CHD). More specifically, LDL-cholesterol is, positively correlated, whereas HDL-cholesterol is, negatively correlated with CHD., Bad cholesterol and good cholesterol :, Cholesterol is a natural metabolite performing a, wide range of functions (membrane structure,, precursor for steroid hormones, bile acids). The, usages good and bad to cholesterol, although, inappropriate, are still in use. The cholesterol in, high concentration, present in LDL, is considered, bad due to its involvement in altherosclerosis, and related complications. Thus, LDL may be, regarded as lethally dangerous lipoprotein. Small, dense LDL (sdLDL) is considered to be the most, dangerous fraction of LDL associated with CHD., On the other hand, HDL cholesterol is good, since its high concentration counteracts, atherogenesis. HDL may be considered as highly, desirable lipoprotein., Affects of lifestyles on serum cholesterol, level : Individual lifestyles and habits certainly, influence serum cholesterol, and thus play a, significant role in the development coronary, heart disease. The parametres such as high blood, pressure, emotional stress, smoking, drinking of, soft water (against hard water), coffee drinking,, lack of exercise, obesity (particlarly of abdomen), elevate serum cholesterol level., , Control of hypercholesterolemia, Several measures are advocated to lower the, plasma cholesterol level, 1. Consumption of PUFA : Dietary intake of, polyunsaturated fatty acids (PUFA) reduces the, plasma cholesterol level. PUFA will help in, , BIOCHEMISTRY, , transport of cholesterol by LCAT mechanism, (described earlier) and its excretion from the, body. The oils with rich PUFA content include, cottonseed oil, soya bean oil, sunflower oil, corn, oil, fish oils etc. Ghee and coconut oil are poor, sources of PUFA., 2. Dietary cholesterol : Dietary cholesterol, influence on plasma cholesterol is minimal., However, avoidance of cholesterol-rich foods is, advocated, and a dietary intake of <300 mg/day, is advised. Certain drugs (e.g. ezetimide) inhibit, intestinal cholesterol absorption., 3. Plant sterols : Certain plant sterols and, their esters (e.g. sitostanol esters) reduce plasma, cholesterol levels. They inhibit the intestinal, absorption of dietary cholesterol., 4. Dietary fiber : Fiber present in vegetables, decreases the cholesterol absorption from the, intestine., 5. Avoiding high carbohydrate diet : Diets, rich in carbohydrates (e.g. sucrose) should be, avoided to control hypercholesterolemia., 6. Impact of lifestyles : Elevation in plasma, cholesterol is obseved in people with smoking,, abdominal obesity, lack of exercise, stress, high, blood pressure, consumption of soft water etc., Therefore, adequate changes in the lifestyles will, bring down plasma cholesterol., 7. Moderate alcohol cosumption : The, beneficial effects of moderate alcohol intake are, masked by the ill effects of chronic alcoholism., Red wine is particularly beneficial due to its, antioxidants, besides low alcohol content., 8. Use of drugs : Drugs such as lovastatin, which inhibit HMG CoA reductase and decrease, cholesterol synthesis are used. Statins currently, in use include atorvastatin, simvastatin,, fluvastatin and pravastatin. Statins are usually, taken at night to ensure maximum effect (HMG, CoA reductase activity at peak about 6 hours, after dark). Certain drugs—cholestyramine and, colestipol—bind with bile acids and decrease, their intestinal reabsorption. This helps in the, conversion of more cholesterol to bile acids and, its excretion through feces. Clofibrate increases, the activity of lipoprotein lipase and reduces the, plasma cholesterol and triacylglycerols.
Page 327 :
317, , Chapter 14 : METABOLISM OF LIPIDS, , Cholesterol, Apoprotein, Phospholipid, , Neutral core, Triacylglycerol, Cholesterol, ester, Shell (coat), , Fig. 14.33 : A general structure of lipoprotein complex., (Note : For the sake of clarity, only a part of the shell, and core are filled with the constituents)., , Hypocholesterolemia, A decrease in the plasma cholesterol,, although less common, is also observed., Hyperthyroidism, pernicious anemia, malabsorption syndrome, hemolytic jaundice etc.,, are some of the disorders associated with, hypocholesterolemia., , LIPOPROTEINS, Lipoproteins are molecular complexes that, consist of lipids and proteins (conjugated, proteins). They function as transport vehicles for, lipids in blood plasma. Lipoproteins deliver the, lipid components (cholesterol, triacylglycerol, etc.) to various tissues for utilization., , 1. Chylomicrons : They are synthesized in, the intestine and transport exogenous (dietary), triacylglycerol to various tissues. They consist of, highest (99%) quantity of lipid and lowest (1%), concentration of protein. The chylomicrons are, the least in density and the largest in size, among, the lipoproteins., 2. Very low density lipoproteins (VLDL) :, They are produced in liver and intestine and are, responsible for the transport of endogenously, synthesized triacylglycerols., 3. Low density lipoproteins (LDL) : They are, formed from VLDL in the blood circulation. They, transport cholesterol from liver to other tissues., 4. High density lipoproteins (HDL) : They are, mostly synthesized in liver. Three different, fractions of HDL (1, 2 and 3) can be identified, by ultracentrifugation. HDL particles transport, cholesterol from peripheral tissues to liver, (reverse cholesterol transport)., 5. Free fatty acids—albumin : Free fatty acids, in the circulation are in a bound form to, albumin. Each molecule of albumin can hold, about 20-30 molecules of free fatty acids., This lipoprotein cannot be separated by, electrophoresis., , (–) Cathode, Origin, Chylomicrons, , Structure of lipoproteins, A lipoprotein basically consists of a neutral, lipid core (with triacylglycerol and/or cholesteryl, ester) surrounded by a coat shell of, phospholipids, apoproteins and cholesterol, (Fig.14.33). The polar portions (amphiphilic) of, phospholipids and cholesterol are exposed on, the surface of lipoproteins so that lipoprotein is, soluble in aqueous solution., , Classification of lipoproteins, Five major classes of lipoproteins are, identified in human plasma, based on their, separation by electrophoresis (Fig.14.34)., , LDL (E-lipoprotein), Mobility, VLDL (pre-E-lipoprotein), , HDL (D-lipoprotein), , (+) Anode, Fig. 14.34 : Electrophoresis of plasma (serum), lipoproteins.
Page 330 :
320, , BIOCHEMISTRY, , LDL receptors and, supply of cholesterol, to tissues, The, most, important, function of LDL is to, supply cholesterol to the, extrahepatic tissues. The, LDL particles bind to the, specific, receptor, pits, (identified as glycoprotein), on the cell membrane. The, shape of the pit is stabilized, by a protein called clathrin., Apo B100 is responsible for, the recognition of LDL, receptor sites., , A, , Liver, , Cholesterol, , CII, , P, C, , E, , Extrahepatic, tissues, , Discoidal, nascent HDL, C, AT, , Cholesterol (C), , LC, Bile acids and, cholesterol, (in bile), , A, CII P C E, CE, HDL, , Fig. 14.36 : Metabolism of high density lipoproteins (P–Phospholipid;, C–Cholesterol; CE–Cholesteryl ester; A, CII, E–Apoproteins; LCAT–Lecithin, cholesterol acyltransferase)., , Deficiency of LDL receptors : A defect in LDL, receptors results in the elevation of plasma LDL,, hence plasma cholesterol. However, plasma, triacylglycerol concentration remains normal., Deficiency of LDL receptors is observed in type, IIa hyperbetalipoproteinemia. This disorder is, associated with a very high risk of, atherosclerosis (particularly of coronary artery)., , METABOLISM OF HDL, High density lipoproteins are synthesized in, the liver as discoidal particles – nascent HDL., They contain free cholesterol and phospholipids, (mostly lecithin) and apoproteins (A, CII, E etc.)., Role of LCAT in HDL metabolism : The, plasma enzyme lecithin-cholesterol acyltransferase (LCAT) catalyses the esterification of, free cholesterol (by fatty acid of lecithin) present, in the extrahepatic tissues and transfers to the, HDL. Apoprotein A promotes the activity of, LCAT. HDL also accepts free cholesterol from, other lipoproteins in circulation and cell, membrane of peripheral tissues (Fig.14.36). Any, free cholesterol taken up by HDL undergoes, LCAT-catalysed esterification. Due to the, addition of cholesterol, HDL particles become, spherical., The HDL particles, with cholesteryl ester, trapped inside, enter the hepatocytes by a, , receptor-mediated endocytosis. In the liver, the, cholesteryl esters are degraded to cholesterol., The latter is utilized for the synthesis of bile acids, and lipoproteins or excreted into bile (as, cholesterol)., , Cardioprotective function of HDL, HDL is a good cholesterol and plays a, cardioprotective role. It is attributed to the, reverse cholesterol transport and removal of, cholesterol from the peripheral tissue. Further,, HDL plays an antioxidant role (due to the, enzyme paroxanase activity) and protects LDL, from getting oxidized. The result is that, atherogenesis and related complications like, heart attack are reduced., , DISORDERS OF PLASMA LIPOPROTEINS, Inherited disorders of lipoproteins are, encountered in some individuals resulting in, primary hyper- or hypolipoproteinemias. These, are due to genetic defects in lipoprotein, metabolism and transport. The secondary, acquired lipoprotein disorders are due to some, other diseases (e.g. diabetes mellitus, nephrotic, syndrome, atherosclerosis, hypothyrodism etc.),, resulting in abnormal lipoprotein pattern, which often resembles the primary inherited, condition.
Page 331 :
321, , Chapter 14 : METABOLISM OF LIPIDS, , TABLE 14.6 Classification and characteristics of hyperlipoproteinemias (hyperlipidemias), Hyperlipoproeinemia Type, , Increased plasma, lipoprotein(s), , Increased plasma, lipid (most), , Probable metabolic, defect, , I, , Chylomicrons, , Triacylglycerols, , Deficiency of lipoprotein, lipase, , May increase, , IIa, , LDL, , Cholesterol, , Deficiency of LDL, receptors, , Very high (mostly in Low cholesterol fat, coronary artery), diet; cholestyramine, , IIb, , LDL and VLDL, , Triacylglycerols, and cholesterol, , Overproduction of, apo-B, , III, , IDL, , Triacylglycerols, and cholesterol, , Abnormality in apo-E, , Very high (mostly in Low fat and low, peripheral vessels) caloric diet; clofibrate, , IV, , VLDL, , Triacylglycerols, , Overproduction of TG, , May or may not, increase, , V, , Chylomicrons and VLDL Triacylglycerols, , Hyperlipoproteinemias, Elevation in one or more of the lipoprotein, fractions, constitutes, hyperlipoproteinemias., These disorders may be either primary or, secondary. Some authors use hyperlipidemias or, dyslipidemias instead of hyperlipoproteinemias., Frederickson’s classification of hyperliporoteinemias—based on the electrophoretic patterns, of plasma lipoproteins—is widely accepted to, understand these disorders. It is given in, Table 14.6 and briefly discussed hereunder., 1. Type I : This is due to familial lipoprotein, lipase deficiency. The enzyme defect causes, increase in plasma chylomicron and triacylglycerol levels., 2. Type IIa : This is also known as hyperbetalipoproteinemia and is caused by a defect in LDL, receptors. Secondary type IIa hyperlipoproteinemia is observed in association with diabetes, mellitus, hypothyroidism, nephrotic syndrome, etc. This disorder is characterized by, hypercholesterolemia., 3. Type IIb : Both LDL and VLDL increase, along with elevation in plasma cholesterol and, triacylglycerol. This is believed to be due to, overproduction of apo B., 4. Type III : This is commonly known as, broad beta disease and characterized by the, appearance of a broad `-band corresponding to, intermediate density lipoprotein (IDL) on, electrophoresis., Biochemistry [21], , Risk of, atherosclerosis, , do, , do, , Suggested, treatment, Low fat diet, , do, , Low fat and low, caloric diet; niacin, do, , 5. Type IV : This is due to overproduction of, endogenous triacylglycerols with a concomitant, rise in VLDL. Type IV disorder is usually, associated with obesity, alcoholism, diabetes, mellitus etc., 6. Type V : Both chylomicrons and VLDL are, elevated. This is mostly a secondary condition,, due to disorders such as obesity, diabetes and, excessive alcohol consumption etc., , Hypolipoproteinemias, Although low levels of plasma lipids (not, HDL!) within the normal range may be beneficial, to the body, very low lipid levels are, undesirable. These are commonly associated, with certain abnormalities, 1. Familial hypobetalipoproteinemia : It is an, inherited disorder probably due to an, impairment in the synthesis of apoprotein B. The, plasma LDL concentration in the affected, individuals is between 10 to 50% of normal, values. This disorder is harmless, and the, individuals have healthy and long life., 2. Abetalipoproteinemia : This is a rare, disorder due to a defect in the synthesis of, apoprotein B. It is characterized by a total, absence of `-lipoprotein (LDL) in plasma., Triacylglycerols are not found in plasma, but, they accumulate in liver and intestine. Serum, cholesterol level is low. Abetalipoproteinemia is, associated with decreased absorption of fat
Page 332 :
322, , BIOCHEMISTRY, , and fat-soluble vitamins. Impairment in physical, growth and mental retardation are commonly, observed., Familial alpha-lipoprotein deficiency (Tangier, disease) : The plasma HDL particles are almost, absent. Due to this, the reverse transport of, cholesterol is severely affected leading to the, accumulation of cholesteryl esters in tissues. An, absence of apoprotein C II—which activates, lipoprotein lipase—is also found. The plasma, triacylglycerol levels are elevated. The affected, individuals are at an increased risk for atherosclerosis., , FATTY LIVER, The normal concentration of lipid (mostly, phospholipid) in liver is around 5%. Liver is not, a storage organ for fat, unlike adipose tissue., However, in certain conditions, lipids—, especially the triacylglycerols—accumulate, excessively in liver, resulting in fatty liver, , (Fig.14.37). In the normal liver, Kupffer cells, contain lipids in the form of droplets. In fatty, liver, droplets of triacylglycerols are found in the, entire cytoplasm of hepatic cells. This causes, impairment in metabolic functions of liver. Fatty, liver is associated with fibrotic changes and, cirrhosis, Fatty liver may occur due to two main, causes., 1. Increased synthesis of triacylglycerols, 2. Impairment in lipoprotein synthesis., 1. Increased triacylglycerol synthesis :, Mobilization of free fatty acids from adipose, tissue and their influx into liver is much higher, than their utilization. This leads to the, overproduction of triacylglycerols and their, accumulation in liver. Diabetes mellitus,, starvation, alcoholism and high fat diet are, associated with increased mobilization of fatty, acids that often cause fatty liver. Alcohol also, inhibits fatty acid oxidation and, thus, promotes, fat synthesis and its deposition., , + Niemann-Pick disease, caused by a defect in the enzyme sphingomyelinase, results in, the accumulation of sphingomyelins in liver and spleen., , + About a dozen glycolipid storage diseases are known. These include Gaucher’s disease, and Krabbe’s disease., , + Hypercholesterolemia is associated with atherosclerosis and coronary heart diseases., Consumption of polyunsaturated fatty acids and fiber decreases cholesterol in, circulation. Drugs—such as lovastatin, cholestyramine, compactin and clofibrate—, reduce plasma cholesterol., , + Cholelithiasis, a cholesterol gall stone disease, is caused by a defect in the absorption, of bile salts from the intestine or biliary tract obstruction., , + High, , density lipoproteins—in association with lecithin-cholesterol acyltransferase (LCAT)—are responsible for the transport and elimination of cholesterol from, the body., , + Hyperlipoproteinemias are a group of disorders caused by the elevation of one or more, of plasma lipoprotein fractions., , + Excessive accumulation of triacylglycerols causes fatty liver which can often be, prevented by the consumption of lipotropic factors (choline, betaine, methionine).
Page 333 :
323, , Chapter 14 : METABOLISM OF LIPIDS, , Adipose, tissue, , Triacylglycerol, , Diabetes, Starvation, Alcohol, , Fat mobilization, Free fatty acids, Alcohol, , Acyl CoA, , Oxidation, , High fat, diet, , Liver, , Diacylglycerol, , Triacylglycerol, FATTY, LIVER, Membrane, synthesis, , Choline, , Choline, deficiency, Phospholipids, Essential, fatty acids, , Nascent, VLDL, , EFA deficiency, Cholesterol, , Protein, synthesis, , Apo B, Cholesterol, (free + ester), , Puromycin, Ethionine, Carbon tetrachloride, , Block in, secretion, , Nascent, VLDL, , VLDL, Fig. 14.37 : Development of fatty liver along with responsible factors., , 2. Impaired synthesis of lipoproteins : The, synthesis of very low density lipoproteins (VLDL), actively takes place in liver. VLDL formation, requires phospholipids and apoprotein B. Fatty, liver caused by impaired lipoprotein synthesis, may be due to :, l, a defect in phospholipid synthesis;, l, a block in apoprotein formation;, , l, , a failure in the formation/secretion of lipoprotein., , Among the three causes, fatty liver due to, impairment in phospholipid synthesis has been, studied in some detail. This is usually associated, with the dietary deficiency of lipotropic factors, such as choline, betaine, inositol etc. (more, details given later). Deficiency of essential fatty
Page 334 :
324, , BIOCHEMISTRY, , acids leads to a decreased formation, of, phospholipids. Further, excessive consumption, of cholesterol competes with essential fatty acids, and impairs phospholipid synthesis., Certain chemicals (e.g. puromycin, ethionine,, carbon, tetrachloride,, chloroform,, lead,, phosphorus etc.) that inhibit protein synthesis, cause fatty liver. This is due to a blockade in the, synthesis of apoprotein B required for VLDL, production., Lipoprotein synthesis and their secretion, require ATP. Decrease in the availability of ATP,, sometimes found in pyridoxine and pantothenic, acid deficiency, impairs lipoprotein formation., The action of ethionine in the development of, fatty liver is believed to be due to a reduction in, the availability of ATP. Ethionine competes with, methionine and traps the available adenosine, (as adenosylethionine)—thereby reducing ATP, levels., , Deficiency of vitamin E is associated with, fatty liver. Selenium acts as a protective agent in, such a condition., Endocrine factors : Certain hormones like, ACTH, insulin, thyroid hormones, adrenocorticoids promote deposition of fat in liver., , LIPOTROPIC FACTORS, These are the substances the deficiency of, which causes fat (triacylglycerol) to accumulate, in liver. This may happen despite the fatty acid, synthesis and uptake by liver being normal., , Important lipotropic factors, These include choline, betaine, methionine, and inositol. Folic acid, vitamin B12, glycine and, serine also serve as lipotropic factors to some, extent., , + Obesity is an abnormal increase in body weight due to excessive fat deposition (>25%)., Overeating, lack of exercise and genetic predisposition play a significant role in the, development of obesity., , + Some individuals with active brown adipose tissue do not become obese despite, overeating, since whatever they eat is liberated as heat due to uncoupling of oxidation, and phosphorylation in the mitochondria., , + A protein namely leptin, produced by the adipose tissue, has been identified in mice., Injection of leptin to obese mice caused reduction in body fat, increased metabolic rate, and increased insulin concentration, besides reduced food intake. Leptin has also been, detected in humans., , + Anorexia nervosa is a psychiatric disorder associated with total loss of appetite—mostly, found in females in the age group 10–30 years., , + Atherosclerosis is characterized by hardening of arteries due to the accumulation of, lipids and other compounds. The probable causes of atherosclerosis include, hyperlipoproteinemias, diabetes mellitus, obesity, high consumption of saturated fat,, lack of exercise and stress., , + Atherosclerosis and coronary heart disease are directly correlated with plasma, cholesterol and LDL, inversely with HDL. Elevation of plasma lipoprotein a suggests, increased risk of CHD., , + Alcoholism is associated with fatty liver, hyperlipidemia and atherosclerosis.
Page 335 :
325, , Chapter 14 : METABOLISM OF LIPIDS, , Action of lipotropic factors, , Body mass index (BMI), , Choline and inositol are components of, phospholipids and, hence, required for their, synthesis. The other lipotropic factors are directly, or indirectly concerned with transmethylation, reactions and, ultimately, the synthesis of, choline. Severe protein deficiency (e.g., kwashiorkor) causes fatty liver. This is due to a, defect in the synthesis of choline as a result of, insufficient amino acid (particularly methionine), supply. In other words the non-availability of, methyl groups may lead to fatty liver (Fig.14.37)., , Clinical obesity is represented by body mass, index. BMI is calculated as the weight (in, kilograms) divided by the height (in meters2)., , Choline deficiency and fatty liver, Several explanations are offered to understand, choline deficiency causing fatty liver :, (1) Decreased, phospholipid, synthesis, (Fig.14.37); (2) Impaired formation of lipoprotein, membrane; (3) Reduced synthesis of carnitine, due to insufficient supply of methyl groups;, (4) Impairment in fatty acid oxidation., , OBESITY, Obesity is an abnormal increase in the body, weight due to excessive fat deposition., , Nutritional basis, Men and women are considered as obese if, their weight due to fat (in adipose tissue),, respectively, exceeds more than 20% and 25%, of body weight. Obesity is basically a disorder of, excess calorie intake, in simple language—, overeating. It has to be remembered that every 7, calories of excess consumption leads to 1 g fat, deposit and increase in body weight., Overeating—coupled with lack of physical, exercise—contribute to obesity., Obesity due to virus infection : It was found, that around 15% of people weighing more than, 120 kg had antibodies to adenovirus-36 in, their blood, implying that this virus infection, (causes cold, diarrhea etc.), by an unknown, mechanism contributes to obesity. Surprisingly,, adenovirus-36 infected individuals have normal, serum cholesterol and other lipid parameters., , Weight (kg), , BMI (kg/m2) =, [height (m)2], , Healthy reference range for BMI is between, 18.5–24.9 kg/m2., l, , Grade I obesity or overweight – BMI 25–30, kg/m2, , l, , Grade II or clinical obesity – BMI > 30 kg/m2, , l, , Grade III or morbid obesity – BMI > 40 kg/m2, , Obesity is associated with many health, complications e.g. type II diabetes, CHD,, hypertension, stroke, arthritis, gall bladder, disease., In recent years, the ratio between waist and, hip sizes (for men < 0.9 and for women < 0.85), is considered as more effective than BMI,, particularly with regard to the risk of heart, diseases. The lower is the waist to hip ratio, the, lower the risk for health complications, and, therefore better is the health., , Genetics, obesity and leptin, There is strong evidence to suggest that, obesity has genetic basis. Thus, a child born to, two obese people has about 75% chances of, being obese. One gene namely ob gene,, expressed in adipocytes (of white adipose tissue), producing a protein called leptin (mol. wt., 16,000 daltons), is associated with obesity., Leptin is regarded as a body weight, regulatory hormone. It binds to a specific, receptor in the brain and functions as a lipostat., When the fat stores in the adipose tissue are, adequate, leptin levels are high. This signals to, restrict the feeding behaviour and limit fat, deposition. Further, leptin stimulates lipolysis, and inhibits lipogenesis. Any genetic defect in, leptin or its receptor will lead to extreme, overeating and obesity. Treatment of such obese, individuals with leptin has been shown to, reverse obesity. During starvation, leptin levels, fall which promote feeding, and fat production, and its deposition.
Page 336 :
326, , Obesity and adipose tissue, Obesity is due to an increase in both the, number and size of adipocytes (of adipose, tissue). There are two types of adipose tissues, 1. White adipose tissue : The fat is mostly, stored and this tissue is metabolically less active., 2. Brown adipose tissue : The stored fat is, less but the tissue is metabolically very active., Brown adipose tissue possesses high, proportion of mitochondria and cytochromes but, low activity of ATP synthase. This is an active, centre for the oxidation of fat and glucose and is, responsible for the diet-induced thermogenesis., The peculiarity of mitochondria of brown, adipose tissue is that the oxidation and, phosphorylation are not coupled. Mitochondrial, oxidation produces more heat and less ATP., A specific protein—namely thermogenin—has, been isolated in the inner membrane of these, mitochondria. Thermogenin functions like an, uncoupler and dissipates the energy in the form, of heat, and thus blocks the formation of ATP., Brown adipose tissue is mostly found in, hibernating animals, and the animals exposed to, cold, besides the newborn. In adult humans,, though not a prominent tissue, it is located in, the thoracic region. It is significant to note that, brown adipose tissue is almost absent in obese, persons. Some individuals are fortunate to have, active brown adipose tissue. They eat and liberate, it as heat, and therefore do not become obese., Pharmacological treatment of obesity : In, recent years, synthetic lipids such as Olestra and, Orlistat are used to treat obesity. They taste like, natural lipids but cannot be digested, and, excreted unchanged., , METABOLIC SYNDROME, Metabolic syndrome (MS) is a cluster of, different conditions that adversely affect the, health. The components contributing to MS, include abdominal obesity, insulin resistance,, dyslipidemia, elevated blood pressure, overnutrition, sedentary lifestyles etc. As per WHO, criteria, metabolic syndrome has the following, characteristics, , BIOCHEMISTRY, , 1. Insulin resistance – identified either as, type 2 diabetes or elevated fasting blood glucose, (>100 mg/dl) or impaired glucose tolerance., 2. And any two of the following, (i) Hypertension (> = 140/90 mm Hg), (ii) Dyslipidemia (serum TG > = 150 mg/dl, or HDL cholesterol <35 mg/dl in men, or <39 mg/dl in women., (iii) BMI >30 kg/m2 or waist : hip ratio of, >0.9 in men or 0.85 in women., Metabolic syndrome can be managed by, healthy habits and change in lifestyles—, restricted balanced diet, adequate intake of fiber,, and antioxidants, exercise, avoiding smoking,, stress-free life etc., , CACHEXIA, This is opposite of what is seen in obesity., Cachexia is characterized by a failure to maintain, normal lipid stores in the body. It involves higher, rate of fat mobilization than deposition., Anorexia nervosa is a total loss of appetite., This is mostly seen in females in the age group, 10-30 years. Surprisingly, majority of the affected, individuals are from wealthy families where food, is aplenty. And some members in these families, may be even obese! Anorexia nervosa is more a, psychiatric disease., , ATHEROSCLEROSIS, Atherosclerosis (Greek: athere—mush) is a, complex disease characterized by thickening or, hardening of arteries due to the accumulation of, lipids (particularly cholesterol, free, and, esterified) collagen, fibrous tissue, proteoglycans,, calcium deposits etc. in the inner arterial wall., Atherosclerosis is a progressive disorder that, narrows and ultimately blocks the arteries., Infarction is the term used to indicate the, stoppage of blood flow resulting in the death of, affected tissue. Coronary arteries—the arteries, supplying blood to heart—are the most, commonly affected leading to myocardial, infarction or heart attacks., Causes of atherosclerosis and CHD : The, development of atherosclerosis and the risk for
Page 337 :
327, , Chapter 14 : METABOLISM OF LIPIDS, , the coronary heart disease (CHD) is directly, correlated with plasma cholesterol and LDL. On, the other hand, plasma HDL is inversely, correlated with CHD., , Disorders that may cause, atherosclerosis, Certain diseases are associated with atherosclerosis. These include diabetes mellitus,, hyperlipoproteinemias, nephrotic syndrome,, hypothyroidism etc. Many other factors like, obesity, high consumption of saturated fat,, excessive smoking, lack of physical exercise,, hypertension, stress etc., are the probable, causes of atherosclerosis., , Relation between HDL and CHD, The increased levels of plasma HDL (good, cholesterol) are correlated with a low incidence, of cardiovascular disorders. Women have higher, HDL and are less prone to heart diseases, compared to men. This is attributed to estrogens, in women. Strenuous physical exercise,, moderate alcohol intake, consumption of, unsaturated fatty acids (vegetable and fish oils),, reduction in body weight—all tend to increase, HDL levels and reduce the risk CHD (see hypercholesterolemia, p-315)., , ALCOHOL METABOLISM, Walker has rightly said ‘alcohol can be a, food, a drug or a poison depending on the dose.’, In small quantities, alcohol relieves tension and, anxiety. Unfortunately, consumption of alcohol, seldom ends with small doses, hence the, beneficial effects are over-shadowed by the, harmful effects., Alcohol (ethanol or ethyl alcohol) is readily, absorbed by the stomach and intestine., Consequently, less than 2% of the alcohol, consumed is excreted through lungs, urine and, sweat., Alcohol gets oxidized in the liver by alcohol, dehydrogenase to acetaldehyde., , CH3 CH2 OH, Alcohol, , Antioxidants and atherosclerosis, Antioxidants, in general, decrease the, oxidation of LDL. There is some evidence, based, on the epidemiological studies that taking of, antioxidants (vitamins E and C or E-carotene), reduces the risk of atherosclerosis, and CHD., , CH3CHO, , NAD+ NADH + H+ Acetaldehyde, , Besides ADH, microsomal ethanol oxidizing, system (MEOS) is also involved in the, metabolism of alcohol. Aldehyde, produced by, the action of either ADH or MEOS, is responsible, for the manifestations of alcohol. The enzyme, aldehyde dehydrogenase converts aldehyde to, acetic acid which then enters Krebs cycle in the, form of acetyl CoA., , Lipoprotein a and CHD, Lipoprotein a (Lp-a) is almost identical in, structure to LDL. Lp-a contains an additional, apoprotein, apo-a. Lp-a inhibits fibrinolysis., Recent studies have shown that elevation of, lipoprotein-a in the plasma (>30 mg/dl) suggests, increased risk of CHD. It is hypothesized that, elevated Lp-a reduces the breakdown of blood, clots by interfering with plasminogen activation., This results in intravascular thrombosis, and, increased risk of heart attacks. Indians have, higher levels of Lp-a compared to Western, population., , Alcohol, dehydrogenase, , CH3 CHO, , Aldehyde, dehydrogenase, , Acetaldehyde NAD +, , NADH + H+, , CH3COOH, Acetic acid, , Since the activity of aldehyde dehydrogenase, is less than that of alcohol dehydrogenase,, acetaldehyde accumulates leading to various, complications. Disulfiram, a drug used for the, treatment of alcoholism, inhibits aldehyde, dehydrogenase., , Biochemical changes in alcoholism, The metabolism of alcohol (by both, dehydrogenases) involves the consumption of, NAD+, and consequently a high NADH/NAD+, ratio. This is mostly responsible for the metabolic, alterations observed in alcoholism. Some of them, are listed., 1. High concentration of NADH favours the, conversion of pyruvate to lactate which may lead, to lactic acidosis.
Page 338 :
328, 2. Hypoglycemia due to reduced gluconeogenesis is observed. This happens as a result, of decreased availability of pyruvate and, oxaloacetate (the latter gets converted to malate, by high NADH)., 3. Citric acid cycle is impaired since the, availability of oxaloacetate and NAD+ is, reduced. As a result, acetyl CoA accumulates, which gets diverted towards ketogenesis,, cholesterologenesis, and fatty acid synthesis., Accumulation of fats leads to fatty liver and, hyperlipidemia., , BIOCHEMISTRY, , 4. Increased concentration of serum uric acid, due to its reduced excretion is observed in, alcoholism. This is due to lactic acidosis., 5. Acetaldehyde, interferes, with, the, functioning of neurotransmitters, with an overall, effect of neurological depression., 6. Acetaldehyde causes headache, nausea,, tachycardia, reduced blood pressure etc., , Effects of chronic alcoholism, Chronic alcoholism is associated with, cirrhosis of liver, neurodegenerative changes,, cardiomyopathy, diuresis, impotence etc., , 1. Triacylglycerols (TG) are the highly concentrated form of energy, stored in adipose tissue., Hormone-sensitive lipase hydrolyses TG to free fatty acids which are transported as, albumin-FFA complexes., 2. Fatty acids are activated (acyl CoA) and transported by carnitine to mitchondria where, they get oxidized (mostly by E-oxidation) to liberate energy. Complete oxidation of one, mole palmitate liberates 129 ATP., 3. Excessive utilization of fatty acids occurs in uncontrolled diabetes mellitus and, starvation. This results in the overproduction of ketone bodies (in liver), namely, acetone, acetoacetic acid and E-hydroxy butyric acid. The last two ketone bodies serve, as energy source for peripheral tissues., 4. Fatty acid biosynthesis occurs from acetyl CoA in the cytosol through the involvement, of a multienzyme complex associated with acyl carrier protein (ACP). The reducing, equivalents (NADPH + H+) are supplied mostly by HMP shunt., 5. Synthesis of triacylglycerols and phospholipids (PL) occurs from glycerol 3-phosphate, and dihydroxyacetone phosphate with the addition of acyl CoA, and activated, nitrogenous bases (for PL)., 6. Cholesterol is synthesized from acetyl CoA in a series of reactions involving HMG CoA,, mevalonate, isoprenoid units and squalene as the intermediates. Cholesterol serves as, a precursor for bile acids, steroid hormones and vitamin D., 7. Lipoproteins are the transport vehicles for lipids in the plasma. Lipoprotein disorders, are associated with abnormalities in their plasma levels. Elevation in LDL and VLDL—, in association with cholesterol and TG—poses a serious health problem with increased, risk of atherosclerosis and CHD., 8. Excessive accumulation of triacylglycerols in liver causes fatty liver, which may be due, to increased production of TG or impairment in lipoprotein (VLDL) synthesis. The, latter is mostly associated with the deficiency of certain substances called lipotropic, factors (e.g. choline, betaine, methionine etc.), 9. Obesity is an abnormal increase in body weight (with more than 25% due to fat)., Among the many causative factors of obesity, lack of active brown adipose tissues, (which burn fat and liberate heat) in these individuals is gaining importance., 10. Atherosclerosis is a complex disease characterized by thickening of arteries due to the, accumulation of lipids. Atherosclerosis and CHD are directly correlated with LDL and, inversely with HDL of plasma.
Page 339 :
Chapter 14 : METABOLISM OF LIPIDS, , 329, , I. Essay questions, 1. Describe the functions and metabolism of phospholipids., 2. Give an account of cholesterol biosynthesis. Add a note on the significance of plasma, cholesterol estimation., 3. Describe in detail the extramitochondrial synthesis of fatty acids., 4. Write about the types, characteristics and metabolism of lipoproteins. Add a note on lipoprotein, disorders., 5. Give an account of fatty acid oxidation., , II. Short notes, (a) Carnitine, (b) LCAT, (c) Fatty liver, (d) Ketone bodies, (e) Lipotropic factors, (f) Acyl carrier, protein, (g) Degradation of cholesterol, (h) HDL, (i) Lipoprotein lipase, (j) Brown adipose tissue., , III. Fill in the blanks, 1. The most predominant lipid component of chylomicrons _____________., 2. Cholesterol synthesis is controlled by feedback inhibition of the enzyme _____________., 3. A compound possessing hydrophobic and hydrophilic groups in its structure is known, as _____________., 4. Niemann-Pick disease is due to a defect in the enzyme _____________., 5. The lipoprotein involved in the reverse cholesterol transport is _____________., 6. The total number of ATP produced by the oxidation of a molecule of palmitic acid is, _____________., 7. The long chain fatty acids (C26—C35) are not oxidized due to the absence of peroxisomes. This, disorder is known as _____________., 8. Acetyl CoA from the mitochondria is transported into the cytosol after its conversion to, _____________., 9. Plasma lipoprotein that is inversely correlated with coronary heart disease is _____________., 10. The fatty acid that is commonly found in the C2 of triacylglycerols is _____________., , IV. Multiple choice questions, 11. The following substance(s) is (are) ketogenic, (a) Fatty acids (b) Leucine (c) Lysine (d) All of them., 12. The lipoprotein possessing the highest quantity of phospholipid, (a) HDL (b) LDL (c) VLDL (d) Chylomicrons., 13. Hypercholesterolemia is observed in the disorder(s), (a) Hypothyroidism (b) Diabetes mellitus (c) Nephrotic syndrome (d) All of them., 14. The two final products in the E-oxidation of odd chain fatty acids are, (a) Acetyl CoA and malonyl CoA (b) Acetyl CoA and acetyl CoA (c) Acetyl CoA and propionyl, CoA (d) Acetyl CoA and succinyl CoA., 15. Hormone sensitive lipase activity is inhibited by the hormone, (a) Epinephrine (b) Insulin (c) Thyroxine (d) Glucocorticoids.
Page 340 :
Section 3, , Metabolisms, , Chapter, , Metabolism of Amino Acids, , 15, Amino acids, , D-Ketoglutarate, Transamination, Glutamate, Keto acids, , Deamination, NH3, Urea, , The amino acids speak :, , “We transaminate and deaminate to liberate ammonia;, That is detoxified in the liver to end product urea;, Greatly important to body are our nitrogen products;, Carbon skeleton is for glucose, fat or fuel.”, , P, , roteins are the most abundant organic, compounds and constitute a major part of, the body dry weight (10-12 kg in adults). They, perform a wide variety of static (structural) and, dynamic (enzymes, hormones, clotting factors,, receptors etc.) functions. About half of the body, protein (predominantly collagen) is present in the, supportive tissue (skeleton and connective) while, the other half is intracellular., , Proteins are nitrogen-containing macromolecules consisting of L-D-amino acids as the, repeating units. Of the 20 amino acids found in, proteins, half can be synthesized by the body, (non-essential) while the rest have to be provided, in the diet (essential amino acids)., The proteins on degradation (proteolysis), release individual amino acids. Amino acids are, not just the structural components of proteins., Each one of the 20 naturally occurring amino, acids undergoes its own metabolism and, performs specific functions. Some of the amino, acids also serve as precursors for the synthesis, of many biologically important compounds (e.g., , melanin, serotonin, creatine etc.). Certain amino, acids may directly act as neurotransmitters, (e.g. glycine aspartate, glutamate). Protein, metabolism is more appropriately learnt as, metabolism of amino acids., , AMINO ACID POOL, An adult has about 100 g of free amino acids, which represent the amino acid pool of the, body. The amino acid pool may be an, oversimplification of the facts, since there is, no single compartment—rather, several compartments exist., , Glutamate and glutamine together constitute, about 50%, and essential amino acids about, 10% of the body pool (100 g). The concentration, of intracellular amino acids is always higher than, the extracellular amino acids. Amino acids enter, the cells against a concentration gradient by, active transport., , 330
Page 341 :
331, , Chapter 15 : METABOLISM OF AMINO ACIDS, , The amino acid pool of, the body is maintained by, the sources that contribute, (input) and the metabolic, pathways that utilize (output), the amino acids (Fig.15.1)., , I. Sources of amino, acid pool, , Body protein, 10-12 kg in adult, , Protein breakdown, (350-400 g/day), , Protein synthesis, (300-400 g/day), , Dietary protein, (40-100 g/day), , Turnover, of, body, Body, protein, intake of dietary, amino acid pool, (100 g), protein and the synthesis of, non-essential amino acids, contribute to the body Synthesis of non- Protein loss from, essential amino body (30-50 g/day), amino acid pool., , Synthesis of non-protein compounds, (30 g/day; creatine, porphyrins,, phospholipids, purines,, pyrimidines etc.), , Carbohydrates, fat, , Energy (10-15% of, body’s daily requirement), , acids (variable), , (a) Protein turnover :, Utilization, Mostly as, Sources, The protein present in the, urea, body is in a dynamic state., It is estimated that about, Urine, 300-400 g of protein per, Fig. 15.1 : Overview of body’s amino acid pool—sources and utilization., day is constantly degraded, and synthesized which, represents body protein, There is no storage form of amino acids as is, turnover. There is a wide variation in the, turnover of individual proteins. For instance, the the case for carbohydrates (glycogen) and lipids, plasma proteins and digestive enzymes are (triacylglycerols). The excess intake of amino, rapidly degraded, their half-lives being in hours acids are metabolized—oxidized to provide, or days. The structural proteins (e.g. collagen) energy, converted to glucose or fat. The amino, have long half-lives, often in months and years. groups are lost as urea and excreted. The protein, consumption in developed countries is much, Control of protein turnover : The turnover of, higher than the recommended dietary allowance, the protein is influenced by many factors. A, (i.e. 1g/kg body weight/day). The daily protein, small polypeptide called ubiquitin (mol. wt., intake by an adult in most countries is 40-100 g., 8,500) tags with the proteins and facilitates, Protein is digested by proteolytic enzymes to, degradation. Certain proteins with amino acid, amino acids which are absorbed in the intestine, sequence proline, glutamine (one letter code E),, and enter the body pool of amino acids., serine and threonine (PEST sequence) are rapidly, (c) Synthesis of non-essential amino acids :, degraded., Ten out of the 20 naturally occurring amino, (b) Dietary protein : There is a regular loss of, acids can be synthesized by the body which, nitrogen from the body due to degradation of, contribute to the amino acid pool., amino acids. In healthy adults, it is estimated, that about 30-50 g of protein is lost everyday, II. Utilization of amino acids, from the body. This amount of protein (30-50 g/, from body pool, day) must, therefore, be supplied daily in the, diet to maintain nitrogen balance. The purpose, (a) Most of the body proteins (300-400 g/day), of dietary protein is to supply amino acids degraded are synthesized from the amino acid, (particularly the essential ones) for the synthesis pool. These include enzymes, hormones,, of proteins and other nitrogen compounds., immunoproteins, contractile proteins etc.
Page 342 :
332, (b) Many important nitrogenous compounds, (porphyrins, purines, pyrimidines, etc.) are, produced from the amino acids. About 30 g of, protein is daily utilized for this purpose., (c) Generally, about 10-15% of body energy, requirements are met from the amino acids., , BIOCHEMISTRY, , Dietary Body, protein protein, , Protein, synthesis, , Synthesis of, N-compounds, , Amino acids, , D-Ketoglutarate, , (d) The amino acids are converted to, carbohydrates, and, fats., This, becomes, predominant when the protein consumption is in, excess of the body requirements., , Transamination, Glutamate, Deamination, NH3, , METABOLISM OF AMINO ACIDS, —GENERAL ASPECTS, The amino acids undergo certain common, reactions like transamination followed by, deamination for the liberation of ammonia. The, amino group of the amino acids is utilized for, the formation of urea which is an excretory end, product of protein metabolism. The carbon, skeleton of the amino acids is first converted to, keto acids (by transamination) which meet one, or more of the following fates., 1. Utilized to generate energy., , Urea, , Keto acids, , Energy, , Non-essential, amino acids, , Glucose Fat, , Fig. 15.2 : An overview of amino acid metabolism., , Salient features of transamination, 1. All transaminases require pyridoxal, phosphate (PLP), a coenzyme derived from, vitamin B6., , 3. Diverted for the formation of fat or ketone, bodies., , 2. Specific transaminases exist for each pair, of amino and keto acids. However, only two—, namely, aspartate transaminase and alanine, transaminase—make a significant contribution, for transamination., , 4. Involved in the production of non-essential, amino acids., , 3. There is no free NH3 liberated, only the, transfer of amino group occurs., , A general picture of amino acid metabolism is, depicted in Fig.15.2., , 4. Transamination is reversible (Fig.15.3)., , 2. Used for the synthesis of glucose., , The details of general and specific metabolic, reactions of amino acids are described in the, following pages., , R1 CH COO–, , R1 C COO–, , +, NH3, , O, , Keto acid-I, , Amino acid-I, , TRANSAMINATION, The transfer of an amino ( NH2) group from, an amino acid to a keto acid is known as, transamination. This process involves the, interconversion of a pair of amino acids and, a pair of keto acids, catalysed by a group, of enzymes called transaminases (recently,, aminotransferases)., , Transminase, PLP, , R2 C COO–, O, Keto acid-II, , R2 C COO–, +, , NH3, Amino acid-II, , Fig. 15.3 : Transamination reaction.
Page 343 :
333, , Chapter 15 : METABOLISM OF AMINO ACIDS, , 5. Transamination is very, important for the redistribution, of, amino, groups, and, production of non-essential, amino acids, as per the, requirement of the cell. It, involves, both, catabolism, (degradation) and anabolism, (synthesis) of amino acids., 6. Transamination diverts, the excess amino acids, towards energy generation., , (A), , CH3 CH COO–, +, , NH3, , Glutamate pyruvate, transaminase, , 8. All amino acids except, lysine, threonine, proline and, hydroxyproline participate in, transamination., 9. Transamination is not, restricted to D-amino groups, only. For instance, G-amino, group, of, ornithine, is, transaminated., , O, Pyruvate, , Alanine, , H3C NH2, , HC O, HO, , CH2 O, , HO, , P, , +, , H3C, , CH2 O, , P, , +, , H3C, , N, , N, H, , H, , Pyridoxamine phosphate, , Pyridoxal phosphate, , 7. The, amino, acids, undergo transamination to, finally concentrate nitrogen in, glutamate. Glutamate is the, only amino acid that undergoes, oxidative deamination to a, significant extent to liberate, free NH3 for urea synthesis., , CH3 C COO–, , +, , NH3, , O, , Glutamate pyruvate, transaminase, , H C COO–, , C COO–, , CH2, , CH2, , CH2, , CH2, , COO–, , COO–, , D-Ketoglutarate, , Glutamate, (B), Enzyme, , R CH COO–, , (CH2)4, +, , N+, , N, H, , H, , C H, , O, , C H, , O, CH2 O, +, , P, , CH2O, +, , Enzyme, , (CH2)4, , 10. Serum transaminases, are important for diagnostic, and, prognostic, purposes., (Refer Chapter 6)., , H3C, , Mechanism of, transamination, , Fig. 15.4 : Mechanism of transamination—(A) Involvement of pyridoxal, phosphate (PLP) in the transfer of amino group, (B) Formation of enzymePLP-Schiff base and amino acid-PLP-Schiff base., Note that when the amino acid binds, enzyme separates., , Transamination occurs in, two stages (Fig.15.4), , N, , H3C, , P, , N, , H, , H, , Enzyme-PLP, Schiff base, , Amino acid, PLP-Schiff base, , 1. Transfer of the amino group to the, coenzyme pyridoxal phosphate (bound to the, coenzyme) to form pyridoxamine phosphate., 2. The amino group of pyridoxamine, phosphate is then transferred to a keto acid to, produce a new amino acid and the enzyme with, PLP is regenerated., , NH2, , All the transaminases require pyridoxal, phosphate (PLP), a derivative of vitamin B6. The, aldehyde group of PLP is linked with H-amino, group of lysine residue, at the active site of the, enzyme forming a Schiff base (imine linkage)., When an amino acid (substrate) comes in, contact with the enzyme, it displaces lysine and, a new Schiff base linkage is formed. The amino
Page 344 :
334, acid-PLP-Schiff base tightly, binds with the enzyme by noncovalent forces. Snell and, Braustein proposed a Ping, Pong Bi Bi mechanism, involving, a, series, of, intermediates (aldimines and, ketimines) in transamination, reaction., , BIOCHEMISTRY, , COO–, COO–, , COO–, , CH2, , CH2, , CH2, H C, , +, NH3, –, , COO, , +, , NAD(P), , GDH, , L-Glutamate, , C NH, , CH2, , H2 O, , +, , GDH, , CH2 + NH4, C O, , COO–, , COO–, , D-Iminoglutarate, , D-Ketoglutarate, , +, , NAD(P)H + H, , CH2, , CH2, , Fig. 15.5 : Oxidation of glutamate by glutamate dehydrogenase (GDH)., , DEAMINATION, The removal of amino group from the amino, acids as NH3 is deamination. Transamination, (discussed above) involves only the shuffling of, amino groups among the amino acids. On the, other hand, deamination results in the liberation, of ammonia for urea synthesis. Simultaneously,, the carbon skeleton of amino acids is converted, to keto acids. Deamination may be either, oxidative or non-oxidative., Although transamination and deamination are, separately discussed, they occur simultaneously,, often involving glutamate as the central, molecule. For this reason, some authors use the, term transdeamination while describing the, reactions of transamination and deamination,, particularly involving glutamate., , I. Oxidative deamination, Oxidative deamination is the liberation of, free ammonia from the amino group of amino, acids coupled with oxidation. This takes place, mostly in liver and kidney. The purpose of, oxidative deamination is to provide NH3 for urea, synthesis and D-keto acids for a variety of, reactions, including energy generation., Role of glutamate dehydrogenase : In the, process of transamination, the amino groups of, most amino acids are transferred to D-ketoglutarate to produce glutamate. Thus, glutamate, serves as a ‘collection centre’ for amino groups, in the biological system. Glutamate rapidly, undergoes oxidative deamination, catalysed by, glutamate dehydrogenase (GDH) to liberate, ammonia. This enzyme is unique in that it can, utilize either NAD+ or NADP+ as a coenzyme., Conversion of glutamate to D-ketoglutarate, , occurs through the formation of an intermediate,, D-iminoglutarate (Fig.15.5)., Glutamate dehydrogenase catalysed reaction, is important as it reversibly links up glutamate, metabolism with TCA cycle through D-ketoglutarate. GDH is involved in both catabolic and, anabolic reactions., Regulation of GDH activity : Glutamate, dehydrogenase is a zinc containing mitochondrial enzyme. It is a complex enzyme, consisting of six identical units with a molecular, weight of 56,000 each. GDH is controlled by, allosteric regulation. GTP and ATP inhibit—, whereas GDP and ADP activate—glutamate, dehydrogenase. Steroid and thyroid hormones, inhibit GDH., After ingestion of a protein-rich meal, liver, glutamate level is elevated. It is converted to, D-ketoglutarate with liberation of NH3. Further,, when the cellular energy levels are low, the, degradation of glutamate is increased to provide, D-ketoglutarate which enters TCA cycle to, liberate energy., Oxidative deamination by amino acid oxidases : L-Amino acid oxidase and D-amino acid, oxidase are flavoproteins, possessing FMN and, FAD,, respectively., They, act, on, the, corresponding amino acids (L or D) to produce, D-keto acids and NH3. In this reaction, oxygen is, reduced to H2O2, which is later decomposed by, catalase (Fig.15.6)., The activity of L-amino acid oxidase is much, low while that of D-amino acid oxidase is high, in tissues (mostly liver and kidney). L-Amino acid, oxidase does not act on glycine and dicarboxylic
Page 345 :
335, , Chapter 15 : METABOLISM OF AMINO ACIDS, , L-Amino acid, , L-Amino acid oxidase, , FMN, , D-Keto acid + NH3, , FMNH2, , undergo, deamination, coupled, desulfhydration to give keto acids., Cysteine, , Desulfhydrases, , with, , Pyruvate, , NH3 + H2S, 1, –, O2, , H2O2, , (c) Deamination of histidine : The enzyme, histidase acts on histidine to liberate NH3 by a, non-oxidative deamination process., , 2, , Catalase, H2O, , Fig. 15.6 : Oxidative deamination of amino acids., , Histidine, , Histidase, Urocanate, NH3, , acids. This enzyme, due to its very low activity,, does not appear to play any significant role in, the amino acid metabolism., Fate of D-amino acids : D-Amino acids are, found in plants and microorganisms. They are,, however, not present in the mammalian proteins., But D-amino acids are regularly taken in the diet, and metabolized by the body. D-Amino acid, oxidase converts them to the respective D-keto, acids by oxidative deamination. The D-keto acids, so produced undergo transamination to be, converted to L-amino acids which participate in, various metabolisms. Keto acids may be oxidized, to generate energy or serve as precursors for, glucose and fat synthesis. Thus, D-amino acid, oxidase is important as it initiates the first step, for the conversion of unnatural D-amino acids, to L-amino acids in the body (Fig.15.7)., , METABOLISM OF AMMONIA, Ammonia is constantly being liberated in the, metabolism of amino acids (mostly) and other, nitrogenous compounds. At the physiological, pH, ammonia exists as ammonium (NH+4) ion., , I. Formation of ammonia, The production of NH3 occurs from the, amino acids (transamination and deamination),, biogenic amines, amino group of purines and, pyrimidines and by the action of intestinal, bacteria (urease) on urea., , II. Transport and storage of NH3, Despite a regular and constant production of, NH3 from various tissues, its concentration in, , II. Non-oxidative deamination, D-Amino acids, , Some of the amino acids can be deaminated, to liberate NH3 without undergoing oxidation, (a) Amino acid dehydrases : Serine, threonine, and homoserine are the hydroxy amino acids., They undergo non-oxidative deamination, catalysed, by, PLP-dependent, dehydrases, (dehydratases)., Serine, Threonine, Homoserine, , Dehydratase, , Respective, D-keto acids, , NH3, , (b) Amino acid desulfhydrases : The sulfur, amino acids, namely cysteine and homocysteine,, , H2 O, , FAD, D-Amino acid oxidase, , +, , NH4, , FADH2, , D-Keto acids, L-Amino acid, D-Keto acid, , Transaminases, , L-Amino acids, , Energy, Glucose, Fat, , Fig. 15.7 : Metabolic fate of D-amino acids.
Page 346 :
336, the circulation is surprisingly low (normal, plasma 10-20 mg/dl). This is mostly, because the body has an efficient, mechanism for NH3 transport and its, immediate utilization for urea synthesis., The transport of ammonia between, various tissues and the liver mostly, occurs in the form of glutamine or, alanine and not as free ammonia. Alanine, is important for NH3 transport from, muscle to liver by glucose-alanine cycle, (Refer Fig.13.13)., , BIOCHEMISTRY, , COO–, , CO NH2, , CH2, , CH2, , +, , NH 4, , CH2, H C, , +, NH3, –, , COO, , Glutamine synthetase, , Glutamine synthetase (a mitochondrial, enzyme) is responsible for the synthesis of, glutamine from glutamate and ammonia. This, reaction is unidirectional and requires ATP and, Mg2+ ions., Glutamine can be deaminated by hydrolysis, to release ammonia by glutaminase (Fig.15.8), an enzyme mostly found in kidney and intestinal, cells., , III. Functions of ammonia, Ammonia is not just a waste product of, nitrogen metabolism. It is involved (directly or, via glutamine) for the synthesis of many, compounds in the body. These include nonessential amino acids, purines, pyrimidines,, amino sugars, asparagine etc. Ammonium ions, (NH4+) are very important to maintain acid-base, balance of the body., , IV. Disposal of ammonia, The organisms, during the course of, evolution, have developed different mechanisms, for the disposal of ammonia from the body. The, animals in this regard are of three different types, , CH2, +, , H C NH3, , 2+, , ATP, , Mg, , COO–, , ADP + Pi, , Glutamine, , Glutamate, , Role of glutamine : Glutamine is a, storehouse of NH3. It is present at the, highest concentration (8 mg/dl in adults), in blood among the amino acids. Glutamine, serves as a storage and transport form of NH3. Its, synthesis mostly occurs in liver, brain and, muscle. Ammonia is removed from the brain, predominantly as glutamine. Glutamine is freely, diffusible in tissues, hence easily transported., , H 2O, , Glutaminase, +, , NH4, , H 2O, , Fig. 15.8 : Synthesis of glutamine and its, conversion to glutamate. (Note : The reactions, are independent and irreversible)., , (a) Ammoniotelic : The aquatic animals, dispose off NH3 into the surrounding water., (b) Uricotelic : Ammonia is converted mostly, to uric acid e.g. reptiles and birds., (c) Ureotelic : The mammals including man, convert NH3 to urea. Urea is a non-toxic and, soluble compound, hence easily excreted., , V. Toxicity of ammonia, Even a marginal elevation in the blood, ammonia concentration is harmful to the brain., Ammonia, when it accumulates in the body,, results in slurring of speech and blurring of the, vision and causes tremors. It may lead to coma, and, finally, death, if not corrected., Hyperammonemia : Elevation in blood NH3, level may be genetic or acquired. Impairment in, urea synthesis due to a defect in any one of the, five enzymes is described in urea synthesis., All these disorders lead to hyperammonemia, and cause hepatic coma and mental retardation., The acquired hyperammonemia may be due to, hepatitis, alcoholism etc. where the urea, synthesis becomes defective, hence NH3, accumulates., Explanation for NH3 toxicity : The reaction, catalysed by glutamate dehydrogenase probably, explains the toxic affects of NH3 in brain, NADPH + H+ NADP+, , D�Ketoglutarate + NH3, , Glutamate
Page 347 :
337, , Chapter 15 : METABOLISM OF AMINO ACIDS, , Accumulation of NH3 shifts the equilibrium, to the right with more glutamate formation,, hence more utilization of D-ketoglutarate. DKetoglutarate is a key intermediate in TCA cycle, and its depleted levels impair the TCA cycle., The net result is that production of energy (ATP), by the brain is reduced. The toxic effects of NH3, on brain are, therefore, due to impairment in, ATP formation., Trapping and elimination of ammonia : When, the plasma level of ammonia is highly elevated,, intravenous administration of sodium benzoate, and phenyllactate is done. These compounds, can respectively condense with glycine and, glutamate to form water soluble products that, can be easily excreted. By this way, ammonia, can be trapped and removed from the body. In, some instances of toxic hyperammonemia,, hemodialysis may become necessary., , UREA CYCLE, Urea is the end product of protein, metabolism (amino acid metabolism). The, nitrogen of amino acids, converted to ammonia, (as described above), is toxic to the body. It is, converted to urea and detoxified. As such, urea, accounts for 80-90% of the nitrogen containing, substances excreted in urine., Urea is synthesized in liver and transported, to kidneys for excretion in urine. Urea cycle is, the first metabolic cycle that was elucidated by, Hans Krebs and Kurt Henseleit (1932), hence it, is known as Krebs-Henseleit cycle. The, individual reactions, however, were described in, more detail later on by Ratner and Cohen., Urea has two amino ( NH2) groups, one, derived from NH3 and the other from aspartate., Carbon atom is supplied by CO2. Urea synthesis, is a five-step cyclic process, with five distinct, enzymes. The first two enzymes are present in, mitochondria while the rest are localized in, cytosol. The details of urea cycle are described, (Figs.15.9 and 15.10)., 1. Synthesis of carbamoyl phosphate :, Carbamoyl phosphate synthase I (CPS I) of, mitochondria catalyses the condensation of, , +, , CO 2 + NH4, , Carbamoyl phosphate, , Citrulline, , Ornithine, , O, NH2 C NH2, Urea, , Aspartate, (R c-NH2 ), , H 2O, Arginine, , Arginosuccinate, , Fumarate, , Fig. 15.9 : Outline of urea cycle. (Note : In the synthesis, of urea one amino group comes from ammonium ion, while the other is from aspartate; carbon is derived from, CO2. This is represented in colours.), , NH4+ ions with CO2 to form carbamoyl, phosphate. This step consumes two ATP and is, irreversible, and rate-limiting. CPS I requires Nacetylglutamate for its activity. Another enzyme,, carbamoyl phosphate synthase II (CPS II)—, involved in pyrimidine synthesis—is present in, cytosol. It accepts amino group from glutamine, and does not require N-acetylglutamate for its, activity., 2. Formation of citrulline : Citrulline is, synthesized from carbamoyl phosphate and, ornithine by ornithine transcarbamoylase., Ornithine is regenerated and used in urea cycle., Therefore, its role is comparable to that of, oxaloacetate in citric acid cycle. Ornithine and, citrulline are basic amino acids. (They are never, found in protein structure due to lack of codons)., Citrulline produced in this reaction is transported, to cytosol by a transporter system., 3. Synthesis of arginosuccinate : Arginosuccinate synthase condenses citrulline with, aspartate to produce arginosuccinate. The, second amino group of urea is incorporated in, this reaction. This step requires ATP which is, cleaved to AMP and pyrophosphate (PPi). The, latter is immediately broken down to inorganic, phosphate (Pi).
Page 349 :
339, , Chapter 15 : METABOLISM OF AMINO ACIDS, , 4. Cleavage of arginosuccinate : Arginosuccinase cleaves arginosuccinate to give, arginine and fumarate. Arginine is the immediate, precursor for urea. Fumarate liberated here, provides a connecting link with TCA cycle,, gluconeogenesis etc., 5. Formation of urea : Arginase is the fifth, and final enzyme that cleaves arginine to yield, urea and ornithine. Ornithine, so regenerated,, enters mitochondria for its reuse in the urea, cycle. Arginase is activated by Co2+ and Mn2+., Ornithine and lysine compete with arginine, (competitive inhibition). Arginase is mostly found, in the liver, while the rest of the enzymes (four), of urea cycle are also present in other tissues., For this reason, arginine synthesis may occur to, varying degrees in many tissues. But only the, liver can ultimately produce urea., , Glutamate, , Acetyl CoA, , Acetate, NAG synthase, , CoA, , NAG hydrolase, , N-Acetylglutamate, , Fig. 15.11 : Formation and degradation of, N-acetylglutamate., , the synthesis of carbamoyl phosphate. The, remaining four enzymes of urea cycle are mostly, controlled by the concentration of their, respective substrates., , Disposal of urea, Overall reaction and energetics, The urea cycle is irreversible and consumes 4, ATP. Two ATP are utilized for the synthesis of, carbamoyl phosphate. One ATP is converted to, AMP and PPi to produce arginosuccinate which, equals to 2 ATP. Hence 4 ATP are actually, consumed., NH4+ + CO2 + Aspartate + 3ATP o Urea, + Fumarate + 2 ADP + 2 Pi + AMP + PPi, , Regulation of urea cycle, The first reaction catalysed by carbamoyl, phosphate synthase I (CPS I) is rate-limiting, reaction or committed step in urea synthesis. CPS, I is allosterically activated by N-acetylglutamate, (NAG). It is synthesized from glutamate and, acetyl CoA by synthase and degraded by a, hydrolase (Fig.15.11)., The rate of urea synthesis in liver is correlated, with the concentration of N-acetylglutamate., High concentrations of arginine increase NAG., The consumption of a protein-rich meal, increases the level of NAG in liver, leading to, enhanced urea synthesis., Carbamoyl phosphate synthase I and, glutamate dehydrogenase are localized in the, mitochondria. They coordinate with each other, in the formation of NH3, and its utilization for, , Urea produced in the liver freely diffuses and, is transported in blood to kidneys, and excreted., A small amount of urea enters the intestine, where it is broken down to CO2 and NH3 by the, bacterial enzyme urease. This ammonia is either, lost in the feces or absorbed into the blood. In, renal failure, the blood urea level is elevated, (uremia), resulting in diffusion of more urea into, intestine and its breakdown to NH3., Hyperammonemia (increased blood NH3) is, commonly seen in patients of kidney failure. For, these patients, oral administration of antibiotics, (neomycin) to kill intestinal bacteria is advised., , Integration between, urea cycle and TCA cycle, Urea cycle is linked with TCA cycle in three, different ways (Fig.15.12). This is regarded as, bicyclic integration between the two cycles., 1. The production of fumarate in urea cycle, is the most important integrating point with TCA, cycle. Fumarate is converted to malate and then, to oxaloacetate in TCA cycle. Oxaloacetate, undergoes transamination to produce aspartate, which enters urea cycle. Here, it combines with, citrulline to produce arginosuccinate. Oxaloacetate is an important metabolite which can, combine with acetyl CoA to form citrate and get
Page 350 :
340, , BIOCHEMISTRY, , +, , NH4 + CO2, , CO2, , Carbamoyl, phosphate, , Ornithine, , Citrulline, Oxaloacetate, Urea cycle, (–4ATP), , Urea, , CO2, TCA cycle, (+12 ATP), , Aspartate, Malate, Arginine, , Arginosuccinate, , Fumarate, , Fumarate, , Fig. 15.12 : Interrelation between urea and tricarboxylic acid (TCA) cycle (Depicted in blue colour)., , finally oxidized. Oxaloacetate can also serve as, a precursor for the synthesis of glucose, (gluconeogenesis)., 2. ATP (12) are generated in the TCA cycle, while ATP (4) are utilized for urea synthesis., 3. Citric acid cycle is an important metabolic, pathway for the complete oxidation of various, metabolites to CO2 and H2O. The CO2 liberated, in TCA cycle (in the mitochondria) can be, utilized in urea cycle., , Metabolic disorders of urea cycle, Metabolic defects associated with each of the, five enzymes of urea cycle have been reported, (Table 15.1). All the disorders invariably lead, to, a, build-up, in, blood, ammonia, (hyperammonemia), leading to toxicity. Other, metabolites of urea cycle also accumulate, which, however, depends on the specific, enzyme defect., The clinical symptoms, associated with defect in urea cycle enzymes, include vomiting, lethargy, irritability, ataxia and, mental retardation., , Blood urea—clinical importance, In healthy people, the normal blood urea, concentration is 10-40 mg/dl. Higher protein, , intake marginally increases blood urea level,, however this is well within normal range. About, 15-30 g of urea (7-15 g nitrogen) is excreted in, urine per day., Blood urea estimation is widely used as a, screening test for the evaluation of kidney (renal), function. It is estimated in the laboratory either, by urease method or diacetyl monoxime (DAM), procedure. Elevation in blood urea may be, broadly classified into three categories., 1. Pre-renal : This is associated with, increased protein breakdown, leading to a, negative nitrogen balance, as observed after, major surgery, prolonged fever, diabetic coma,, thyrotoxicosis etc. In leukemia and bleeding, disorders also, blood urea is elevated., , TABLE 15.1 Metabolic defects in urea cycle, , Defect, , Enzyme involved, , Hyperammonemia type I, , Carbamoyl phosphate synthase I, , Hyperammonemia type II, , Ornithine transcarbamoylase, , Citrullinemia, , Arginosuccinate synthase, , Arginosuccinic aciduria, , Arginosuccinase, , Hyperargininemia, , Arginase
Page 351 :
341, , Chapter 15 : METABOLISM OF AMINO ACIDS, , 2. Renal : In renal disorders like acute glomerulonephritis, chronic nephritis, nephrosclerosis,, polycystic kidney, blood urea is increased., 3. Post-renal : Whenever there is an obstruction in the urinary tract (e.g. tumors, stones,, enlargement of prostate gland etc.), blood urea is, elevated. This is due to increased reabsorption of, urea from the renal tubules., , TABLE 15.2 A summary of the specialized, products formed/contributed by amino acids, , Amino acid, , Specialized product(s), , Glycine, , Creatine, glutathione, heme,, purines, conjugated bile acids., , Tyrosine, , Thyroxine, triiodothyronine,, epinephrine, norepinephrine,, dopamine, melanin., , Tryptophan, , NAD+, NADP+ (coenzymes of, niacin), serotonin, melatonin., , Methionine, , Active methionine, creatine,, epinephrine, polyamines., , Cysteine, , Glutathione, taurine, coenzyme A,, active sulfate., , Non-protein nitrogen (NPN), , Histidine, , Histamine, , As is obvious from the name, the term NPN, refers to all the nitrogen-containing substances, other than proteins. These include urea (most, abundant), creatinine, creatine, uric acid,, peptides, amino acids etc. In healthy persons,, NPN concentration in blood is 20-40 mg/dl., , Arginine, , Creatine, nitric oxide, , Lysine, , Carnitine, , Glutamate, , a-Amino butyric acid, glutathione,, a-carboxyglutamate., , Glutamine, , Purines, pyrimidines, amino sugars., , Aspartate, , Purines, pyrimidines, , Serine, , Phosphatidylserine,, sphingomyelins, choline., , `-Alanine, , Coenzyme A, , The term ‘uremia’ is used to indicate, increased blood urea levels due to renal failure., Azotemia represents an elevation in blood urea/, or other nitrogen metabolites which may or may, not be associated with renal diseases., , The molecular weight of urea is 60 and about, half of it (28) is contributed by the two nitrogen, atoms. Thus, if blood urea concentration is 60, mg, then about half of it—28 mg—is blood urea, nitrogen (BUN). Therefore,, 1, 2, , BUN, , =, , NPN, , = 2 BUN, , NPN, , In some countries, estimations of BUN or, NPN are used rather than blood urea for, assessing kidney function. The normal range for, ratio of BUN to serum creatinine is 10:1 to 15:1., , METABOLISM OF, INDIVIDUAL AMINO ACIDS, In the preceding pages, the general aspects of, amino acid metabolism have been discussed. A, summary of the biologically important or, specialized products obtained from or, contributed by the amino acids is given in the, Table 15.2. The metabolism of individual amino, acids with special emphasis on the specialized, products is described next., , GLYCINE, Glycine (Gly, G) is a non-essential, optically, inactive and glycogenic (precursor for glucose), amino acid. It is indispensable for chicks. The, outline of glycine metabolism is depicted in, Fig.15.13. Glycine is actively involved in the, synthesis of many specialized products (heme,, purines, creatine etc.) in the body, besides its, incorporation into proteins, synthesis of serine, and glucose and participation in one-carbon, metabolism. Glycine is the most abundant amino, acid normally excreted into urine (0.5–1.0 g/g, creatinine)., , Glycine in proteins, Glycine is one among the commonest amino, acids found in protein structure. Being small and, non-polar, glycine is mostly present in the, interior structure of protein. Collagen contains, very high (about 30%) content of glycine.
Page 354 :
344, , BIOCHEMISTRY, +, , NH2, H2N C NH, (CH2)3, , +, , +, , H3N CH2 COO–, , H C NH3, –, , Glycine, , COO, Arginine, , Urea, cycle, , Arginine-glycine, transamidinase, , Ornithine, +, , NH2, H2 N C, , N CH2 COO–, H, , Guanidoacetate, S-Adenosylmethionine ( CH3), , Guanidoacetate, methyltransferase, , S-Adenosylhomocysteine, , +, , NH2, H2 N C, H2O, , H, , O, , N, HN C, N, , Creatine, , ATP, Creatine kinase, , ADP, CH2, , CH3, Pi, Urine, , NH, H 2N C, , Creatinine, , The amount of creatinine excreted is proportional to total creatine phosphate content of the, body and, in turn, the muscle mass. The daily, excretion of creatinine is usually constant., Creatinine coefficient is defined as the mg of, creatinine and creatine (put together) excreted, per kg body weight per day. For a normal adult, man, the value is 24-26 mg, while for a woman,, it is 20-22 mg., Increased output of creatine in urine is, referred to as creatinuria. Creatinuria is observed, in muscular dystrophy, diabetes mellitus,, hyperthyroidism, starvation etc., , Metabolic disorders of glycine, , N CH2 COO–, CH3, , C, , Estimation of serum creatinine (along with, blood urea) is used as a diagnostic test to assess, kidney function. Serum creatinine concentration, is not influenced by endogenous and exogenous, factors, as is the case with urea. Hence, some, workers consider serum creatinine as a more, reliable indicator of renal function., , P, , N CH2 COO–, CH3, , Creatine phosphate, , Fig. 15.16 : Metabolism of creatine., , Creatine and creatinine—clinical importance :, The normal concentrations of creatine and creatinine in human serum and urine are as follows, Serum, Creatine — 0.2–0.6 mg/dl, Creatinine — 0.6–1 mg/dl, Urine, Creatine — 0–50 mg/day, Creatinine — 1–2 g/day, , 1. Glycinuria : This is a rare disorder. Serum, glycine concentration is normal, but very high, amount of it (normal 0.5-1 g/day) is excreted in, urine. It is believed that glycinuria is due to a, defective renal reabsorption. Glycinuria is, characterized by increased tendency for the, formation of oxalate renal stones. However,, urinary oxalate level is normal in these patients., 2. Primary hyperoxaluria : This disorder is, characterized by increased urinary oxalate, resulting in oxalate stones. Deposition of oxalate, (oxalosis) in various tissues is observed. The, urinary oxalate is of endogenous origin and not, due to dietary consumption of oxalate. Primary, hyperoxaluria is due to a defect in glycine, transaminase coupled with impairment in, glyoxalate oxidation to formate., It is now known that primary hyperoxaluria is, mainly due to a defect in protein targeting (i.e., defect in transport of protein from one, compartment to another). As a result, the, enzyme glycine transaminase is found in, mitochondria instead of its normal distribution in, peroxisomes.
Page 355 :
345, , Chapter 15 : METABOLISM OF AMINO ACIDS, , In vitamin B6 deficiency, urinary oxalate is, elevated which can be corrected by B6 supplementation. However, B6 administration has no, effect on endogenous hyperoxaluria., , PHENYLALANINE AND TYROSINE, Phenylalanine (Phe, F) and tyrosine (Tyr, Y), are structurally related aromatic amino acids., Phenylalanine is an essential amino acid while, tyrosine, is, non-essential., Besides, its, incorporation into proteins, the only function of, phenylalanine is its conversion to tyrosine. For, this reason, ingestion of tyrosine can reduce the, dietary requirement of phenylalanine. This, phenomenon is referred to as ‘sparing action’ of, tyrosine on phenylalanine., The predominant metabolism of phenylalanine occurs through tyrosine. Tyrosine is, incorporated into proteins and is involved in the, synthesis of a variety of biologically important, , compounds—epinephrine,, norepinephrine,, dopamine (catecholamines), thyroid hormones—, and the pigment melanin (Fig.15.17). During the, course of degradation, phenylalanine and, tyrosine are converted to metabolites which can, serve as precursors for the synthesis of glucose, and fat. Hence, these amino acids are both, glucogenic and ketogenic. Biochemists attach, special significance to phenylalanine and, tyrosine metabolism for two reasons—synthesis, of biologically important compounds and the, metabolic disorders due to enzyme defects., , Conversion of phenylalanine, to tyrosine, Under normal circumstances, the degradation, of phenylalanine mostly occurs through tyrosine., Phenylalanine is hydroxylated at para-position, by phenylalanine hydroxylase to produce, tyrosine (p-hydroxy phenylalanine). This is an, irreversible, reaction, and, requires, the, participation of a specific coenzyme biopterin, , + About 300–400 g of protein per day is constantly degraded and synthesized in the, human body., , + The amino acids are mainly utilized for protein biosynthesis, production of specialized, products (creatine, porphyrin, amines, purines, pyrimidines) and generation of energy., , + Glutamate is the collection centre for the amino groups in the biological system while, glutamine is the storehouse of NH3. Free NH3 can be liberated predominantly from, glutamate., , + Ammonia accumulation in blood is toxic to brain causing slurring of speech, blurring, of vision, tremors and even death. Mammals convert NH3 to urea, a non-toxic excretory, product. Metabolic defects in urea cycle enzymes result in hyperammonemia., , + Dietary consumption of a protein rich meal increases the level of N-acetylglutamate in, liver which enhances urea production., , + Primary hyperoxaluria—a metabolic disorder due to a defect in the enzyme glycine transaminase—is characterized by elevated urinary oxalate and the formation of oxalate stones., , + Blood urea estimation is commonly used to assess renal function. Elevation of blood, urea level (normal 10–40 mg/dl) is associated with several disorders which may be prerenal (diabetic coma), renal (acute glomerulonephritis) and post-renal (tumors or stones, in the urinary tract)., , + Estimation of serum creatinine (normal < 1 mg/dl) is considered to be a more reliable, indicator for the evaluation of kidney function.
Page 360 :
350, , BIOCHEMISTRY, , different enzyme systems exist to convert, tyrosine to DOPA., , CH2 CH COO–, , HO, , +, , NH3, , DOPA undergoes PLP-dependent decarboxylation to give dopamine which, in turn, is, hydroxylated to produce norepinephrine., Methylation of norepinephrine by S-adenosylmethionine gives epinephrine. The difference, between epinephrine and norepinephrine is only, a methyl group (remember that norepinephrine, has no methyl group)., , Tyrosine, O2, H4-Biopterin, , Tyrosine, hydroxylase, , H2-Biopterin, H2O, , HO, , CH2 CH COO–, , HO, , +, NH3, , Dihydroxyphenylalanine (DOPA), , PLP Aromatic amino acid, CO2, , decarboxylase, , HO, HO, , CH2 CH2, , +, NH3, , Dopamine, O2, Ascorbate, Dehydroascorbate, , Dopamine, E-hydroxylase, , H2O, , +, , CH CH2 NH3, OH, Norepinephrine, , S-Adenosylmethionine (, , CH3), , S-Adenosylhomocysteine, , Phenylethanolamine, N-methyltransferase, , HO, HO, , Functions of catecholamines : Norepinephrine and epinephrine regulate carbohydrate, and lipid metabolisms. They stimulate the, degradation of triacylglycerol and glycogen., They cause an increase in the blood pressure., Dopamine and norepinephrine serve as, neurotransmitters in the brain and autonomous, nervous system., , Dopamine and Parkinson’s disease, , HO, HO, , There exists tissue specificity in the formation, of catecholamines. In adrenal medulla, synthesis, of, the, hormones,, norepinephrine, and, epinephrine is prominent. Norepinephrine is, produced in certain areas of the brain while, dopamine is predominantly synthesized in, substantia nigra and coeruleus of brain., , CH CH2 N CH3, OH, , H, , Epinephrine, , Fig. 15.22 : Metabolism of tyrosine-synthesis of, catecholamines (dopamine, norepinephrine,, epinephrine; PLP–pyridoxal phosphate)., , Parkinson’s disease is a common disorder in, many elderly people, with about 1% of the, population above 60 years being affected. It is, characterized by muscular rigidity, tremors,, expressionless face, lethargy, involuntary, movements etc., Biochemical basis : The exact biochemical, cause of this disorder has not been identified., Parkinson’s disease is, however, linked with a, decreased production of dopamine. The disease, is due to degeneration of certain parts of the, brain (substantia nigra and locus coeruleus),, leading to the impairment in the synthesis of, dopamine., Treatment : Dopamine cannot enter the brain,, hence its administration is of no use. DOPA, (levodopa or L-dopa) is used in the treatment of, Parkinson’s disease. In the brain, DOPA is, decarboxylated to dopamine which alleviates the
Page 361 :
351, , Chapter 15 : METABOLISM OF AMINO ACIDS, , symptoms of this disorder. Unfortunately,, dopamine synthesis occurs in various other, tissues and results in side-effects such as nausea,, vomiting, hypretension etc. Administration of, dopa analogs—that inhibit dopa decarboxylase, (in various tissues) but not enter brain (due to, blood-brain barrier)—are found to be effective., Carbidopa and J-methyl-dopa (dopa analogs) are, administered along with dopa for the treatment, of Parkinson’s disease., , Phenylalanine, hydroxylase, Phenylalanine, Transaminase, Phenylpyruvate, +, , NADH + H, , Phenylalanine metabolism in PKU :, Phenylketonuria primarily causes the accumulation of phenylalanine in tissues and blood, and, results in its increased excretion in urine. Due to, disturbances in the routine metabolism, phenylalanine is diverted to alternate pathways, (Fig.15.23), resulting in the excessive production, of phenylpyruvate, phenylacetate, phenyllactate, and phenylglutamine. All these metabolites are, excreted in urine in high concentration in PKU., Phenylacetate gives the urine a mousey odour., The name phenylketonuria is coined due to, the fact that the metabolite phenylpyruvate is a, keto acid (C6H5CH2 CO COO–) excreted in, urine in high amounts., , +, , CO2, , Phenylacetate, , NAD+, Phenyllactate, , Glutamine, H 2O, , Several enzyme defects in phenylalanine/, tyrosine degradation leading to metabolic, disorders are known. In Fig.15.19, the deficient, enzymes and the respective inborn errors are, depicted and they are discussed here under., , Phenylketonuria (PKU) is the most common, metabolic disorder in amino acid metabolism., The incidence of PKU is 1 in 10,000 births. It is, due to the deficiency of the hepatic enzyme,, phenylalanine hydroxylase, caused by an, autosomal recessive gene. In recent years, a, variant of PKU—due to a defect in, dihydrobiopterin reductase (relatively less)—has, been reported. This enzyme deficiency impairs, the synthesis of tetrahydrobiopterin required for, the action of phenylalanine hydroxylase (See, Fig.15.18). The net outcome in PKU is that, phenylalanine is not converted to tyrosine., , +, NADH + H, , NAD, , DISORDERS OF TYROSINE, (PHENYLALANINE) METABOLISM, , Phenylketonuria, , Tyrosine, , Phenylacetylglutamine, , Fig. 15.23 : Metabolites that accumulate in, phenylketonuria ( –Block in phenylketonuria)., , Clinical/biochemical manifestations of PKU :, The disturbed metabolism of phenylalanine—, resulting in the increased concentration of, phenylalanine and its metabolites in the body—, causes many clinical and biochemical, manisfestations., 1. Effects on central nervous system : Mental, retardation, failure to walk or talk, failure of, growth, seizures and tremor are the characteristic, findings in PKU. If untreated, the patients show, very low IQ (below 50). The biochemical basis, of mental retardation in PKU is not well, understood. There are, however, many, explanations offered, l, , l, , l, , Accumulation of phenylalanine in brain, impairs the transport and metabolism of other, aromatic amino acids (tryptophan and, tyrosine)., The synthesis of serotonin (an excitatory, neurotransmitter), from, tryptophan, is, insufficient. This is due to the competition of, phenylalanine and its metabolites with, tryptophan that impairs the synthesis of, serotonin., Defect in myelin formation is observed in PKU, patients., , 2. Effect on pigmentation : Melanin is the, pigment synthesized from tyrosine by tyrosinase.
Page 362 :
352, Accumulation of phenylalanine competitively, inhibits tyrosinase and impairs melanin, formation. The result is hypopigmentation that, causes light skin colour, fair hair, blue eyes etc., Diagnosis of PKU : PKU is mostly detected by, screening the newborn babies for the increased, plasma levels of phenylalanine (PKU, 20–65 mg/, dl; normal 1–2mg/dl). This is usually carried out, by Guthrie test, which is a bacterial (Bacillus, subtilis) bioassay for phenylalanine. The test is, usually performed after the baby is fed with, breast milk for a couple of days by testing, elevated levels of phenylalanine. Phenylpyruvate, in urine can be detected by ferric chloride test, (a green colour is obtained). This test is not, specific, since many other compounds give a, false positive test. Prenatal diagnosis of PKU can, also be done by using cultured amniotic cells., Treatment of PKU : The maintenance of, plasma phenylalanine concentration within the, normal range is a challenging task in the, treatment of PKU. This is done by selecting foods, with low phenylalanine content and/or feeding, synthetic amino acid preparations, low in, phenylalanine. Dietary intake of phenylalanine, should be adjusted by measuring plasma levels., Early diagnosis (in the first couple of months of, baby’s life) and treatment for 4–5 years can, prevent the damage to brain. However, the, restriction to protein diet should be continued, for many more years in life. Since the amino, acid tyrosine cannot be synthesized in PKU, patients, it becomes essential and should be, provided in the diet in sufficient quantity., In some seriously affected PKU patients,, treatment includes administration of 5-hydroxytryptophan and dopa to restore the synthesis, of serotonin and catecholamines. PKU patients, with tetrahydrobiopterin deficiency require, tetrahydrobiopterin supplementation., , Tyrosinemia type II, This disorder—also known as RichnerHanhart syndrome, is due to a defect in the, enzyme tyrosine transaminase. The result is a, blockade in the routine degradative pathway of, tyrosine. Accumulation and excretion of tyrosine, and its metabolites—namely p-hydroxyphenyl-, , BIOCHEMISTRY, , pyruvate, p-hydroxyphenyllactate, phydroxyphenylacetate, N-acetyltyrosine—and tyramine, are observed., Tyrosinemia type II is characterized by skin, (dermatitis) and eye lesions and, rarely, mental, retardation. A disturbed self-coordination is seen, in these patients., , Neonatal tyrosinemia, The absence of the enzyme p-hydroxyphenylpyruvate, dioxygenase, causes, neonatal, tyrosinemia. This is mostly a temporary condition, and usually responds to ascorbic acid. It is, explained that the substrate inhibition of the, enzyme is overcome by the presence of ascorbic, acid., , Alkaptonuria (Black urine disease), Alkaptonuria has great historical importance., It was first described by Lusitanus in 1649 and, characterized in 1859. Garrod conceived the, idea of inborn errors of metabolism from his, observation on alkaptonuria. The prevalance of, this autosomal recessive disorder is 1 in 25,000., Enzyme defect : The defective enzyme in, alkaptonuria is homogentisate oxidase in tyrosine, metabolism (See Fig.15.19). Homogentisate, accumulates in tissues and blood, and is excreted, into urine. Homogentisate, on standing, gets, oxidized to the corresponding quinones, which, polymerize to give black or brown colour. For, this reason, the urine of alkaptonuric patients, resembles coke in colour., Biochemical manifestations : Homogentisate, gets oxidized by polyphenol oxidase to benzoquinone acetate which undergoes polymerization to produce a pigment called alkapton, (Fig.15.24). Alkapton deposition occurs in, connective tissue, bones and various organs, (nose, ear etc.) resulting in a condition known as, ochronosis. Many alkaptonuric patients suffer, from arthritis and this is believed to be due to, the deposition of pigment alkapton (in the joints),, produced from homogentisate., Diagnosis : Change in colour of the urine on, standing to brown or dark has been the simple, traditional method to identify alkaptonuria.
Page 363 :
353, , Chapter 15 : METABOLISM OF AMINO ACIDS, , Homogentisate, Polyphenol oxidase, Benzoquinone acetate, Polymerization, Alkapton, , Binds to tissues, , About 150 genes have been identified in mice., The melanin synthesis can be influenced by a, variety of factors. Many possible causes (rather, explanations) for albinism have been identified, 1. Deficiency or lack of the enzyme tyrosinase., 2. Decrease in melanosomes of melanocytes., 3. Impairment in melanin polymerization., 4. Lack of protein matrix in melanosomes., , Fig. 15.24 : Conversion of homogentisate to alkapton., , 5. Limitation of substrate (tyrosine) availability., The urine gives a positive test with ferric chloride, and silver nitrate. This is due to the strong, reducing activity of homogentisate. Benedict’s, test—employed for the detection of glucose and, other reducing sugars—is also positive with, homogentisate., Treatment : Alkaptonuria is not a dangerous, disorder and, therefore, does not require any, specific treatment. However, consumption of, protein diet with relatively low phenylalanine, content is recommended., , Tyrosinosis or tyrosinemia type I, This is due to the deficiency of the enzymes, fumarylacetoacetate hydroxylase and/or maleylacetoacetate isomerase. Tyrosinosis is a rare but, serious disorder. It causes liver failure, rickets,, renal tubular dysfunction and polyneuropathy., Tyrosine, its metabolites and many other amino, acids are excreted in urine., In acute tyrosinosis, the infant exhibits, diarrhea, vomiting, and ‘cabbage-like’ odor., Death may even occur due to liver failure within, one year. For the treatment, diets low in, tyrosine, phenylalanine and methionine are, recommended., , Albinism, Albinism (Greek: albino—white) is an inborn, error, due to the lack of synthesis of the pigment, melanin. It is an autosomal recessive disorder, with a frequency of 1 in 20,000., Biochemical basis : The colour of skin and, hair is controlled by a large number of genes., , 6. Presence of inhibitors of tyrosinase., The most common cause of albinism is a, defect in tyrosinase, the enzyme most responsible, for the synthesis of melanin (See Fig.15.20)., Clinical manifestations : The most important, function of melanin is the protection of the body, from sun radiation. Lack of melanin in albinos, makes them sensitive to sunlight. Increased, susceptibility to skin cancer (carcinoma) is, observed. Photophobia (intolerance to light) is, associated with lack of pigment in the eyes., However, there is no impairment in the eyesight, of albinos., , Hypopigmentation, In some individuals, a reduced synthesis of, melanin (instead of total lack) is often observed., Hypopigmentation disorders may be either, diffuse or localized., A good example of diffuse hypopigmentation, is oculocutaneous albinism which is mostly due, to mutations in the tyrosinase gene. The degree, of hypopigmentation depends on the type and, severity of mutated genes., , Vitiligo and leukoderma are the important, among the localized hypopigmentation disorders., Vitiligo is an acquired progressive disease with, loss of pigmentation around mouth, nose, eyes, and nipples. Leukoderma is comparable with, vitiligo, but lack of pigmentation usually begins, with hands and then spreads., Greying of hair is due to lack of melanin, synthesis which usually occurs as a result of, disappearance of melanocytes from the hair roots.
Page 366 :
356, NAD+ Pathway : Tryptophan is not a, precursor for the synthesis of free niacin., Quinolinate undergoes decarboxylation and is, converted to nicotinate mononucleotide by the, enzyme quinolinate phosphoribosyl transferase, (QPRT). The synthesis of NAD+ and NADP+ from, nicotinate mononucleotide is similar to that from, niacin as described in vitamins (Refer Fig.7.21)., Conversion of tryptophan to indole acetate :, Tryptophan, undergoes, deamination, and, decarboxylation to produce indolepyruvate and, tryptamine, respectively. Both these compounds, are converted to indoleacetate (Fig.15.27) and, excreted in urine., , II. Serotonin pathway, Serotonin or 5-hydroxytryptamine (5HT) is a, neurotransmitter, synthesized from tryptophan., Normally, about 1% of the tryptophan is, converted to serotonin. The production of 5HT, occurs in the target tissues., Synthesis of serotonin : In mammals, the, largest amount of serotonin is synthesized in the, intestinal cells. The formation of serotonin is, comparable, with, the, production, of, catecholamines. Tryptophan is first hydroxylated, at 5th carbon by tryptophan hydroxylase. This, enzyme requires tetrahydrobiopterin as a, cofactor. 5-Hydroxytryptophan is decarboxylated, by aromatic amino acid decarboxylase (PLPdependent) to give serotonin (Fig.15.27)., , Platelets contain high concentration of 5HT,, the significance of which is not clear. As, such, platelets cannot synthesize serotonin., Degradation of serotonin : Monoamine, oxidase (MAO) degrades serotonin to 5hydroxyindoleacetate (5HIA) which is excreted, in urine., Functions of serotonin : Serotonin is a neurotransmitter and performs a variety of functions., 1. Serotonin is a powerful vasoconstrictor, and results in smooth muscle contraction in, bronchioles and arterioles., 2. It is closely involved in the regulation of, cerebral activity (excitation)., , BIOCHEMISTRY, , 3. Serotonin controls the behavioural, patterns, sleep, blood pressure and body, temperature., 4. Serotonin evokes the release of peptide, hormones from gastrointestinal tract., 5. It is also necessary for the motility of GIT, (peristalsis)., Serotonin and brain : The brain itself, synthesizes 5HT which is in a bound form. The, outside serotonin cannot enter the brain due to, blood-brain barrier. Primarily, serotonin is a, stimulator (excitation) of brain activity, hence, its deficiency causes depression. Serotonin level, is decreased in psychosis patients., , Defects in monoamine oxidase gene (lowered, MAO activity) are linked to violent behaviour, and slight mental retardation., Effect of drugs on serotonin : The drug,, iproniazid (isopropyl isonicotinyl hydrazine), inhibits MAO and elevates serotonin levels,, therefore, this drug is a psychic stimulant. On, the other hand, reserpine increases the, degradation of serotonin, hence acts as a, depressant drug. Lysergic acid diethylamide, (LSD) competes with serotonin and, therefore,, acts as a depressant., Malignant carcinoid syndrome : Serotonin is, produced by argentaffin cells of gastrointestinal, tract. When these cells undergo uncontrolled, growth, they develop into a tumor called, malignant carcinoid or argentaffinomas. The, patients exhibit symptoms like respiratory, distress, sweating, hypertension etc., Normally about 1% of the tryptophan is, utilized for serotonin synthesis. In case of, carcinoid syndrome, very high amount (up to, 60%) of tryptophan is diverted for serotonin, production. This disturbs the normal tryptophan, metabolism and impairs the synthesis of NAD+, and NADP+. Hence, the patients of carcinoid, syndrome develop symptoms of pellagra (niacin, deficiency). Further, negative nitrogen balance, is also observed.
Page 368 :
358, , BIOCHEMISTRY, , Diagnosis : The excretion of 5-hydroxy indole, acetate in urine is tremendously elevated (upto, 500mg/day against normal <5 mg/day) in, carcinoid syndrome. The estimation of 5 HIA in, urine is used for the diagnosis of this disorder. In, general, urine concentration of 5 HIA above 25, mg/day should be viewed with caution as it may, be suggestive of carcinoid syndrome. Sufficient, precaution should, however, be taken for sample, collection. During the course of urine collection,, the patients should not ingest certain foods, (banana, tomato etc.) that increase urine 5 HIA., , Melatonin, Melatonin is a hormone, mostly synthesized, by the pineal gland. Serotonin—produced from, tryptophan—is acted upon by serotonin, N-acetylase (the rate limiting enzyme), to give, N-acetylserotonin., The, latter, undergoes, methylation, S-adenosylmethionine being the, methyl group donor to produce melatonin or, N-acetyl 5-methoxyserotonin (Fig.15.27). The, synthesis and secretion of melatonin from pineal, gland is controlled by light., , Functions of melatonin, 1. Melatonin is involved in circadian, rhythms or diurnal variations (24 hr cyclic, process) of the body. It plays a significant role in, sleep and wake process., 2. Melatonin inhibits the production of, melanocyte stimulating hormone (MSH) and, adrenocorticotropic hormone (ACTH)., 3. It has some inhibitory effect on ovarian, functions., 4. Melatonin also, transmitter function., , performs, , a, , neuro-, , Hartnup’s disease, This disorder was first described in the family, of Hartnup, hence the name—Hartnup’s disease., It is a hereditary disorder of tryptophan, metabolism. The clinical symptoms include, dermatitis, ataxia, mental retardation etc., Hartnup’s disease is characterized by low plasma, levels of tryptophan and other neutral amino, , acids and their elevated urinary excretion., Increased urinary output of indoleacetic acid and, indolepyruvic acid is also observed., Pellagra-like symptoms are common in these, patients. There is an impairment in the synthesis, of NAD+ and serotonin from tryptophan. Some, authors (earlier) attributed Hartnup’s disease to a, defect in the enzyme tryptophan pyrrolase. This,, however, does not appear to be true. Hartnup’s, disease is now believed to be due to an, impairment in the absorption and/or transport, of tryptophan and other neutral amino acids, from the intestine, renal tubules and, probably, brain. Some more details on Hartnup’s disease, are given under digestion and absorption, (Chapter 8)., , SULFUR AMINO ACIDS, The sulfur-containing amino acids are methionine, cysteine and cystine. Among these, only, methionine is essential. It serves as a precursor, for the synthesis of cysteine and cystine which, are, therefore, non-essential. Cysteine can spare, the requirement of methionine in the diet., Cysteine and cystine are interconvertible. Cystine, is found exclusively in proteins. Methionine and, cysteine, besides being present in proteins, are, involved in many important metabolic reactions, (Fig.15.28). Methionine is also required for the, initiation of protein biosynthesis. The sulfurcontaining amino acids are almost an exclusive, dietary source of sulfur to the body., , METABOLISM OF METHIONINE, Methionine, (or, sulfur, amino, acids), metabolism may be divided into three parts., 1. Utilization of methionine for transmethylation reactions., 2. Conversion of methionine to cysteine and, cystine., 3. Degradation of cysteine and its conversion, to specialized products.
Page 373 :
363, , Chapter 15 : METABOLISM OF AMINO ACIDS, , ONE-CARBON METABOLISM, Amino acid metabolism is particularly, important for the transfer or exchange of onecarbon units. The following one-carbon, fragments are encountered in the biological, reactions, which constitute one-carbon pool, Methyl, Hydroxymethyl, Methylene, Methenyl, Formyl, Formimino, , (, (, (, (, (, (, , CH3), CH2OH), CH2), CH ), CH O), CH NH), , [Note : It may be stated here that CO2 is also, a one-carbon unit. Carbon dioxide is involved, (carboxylation) in many biochemical reactions,, which are dependent on biotin. For instance,, conversion of pyruvate to oxaloacetate in, gluconeogenesis. Most of the authors, however,, ignore CO2 as one-carbon unit and do not even, consider it worth mentioning. This would be, unfair to CO2!], Tetrahydrofolate (THF) is a versatile, coenzyme that actively participates in onecarbon metabolism. With regard to the transfer, of methyl groups from S-adenosylmethionine,, vitamin B12 is also involved besides THF., The one-carbon unit covalently binds with, THF at position N5 or N10 or on both N5 and, N10 of pteroyl structure of folate. The details of, different one-carbon units binding with THF and, the structures of THF derivatives are given under, vitamin-folic acid (Chapter 7)., The one-carbon metabolism is rather, complex, involving many reactions. For the sake, of better understanding, it is divided into, generation and utilization of one-carbon units,, and the role of methionine and vitamin B12., , 2. Histidine contributes formimino fragment, to produce N5-formimino THF., 3. When serine is converted to glycine, N5,, THF is formed. This is the most, predominant entry of one carbon units into one, carbon pool., N10-methylene, , 4. Choline and betaine contribute to the, formation of N5-methyl THF., The different derivatives of THF carrying onecarbon units are interconvertible, and this is, metabolically significant for the continuity of, one- carbon pool (Fig.15.32)., , II. Utilization of one-carbon, moieties, One-carbon fragments from THF are used for, the synthesis of a wide variety of compounds., These include purines, formylmethionine tRNA, (required for initiation of protein synthesis),, glycine, pyrimidine nucleotide (thymidylate) etc., , III. Role of methionine and B12, in one-carbon metabolism, Methyl ( CH3) group is an important onecarbon unit. The role of active methionine as, methyl donor in transmethylation reactions is, already described. After the release of methyl, group, methionine is converted to homocysteine., For the regeneration of methionine, free, homocysteine and N5-methyl THF are required, and, this, reaction, is, dependent, on, methylcobalamin (vitamin B12). The one-carbon, pool, under the control of THF, is linked with, methionine, metabolism, (transmethylation), through vitamin B12. Hence vitamin B12 is also, involved in one-carbon metabolism., , BRANCHED CHAIN AMINO ACIDS, , I. Generation of one-carbon units, Many compounds (particularly amino acids), act as donors of one-carbon fragments, 1. The formate released from glycine and, tryptophan metabolism combines with THF to, form N10-formyl THF., , Valine, leucine and isoleucine are the, branched chain and essential amino acids. These, three amino acids initially undergo a common, pathway and then diverge to result in different, end products. Based on the products obtained, from the carbon skeleton, the branched
Page 375 :
365, , Chapter 15 : METABOLISM OF AMINO ACIDS, , to, propionyl, CoA,, a, precursor, for, glucose., Leucine produces acetyl, CoA and acetoacetate, the, substrates for fatty acid, synthesis., Isoleucine, is, degraded to propionyl CoA, and acetyl CoA. Thus, valine, is glycogenic and leucine is, ketogenic while isoleucine is, both, glycogenic, and, ketogenic., , Valine, leucine, isoleucine, Branched chain, amino acid, transaminase, Corresponding D-keto acids, (D-ketoisovalerate, D-ketoisocaproate,, D-keto E-methyl valerate, , +, , TPP, NAD , CoASH, CO2, , Corresponding D, E-unsaturated acyl CoA, thioesters (isobutyryl CoA, isovaleryl CoA,, D-methylbutyryl CoA), , Metabolic defects, of branched chain, amino acids, 1. Maple syrup urine, disease (MSUD) : This is a, metabolic, disorder, of, branched chain amino acids., The urine of the affected, individuals smells like maple, syrup or burnt sugar—hence, the name., , D-Keto acid, dehydrogenase, complex, , FAD, FADH2, , Methylacrylyl CoA, , Propionyl CoA, , Acyl CoA dehydrogenase, (2 enzymes?), , E-Methylcrotonyl CoA, , HMG CoA, , Triglyl CoA, , Methylacetoacetyl CoA, , Acetyl CoA, Methylmalonyl CoA, , Acetoacetate, , Acetyl CoA, Propionyl CoA, , Enzyme defect : Maple, Glucose, Fat, Glucose, Fat, syrup urine disease is due to, a defect in the enzyme, Fig. 15.33 : Summary of branched chain amino acid metabolism ( –Defect in, D-keto acid dehydrogenase results in maple syrup urine disease; HMG CoA–, branched chain D-keto acid, E-Hydroxy E-methyl-glutaryl CoA; Valine is glycogenic, leucine is ketogenic,, dehydrogenase. This causes, while isoleucine is both)., a blockade in the conversion, of D-keto acids to the, respective acyl CoA thioesters. The plasma and, Diagnosis and treatment : An early diagnosis, urine concentrations of branched amino acids by enzyme analysis—preferably within the first, and their keto acids are highly elevated. This week of life—is ideal. Estimation of urinary, disease is also known as branched chain branched amino acids and keto acids will also, ketonuria., help in diagnosis., Biochemical complications and symptoms :, l, , l, , l, , l, , Accumulation of branched chain amino acids, causes an impairment in transport and, function of other amino acids., Protein biosynthesis is reduced., Branched chain amino acids competitively, inhibit glutamate dehydrogenase., The disease results in acidosis, lethargy,, convulsions, mental retardation, coma and,, finally, death within one year after birth., , The treatment is to feed a diet with low (or, no) content of branched amino acids. Mild, variants of MSUD respond to high doses of, thiamine. In severe cases of MSUD, liver, transplantation is required., 2. Intermittent branched chain ketonuria :, This is a less severe variant form of maple syrup, urine disease. The enzyme defect is the same—, D-keto acid dehydrogenase. As such, there is an, impairment and no total blockade in the, conversion of D-keto acids to their respective
Page 376 :
366, acyl CoA thioesters. The symptoms are not as, severe as in maple syrup urine disease. Careful, diet planning is adequate to ovecome this, disorder., 3. Isovaleric acidemia : This is a specific, inborn error of leucine metabolism. It is due to, a defect in the enzyme isovaleryl CoA, dehydrogenase. The conversion of isovaleryl, CoA to methylcrotonyl CoA is impaired. The, excretion of isovalerate is high in urine. The, affected individuals exhibit a ‘cheesy’ odor in, the breath and body fluids. The symptoms, include acidosis and mild mental retardation., 4. Hypervalinemia : This inborn error is, characterized by increased plasma concentration, of valine while leucine and isoleucine levels, remain normal. The transamination of valine, alone is selectively impaired., , HISTIDINE, PROLINE AND ARGININE, The metabolism of histidine, proline and, arginine is considered together, as all the three, are converted to glutamate and metabolized, (Fig.15.34)., , Histidine, The metabolism of histidine is important for, the generation of one-carbon unit, namely, formimino group. The enzyme histidase acts on, histidine to split off ammonia. Urocanate formed, in this reaction is acted upon by urocanase to, produce 4-imidazole 5-propionate. Imidazole, ring of the product is cleaved by a hydrolase to, give N-formiminoglutamate (FIGLU). Tetrahydrofolate (THF) takes up the formimino group, to form N5-formimino THF, and glutamate is, liberated. Deficiency of folate blocks this, reaction and causes elevated excretion of FIGLU, in urine. Histidine loading test is commonly, employed to assess folate deficiency., Histidine, on decarboxylation, gives the, corresponding amine—histamine. Histamine, regulates HCl secretion by gastric mucosa, (Table 15.7). Excessive production of histamine, causes asthma and allergic reactions., , BIOCHEMISTRY, , Histidinemia : The frequency of histidinemia is, 1 in 20,000. It is due to a defect in the enzyme, histidase. Histidinemia is characterized by elevated, plasma histidine levels and increased excretion of, imidazole pyruvate and histidine in urine. Most of, the patients of histidinemia are mentally retarded, and have defect in speech. No treatment will, improve the condition of the patients., , Proline, Proline is oxidized to pyrroline 5-carboxylate, which undergoes a non-enzymatic conversion to, glutamate 5-semialdehyde. The latter is, converted to glutamate and then transaminated, to D-ketoglutarate. The five carbons of proline, are converted to D-ketoglutarate., Hyperprolinemia type I : It is due to a defect, in the enzyme proline oxidase (proline, dehydrogenase)., Another metabolic disorder—hyperprolinemia, type II—associated with hydroxyproline metabolism is also reported., , Arginine, Arginine is cleaved by arginase to liberate, urea and produce ornithine. Ornithine undergoes, transamination of G-amino group to form, glutamate J-semialdehyde which is converted to, glutamate. Hyperargininemia is an inborn error, in arginine metabolism due to a defect in the, enzyme arginase., Nitric oxide (NO) : Arginine is the substrate, for the production of nitric oxide (NO), a wonder, molecule with a wide range of functions. The, enzyme nitric oxide synthase (three isoenzymes, known) cleaves the nitrogen from the guanidino, group of arginine to form NO. This reaction, requires NADPH, FMN, FAD, heme and, tetrahydrobiopterin. NO has a very short half-life, (about 5 seconds)., +, , NH 2, , NH 2, , C O, , C NH2, NO synthase, , HN, (CH2)3, H C, , +, NH3, –, , COO, , Arginine, , O2, , NO, , HN, (CH2)3, +, , H C NH3, COO–, Citrulline
Page 378 :
368, , BIOCHEMISTRY, , (EDRF) which causes smooth muscle relaxation, is none other than a gas, NO., Nitric oxide acts as a mediator for several, biological functions., 1. NO functions as a vasodilator and causes, relaxation of smooth muscles., 2. It is a key molecule in the regulation of, blood flow and the blood pressure (inhibitors of, NO synthesis markedly raise blood pressure)., 3. NO acts as an inhibitor of platelet, aggregation and adhesion., 4. It functions as a messenger molecule of, the nervous system (neurotransmitter)., 5. NO mediates the bactericidal actions of, macrophages., 6. It is involved in the erection of penis., , Lysine, D-Ketoglutarate, , Saccharopine, dehydrogenase, , Saccharopine, Glutamate, , Saccharopine, dehydrogenase, , D-Aminoadipate, G-semialdehyde, Semialdehyde, dehydrogenase, D-Aminoadipate, Aminotransferase, D-Ketoadipate, Dehydrogenase, , Mechanism of action : Nitric oxide promotes, the synthesis of cGMP. It is believed that some, of the actions of NO are mediated through, cGMP and protein kinase G., , Glutaryl CoA, , Agmatine : It is a derivative of arginine, produced in the brain. Agmatine possesses, antihypertensive properties., , Crotonyl CoA, , Dehydrogenase,, Decarboxylase, , Acetyl CoA, , Fig. 15.35 : Summary of lysine metabolism., , LYSINE, Lysine is an essential amino acid. Cereal, proteins are deficient in lysine. It does not, participate in transamination reactions. Some of, the lysine residues in protein structure are, present as hydroxylysine, methyllysine or, acetyllysine. H-Amino group of lysine, forming, salt bridges is necessary for the maintenance of, structural conformation of protein., Lysine is a ketogenic amino acid. The, summary of lysine metabolism is depicted in, Fig.15.35., , Synthesis of carnitine, Some of the lysine residues in proteins are, found in methylated form. The methyl groups, are obtained from active methionine (SAM). Such, proteins on degradation (by proteolysis) will, release the methyllysines. The trimethyllysine, , serves as a precursor for the synthesis of, carnitine, a compound involved in the transport, of fatty acids to mitochondria for oxidation. It, should be noted that free lysine is not, methylated, hence it will not be a substrate for, carnitine formation., Synthesis of carnitine from trimethyllysine is a, 4-step reaction involving oxidation, splitting off, glycine residue, dehydrogenation and, finally,, oxidation (Fig.15.36)., , Biochemical importance of carnitine, Carnitine plays a key role in the fatty acid, oxidation (Chapter 15)., Human requirements of carnitine are usually, met with the endogeneous biosynthesis and the, dietary supply. Good sources of carnitine include, meat, fish, poultry and dairy products.
Page 382 :
372, , BIOCHEMISTRY, , 3. On, decarboxylation, (PLP-dependent), serine forms ethanolamine which is the precursor, for choline synthesis., COO–, +, , +, , HC NH3, CH2OH, , H 2 C NH3, CO2, , CH2OH, Ethanolamine, , Serine, , 4. Serine is utilized for the synthesis of, cysteine (See Fig.15.30). It may be noted that the, entire cysteine molecule is derived from serine, except the sulfur that comes from homocysteine., 5. Serine is involved in the formation of, selenocysteine, the 21st amino acid found in, certain proteins., , Threonine undergoes deamination (by, threonine dehydratase) to D-ketobutyrate which, is converted to propionyl CoA. Threonine can be, cleaved to glycine and acetaldehyde by serine, hydroxymethyltransferase., Dehydrogenation, followed by decarboxylation of threonine results, in aminoacetone which may be converted to, pyruvate or lactate., , FATE OF CARBON SKELETON, OF AMINO ACIDS, , 6. Serine directly participates in the synthesis, of phospholipid-phosphatidyl serine (details, described in lipid metabolism, Chapter 14)., , The metabolic reactions of individual amino, acids are described above. After the removal of, amino groups, the carbon skeleton of amino, acids is converted to intermediates of TCA cycle, or their precursors. The carbon skeleton finally, has one or more of the following fates, , 7. Serine is also involved in the synthesis of, sphingomyelins and cephalins., , 1. Oxidation via TCA cycle to produce, energy (about 10-15% of body needs)., , 8. In the structure of proteins, serine ( OH, group) serves as a carrier of phosphate which is, involved in the regulation of many enzyme, activities., , 2. Synthesis of glucose., 3. Formation of lipids—fatty acids and ketone, bodies., 4. Synthesis of non-essential amino acids., , THREONINE, Threonine is an essential hydroxy amino acid., It is glycogenic and does not participate in, transamination reactions. Threonine is often a, carrier of phosphate group in the protein, structure. The outline of threonine metabolism is, depicted in Fig.15.42., , Proteins, D-Ketobutyrate, , Propionyl CoA, , T, H, R, E, O, N, I, N, E, , The carbon skeletons of the 20 standard (or, more) amino acids (or the amino acids of, proteins) are degraded to one of the following, seven products—pyruvate, D-ketoglutarate,, succinyl CoA, fumarate, oxaloacetate, acetyl, CoA and acetoacetate. Some authors use the, term amphibolic (Greek: amphiboles—uncertain), intermediates to these compounds due to their, multiple metabolic functions., The amino acids are classified into two, groups, based on the nature of the metabolic, end products of carbon skeleton., , Glycine +, Acetaldehyde, Aminoacetone, Pyruvate, Lactate, , Methylglyoxyl, , Fig. 15.42 : Overview of threonine metabolism., , 1. Glycogenic (glucogenic) amino acids :, These are the amino acids whose carbon, skeleton is finally degraded to pyruvate or one of, the intermediates of TCA cycle (D-ketoglutarate,, succinyl CoA, fumarate and oxaloacetate). These, intermediates serve as good substrates for, gluconeogenesis leading to the formation of, glucose or glycogen.
Page 384 :
374, , BIOCHEMISTRY, , l, , Fumarate : Phenylalanine and tyrosine., , l, , Oxaloacetate : Asparagine and aspartate., , l, , Acetyl CoA and acetoacetate : Phenylalanine,, tyrosine, tryptophan, isoleucine, leucine and, lysine., , Leucine and lysine are only ketogenic, since, they produce acetoacetate or acetyl CoA., , Biosynthesis of non-essential, amino acids, Of the 20 amino acids, about half of them are, non-essential in the diet, as they can be, synthesized in human body. This is carried out, , by the biosynthesis of carbon skeleton, followed, by the addition of amino group via, transamination. In the Table 15.5, the sources of, carbon skeleton for the synthesis of non-essential, amino acids are given., , Inborn errors of amino acid, metabolism—a summary, Several inherited disorders are associated with, amino acid metabolism. The details of these, metabolic disorders are described in the, respective amino acids. Table 15.6 gives a, summary of the inborn errors of amino acid, metabolism., , + Melanin—the pigment of skin, hair and eyes—is produced from tyrosine. Lack of, melanin synthesis (mostly due to a deficiency of tyrosinase) causes albinism., , + Parkinson’s disease—a common disorder of the elderly—is linked with decreased, synthesis of dopamine. It is characterized by muscular rigidity, tremors, lethargy etc., , + Phenylketonuria, due to a defect in the enzyme phenylalanine hydroxylase, is, characterized by failure of growth, seizures and mental retardation (low IQ)., , + Alkaptonuria causes the accumulation of homogentisate which undergoes oxidation followed by polymerization to produce the pigment alkapton. Deposition of, alkapton in connective tissue, causes ochronosis which is associated with arthritis., , + Serotonin, an excitatory neurotransmitter, is synthesized from tryptophan. Psychic, stimulant drugs (iproniazid) elevate serotonin levels while depressant drugs (LSD), decrease., , + Malignant carcinoid syndrome, a tumor of argentaffin cells of gastrointestinal tract, is, characterized by tremendously increased production of serotonin. This disorder can be, diagnosed by the elevated levels of 5-hydroxyindoleacetate in urine., , + Melatonin, produced from serotonin, is involved in circadian rhythms or diurnal, variations, i.e., maintenance of body’s biological clock., , + Homocysteine has been implicated as a risk factor in the onset of coronary heart diseases., + Histidine loading test, characterized by elevated excretion of N-formiminoglutamate, (FIGLU) is commonly employed to assess the deficiency of the vitamin, folic acid., , + Nitric oxide (NO), synthesized from arginine, is involved in several biological functions—, vasodilation, platelet aggregation, neurotransmission and bactericidal action. NO is, used in the treatment of chronic obstructive pulmonary disease (COPD)., , + J-Aminobutyric acid (GABA), produced from glutamate, is an inhibitory neurotransmitter. Low levels of GABA result in convulsions., , + The carbon skeleton of amino acids may be converted to glucose (glycogenic) or fat, (ketogenic), besides being responsible for the synthesis of non-essential amino acids., , + Polyamines (spermine, putrescine) are involved in the synthesis of DNA, RNA and, proteins and, thus, they are essential for cell growth and differentiation.
Page 385 :
375, , Chapter 15 : METABOLISM OF AMINO ACIDS, , TABLE 15.5 The sources of carbon skeleton for, the biosynthesis of non-essential amino acids, , Amino acid, , Source(s) of carbon skeleton, , Glycine, , Serine, , Alanine, , Pyruvate, , Serine, , 3-Phosphoglycerate, , Aspartic acid, Asparagine, Glutamic acid, Glutamine, Proline, , and spermidine are the biologically important, polyamines. Spermine and spermidine were, originally detected in human semen (sperms),, hence they are so named., , Biosynthesis, , Intermediates of, Krebs cycle, , Cysteine, , Serine (sulfur donated, by methionine), , Tyrosine, , Phenylalanine, , Amino aciduria, The term amino aciduria is generally used to, indicate the urinary excretion of amino acids. It, is frequently associated with defects in amino, acid metabolism. Most of the amino acidurias, manifest in mental retardation., , AMINO ACIDS AS, NEUROTRANSMITTERS, A neurotransmitter is an extracellular, massenger that can transmit an extracellular, message from a neuron to cells. Certain amino, acids and or their derivatives can serve as, neurotransmitters e.g. glycine, glutamate,, serononin, GABA (Table 15.7)., , Ornithine and S-adenosylmethionine are the, precursors for polyamine synthesis. It should,, however, be noted that only the four-carbon, moiety of SAM (not the methyl group) is, involved in polyamine formation. Ornithine, decarboxylase acts on ornithine to split off CO2, and produce putrescine (Fig.15.44). The enzyme, ornithine decarboxylase has the shortest halflife (about 10 minutes) among the known, mammalian enzymes. It regulates polyamine, synthesis. The activity of this enzyme is, increased by hormones like corticosteroids,, testosterone and growth hormone., Putrescine is converted to spermidine and, then spermine with the involvement of SAM., S-Adenosylmethionine is first decarboxylated to, give decarboxylated SAM. SAM decarboxylase is, a rare example of an enzyme that does not, require pyridoxal phosphate as coenzyme. An, amino acid residue bound to pyruvate is, believed to function as a cofactor. The, propylamino group of decarboxylated SAM is, transferred to putrescine to give spermidine., Synthesis of spermine requires one more, molecule of decarboxylated SAM and this, reaction is catalysed by spermine synthase., , BIOGENIC AMINES, Degradation of polyamines, In general, the decarboxylation of amino, acids or their derivatives results in the formation, of amines., NH2, R CH COOH, Amino acid, , Decarboxylase (PLP), CO2, , R CH2 NH2, Amine, , A summary of the biogenic amines derived, from different amino acids and their major, functions are given in Table 15.8., , The enzyme polyamine oxidase (of liver, peroxisomes) oxidizes spermine to spermidine, and then to putrescine. Spermidine and, putrescine are excreted in urine in a conjugated, form, as acetylated derivatives. Some amount of, putrescine is also oxidized to NH3 and CO2., , Functions of polyamines, , POLYAMINES, , 1. Polyamines are basic in nature and possess, multiple positive charges. Hence they are readily, associated with nucleic acids (DNA and RNA)., , Polyamines (Greek: poly—many) possess, multiple amino groups. Putrescine, spermine, , 2. They are involved in the synthesis of DNA,, RNA and proteins.
Page 388 :
378, , BIOCHEMISTRY, , 1. The body proteins are in a dynamic state (degradation and synthesis) and there is an, active amino acid pool (100 g) maintained for this purpose., 2. The amino acids undergo transamination and deamination to liberate ammonia for the, synthesis of urea, the end product of protein metabolism., 3. Besides being present as structural components of proteins, amino acids participate in, the formation of several biologically important compounds., 4. Glycine is involved in the synthesis of creatine, heme, purines, glutathione etc., 5. Phenylalanine is hydroxylated to tyrosine, which is a precursor for the production of, skin pigment (melanin), catecholamines (dopamine, epinephrine and norepinephrine), and thyroid hormones (T3 and T4)., 6. Tryptophan is converted to NAD+ and NADP+, the coenzymes of niacin, serotonin, (a neurotransmitter) and melatonin., 7. The active methionine (SAM) is a donor of methyl group (transmethylation) for the, synthesis of many biological compounds (epinephrine, choline, methylcytosine etc.)., 8. Many amino acids contribute to one-carbon fragments (formyl, formimino, methylene, etc.) for participation in one-carbon metabolism—which is mostly under the control of, tetrahydrofolate., 9. The carbon skeleton of amino acids is involved either in the synthesis of glucose, (glycogenic) or fat (ketogenic), or both-glucose and fat., 10. Many inborn errors (mostly due to enzyme defects) in amino acid metabolism have, been identified. These include phenylketonuria (defect-phenylalanine hydroxylase),, albinism (defect-tyrosinase), maple syrup urine disease (defect�D-keto acid dehydrogenase) etc.
Page 389 :
Chapter 15 : METABOLISM OF AMINO ACIDS, , 379, , I. Essay questions, 1. Describe the reactions in the synthesis of urea., 2. Give an account of the formation of specialized products from glycine., 3. Discuss the metabolism of phenylalanine and tyrosine., 4. Describe the fate of carbon skeleton of amino acids., 5. Write briefly on various inborn errors of amino acid metabolism., , II. Short notes, (a) Amino acid pool, (b) Transmethylation, (c) Transamination, (d) Deamination, (e) Ammonia, toxicity, (f) One-carbon metabolism, (g) Albinism, (h) Serotonin, (i) Glutamate and glutamine,, (j) Polyamines., , III. Fill in the blanks, 1. The coenzyme that participates in transamination reactions is ______________., 2. The most important enzyme involved in oxidative deamination is ______________., 3. N-Acetylglutamate is required for the activation of the enzyme ______________., 4. Primary hyperoxaluria is due to a defect in the enzyme ______________., 5. The cofactor required for the conversion of phenylalanine to tyrosine is ______________., 6. Parkinson’s disease is linked with decreased synthesis of ______________., 7. The metabolite excreted (elevated) in alkaptonuria is ______________., 8. The disease in which very high amount of tryptophan (nearly 60%) is converted to serotonin, is ______________., 9. The mammalian enzyme with the shortest half-life (about 10 minutes) is ______________., 10. The branched chain amino acid that is only ketogenic is ______________., , IV. Multiple choice questions, 11. The synthesis of urea occurs in, (a) Kidney (b) Liver (c) Muscle (d) Brain., 12. The amino acid required for the formation of glutathione, (a) Glycine (b) Cysteine (c) Glutamate (d) All of them., 13. In the synthesis of cysteine, the carbon skeleton is provided by, (a) Serine (b) Methionine (c) Glutamate (d) Alanine., 14. The amino acids are said to be ketogenic when the carbon skeleton is finally degraded to, (a) Succinyl CoA (b) Fumarate (c) Acetyl CoA (d) Pyruvate., 15. The amino acid that does not participate in transamination, (a) Lysine (b) Glutamate (c) Alanine (d) Tryptophan.
Page 390 :
Section 3, , Metabolisms, , Chapter, , Integration of Metabolism, , 16, , The energy metabolism speaks :, Glycogen, Liver, , Adipose tissue, Glucose, , HMP Pyruvate, shunt, , Glucose, , Acetyl CoA, , HMP shunt, Glycerol, , Pyruvate, , Fatty acids, , Triacylglycerol, , Acetyl CoA, NH3, , Amino acids, , Urea, , Proteins, , Krebs, cycle, , Triacylglycerol, , Fatty acids, , Krebs, cycle, , Glucose, , Muscle, , Fatty acids, , Amino acids, , Acetyl CoA, , Proteins, , Krebs, cycle, , Brain, Pyruvate, , Pyruvate, , Acetyl CoA, , Glucose, , Glucose, , Krebs, cycle, , Glycogen, , “Carbohydrate, fat, protein metabolisms integrate;, Cells, tissues and organs coordinate;, To meet the body’s fuel demands;, The essential requisite for existence.”, , M, , etabolism is a continuous process, with, thousands of reactions, simultaneously, occurring in the living cell. However,, biochemists prefer to present metabolism in the, form of reactions and metabolic pathways. This, is done for the sake of convenience in, presentation and understanding., In the preceeding three chapters (13-15), we, have learnt the metabolism of carbohydrates,, lipids and amino acids. We shall now consider, the organism as a whole and integrate the, metabolism with particular reference to energy, demands of the body., , Energy demand and supply, The organisms possess variable energy, demands, hence the supply (input) is also equally, variable. The consumed metabolic fuel may be, burnt (oxidized to CO2 and H2O) or stored to, meet the energy requirements as per the body, needs. ATP serves as the energy currency of the, cell in this process (Fig.16.1)., , O2, Energy supply, (variable), , CO2 + H 2O, Stored, energy, , ADP + Pi, , ATP, , Energy demands, (variable), , Fig. 16.1 : A summary of body’s energy supply and, demands. (Note : ATP serves as the energy currency)., , The humans possess enormous capacity for, food consumption. It is estimated that one can, consume as much as 100 times his/her basal, requirements! Obesity, a disorder of overnutrition mostly prevalent in affluent societies, is, primarily a consequence of overconsumption., , Integration of major metabolic, pathways of energy metabolism, An overview of the interrelationship between, the important metabolic pathways, concerned, , 380
Page 391 :
381, , Chapter 16 : INTEGRATION OF METABOLISM, , Glucose, , Triacylglycerols, , Glycogen, Glycogenesis, , Glycogenolysis, , Glucose, 6-phosphate, , Gluconeogenesis, , Fatty acids, , Glycolysis, , HMP, Shunt, , ATP, , Fatty acid, synthesis, NAD, , PH, , Acetyl CoA, , Pyruvate, , Ketogenic, amino acids, , `-Oxidation, , NADH, FADH2, Oxidative, phosphorylation, , Citric acid, cycle, , NADH, FADH2, , Glucogenic, amino acids, , ADP + Pi, , H2O, , ATP, , Fig. 16.2 : An overview of integration of metabolic pathways of, energy metabolism (HMP shunt–Hexose monophosphate shunt)., , with fuel metabolism depicted in Fig.16.2, is, briefly described here. For detailed information, on these metabolic pathways, the reader must, refer the respective chapters., 1. Glycolysis : The degradation of glucose to, pyruvate (lactate under anaerobic condition), generates 8 ATP. Pyruvate is converted to acetyl, CoA., 2. Fatty acid oxidation : Fatty acids undergo, sequential degradation with a release of, 2-carbon fragment, namely acetyl CoA. The, energy is trapped in the form of NADH and, FADH2., 3. Degradation of amino acids : Amino, acids, particularly when consumed in excess, than required for protein synthesis, are degraded, and utilized to meet the fuel demands of the, body. The glucogenic amino acids can serve as, precursors for the synthesis of glucose via the, formation of pyruvate or intermediates of citric, acid cycle. The ketogenic amino acids are the, precursors for acetyl CoA., 4. Citric acid cycle : Acetyl CoA is the key, and common metabolite, produced from, , different fuel sources (carbohydrates, lipids,, amino acids). Acetyl CoA enters citric acid, (Krebs) cycle and gets oxidized to CO2. Thus,, citric acid cycle is the final common metabolic, pathway for the oxidation of all foodstuffs. Most, of the energy is trapped in the form of NADH, and FADH2., 5. Oxidative phosphorylation : The NADH, and FADH2, produced in different metabolic, pathways, are finally oxidized in the electron, transport chain (ETC). The ETC is coupled with, oxidative phosphorylation to generate ATP., 6. Hexose monophosphate shunt : This, pathway is primarily concerned with the, liberation of NADPH and ribose sugar. NADPH, is utilized for the biosynthesis of several, compounds, including fatty acids. Ribose is an, essential component of nucleotides and nucleic, acids (Note : DNA contains deoxyribose)., 7. Gluconeogenesis : The synthesis of, glucose, from, non-carbohydrate, sources, constitutes gluconeogenesis. Several compounds, (e.g. pyruvate, glycerol, amino acids) can serve, as precursors for gluconeogenesis.
Page 392 :
382, , BIOCHEMISTRY, , 8. Glycogen metabolism : Glycogen is the, storage form of glucose, mostly found in liver, and muscle. It is degraded (glycogenolysis) and, synthesized (glycogenesis) by independent, pathways. Glycogen effectively serves as a fuel, reserve to meet body needs, for a brief period, (between meals)., , Regulation of metabolic pathways, The metabolic pathways, in general, are, controlled by four different mechanisms, 1. The availability of substrates, 2. Covalent modification of enzymes, 3. Allosteric regulation, 4. Regulation of enzyme synthesis., The details of these regulatory processes are, discussed under the individual metabolic, pathways, in the respective chapters., , ORGAN SPECIALIZATION AND, METABOLIC INTEGRATION, The various tissues and organs of the body, work in a well coordinated manner to meet its, metabolic demands. The major organs along, with their most important metabolic functions, in, a well-fed absorptive state (usually 2-4 hours, after food consumption), are described., , Liver, The liver is specialized to serve as the body’s, central metabolic clearing house. It processes, and distributes the nutrients to different tissues, for utilization. After a meal, the liver takes up the, carbohydrates, lipids and most of the amino, acids, processes them and routes to other tissues., The major metabolic functions of liver, in an, absorptive state, are listed, 1. Carbohydrate metabolism : Increased, glycolysis, glycogenesis and hexose monophosphate shunt and decreased gluconeogenesis., 2. Lipid metabolism : Increased synthesis of, fatty acids and triacylglycerols., , 3. Protein metabolism : Increased degradation, of amino acids and protein synthesis., , Adipose tissue, Adipose tissue is regarded as the energy, storage tissue. As much as 15 kg. of, triacylglycerol (equivalent to 135,000 Cal) is, stored in a normal adult man. The major, metabolic functions of adipose tissue in an, absorptive state are listed here., 1. Carbohydrate metabolism : The uptake of, glucose is increased. This follows an increase in, glycolysis and hexose monophosphate shunt., 2. Lipid metabolism : The synthesis of fatty, acids and triacylglycerols is increased. The, degradation of triacylglycerols is inhibited., , Skeletal muscle, The metabolism of skeletal muscle is rather, variable depending on its needs. For instance,, the resting muscle of the body utilizes about, 30% of body’s oxygen consumption. However,, during strenuous exercise, this may be as high as, 90%. The important metabolic functions of, skeletal muscle in an absorptive state are listed., 1. Carbohydrate metabolism : The uptake of, glucose is higher, and glycogen synthesis is, increased., 2. Lipid metabolism : Fatty acids taken up, from the circulation are also important fuel, sources for the skeletal muscle., 3. Protein metabolism : Incorporation of, amino acids into proteins is higher., , Brain, The human brain constitutes about 2% of the, body’s weight. But it utilizes as much as 20% of, the oxygen consumed by the body. Being a vital, organ, special priority is given to the metabolic, needs of the brain., 1. Carbohydrate metabolism : In an, absorptive state, glucose is the only fuel source, to the brain. About 120 g of glucose is utilized, per day by an adult brain. This constitutes about, 60% of the glucose consumed by the body at
Page 393 :
383, , Chapter 16 : INTEGRATION OF METABOLISM, , TABLE 16.1 Energy relationship in major mammalian organs (tissues), , Organ/Tissue, , Energy compound(s) preferably utilized, , Energy compound(s) exported, , Liver, , Amino acids, glucose, fatty acids, , Glucose, fatty acids, ketone bodies, , Adipose tissue, , Fatty acids, , Fatty acids, glycerol., , Skeletal muscle, , Fatty acids, , None, , Glucose, , Lactate, , Glucose, ketone bodies (in starvation), , None, , Brain, , rest. It is estimated that about 50% of the, energy consumed by brain is utilized by, plasma membrane Na+-K+-ATPase to maintain, membrane potential required for nerve impulse, transmission., , reserve of the body. The survival time of an, individual on starvation is mostly dependent on, his/her fat stores. And for this reason, obese, individuals can survive longer than lean, individuals without consuming food., , 2. Lipid metabolism : The free fatty acids, cannot cross the blood-brain barrier, hence their, contribution for the supply of energy to the brain, is insignificant. Further, in a fed state, ketone, bodies are almost negligible as fuel source to the, brain. However, brain predominantly depends, on ketone bodies during prolonged starvation, (details given later)., , Protein is basically a structural constituent,, mostly present in the muscle. However, during, starvation, protein can also meet the fuel, demands of the body. It is estimated that about, 1/ rd of the body’s protein can be utilized, 3, towards energy needs without compromising the, vital functions., , The metabolic interrelationship among the, major tissues in an absorptive state are given in, Fig.16.3. The fuel sources that are preferably, utilized by the major organs and the compounds, exported from them are listed in Table 16.1., , METABOLISM IN STARVATION, Starvation may be due to food scarcity or the, desire to rapidly lose weight or certain clinical, conditions (e.g. surgery, burns etc.). Starvation is, a metabolic stress which imposes certain, metabolic compulsions on the organism. The, metabolism is reorganized to meet the new, demands of starvation., Glucose is the fuel of choice for brain and, muscle. Unfortunately, the carbohydrate reserve, of the body is so low that it cannot meet the, energy requirements even for a day. The fuel, stores (or energy reserves) of a 70 kg normal, man are given in Table 16.2. Triacylglycerol (fat), of adipose tissue is the predominant energy, , Starvation is associated with a decrease in, insulin level and an increase in glucagon. The, metabolic changes during starvation are, discussed with reference to the major organs/, tissues., , Liver in starvation, 1. Carbohydrate metabolism : An important, function of liver is to act as a blood glucose, buffering organ. The action of liver is to suit the, metabolic needs of the body. During starvation,, increased, gluconeogenesis, and, elevated, glycogen degradation furnish glucose to the, needy tissues (mostly brain)., , TABLE 16.2 Energy reserves of a normal 70 kg man, Energy source, (main storage, tissue), , Weight, (kg), , Energy, equivalent, (in Cal), , Triacylglycerol, (adipose tissue), , 15, , 135,000, , Protein (muscle), , 6, , 24,000, , Glycogen (muscle, liver), , 0.2, , 800
Page 394 :
384, , BIOCHEMISTRY, , Glycogen, Liver, , Adipose tissue, Glucose, , HMP Pyruvate, shunt, NH3, , Amino acids, , Urea, , Proteins, , Glucose, , Acetyl CoA, , Krebs, cycle, , Fatty acids, , Triacylglycerol, , HMP shunt, , Pyruvate, , Glycerol, , Acetyl CoA, , Triacylglycerol, , Fatty acids, , Krebs, cycle, , Glucose, , Muscle, , Amino acids Acetyl CoA, , Proteins, , Brain, , Fatty acids, , Krebs, cycle, , Pyruvate, , Pyruvate, , Acetyl CoA, , Glucose, , Glucose, , Krebs, cycle, , Glycogen, , Fig. 16.3 : Metabolic interrelationship among the major tissues in a well fed state, (HMP shunt–Hexose monophosphate shunt)., , ☞ Biochemists, for their convenience, learn body chemical processes in terms of individual, metabolic reactions and pathways, although thousands of reactions simultaneously, occur in a living cell., , ☞ The metabolic pathways in various tissues and organs are well coordinated to meet the, demands of the body., , ☞ Liver is appropriately regarded as the body’s ‘central metabolic clearing house’ while, adipose tissues constitute the energy (fat) storehouse., , ☞ Brain is a vital metabolic organ that consumes about 20% of body’s oxygen, although, it constitutes only 2% of body weight., , ☞ The metabolism in starvation is reorganized to meet the body’s changed demands and, metabolic compulsions., , ☞ Under normal circumstances, glucose is the only fuel source to brain. However, during, starvation, the brain slowly gets adapted to use ketone bodies for energy needs.
Page 395 :
385, , Chapter 16 : INTEGRATION OF METABOLISM, , Glycogen, Liver, , Glucose, , Glucose, , Pyruvate, , Acetyl CoA, , Krebs, cycle, , Ketone bodies, , Adipose tissue, , Acetyl CoA, , Fatty acids, , Krebs, cycle, , Fatty acids, , Glycerol, , Triacylglycerol, Gluconeogenesis, , Ketone, bodies, Glycerol,, Amino acids, , Muscle, Fatty acids, Brain, , Amino acids, , Acetyl CoA, , Proteins, , Krebs, cycle, , Ketone bodies, , Ketone bodies, Pyruvate, , Acetyl CoA, , Glucose, , Krebs, cycle, , Fig. 16.4 : Metabolic interrelationship among major tissues during starvation., , 2. Lipid metabolism : Fatty acid oxidation is, increased with an elevated synthesis of ketone, bodies. This is due to the fact that TCA (Krebs), cycle cannot cope up with the excess production, of acetyl CoA, hence the latter is diverted for, ketone body synthesis., , Ketone bodies (primarily `-hydroxybutyrate), effectively serve as fuel source for the peripheral, tissues. The brain slowly adapts itself to use, ketone bodies. Thus, after a 3-day fast, about, 1/ rd of the brain’s fuel demands are met by, 3, ketone bodies, while, after 40 days’ starvation,, they countribute to about 70% of energy needs., , Adipose tissue in starvation, 1. Carbohydrate metabolism : Glucose, uptake and its metabolism are lowered., 2. Lipid metabolism : The degradation, of triacylglycerol is elevated, leading to an, increased release of fatty acids from the adipose, tissue which serve as fuel source for various, tissues (brain is an exception). The glycerol, liberated in lipolysis serves as a precursor for, glucose synthesis by liver. The synthesis of fatty, acids and triacylglycerols is totally stopped in, adipose tissue.
Page 396 :
386, , BIOCHEMISTRY, , Skeletal muscle in starvation, , Brain in starvation, , 1. Carbohydrate metabolism : Glucose, uptake and its metabolism are very much, depressed., , As already stated, glucose is the preferred fuel, source by brain. During the first 2 weeks of, starvation, the brain is mostly dependent on, glucose, supplied by liver gluconeogenesis. This,, in turn, is dependent on the amino acids released, from the muscle protein degradation. Starvation, beyond 3 weeks generally results in a marked, increase in plasma ketone bodies. By this time,, the brain adapts itself to depend on ketone, bodies for the energy needs., , 2. Lipid metabolism : Both fatty acids and, ketone bodies are utilized by the muscle as fuel, source. However, on prolonged starvation, beyond 3 weeks, the muscle adapts to, exclusively utilize fatty acids. This further, increases the level of ketone bodies in the, circulation., 3. Protein metabolism : During the early, period of starvation, muscle proteins are, degraded to liberate the amino acids which are, effectively utilized by the liver for glucose, synthesis (gluconeogenesis). On prolonged, starvation, however, protein breakdown is, reduced., , The metabolic interrelationship among the, major organs in starvation are depicted in, Fig.16.4. The biochemical changes that occur, during starvation are such that an adequate, supply of fuel molecules is maintained to, various tissues to meet the energy demands., This is a natural adaptation for the survival of the, organism., , 1. The metabolism of carbohydrates, lipids and proteins is integrated to meet the energy, and metabolic demands of the organism. The metabolic pathways—glycolysis, fatty acid, oxidation, citric acid cycle and oxidative phosphorylation—are directly concerned with, the generation of ATP. Gluconeogenesis, glycogen metabolism, hexose monophosphate, shunt and amino acid degradation are also associated with energy metabolism., 2. The organs/tissues, with their respective specializations, coordinate with each other to, meet the metabolic demands of the organism as a whole. Liver is specialized to serve, as the body’s central metabolic clearing house. It processes and distributes the nutrients, to different tissues for their utilization. Adipose tissue is primarily a storage organ of, fat. The major bulk of the body protein is located in the muscle tissue., 3. Brain is a specialized organ which, in the normal situation, is exclusively dependent on, the supply of glucose (120 g/day) for its fuel needs., 4. Starvation is a metabolic stress, as it imposes certain metabolic compulsions on the, organism. The stored fat of adipose tissue and the muscle protein are degraded and, utilized to meet the body’s fuel demands. Brain gradually adapts itself to use ketone, bodies (instead of glucose) for its energy requirements. Starvation is, thus, associated, with metabolic reorganization for the survival of the organism.
Page 397 :
Section 3, , Metabolisms, , Chapter, , Metabolism of Nucleotides, , 17, O, HN, , The uric acid speaks :, H, N, O, , O, , N, H, , N, H, , “I am the end product of purines;, An increase in my production causes gout;, Inflammation of joints is the symptom,, And administration of allopurinol a relief., , N, , ucleotides consist of a nitrogenous base, a, pentose and a phosphate. The pentose, sugar is D-ribose in ribonucleotides of RNA while, in deoxyribonucleotides (deoxynucleotides) of, DNA, the sugar is 2-deoxy D-ribose. Nucleotides, participate in almost all the biochemical, processes, either directly or indirectly. They are, the structural components of nucleic acids (DNA,, RNA), coenzymes, and are involved in the, regulation of several metabolic reactions., , CO2 Glycine, C, Aspartate, N, , 3. N3 and N9 are obtained from amide group, of glutamine., , C, , 5, , C, 4, , 2, 3, , N, , C, , N, , 7, , 8C, 9, , 10, , N -Formyl, THF, , N, , Fig. 17.1 : The sources of individual atoms, in purine ring. (Note : Same colours are, used in the synthetic pathway Fig. 17.2)., , 4. C4, C5 and N7 are contributed by glycine., , Many compounds contribute to the purine, ring of the nucleotides (Fig.17.1)., , 2. C2 and C8 arise from formate of N10formyl THF., , -Formyl, THF, , 6, , Glutamine, , BIOSYNTHESIS OF PURINE, RIBONUCLEOTIDES, , 1. N1 of purine is derived from amino group, of aspartate., , 10, , N1, , 5. C6 directly comes from CO2., It should be remembered that purine bases, are not synthesized as such, but they are formed, as ribonucleotides. The purines are built upon a, pre-existing ribose 5-phosphate. Liver is the, major site for purine nucleotide synthesis., Erythrocytes, polymorphonuclear leukocytes and, brain cannot produce purines., , 387
Page 400 :
390, , BIOCHEMISTRY, , 11. The final reaction, catalysed, by, cyclohydrolase leads to ring, closure with an elimination, of water molecule. The, product obtained is inosine, monophosphate (IMP), the, parent purine nucleotide, from which other purine, nucleotides can be synthesized., , O, , N, , Folic acid (THF) is, essential for the synthesis, of, purine, nucleotides, (reactions 4 and 10)., Sulfonamides, are, the, structural analogs of paraaminobenzoic, acid, (PABA). These sulfa drugs, can be used to inhibit the, synthesis of folic acid by, microorganisms., This, indirectly reduces the, synthesis of purines and,, therefore, the nucleic acids, (DNA, and, RNA)., Sulfonamides have no, influence on humans,, since folic acid is not, synthesized, and, is, supplied through diet., , N, , Ribose 5-P, Inosine monophosphate (IMP), Aspartate + GTP, +, , GDP + Pi, , Adenylsuccinate, synthetase, , IMP dehydrogenase, , NAD, + H2O, , NADH + H+, –, , Inhibitors of, purine synthesis, , N, , HN, , –, , O, , OOC CH2 CH COO, NH, , N, , HN, N, , N, , O, N, , N, , Ribose 5-P, Adenylsuccinate, , Fumarate, , N, H, , Ribose 5-P, Xanthosine monophosphate, (XMP), Glutamine, + ATP + H2O, GMP synthetase, , Adenylsuccinase, Glutamate +, AMP + PPi, O, , NH2, N, , N, , N, , HN, N, , N, , N, , Ribose 5-P, Adenosine monophosphate, (AMP), , H2N, , N, , N, , Ribose 5-P, Guanosine monophosphate, (GMP), , Fig. 17.3 : Synthesis of AMP and GMP from inosine monophosphate., , The structural analogs of folic acid (e.g., methotrexate) are widely used to control cancer., They inhibit the synthesis of purine nucleotides, (reaction 4 and 10) and, thus, nucleic acids. Both, these reactions are concerned with the transfer of, one-carbon moiety (formyl group). These, inhibitors also affect the proliferation of normally, growing cells. This causes many side-effects, including anemia, baldness, scaly skin etc., , Synthesis of AMP, and GMP from IMP, Inosine monophosphate is the immediate, precursor for the formation of AMP and GMP, , (Fig.17.3). Aspartate condenses with IMP in the, presence of GTP to produce adenylsuccinate, which, on cleavage, forms AMP., For the synthesis of GMP, IMP undergoes, NAD+ dependent dehydrogenation to form, xanthosine monophosphate (XMP). Glutamine, then transfers amide nitrogen to XMP to produce, GMP., 6-Mercaptopurine is an inhibitor of the, synthesis of AMP and GMP. It acts on, the, enzyme, adenylsuccinase, (of, AMP, pathway) and IMP dehydrogenase (of GMP, pathway).
Page 402 :
392, , P, , BIOCHEMISTRY, , O, , P, , O, , O H2C, H, , Base, , P, , Ribonucleotide reductase, , O, , P, , H, , H, , H, H2O, OH OH, Ribonucleoside Thioredoxin, Thioredoxin, diphosphate (2SH, reduced), (S S , oxidized), (ADP, GDP,, Thioredoxin, CDP, UDP), reductase, +, , NADP, , O, , O H2C, H, H, , Base, H, H, , OH H, Deoxyribonucleoside, diphosphate, (dADP, dGDP,, dCDP, dUDP), , +, , NADPH + H, , Fig. 17.6 : Formation of deoxyribonucleotides from ribonucleotides., , The salvage pathway is particularly important, in certain tissues such as erythrocytes and brain, where de novo (a new) synthesis of purine, nucleotides is not operative., A defect in the enzyme HGPRT causes LeschNyhan syndrome (details given later)., , Regulation of purine, nucleotide biosynthesis, The purine nucleotide synthesis is well, coordinated to meet the cellular demands. The, intracellular concentration of PRPP regulates, purine synthesis to a large extent. This, in turn,, is dependent on the availability of ribose, 5-phosphate and the enzyme PRPP synthetase., PRPP glutamyl amidotransferase is controlled, by a feedback mechanism by purine nucleotides., That is, if AMP and GMP are available in, adequate amounts to meet the cellular, requirements, their synthesis is turned off at the, amidotransferase reaction., Another important stage of regulation is in the, conversion of IMP to AMP and GMP. AMP, inhibits adenylsuccinate synthetase while GMP, inhibits IMP dehydrogenase. Thus, AMP and, GMP control their respective synthesis from IMP, by a feedback mechanism., , Conversion of ribonucleotides, to deoxyribonucleotides, The synthesis of purine and pyrimidine, deoxyribonucleotides, occurs, from, ribonucleotides by a reduction at the C2 of ribose, , moiety (Fig.17.6). This reaction is catalysed by a, multisubunit (two B1 and two B2 subunits), enzyme, ribonucleotide reductase., Supply of reducing equivalents : The enzyme, ribonucleotide reductase itself provides the, hydrogen atoms needed for reduction from its, sulfhydryl groups. The reducing equivalents, in, turn, are supplied by thioredoxin, a monomeric, protein with two cysteine residues., NADPH-dependent thioredoxin reductase, converts the oxidized thioredoxin to reduced, form which can be recycled again and again., Thioredoxin thus serves as a protein cofactor in, an enzymatic reaction., Regulation of deoxyribonucleotide synthesis :, Deoxyribonucleotides are mostly required for the, synthesis of DNA. The activity of the enzyme, ribonucleotide reductase maintains the adequate, supply of deoxyribonucleotides., The drug hydroxyurea inhibits ribonucleotide, reductase by destroying free radicals required by, this enzyme. Hydroxyurea is used in the, treatment of cancers such as chronic, myologenous leukemia., , DEGRADATION OF, PURINE NUCLEOTIDES, The end product of purine metabolism in, humans is uric acid. The sequence of reactions, in purine nucleotide degradation is given in, Fig.17.7.
Page 404 :
394, , BIOCHEMISTRY, , Most animals (other than primates) however,, oxidize uric acid by the enzyme uricase to, allantoin, where the purine ring is cleaved., Allantoin is then converted to allantoic acid and, excreted in some fishes (Fig.17.8). Further, degradation of allantoic acid may occur to, produce urea (in amphibians, most fishes and, some molluscs) and, later, to ammonia (in, marine invertebrates)., , Uric acid, Uricase, Allantoin, Allantoinase, Allantoic acid, Glyoxylate, , Allantoicase, Urea, Urease, , DISORDERS OF, PURINE METABOLISM, , Ammonia, , Fig. 17.8 : Degradation of uric acid in, animals other than man., , Hyperuricemia and gout, , 1. The nucleotide monophosphates (AMP,, IMP and GMP) are converted to their respective, nucleoside forms (adenosine, inosine and, guanosine) by the action of nucleotidase., 2. The amino group, either from AMP or, adenosine, can be removed to produce IMP or, inosine, respectively., 3. Inosine and guanosine are, respectively,, converted to hypoxanthine and guanine (purine, bases) by purine nucleoside phosphorylase., Adenosine is not degraded by this enzyme,, hence it has to be converted to inosine., 4. Guanine undergoes, guanase to form xanthine., , deamination, , by, , 5. Xanthine oxidase is an important enzyme, that converts hypoxanthine to xanthine, and, xanthine to uric acid. This enzyme contains, FAD, molybdenum and iron, and is exclusively, found in liver and small intestine. Xanthine, oxidase liberates H2O2 which is harmful to the, tissues. Catalase cleaves H2O2 to H2O and O2., , Uric acid (2,6,8-trioxypurine) is the final, excretory product of purine metabolism in, humans. Uric acid can serve as an important, antioxidant by getting itself converted (nonenzymatically) to allantoin. It is believed that the, antioxidant role of ascorbic acid in primates is, replaced by uric acid, since these animals have, lost the ability to synthesize ascorbic acid., , Uric acid is the end product of purine, metabolism, in, humans., The, normal, concentration of uric acid in the serum of adults, is in the range of 3-7 mg/dl. In women, it is, slightly lower (by about 1 mg) than in men. The, daily excretion of uric acid is about 500-700 mg., Hyperuricemia refers to an elevation in the, serum uric acid concentration. This is sometimes, associated with increased uric acid excretion, (uricosuria)., , Gout is a metabolic disease associated with, overproduction of uric acid. At the physiological, pH, uric acid is found in a more soluble form as, sodium urate. In severe hyperuricemia, crystals, of sodium urate get deposited in the soft tissues,, particularly in the joints. Such deposits are, commonly known as tophi. This causes, inflammation in the joints resulting in a painful, gouty arthritis. Sodium urate and/or uric acid, may also precipitate in kidneys and ureters that, results in renal damage and stone formation., Historically, gout was found to be often, associated with high living, over-eating and, alcohol consumption. In the previous centuries,, alcohol was contaminated with lead during its, manufacture and storage. Lead poisoning leads, to kidney damage and decreased uric acid, excretion causing gout. In general, a diet rich is, meat and seafoods is associated with increased, risk of gout., The prevalence of gout is about 3 per 1,000, persons, mostly affecting males. Post-menopausal
Page 405 :
395, , Chapter 17 : METABOLISM OF NUCLEOTIDES, , women, however, are as susceptible as, men for this disease. Gout is of two, types—primary and secondary., 1. Primary gout : It is an inborn, error, of, metabolism, due, to, overproduction of uric acid. This is, mostly related to increased synthesis of, purine nucleotides. The following are, the important metabolic defects, (enzymes) associated with primary gout, (Fig.17.9), ●, , ●, , ●, , ●, , Glycogen, Glucose, 6-phosphate, , 2GSH, , NADP+, Glutathione, reductase, , G-S-S-G, , HMP shunt, , NADPH, + H+, Ribose 5-phosphate, , PRPP synthetase, , PRPP, , Glutamine, , PRPP glutamylamidotransferase, , PRPP glutamylamidotransferase :, The lack of feedback control of this, enzyme by purine nucleotides also, leads to their elevated synthesis., , Glucose 6-phosphatase deficiency :, In type I glycogen storage disease, (von Gierke’s), glucose 6-phosphate, cannot be converted to glucose, due to the deficiency of glucose, 6-phosphatase. This leads to the, , Glucose, , Glucose, , PRPP synthetase : In normal, circumstances, PRPP synthetase is, under feedback control by purine, nucleotides, (ADP, and, GDP)., However, variant forms of PRPP, synthetase—which are not subjected, to feedback regulation—have been, detected. This leads to the increased, production of purines., , HGPRT deficiency : This is an, enzyme of purine salvage pathway,, and its defect causes Lesch-Nyhan, syndrome. This disorder is associated, with, increased, synthesis, of, purine nucleotides by a two-fold, mechanism., Firstly,, decreased, utilization of purines (hypoxanthine, and guanine) by salvage pathway,, resulting in the accumulation and, diversion of PRPP for purine, nucleotides. Secondly, the defect in, salvage pathway leads to decreased, levels of IMP and GMP causing, impairment in the tightly controlled, feedback, regulation, of, their, production., , Glucose, 6-phosphatase, , 5-Phosphoribosylamine, , HGPRT, , Hypoxanthine, , Guanine, , Inosine monophosphate, HGPRT, , GMP, , AMP, , Adenine, , Hypoxanthine, Xanthine oxidase, , Allopurinol, inhibits, , Xanthine, , Xanthine oxidase, , Uric acid, , Fig. 17.9 : Summary of possible enzyme alterations causing gout, ( B–Increased enzyme activity; 9–Decreased enzyme activity;, GSH–Reduced glutathione; G-S-S-G–Oxidized glutathione;, PRPP–Phosphoribosyl pyrophosphate; HGPRT–Hypoxanthineguanine phosphoribosyltransferase).
Page 406 :
396, increased utilization of glucose 6-phosphate, by hexose monophosphate shunt (HMP shunt),, resulting in elevated levels of ribose, 5-phosphate and PRPP and, ultimately, purine, overproduction. von Gierke’s disease is also, associated with increased activity of, glycolysis. Due to this, lactic acid accumulates, in the body which interferes with the uric acid, excretion through renal tubules., ●, , Elevation of glutathione reductase : Increased, glutathione reductase generates more NADP+, which is utilized by HMP shunt. This causes, increased ribose 5-phosphate and PRPP, synthesis., , Among the five enzymes described, the first, three are directly involved in purine synthesis., The remaining two indirectly regulate purine, production. This is a good example to show how, an abnormality in one metabolic pathway, influences the other., 2. Secondary gout : Secondary hyperuricemia, is due to various diseases causing increased, synthesis or decreased excretion of uric acid., Increased degradation of nucleic acids (hence, more uric acid formation) is observed in various, cancers (leukemias, polycythemia, lymphomas,, etc.) psoriasis and increased tissue breakdown, (trauma, starvation etc.)., The disorders associated with impairment in, renal function cause accumulation of uric acid, which may lead to gout., , Uric acid pool in gout, By administration of uric acid isotope (N15),, the miscible uric acid pool can be calculated. It, is around 1,200 mg in normal subjects. Uric acid, pool is tremendously increased to 3,000 mg. or, even more, in patients suffering from gout., , BIOCHEMISTRY, , O, N, , HN, , N, N, H, Hypoxanthine, O, , O, , HN, N, , Xanthine, oxidase, , N, H, Allopurinol, N, , HN, N, O, , N, N, H, H, Alloxanthine, , Fig. 17.10 : Structures of hypoxanthine, and its structural analogs., , oxidase. This type of inhibition is referred to as, suicide inhibition (For more details, Refer, Chapter 6)., Inhibition of xanthine oxidase by allopurinol, leads to the accumulation of hypoxanthine and, xanthine. These two compounds are more, soluble than uric acid, hence easily excreted., Besides the drug therapy, restriction in dietary, intake of purines and alcohol is advised., Consumption of plenty of water will also be, useful., The anti-inflammatory drug colchicine is used, for the treatment of gouty arthritis. Other antiinflammatory drugs—such as phenylbutazone,, indomethacin, oxyphenbutazone, corticosteroids—, are also useful., , Pseudogout, The clinical manifestations of pseudogout are, similar to gout. But this disorder is caused by the, deposition of calcium pyrophosphate crystals in, joints. Further, serum uric acid concentration is, normal in pseudogout., , Treatment of gout, The drug of choice for the treatment of, primary gout is allopurinol. This is a structural, analog of hypoxanthine that competitively, inhibits the enzyme xanthine oxidase. Further,, allopurinol is oxidized to alloxanthine by, xanthine oxidase (Fig.17.10). Alloxanthine, in, turn, is a more effective inhibitor of xanthine, , Lesch-Nyhan syndrome, This disorder is due to the deficiency of, hypoxanthine-guanine phosphoribosyltransferase, (HGPRT), an enzyme of purine salvage pathway, (See Fig.17.5). It was first described in 1964 by, Michael Lesch (a medical student) and William, L. Nyhan (his teacher).
Page 407 :
397, , Chapter 17 : METABOLISM OF NUCLEOTIDES, , Lesch-Nyhan syndrome is a sex-linked, metabolic disorder since the structural gene for, HGPRT is located on the X-chromosome., It affects only the males and is characterized by, excessive uric acid production (often gouty, arthritis), and neurological abnormalities such as, mental retardation, aggressive behavior, learning, disability etc. The patients of this disorder have, an irresistible urge to bite their fingers and lips,, often causing self-mutilation., The overproduction of uric acid in LeschNyhan syndrome is explained. HGPRT, deficiency results in the accumulation of PRPP, and decrease in GMP and IMP, ultimately, leading to increased synthesis and degradation, of purines (more details given under primary, gout)., The biochemical basis for the neurological, symptoms observed in Lesch-Nyhan syndrome is, not clearly understood. This may be related to, the dependence of brain on the salvage pathway, for de novo synthesis of purine nucleotides. Uric, acid is not toxic to the brain, since patients with, severe hyperuricemia (not related to HGPRT, deficiency) do not exhibit any neurological, symptoms. Further, allopurinol treatment that, , helps to decrease uric acid production, has no, affect on the neurological manifestations in these, patients., , Immunodeficiency diseases, associated with purine metabolism, Two different immunodeficiency disorders, associated with the degradation of purine, nucleosides are identified. The enzyme defects, are adenosine deaminase and purine nucleoside, phosphorylase, involved in uric acid synthesis, (See Fig.17.7)., The deficiency of adenosine deaminase (ADA), causes severe combined immunodeficiency, (SCID) involving T-cell and usually B-cell, dysfunction. It is explained that ADA deficiency, results in the accumulation of dATP which is an, inhibitor of ribonucleotide reductase and,, therefore, DNA synthesis and cell replication., The deficiency of purine nucleotide phosphorylase is associated with impairment of T-cell, function but has no effect on B-cell function., Uric acid synthesis is decreased and the tissue, levels of purine nucleosides and nucleotides are, higher. It is believed that dGTP inhibits the, development of normal T-cells., , ☞ Folic acid is essential for the synthesis of purine nucleotides. Folic acid analogs, (methotrexate) are employed to control cancer., , ☞ The salvage pathway, involving the direct conversion of purines to corresponding, nucleotides, is important in tissues—brain and erythrocytes., , ☞ Gout is the disorder associated with the overproduction of uric acid, the end product, of purine metabolism. Allopurinol is the drug of choice for the treatment of gout., , ☞ Lesch-Nyhan syndrome is caused by a defect in the enzyme hypoxanthine-guanine phosphoribosyltransferase. The patients have an irresistible urge to bite their fingers and lips., , ☞ A defect in the enzyme adenosine deaminase (ADA) results in severe combined, immunodeficiency (SCID) involving both T-cell and B-cell dysfunction. A girl suffering, from SCID was cured by transferring ADA gene (in 1990) and that was the first attempt, for gene therapy in modern medicine., , ☞ Orotic aciduria, a metabolic defect in pyrimidine biosynthesis, is characterized by, anaemia and retarded growth, besides the excretion of orotic acid in urine.
Page 410 :
400, , Degradation of, pyrimidine nucleotides, The pyrimidine nucleotides undergo similar, reactions (dephosphorylation, deamination and, cleavage of glycosidic bond) like that of purine, nucleotides to liberate the nitrogenous bases—, cytosine, uracil and thymine. The bases are then, degraded to highly soluble products—`-alanine, and `-aminoisobutyrate. These are the amino, acids which undergo transamination and other, reactions to finally produce acetyl CoA and, succinyl CoA., , Salvage pathway, The pyrimidines (like purines) can also serve, as precursors in the salvage pathway to be, converted to the respective nucleotides. This, reaction, is, catalysed, by, pyrimidine, phosphoribosyltransferase which utilizes PRPP as, the source of ribose 5-phosphate., , BIOCHEMISTRY, , orotate, phosphoribosyl, transferase, and, OMP decarboxylase of pyrimidine synthesis, (Fig.17.12). Both these enzyme activities are, present on a single protein as domains, (bifunctional enzyme)., Feeding diet rich in uridine and/or cytidine is, an effective treatment for orotic aciduria. These, compounds provide (through phosphorylation), pyrimidine nucleotides required for DNA and, RNA synthesis. Besides this, UTP inhibits, carbamoyl phosphate synthetase II and blocks, synthesis of orotic acid., Reye’s syndrome : This is considered as a, secondary orotic aciduria. It is believed that a, defect in ornithine transcarbamoylase (of urea, cycle) causes the accumulation of carbamoyl, phosphate. This is then diverted for the increased, synthesis and excretion of orotic acid., , Disorders of pyrimidine metabolism, , Biosynthesis of, nucleotide coenzymes, , Orotic aciduria : This is a rare metabolic, disorder characterized by the excretion of orotic, acid in urine, severe anemia and retarded, growth. It is due to the deficiency of the enzymes, , The nucleotide coenzymes FMN, FAD, NAD +, NADP+ and coenzyme A are synthesized from the, B-complex vitamins. Their formation is described, under the section on vitamins (Chapter 7).
Page 411 :
Chapter 17 : METABOLISM OF NUCLEOTIDES, , 1. Nucleotides participate in a wide variety of reactions in the living cells—synthesis of, DNA and RNA; as constituents of many coenzymes; in the regulation of metabolic, reactions etc., 2. Purine nucleotides are synthesized in a series of reactions starting from ribose, 5-phosphate. Glycine, glutamine, aspartate, formate and CO2 contribute to the synthesis, of purine ring., 3. Purine nucleotides can also be synthesized from free purines by a salvage pathway. The, defect in the enzyme HGPRT causes Lesch-Nyhan syndrome., 4. Deoxyribonucleotides are formed from ribonucleotides by a reduction process catalysed, by ribonucleotide reductase. Thioredoxin is the protein cofactor required for this reaction., 5. Purine nucleotides are degraded to uric acid, the excretory product in humans. Uric acid, serves as a natural antioxidant in the living system., 6. Uric acid in many animal species (other than primates) is converted to more soluble, forms such as allantoin, allantoic acid etc., and excreted., 7. Gout is a metabolic disease associated with overproduction of uric acid. This often leads, to the accumulation of sodium urate crystals in the joints, causing painful gouty, arthritis. Allopurinol, an inhibitor of xanthine oxidase, is the drug used for the, treatment of gout., 8. Pyrimidine nucleotides are synthesized from the precursors aspartate, glutamine and, CO2, besides ribose 5-phosphate., 9. Orotic aciduria is a defect in pyrimidine synthesis caused by the deficiency of orotate, phosphoribosyltransferase and OMP decarboxylase. Diet rich in uridine and/or cytidine, is an effective treatment for orotic aciduria., 10. Pyrimidines are degraded to amino acids, namely `-alanine and `-aminoisobutyrate, which are then metabolized., , 401
Page 412 :
402, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the catabolism of purine nucleotides and the associated metabolic disorders., 2. Write an account of the biosynthesis of inosine monophosphate., 3. Discuss the synthesis and degradation of pyrimidines., 4. Describe the role of PRPP in purine and pyrimidine synthesis., 5. Write an account of salvage pathway in purine nucleotide synthesis. Add a note on LeschNyhan syndrome., , II. Short notes, (a) Gout, (b) PRPP, (c) Synthesis of deoxyribonucleotides, (d) Functions of nucleotides, (e) Immunodeficiency diseases in purine metabolism, (f) Orotic aciduria, (g) Carbamoyl phosphate synthetase, II, (h) HGPRT, (I) Degradation of uric acid in different animals, (j) Regulation of purine synthesis, (k) Inhibitors of purine synthesis., , III. Fill in the blanks, 1. The amino acids required for the synthesis of purines and pyrimidines are ________________., 2. The enzyme xanthine oxidase is inhibited by ____________________., 3. Tophi are mostly made up of ____________________., 4. Hypouricemia is due to the deficiency of the enzyme ____________________., 5. The disorder in which the patients have an irresistible urge to bite their fingers and lips is, ____________________., 6. The cofactor required by the enzyme ribonucleotide reductase is ____________________., 7. The ‘parent’ nucleotide synthesized in the biosynthesis of purines is ____________________., 8. Xanthine oxidase converts allopurinol to ____________________., 9. The amino acid that contributes to the synthesis of more than half of the pyrimidine ring, ___________________., 10. The regulatory enzyme in the pyrimidine biosynthesis in animals is ____________________., , IV. Multiple choice questions, 11. Name the enzyme associated with hyperuricemia, (a) PRPP synthetase (b) HGPRT (c) Glucose 6-phosphatase (d) All of them., 12. An enzyme of purine metabolism associated with immunodeficiency disease, (a) Adenosine deaminase (b) Xanthine oxidase (c) PRPP synthetase (d) HGPRT., 13. Orotic aciduria can be treated by a diet rich in, (a) Adenine (b) Guanine (c) Uridine (d) Any one of them., 14. The end product of purine metabolism in humans is, (a) Xanthine (b) Uric acid (c) Urea (d) Allantoin., 15. The nitrogen atoms in the purine ring are obtained from, (a) Glycine (b) Glutamine (c) Aspartate (d) All of them.
Page 413 :
Section 3, , Metabolisms, , Chapter, , Mineral Metabolism, , 18, , The mineral, calcium speaks :, Intestine Ca, , PTH, , Calcitriol, , Vitamin D, , Plasma Ca, (9-11 mg/dl), Calcitriol, Calcitonin, , PTH, PTH, , Bone Ca, , Renal, tubular Ca, , “I am the most abundant mineral;, Calcify and strengthen bones, teeth……, Coagulate blood and contract muscle;, Regulated by calcitriol, PTH and calcitonin.”, , T, , he mineral (inorganic) elements constitute, only a small proportion of the body weight., There is a wide variation in their body content., For instance, calcium constitutes about 2% of, body weight while cobalt about 0.00004%., , General functions, Minerals perform several vital functions which, are absolutely essential for the very existence of, the organism. These include calcification of, bone, blood coagulation, neuromuscular, irritability, acid-base equilibrium, fluid balance, and osmotic regulation., Certain minerals are integral components of, biologically important compounds such as, hemoglobin (Fe), thyroxine (I), insulin (Zn) and, vitamin B12 (Co). Sulfur is present in thiamine,, biotin, lipoic acid and coenzyme A. Several, minerals participate as cofactors for enzymes in, metabolism (e.g. Mg, Mn, Cu, Zn, K). Some, elements are essential constituents of certain, enzymes (e.g. Co, Mo, Se)., , Classification, The minerals are classified as principal, elements and trace elements., The seven principal elements (macrominerals) constitute 60-80% of the body’s, inorganic material. These are calcium,, phosphorus, magnesium, sodium, potassium,, chloride and sulfur., The principal elements are required in, amounts greater than 100 mg/day., The (microminerals) are required in amounts, less than 100 mg/day. They are subdivided into, three categories, 1. Essential trace elements : Iron, copper,, iodine, manganese, zinc, molybdenum, cobalt,, fluorine, selenium and chromium., 2. Possibly essential trace elements : Nickel,, vanadium, cadmium and barium., 3. Non-essential trace elements : Aluminium,, lead, mercury, boron, silver, bismuth etc., , 403
Page 414 :
404, , BIOCHEMISTRY, , TABLE 18.1 A summary of the major characteristics of principal elements (macroelements), , Element, , Major, functions, , Deficiency, disease/symptoms, , Recommended, dietary allowance, , Major sources, , Calcium, , Constituent of bones and, teeth; muscle contraction,, nerve transmission, , Rickets; osteomalacia,, osteoporosis, , 0.8–1.0 g/d, , Milk and milk products, leafy, vegetables, beans, , Phosphorus, , Constituent of bones and, teeth; in the formation of, high energy phosphates,, nucleic acids, nucleotide, coenzymes., , Rickets,, osteomalacia, , 0.8–1.0 g/d, , Milk, cereals, leafy vegetables, , Magnesium, , Constituent of bones and, teeth; cofactor for enzymes, e.g. kinases., , Neuromuscular weakness,, irritation, , 300–350 mg/d, , Cereals, vegetables, fruits,, milk, , Sodium, , Chief cation of extracellular, fluids; acid-base balance,, osmotic pressure; nerve, and muscle function, , Almost unknown on normal, diet, , 5–10 g/d, , Table salt, salt added foods, , Potassium, , Chief cation of intracellular, fluids; acid-base balance;, osmotic pressure; muscle, function, , Muscular weakness, mental, confusion, , 3–4 g/d, , Fruits, nuts, vegetables, , Chlorine, , Regulation of acid-base, balance; formation of HCl, , Almost unknown on normal, diet, , 5–10 g/d, , Table salt, , Sulfur, , Constituent of sulfur, Almost unknown, containing amino acids,, certain vitamins (thiamine,, biotin) and other compounds, (heparin, chondroitin sulfate)., , —, , Sulfur containing amino acids, , A summary of the major characteristics of, principal elements and trace elements is, respectively given in Tables 18.1 and 18.2. The, individual elements are described next., , the formation (of hydroxyapatite) and physical, strength of skeletal tissue. Bone is regarded as a, mineralized connective tissue. Bones which are, in a dynamic state serve as reservoir of Ca., Osteoblasts are responsible for bone formation, while osteoclasts result in demineralization., , CALCIUM, , 2. Muscle contraction : Ca2+ interacts with, troponin C to trigger muscle contraction., Calcium also activates ATPase, increases the, interaction between actin and myosin., , Calcium is the most abundant among the, minerals in the body. The total content of, calcium in an adult man is about 1 to 1.5 kg. As, much as 99% of it is present in the bones and, teeth. A small fraction (1%) of the calcium, found, outside the skeletal tissue, performs a wide, variety of functions., , Biochemical functions, 1. Development of bones and teeth :, Calcium, along with phosphate, is required for, , 3. Blood coagulation : Several reactions in, the cascade of blood clotting process are, dependent on Ca2+(factor IV)., 4. Nerve transmission : Ca2+ is necessary for, the transmission of nerve impulse., 5. Membrane integrity and permeability :, Ca2+ influences the membrane structure and, transport of water and several ions across it.
Page 415 :
405, , Chapter 18 : MINERAL METABOLISM, , TABLE 18.2 A summary of the major characteristics of trace elements (microelements), , Element, , Major functions, , Deficiency, disease/symptoms, , Recommended, dietary allowance, , Major sources, , Iron, , Constituent of heme, e.g. hemoglobin, myoglobin,, cytochromes; involved in O2, transport and biological, oxidation., , Hypochromic, microcytic, anemia, , 10–15 mg/d, , Organ meats (liver, heart),, leafy vegetables, iron cookware, , Copper, , Constituent of enzymes, e.g. cytochrome C oxidase,, catalase, tyrosinase; in iron, transport., , Anemia, Menke s disease, , 2–3 mg/d, , Organ meats cereals, leafy, vegetables, , Iodine, , Constituent of thyroxine and, triiodothyronine, , Cretinism, goiter, myxedema, , 150–200 +g/d, , Iodized salt, sea foods, , Manganese, , Cofactor for enzymes, e.g. arginase, pyruvate, carboxylase; glycoprotein, synthesis., , Almost unknown, , 2–9 mg/d, , Cereals, leafy vegetables, , Zinc, , Cofactor for enzymes, e.g. alcohol dehydrogenase,, carbonic anhydrase,, lactate dehydrogenase., , Growth retardation, poor, 10–15 mg/d, wound healing, hypogonadism, , Meat, fish, milk, , Mol bdenum, , Constituent of enzymes, e.g. xanthine oxidase, , Almost unknown, , 75–250 +g/d, , Vegetables, , Cobalt, , Constituent of vitamin B12,, required for the formation, of erythrocytes, , Pernicious anemia (as in, vitamin B12 deficiency), , 5–8 +g/d, , Foods of animal origin, , Fluorine, , Helps in the proper formation, of bones and teeth, , Dental caries, osteoporosis, , 2–4 mg/d, , Drinking water, , Selenium, , Involved in antioxidant, function along with vitamin E;, constituent of glutathione, peroxidase and selenocysteine, , Muscular degeneration,, cardiomyopathy, , 50–200 +g/d, , Organ meats, sea foods, , Chromium, , Promotes insulin function, (as glucose tolerance factor), , Impaired glucose tolerance, , 10–100 +g/d, , Brewer s yeast, meat, whole, grains, , 6. Activation of enzymes : Ca2+ is needed, for the direct activation of enzymes such as, lipase (pancreatic), ATPase and succinate, dehydrogenase., 7. Calmodulin mediated action of Ca2+ :, Calmodulin (mol. wt. 17,000) is a calcium, binding regulatory protein. Ca-calmodulin, complex activates certain enzymes e.g., adenylate cyclase, Ca2+ dependent protein, kinases., 8. Calcium as intracellular messenger :, Certain hormones exert their action through the, , mediation of Ca2+ (instead of cAMP). Calcium is, regarded as a second messenger for such, hormonal action e.g. epinephrine in liver, glycogenolysis. Calcium serves as a third, messenger for some hormones e.g. antidiuretic, hormone (ADH) acts through cAMP, and then, Ca2+., 9. Release of hormones : The release of, certain hormones (insulin, PTH, calcitonin) from, the endocrine glands is facilitated by Ca2+., 10. Secretory processes : Ca2+ regulates, microfilament and microtubule mediated
Page 416 :
406, , BIOCHEMISTRY, , processes such as endocytosis, exocytosis and, cell motility., 11. Contact inhibition : Calcium is believed, to be involved in cell to cell contact and, adhesion of cells in a tissue (Refer p. 692 also)., The cell to cell communication may also require, Ca2+., 12. Action on heart : Ca2+, myocardium and prolongs systole., , acts, , Ionized Ca, (biologically active), , Ca complexed with, citrate, phosphate,, bicarbonate, , 50%, 10%, 40%, , on, Protein-bound, non-diffusible Ca, , Dietary requirements, Adult men and women —, , 800 mg/day, , Women during, pregnancy, lactation, and post-menopause, , —, , 1.5 g/day, , Children (1-18 yrs.), , —, , 0.8–1.2 g/day, , Infants (<1 year), , — 300–500 mg/day, , Fig. 18.1 : Different forms of circulating calcium., , Factors inhibiting Ca absorption, , Sources, Best sources, , —, , Milk and milk products, , Good sources, , —, , Beans, leafy vegetables,, fish, cabbage, egg yolk., , Absorption, The absorption of calcium mostly occurs in, the duodenum by an energy dependent active, process. It is influenced by several factors., , 1. Phytates and oxalates form insoluble salts, and interfere with Ca absorption., 2. High content of dietary phosphate results, in the formation of insoluble calcium phosphate, and prevents Ca uptake. The dietary ratio of Ca, and P—between 1 : 2 and 2 : 1—is ideal for, optimum Ca absorption by intestinal cells., 3. The free fatty acids react with Ca to form, insoluble calcium soaps. This is particularly, observed when the fat absorption is impaired., 4. Alkaline, condition, (high, unfavourable for Ca absorption., , pH), , is, , 5. High content of dietary fiber interferes with, Ca absorption., , Factors promoting Ca absorption, , Plasma calcium, , 1. Vitamin D (through its active form, calcitriol) induces the synthesis of calcium, binding protein in the intestinal epithelial cells, and promotes Ca absorption., , Most of the blood Ca is present in the plasma, since the blood cells contain very little of it. The, normal concentration of plasma or serum Ca is, 9-11 mg/dl (4.5-5.5 mEq/l). About half of this, (5 mg/dl) is in the ionized form which is, functionally the most active (Fig.18.1). At least 1, mg/dl serum Ca is found in association with, citrate and/or phosphate. About 40% of serum, Ca (4-5mg/dl) is bound to proteins, mostly, albumin and, to a lesser extent, globulin. Ionized, and citrate (or phosphate) bound Ca is diffusible, from blood to the tissues while protein bound Ca, is non-diffusible. In the usual laboratory, determination of serum Ca, all the three fractions, are measured together., , 2. Parathyroid hormone enhances Ca, absorption through the increased synthesis of, calcitriol., 3. Acidity (low pH) is more favourable for Ca, absorption., 4. Lactose promotes calcium uptake by intestinal cells., 5. The amino acids lysine and arginine, facilitate Ca absorption.
Page 417 :
407, , Chapter 18 : MINERAL METABOLISM, , Intestine Ca, , PTH, , Calcitriol, , Vitamin D, , Plasma Ca, (9-11 mg/dl), Calcitriol, Calcitonin, , PTH, PTH, , Bone Ca, , Renal, tubular Ca, , Fig. 18.2 : Overview of calcium homeostasis, (PTH–Parathyroid hormone)., , FACTORS REGULATING, PLASMA Ca LEVEL, As already stated, calcium is almost, exclusively present in blood plasma (or serum)., The hormones—calcitriol, parathyroid hormone, (PTH) and calcitonin are the major factors that, regulate the plasma calcium (homeostasis of Ca;, Fig.18.2) within a narrow range (9-11 mg/dl)., , Calcitriol, The physiologically active form of vitamin D is, a hormone, namely calcitriol or 1,25-dihydroxycholecalciferol (1,25 DHCC). The synthesis of, calcitriol and its wide range of biochemical, actions are described under Vitamins (Chapter 7)., Calcitriol induces the synthesis of a specific, calcium binding protein in the intestinal cells., This protein increases the intestinal absorption of, calcium as well as phosphate. Thus blood Ca, level is increased by calcitriol (the active vitamin, D). Furthermore, calcitriol stimulates calcium, uptake by osteoblasts of bone and promotes, calcification or mineralization (deposition of, calcium phosphate) and remodelling., , Parathyroid hormone, Parathyroid hormone (PTH) is secreted by two, pairs of parathyroid glands that are closely, associated with thyroid glands. Parathyroid, hormone (mol. wt. 95,000) is a single chain, polypeptide, containing 84 amino acids. It is, originally synthesized as preproPTH which is, , degraded to proPTH and, finally, to active PTH., The rate of formation (by degradation of proPTH), and the secretion of PTH are promoted by low, Ca2+ concentration. Thus, the release of PTH, from parathyroid glands is under the negative, feedback regulation of serum Ca2+., Mechanism of action of PTH : PTH binds to, a membrane receptor protein on the target cell, and activates adenylate cyclase to liberate, cAMP. This, in turn, increases intracellular, calcium that promotes the phosphorylation of, proteins (by kinases) which, finally brings about, the biological actions. PTH has 3 independent, tissues—bone, kidneys and intestine—to exert its, action. The prime function of PTH is to elevate, serum calcium level., Action on the bone : PTH causes, decalcification or demineralization of bone, a, process carried out by osteoclasts. This is, brought out by PTH stimulated increased activity, of, the, enzymes, pyrophosphatase, and, collagenase. These enzymes result in bone, resorption. Demineralization ultimately leads to, an increase in the blood Ca level. The action of, PTH on bone is quantitatively very significant to, maintain Ca homeostasis. It must, however, be, noted that this is being done at the expense of, loss of Ca from bone, particularly in dietary Ca, deficiency., Action on the kidney : PTH increases the Ca, reabsorption by kidney tubules. This is the most, rapid action of PTH to elevate blood Ca levels., However, quantitatively, this is less important, compared to the action of PTH on bone., PTH promotes the production of calcitriol, (1,25 DHCC) in the kidney by stimulating, 1-hydroxylation of 25-hydroxycholecalciferol., Action on the intestine : The action of PTH, on the intestine is indirect. It increases the, intestinal absorption of Ca by promoting the, synthesis of calcitriol., , Calcitonin, Calcitonin is a peptide containing 32 amino, acids. It is secreted by parafollicular cells of, thyroid gland. The action of CT on calcium, metabolism is antagonistic to that of PTH. Thus,
Page 418 :
408, calcitonin promotes calcification by increasing, the activity of osteoblasts. Further, calcitonin, decreases bone reabsorption and increases the, excretion of Ca into urine. CT, therefore, has a, decreasing influence on blood calcium., , Importance of Ca : P ratio, The ratio of plasma Ca : P is important for, calcification of bones. The product of Ca × P (in, mg/dl) in children is around 50 and in adults, around 40. This product is less than 30 in rickets., , Excretion of calcium, Calcium is excreted partly through the, kidneys and mostly through the intestine. The, renal threshold for serum Ca is 10 mg/dl., Calcium gets excreted into urine beyond this, concentration., Excretion of Ca into the feces is a continuous, process and this is increased in vitamin D, deficiency., , Calcium in the teeth, The teeth calcium is not subjected to, regulation as observed for bone calcium. Thus, the adult teeth, once formed, do not undergo, decalcification to meet the body needs of, calcium. However, proper calcification of teeth, is important in the growing children., , BIOCHEMISTRY, , excretion of Ca and P, often resulting in the, formation of urinary calculi, is also observed in, these patients., The determination of ionized serum calcium, (elevated to 6-9mg/dl) is more useful for the, diagnosis of hyperparathyroidism. It has been, observed that some of the patients may have, normal levels of total calcium in the serum but, differ with regard to ionized calcium., The symptoms of hypercalcemia include, lethargy, muscle weakness, loss of appetite,, constipation, nausea, increased myocardial, contractility and susceptibility to fractures., , Hypocalcemia, Hypocalcemia is a more serious and life, threatening condition. It is characterized by, a fall in the serum Ca to below 7 mg/dl,, causing tetany. The symptoms of tetany include, neuromuscular irritability, and convulsions., Hypocalcemia is mostly due to hypoparathyroidism. This may happen after an accidental, surgical removal of parathyroid glands or due to, an autoimmune disease., Treatment : Supplementation of oral calcium, with vitamin D is commonly employed. In, severe cases of hypocalcemia, calcium, gluconate is intravenously administered., , Rickets, DISEASE STATES, The blood Ca level is maintained within a, narrow range by the homeostatic control, most, predominantly by PTH. Hence abnormalities in, Ca metabolism are mainly associated with, alterations in PTH., , Hypercalcemia, Elevation in serum Ca level (normal 9–11, mg/dl) is hypercalcemia. Hypercalcemia is, associated with hyperparathyroidism caused by, increased activity of parathyroid glands., Decrease in serum phosphate (due to increased, renal losses) and increase in alkaline, phosphatase activity are also found in, hyperparathyroidism. Elevation in the urinary, , Rickets is a disorder of defective calcification, of bones. This may be due to a low levels of, vitamin D in the body or due to a dietary, deficiency of Ca and P — or both. The, concentrations of serum Ca and P may be low, or normal. An increase in the activity of, alkaline phosphatase is a characteristic feature, of rickets., , Renal rickets, Renal rickets is associated with damage to, renal tissue, causing impairment in the synthesis, of calcitriol. It does not respond to vitamin D in, ordinary doses, therefore, some workers regard, this as vitamin D resistant rickets. Renal rickets, can be treated by administration of calcitriol.
Page 419 :
409, , Chapter 18 : MINERAL METABOLISM, , Osteoporosis, Osteoporosis is characterized by demineralization of bone resulting in the progressive loss, of bone mass., Occurrence : The elderly people (over 60 yr.), of both sexes are at risk for osteoporosis., However, it more predominantly occurs in the, post-menopausal women. Osteoporosis results in, frequent bone fractures which are a major cause, of disability among the elderly. It is estimated, that more than 50% of the fractures in USA are, due to this disorder. Osteoporosis may be, regarded as a silent thief., Etiology : The etiology of osteoporosis is, largely unknown, but it is believed that several, causative factors may contribute to it. The ability, to produce calcitriol from vitamin D is, decreased with age, particularly in the, postmenopausal women. Immobilized or, sedentary individuals tend to decrease bone, mass while those on regular exercise tend to, increase bone mass. Deficiency of sex hormones, (in women) has been implicated in the, development of osteoporosis., Treatment : Estrogen administration along, with calcium supplementation (in combination, with vitamin D) to postmenopausal women, reduces the risk of fractures. Higher dietary, intake of Ca (about 1.5 g/day) is recommended, for elderly people., , Osteopetrosis, (marble bone disease), Osteopetrosis is characterized by increased, bone density. This is primarily due to inability to, resorb bone. This disorder is mostly observed in, association with renal tubular acidosis (due to a, defect in the enzyme carbonic anhydrase) and, cerebral calcification., , the bones and teeth. About 10% of body P is, found in muscles and blood in association with, proteins, carbohydrates and lipids. The, remaining 10% is widely distributed in various, chemical compounds., , Biochemical functions, 1. Phosphorus is essential for the development, of bones and teeth., 2. It plays a central role for the formation and, utilization of high-energy phosphate compounds, e.g. ATP, GTP, creatine phosphate etc., 3. Phosphorus is required for the formation of, phospholipids, phosphoproteins and nucleic, acids (DNA and RNA)., 4. It is an essential component of several, nucleotide coenzymes e.g. NAD+, NADP+,, pyridoxal phosphate, ADP, AMP., 5. Several proteins and enzymes are activated, by phosphorylation., 6. Phosphate buffer system is important for, the maintenance of pH in the blood (around 7.4), as well as in the cells., , Dietary requirements, The recommended dietary allowance (RDA), of phosphate is based on the intake of calcium., The ratio of Ca : P of 1 : 1 is recommended (i.e., 800 mg/day) for an adult. For infants, however,, the ratio is around 2 : 1, which is based on the, ratio found in human milk. Calcium and, phosphate are distributed in the majority of, natural foods in 1 : 1 ratio. Therefore, adequate, intake of Ca generally takes care of the P, requirement also., , Sources, Milk, cereals, leafy vegetables, meat, eggs., , PHOSPHORUS, An adult body contains about 1 kg phosphate, and it is found in every cell of the body. Most of, it (about 80%) occurs in combination with Ca in, , Absorption, Phosphate absorption occurs from jejunum, 1. Calcitriol promotes, along with calcium., , phosphate, , uptake
Page 420 :
410, 2. Absorption of phosphorus and calcium is, optimum when the dietary Ca : P is between, 1 : 2 and 2 : 1., 3. Acidity favours while phytate decreases, phosphate uptake by intestinal cells., , Serum phosphate, The phosphate level of the whole blood is, around 40 mg/dl while serum contains about 34 mg/dl. This is because the RBC and WBC have, very high content of phosphate., The serum phosphate may exist as free ions, (40%) or in a complex form (50%) with cations, such as Ca2+, Mg2+, Na+, K+. About 10% of, serum phosphate is bound to proteins. It is, interesting to note that the fasting serum, phosphate levels are higher than the postprandial. This is attributed to the fact that, following the ingestion of carbohydrate, (glucose), the phosphate from the serum is drawn, by the cells for metabolism (phosphorylation, reactions)., , BIOCHEMISTRY, , MAGNESIUM, The adult body contains about 20 g, magnesium, 70% of which is found in bones in, combination with calcium and phosphorus. The, remaining 30% occurs in the soft tissues and, body fluids., , Biochemical functions, 1. Magnesium is required for the formation, of bones and teeth., 2. Mg2+ serves as a cofactor for several, enzymes requiring ATP e.g. hexokinase,, glucokinase, phosphofructokinase, adenylate, cyclase., 3. Mg2+ is necessary for proper neuromuscular function. Low Mg2+ levels lead to, neuromuscular irritability., , Dietary requirements, Adult man, , — 350 mg/day, , Adult woman — 300 mg/day, , Excretion, About 500 mg phosphate is excreted in urine, per day. The renal threshold is 2 mg/dl. The, reabsorption of phosphate by renal tubules is, inhibited by PTH., , Disease states, 1. Serum phosphate level is increased in, hypoparathyroidism and decreased in hyperparathyroidism., 2. In severe renal diseases, serum phosphate, content is elevated causing acidosis., 3. Vitamin D deficient rickets is characterized, by decreased serum phosphate (1–2 mg/dl)., 4. Renal rickets is associated with low serum, phosphate levels and increased alkaline, phosphatase activity., 5. In diabetes mellitus, serum content of, organic phosphate is lower while that of, inorganic phosphate is higher., , Sources, Cereals, nuts, beans, vegetables (cabbage,, cauliflower), meat, milk, fruits., , Absorption, Magnesium is absorbed by the intestinal cells, through a specific carrier system. About 50% of, the dietary Mg is normally absorbed., Consumption of large amounts of calcium,, phosphate and alcohol diminishes Mg, absorption. PTH increases Mg absorption., , Serum Mg, Normal serum concentration of Mg is 2–3 mg/, dl. It is present in the ionized form (60%), in, combination with other ions (10%) and bound to, proteins (30%)., , Disease states, 1. Magnesium deficiency causes neuromuscular irritation, weakness and convulsions.
Page 421 :
411, , Chapter 18 : MINERAL METABOLISM, , These symptoms are similar to that observed in, tetany (Ca deficiency) which are relieved only, by Mg. Malnutrition, alcoholism and cirrhosis of, liver may lead to Mg deficiency., 2. Low levels of Mg may be observed in, uremia, rickets and abnormal pregnancy., , Absorption, Sodium is readily absorbed in the gastrointestinal tract and, therefore, very little of it (< 2%) is, normally found in feces. However, in diarrhea,, large quantities of sodium is lost in feces., , Plasma sodium, SODIUM, Sodium is the chief cation of the extracellular, fluid. About 50% of body sodium is present in, the bones, 40% in the extracellular fluid and the, remaining (10%) in the soft tissues., , Biochemical functions, 1. In association with chloride and, bicarbonate, sodium regulates the body’s acidbase balance., 2. Sodium is required for the maintenance of, osmotic pressure and fluid balance., 3. It is necessary for the normal muscle, irritability and cell permeability., 4. Sodium is involved in the intestinal, absorption of glucose, galactose and amino, acids., 5. It is necessary, maintaining heart beat., , for, , initiating, , and, , Dietary requirements, For normal individuals, the requirement of, sodium is about 5-10 g/day which is mainly, consumed as NaCl. For persons with a family, history of hypertension, the daily NaCl intake, should be less than 5 g. For patients of, hypertension, around 1 g/day is recommended., It may be noted that 10 g of NaCl contains 4 g, of sodium. The daily consumption of Na is, generally higher than required due to its flavour., , Sources, The common salt (NaCl) used in the cooking, medium is the major source of sodium. The, ingested foods also contribute to sodium. The, good sources of sodium include bread, whole, grains, leafy vegetables, nuts, eggs and milk., , In the plasma (serum), the normal, concentration of sodium is 135-145 mEq/l., Sodium is an extracellular cation, therefore, the, blood cells contain much less (35 mEq/l). The, mineralocorticoids, secreted by adrenal cortex,, influence sodium metabolism. A decrease in, plasma sodium and an increase in its urinary, excretion are observed in adrenocortical, insufficiency., , Excretion, Kidney is the major route of sodium excretion, from the body. As much as 800 g Na/day is, filtered by the glomeruli, 99% of this is, reabsorbed by the renal tubules by an active, process. This is controlled by aldosterone., Extreme sweating also causes considerable, amount of sodium loss from the body. There is,, however, individual variation in sodium loss, through sweat., , Disease states, 1. Hyponatremia : This is a condition in, which the serum sodium level falls below the, normal. Hyponatremia may occur due to, diarrhea, vomiting, chronic renal diseases,, adrenocortical insufficiency (Addison’s disease)., Administration of salt free fluids to patients may, also cause hyponatremia. This is due to, overhydration., Decreased, serum, sodium, concentration is also observed in edema, which occurs in cirrhosis or congestive heart, failure., The manifestations of hyponatremia include, reduced blood pressure and circulatory failure., 2. Hypernatremia : This condition is, characterized by an elevation in the serum, sodium level. The symptoms include increase in
Page 422 :
412, , BIOCHEMISTRY, , blood volume and blood pressure. It may occur, due to hyperactivity of adrenal cortex (Cushing’s, syndrome),, prolonged, administration, of, cortisone, ACTH and/or sex hormones. Loss of, water from the body causing dehydration, as it, occurs in diabetes insipidus, results in, hypernatremia., Rapid, administration, of, sodium salts also increases serum sodium, concentration. It may be noted that in, pregnancy, steroid and placental hormones, cause sodium and water retention in the body,, leading to edema., In edema, along with water, sodium, concentration in the body is also elevated., Administration of diuretic drugs increases, the urinary output of water along with, sodium. In the patients of hypertension and, congestive cardiac failure salt (Na+) restriction, is advocated., , Sources, Banana, orange, pineapple, potato, beans,, chicken, and liver. Tender coconut water is a, rich source of potassium., , Absorption, The absorption of K+ from the gastrointestinal, tract is very efficient (90%) and very little is lost, through feces. However, in subjects with, diarrhea, a good proportion of K+ is lost in the, feces., , Plasma potassium, The plasma (serum) concentration of, potassium is 3.4-5.0 mEq/l. The whole blood, contains much higher level of K+ (50 mEq/l),, since it is predominantly an intracellular cation., Care should, therefore, be taken to avoid, hemolysis of RBC for the estimation of serum K+., , Excretion, POTASSIUM, Potassium is the principal intracellular cation., It is equally important in the extracellular fluid, for specific functions., , Biochemical functions, 1. Potassium maintains intracellular osmotic, pressure., 2. It is required for the regulation of acidbase balance and water balance in the cells., 3. The enzyme pyruvate kinase (of glycolysis), is dependent on K+ for optimal activity., 4. Potassium is required for the transmission, of nerve impulse., 5. Adequate intracellular concentration K+ is, necessary for proper biosynthesis of proteins by, ribosomes., 6. Extracellular K+ influences cardiac muscle, activity., , Dietary requirements, About 3-4 g/day., , Potassium is mainly excreted through urine., The maintenance of body acid-base balance, influences K+ excretion. Aldosterone increases, excretion of potassium., , Disease states, Serum potassium concentration is maintained, within a narrow range. Either high or low, concentrations are dangerous since potassium, effects the contractility of heart muscle., Hypokalemia : Decrease in the concentration, of serum potassium is observed due to, overactivity of adrenal cortex (Cushing’s, syndrome), prolonged cortisone therapy,, intravenous administration of K+-free fluids,, treatment of diabetic coma with insulin,, prolonged diarrhea and vomiting., The symptoms of hypokalemia include, irritability, muscular weakness, tachycardia,, cardiomegaly and cardiac arrest. Changes in the, ECG are observed (flattened waves with inverted, T wave)., Hyperkalemia : Increase in the concentration, of serum potassium is observed in renal failure,
Page 423 :
413, , Chapter 18 : MINERAL METABOLISM, , adrenocortical insufficiency (Addison’s disease),, diabetic coma, severe dehydration, intravenous, administration of fluids with excessive potassium, salts., The manifestations of hyperkalemia include, depression of central nervous system, mental, confusion, numbness, bradycardia with reduced, heart sounds and, finally, cardiac arrest. Changes, in ECG are also observed (elevated T wave)., , contains higher level of Cl– (125 mEq/l). This is, due to the fact that protein content is low in CSF, and, therefore, Cl– is higher in order to maintain, Donnan membrane equilibrium., , Excretion, There exists a parallel relationship between, excretion of chloride and sodium. The renal, threshold for Cl– is about 110 mEq/l., , Disease states, CHLORINE, Chlorine is a constituent of sodium chloride., Hence, the metabolism of chlorine and sodium, are intimately related., , Biochemical functions, 1. Chloride is involved in the regulation of, acid-base equilibrium, fluid balance and osmotic, pressure. These functions are carried out by the, interaction of chloride with Na+ and K+., 2. Chloride is necessary for the formation of, HCl in the gastric juice., 3. Chloride shift involves the active participation of Cl–., 4. The enzyme salivary amylase is activated, by chloride., , Dietary requirements, The daily requirement of chloride as NaCl is, 5-10 g. Adequate intake of sodium will satisfy, the chloride requirement of the body., , Sources, , 1. Hypochloremia : A reduction in the serum, Cl– level may occur due to vomiting, diarrhea,, respiratory alkalosis, Addison’s disease and, excessive sweating., 2. Hyperchloremia : An increase in serum, Cl– concentration may be due to dehydration,, respiratory acidosis and Cushing’s syndrome., , SULFUR, Sulfur of the body is mostly present in the, organic form. Methionine, cysteine and cystine, are the three sulfur-containing amino acids, present in the proteins. Generally, proteins, contain about 1% sulfur by weight., , Biochemical functions, 1. Sulfur-containing amino acids are very, essential for the structural conformation and, biological functions of proteins (enzymes,, hormones, structural proteins etc.). The disulfide, linkages ( S S ) and sulfhydryl groups ( SH), are largely responsible for this., , Common salt as cooking medium, whole, grains, leafy vegetables, eggs and milk., , 2. The vitamins thiamine, biotin, lipoic acid,, and coenzyme A of pantothenic acid contain, sulfur., , Absorption, , 3. Heparin, chondroitin sulfate, glutathione,, taurocholic acid are some other important sulfurcontaining compounds., , In normal circumstances, chloride is almost, totally absorbed in the gastrointestinal tract., , Plasma chloride, The normal plasma concentration of chloride, is 95-105 mEq/l. Cerebrospinal fluid (CSF), , 4. Phosphoadenosine phosphosulfate (PAPS), is the active sulfate utilized for several reactions, e.g. synthesis of glycosaminoglycans, detoxification mechanism.
Page 424 :
414, , BIOCHEMISTRY, , 5. The, sulfur-containing, amino, acid, methionine (as S–adenosylmethionine) is actively, involved in transmethylation reactions., , 4. Iron is associated with effective immunocompetence of the body., , Dietary requirements, Dietary requirements and sources, , Adult man, , — 10 mg/day, , There is no specific dietary requirement for, sulfur. Adequate intake of sulfur-containing, essential amino acid methionine will meet the, body needs. Food proteins rich in methionine, and cysteine are the sources of sulfur., , Menstruating woman, , — 18 mg/day, , Pregnant and lactating woman — 40 mg/day, , Sources, Rich sources — Organ meats (liver, heart,, kidney)., , Excretion, The sulfur from different compounds is, oxidized in the liver to sulfate and excreted in, urine. The urine contains inorganic sulfate, (80%), organic or conjugated or ethereal sulfate, (10%) and unoxidized sulfur (10%). The, unoxidized sulfur is in the form of sulfurcontaining amino acids, thiocyanates etc., , IRON, The total content of iron in an adult body is, 3-5 g. About 70% of this occurs in the, erythrocytes of blood as a constituent of, hemoglobin. At least 5% of body iron is present, in myoglobin of muscle. Heme is the most, predominant iron-containing substance. It is a, constituent, of, several, proteins/enzymes, (hemoproteins)—hemoglobin,, myoglobin,, cytochromes, xanthine oxidase, catalase,, tryptophan pyrrolase, peroxidase. Certain other, proteins contain non-heme iron e.g. transferrin,, ferritin, hemosiderin., , Biochemical functions, 1. Iron mainly exerts its functions through the, compounds in which it is present. Hemoglobin, and myoglobin are required for the transport of, O2 and CO2., 2. Cytochromes and certain non-heme, proteins are necessary for electron transport, chain and oxidative phosphorylation., 3. Peroxidase, the lysosomal enzyme, is, required for phagocytosis and killing of bacteria, by neutrophils., , Good sources — Leafy vegetables, pulses,, cereals, fish, apples, dried, fruits, molasses., Poor sources — Milk,, rice., , wheat,, , polished, , Absorption, transport and storage, Iron is mainly absorbed in the stomach and, duodenum. In normal people, about 10% of, dietary iron is usually absorbed. However, in, iron deficient (anemic) individuals and growing, children, a much higher proportion of dietary, iron is absorbed to meet the increased body, demands., Iron is mostly found in the foods in ferric form, (Fe3+), bound to proteins or organic acids. In the, acid medium provided by gastric HCl, the Fe3+, is released from foods. Reducing substances, such as ascorbic acid (vitamin C) and cysteine, convert ferric iron (Fe3+) to ferrous form (Fe2+)., Iron in the ferrous form is soluble and readily, absorbed., , Factors affecting Fe absorption, 1. Acidity, ascorbic acid, alcohol, fructose, and cysteine promote iron absorption., 2. In iron deficiency anemia, Fe absorption is, increased to 2-10 times that of normal., 3. Small peptides and amino acids favour, iron uptake., 4. Phytate (found in cereals) and oxalate, (found in leafy vegetables) interfere with Fe, absorption.
Page 425 :
415, , Chapter 18 : MINERAL METABOLISM, , Lumen of GIT, Food Fe, , Mucosal cells of GIT, Apoferritin, , HCl, organic acids, Fe3+, Ascorbic acid, cysteine, Fe2+, , Apotransferrin, , Ferritin, (Fe3+), Fe3+, Ferroxidase, , Tissues, , Plasma, , Liver, Ferritin, Hemosiderin, , Transferrin, (Fe3+), Ferroreductase, Fe2+, , Fe2+, , Fe3+, , Bone marrow (Hb), , Ceruloplasmin, or ferroxidase II, Fe2+, , Muscle (Mb), Other tissues, (Cyts & NHI), , Fig. 18.3 : Iron absorption and transport (GIT–Gastrointestinal tract;, Hb–Hemoglobin; Mb–Myoglobin; Cyts–Cytochromes; NHI–Non-heme iron)., , 5. A diet with high phosphate content, decreases Fe absorption while low phosphate, promotes., 6. Impaired absorption of iron is observed in, malabsorption syndromes such as steatorrhea., 7. In patients with partial or total surgical, removal of stomach and/or intestine, iron, absorption is severely impaired., Iron in the mucosal cells : The iron (Fe2+), entering the mucosal cells by absorption is, oxidized to ferric form (Fe3+) by the enzyme, ferroxidase. Fe3+ then combines with apoferritin, to form ferritin which is the temporary storage, form of iron. From the mucosal cells, iron may, enter the blood stream (which mainly depends, on the body needs) or lost when the cells are, desquamated., Transport of Fe in the plasma : The iron, liberated from the ferritin of mucosal cells enters, the plasma in ferrous state. Here, it is oxidized to, ferric form by a copper-containing protein,, ceruloplasmin which possesses ferroxidase, activity. Another cuproprotein ferroxidase II also, helps for the conversion of Fe2+ to Fe3+., Ferric iron then binds with a specific ironbinding protein, namely transferrin or, siderophilin (a glycoprotein with mol. wt., 90,000). Each transferrin molecule can bind with, two atoms of ferric iron (Fe3+). The plasma, transferrin (concentration 250 mg/dl) can bind, with 400 mg of iron/dl plasma. This is known as, total iron binding capacity (TIBC) of plasma., , Storage of iron : Iron is stored in liver, spleen, and bone marrow in the form of ferritin. In the, mucosal cells, ferritin is the temporary storage, form of iron. A molecule of apoferritin (mol. wt., 500,000) can combine with 4,000 atoms of iron., The maximum iron content of ferritin on weight, basis is around 25%., , Hemosiderin is another iron storage protein, which can hold about 35% of iron by weight., Hemosiderin accumulates in the body (spleen,, liver) when the supply of iron is in excess of, body demands., , Iron is a one-way substance, Iron metabolism is unique as it operates, in a closed system. It is very efficiently, utilized and reutilized by the body. Further, iron, losses from the body are minimal (< 1 mg/day), which may occur through bile, sweat, hair, loss etc. Iron is not excreted into urine., Thus, iron differs from the vitamins or, other organic and inorganic substances which, are either inactivated or excreted during, the course of metabolic function. Hence, iron, is appropriately regarded as a one-way, substance., Iron entry into the body is controlled at the, absorption level, depending on the body needs., Thus the periodical blood loss in menstruating, women increases its requirements. Increased iron, demands are also observed in pregnancy,, lactation, and in growing children.
Page 426 :
416, , BIOCHEMISTRY, , Iron metabolism, A general overview of iron, metabolism is depicted in Fig.18.4., It shows the distribution of iron in, the body and its efficient, reutilization. It may be noted that, 1-2 mg of iron is absorbed per day, to replace the loss., , Disease states, 1. Iron deficiency anemia :, This is the most prevalent, nutritional disorder worldover,, including the well developed, countries (e.g. USA). Several, factors may contribute to iron, deficiency anemia. These include, inadequate intake or defective, absorption of iron, chronic blood, loss, repeated pregnancies and, hookworm infections., , Myoglobin, Body stores, , and other, compounds, 300 mg Fe, , 1,000 mg Fe, , 5, , m, , g/, , y, , da, , da, , g/, , y, , 5, , Food Fe, , Absorption, (1-2 mg/day), , m, , Plasma transferrin, 4 mg Fe, , Utilization for synthesis, (20 mg/day), , Fe lost, (1-2 mg/day), , Release by degradation, (20 mg/day), , Erythrocyte, hemoglobin, 2,500 mg Fe, , Fig. 18.4 : A general overview of iron metabolism., , Strict vegetarians are more, prone for iron deficieny anemia., This is due to the presence of inhibitors of iron, absorption in the vegetarian foods, besides the, relatively low content of iron., Iron deficiency anemia mostly occurs in, growing children, adolescent girls, pregnant and, lactating women. It is characterized by, microcytic hypochromic anemia with reduced, blood hemoglobin levels (<12 g/dl). The other, manifestations include apathy (dull and inactive),, sluggish metabolic activities, retarded growth, and loss of appetite., , 3. Hemochromatosis : This is a rare disease, in which iron is directly deposited in the tissues, (liver, spleen, pancreas and skin). Hemosiderosis, is sometimes accompanied by hemochromatosis., Bronzed-pigmentation of the skin, cirrhosis of, liver, pancreatic fibrosis are the manifestations of, this disorder. Hemochromatosis causes a, condition known as bronze diabetes., , Treatment : Iron deficiency is treated by, supplementation of iron along wih folic acid and, vitamin C., , COPPER, , 2. Hemosiderosis : This is a less common, disorder and is due to excessive iron in the body., It is commonly observed in subjects receiving, repeated blood transfusions over the years, e.g., patients of hemolytic anemia, hemophilia. As, already stated, iron is a one-way compound,, once it enters the body, it cannot escape., Excessive iron is deposited as ferritin and, hemosiderin., Hemosiderosis is commonly observed among, the Bantu tribe in South Africa. This is attributed, , to a high intake of iron from their staple diet, corn and their habit of cooking foods in iron, pots., , The body contains about 100 mg copper, distributed in different organs. It is involved in, several important functions., , Biochemical functions, 1. Copper is an essential constituent of, several enzymes. These include cytochrome, oxidase,, catalase,, tyrosinase,, superoxide, dismutase, monoamine oxidase, ascorbic acid, oxidase, ALA synthase, phenol oxidase and
Page 427 :
417, , Chapter 18 : MINERAL METABOLISM, , uricase. Due to its presence in a wide variety of, enzymes, copper is involved in many metabolic, reactions., 2. Copper is necessary for the synthesis of, hemoglobin (Cu is a constituent of ALA synthase,, needed for heme synthesis)., 3. Lysyl oxidase (a copper-containing enzyme), is required for the conversion of certain lysine, residues of collagen and elastin to allysine which, are necessary for cross-linking these structural, proteins., 4. Ceruloplasmin serves as ferroxidase and is, involved in the conversion of iron from Fe2+ to, Fe3+ in which form iron (transferrin) is, transported in plasma., 5. Copper is necessary for the synthesis of, melanin and phospholipids., 6. Development of bone and nervous system, (myelin) requires Cu., 7. Certain copper-containing non-enzymatic, proteins have been identified, although their, functions are not clearly known. These include, hepatocuprein (storage form in liver), cerebrocuprein (in brain) and hemocuprein (in RBC)., 8. Hemocyanin, a copper protein complex in, invertebrates, functions like hemoglobin for O2, transport., , Plasma copper, The copper concentration of plasma is about, 100-200 mg/dl. Most of this (95%) is tightly, bound to ceruloplasmin while a small fraction, (5%) is loosely held to albumin. Normal, concentration of serum ceruloplasmin is 25-50, mg/dl. It contains about 0.34% copper (6-8, atoms of Cu per molecule, half in Cu2+ state and, the other half in Cu+ state)., , Disease states, 1. Copper deficiency : Severe deficiency of, copper causes demineralization of bones,, demyelination of neural tissue, anemia, fragility, of arteries, myocardial fibrosis, hypopigmentation of skin, greying of hair., 2. Menke’s disease : This disorder is due to a, defect in the intestinal absorption of copper. It is, possible that copper may be trapped by metallothionein in the intestinal cells. The symptoms, of Menke’s disease include decreased copper in, plasma and urine, anemia and depigmentation, of hair., 3. Wilson’s, disease, (hepatolenticular, degeneration) : It is a rare disorder (1 : 50,000), of abnormal copper metabolism and is, characterized by the following manifestations., ●, , Dietary requirements, Adults, , — 2-3 mg/day, , ●, , Infants and children — 0.5-2 mg/day, , Sources, Liver, kidney, meat, egg yolk, cereals, nuts, and green leafy vegetables. Milk is a poor, source., , Absorption, About 10% of dietary copper is absorbed,, mainly in the duodenum. Metallothionein is a, transport protein that facilitates copper, absorption. Phytate, zinc and molybdenum, decrease copper uptake., , ●, , ●, , Copper is deposited in abnormal amounts in, liver and lenticular nucleus of brain. This may, lead to hepatic cirrhosis and brain necrosis., Low levels of copper and ceruloplasmin in, plasma (reference range 20–50 mg/dl) with, increased excretion of copper in urine., Copper deposition in kidney causes renal, damage. This leads to increased excretion of, amino, acids,, glucose,, peptides, and, hemoglobin in urine., Intestinal absorption of copper is very high,, about 4-6 times higher than normal., , Probable causes of Wilson’s disease : The, following explanations are offered to understand, the etiology of this disease., 1. A failure to synthesize ceruloplasmin or an, impairment in the binding capacity of copper to
Page 428 :
418, , BIOCHEMISTRY, , this protein or both. As a result of this, copper is, free in the plasma which easily enters the tissues, (liver, brain, kidney), binds with the proteins and, gets deposited. The albumin bound copper is, either normal or increased., , About 80% of body’s iodine is stored in the, organic, form, as, iodothyroglobulin, (a, glycoprotein) in the thyroid gland. This protein, contains, thyroxine,, diiodotyrosine, and, triiodothyronine in different proportions., , 2. A mutation in the gene encoding copper, binding ATPase is believed to be responsible for, Wilson’s disease. Defect in this ATPase reduces, intestinal excretion of Cu through bile., , Excretion of iodine mostly occurs through, kidney. It is also excreted through saliva, bile,, skin, and milk (in lactating women)., , Treatment : Administration of penicillamine,, a naturally occurring copper chelating agent, is, used for the treatment of Wilson’s disease., , Plasma iodine, , IODINE, The total body contains about 20 mg iodine,, most of it (80%) being present in the thyroid, gland. Muscle, salivary glands and ovaries also, contain some amount of iodine., , Biochemical functions, The only known function of iodine is its, requirement for the synthesis of thyroid, hormones, namely,, thyroxine (T4) and, triiodothyronine (T3). These hormones are, involved in several biochemical functions, (Chapter 19). Functionally, T3 is more active, than T4., , Dietary requirements, Adults, , —, , Pregnant women, , —, , 100-150 +g/day, 200 +g/day, , Sources, Seafoods, drinking water, vegetables, fruits, (grown on seaboard). High altitudes are deficient, in iodine content in water as well as soil. Plant, and animal foods of these areas, therefore,, contain lesser amount of iodine. In these regions,, iodine is added to drinking water or to table salt., , Absorption, storage and excretion, Iodine as iodide is mainly absorbed from the, small intestine. Normally, about 30% of dietary, iodine is taken up by the intestinal cells. Iodine, absorption also occurs through skin and lungs., , The normal concentration of plasma iodine is, 4-10 mg/dl. Most of this is present as protein, bound iodine (PBI) and represents the iodine, contained in the circulating thyroid hormones., PBI level decreases in hypothyroidism and, increases in hyperthyroidism. RBC do not, contain iodine., , Disease states, The disorders of iodine metabolism—simple, goiter and toxic goiter—are discussed in detail, under thyroid hormones (Chapter 19)., , MANGANESE, The total body content of manganese is about, 15 mg. The liver and kidney are rich in Mn., Within the cells, Mn is mainly found in the, nuclei in association with nucleic acids., , Biochemical functions, 1. Mn serves as a cofactor for several, enzymes. These include arginase, pyruvate, carboxylase,, isocitrate, dehydrogenase,, superoxide dismutase (mitochondrial) and, peptidase., 2. Mn is required for the formation of bone,, proper reproduction and normal functioning of, nervous system., 3. Mn is necessary for the synthesis of, mucopolysaccharides and glycoproteins., 4. Hemoglobin synthesis involves Mn., 5. Mn inhibits lipid peroxidation., 6. Mn is necessary for cholesterol biosynthesis.
Page 429 :
419, , Chapter 18 : MINERAL METABOLISM, , Dietary requirements, The exact requirement of Mn is not known., About 2-9 mg/day is recommended for an adult., , Sources, , 3. The storage and secretion of insulin from, the `-cells of pancreas require Zn., 4. Zn is necessary to maintain the normal, levels of vitamin A in serum. Zn promotes the, synthesis of retinol binding protein., , Cereals, nuts, leafy vegetables and fruits. Tea, is a rich source of Mn., , 5. It is required for wound healing. Zn, enhances cell growth and division, besides, stabilizing biomembranes., , Absorption, , 6. Gusten, a zinc containing protein of the, saliva, is important for taste sensation., , About 3-4% of dietary Mn is normally, absorbed in the small intestine. Iron inhibits Mn, absorption., , 7. Zn is essential for proper reproduction., , Dietary requirements, Serum Mn, Manganese in the serum is bound to a specific, carrier protein—transmagnanin (a `-globulin)., The normal blood contains about 5-20 mg/dl., , Zinc requirement for an adult is 10-15 mg/, day. It is increased (by about 50%) in pregnancy, and lactation., , Sources, Disease states, , Meat, fish, eggs, milk, beans, nuts., , Mn deficiency in animals causes, , Absorption, , 1. Retarded growth, bone deformities and, in, severe deficiency, sterility., 2. Accumulation of fat in liver., 3. Increased activity, phosphatase, and, , of, , serum, , alkaline, , 4. Diminished activity of `-cells of pancreas, (low insulin)., , Zinc is absorbed mainly in the duodenum. Zn, from the animal sources is better absorbed than, the vegetable sources. Zn absorption appears to, be dependent on a transport protein—metallothionein. Phytate, calcium, copper and iron, interfere while small peptides and amino acids, promote Zn absorption., , Serum Zn, ZINC, The total content of zinc in an adult body is, about 2 g. Prostate gland is very rich in Zn (100, mg/g). Zinc is mainly an intracellular element., , Biochemical functions, 1. Zn is an essential component of several, enzymes e.g. carbonic anhydrase, alcohol, dehydrogenase, alkaline phosphatase, carboxypeptidase, superoxide dismutase (cytosolic)., 2. Zinc may be regarded as an antioxidant, since the enzyme superoxide dismutase (Zn, containing) protects the body against free radical, damage., , The concentration of Zn in serum is about, 100 mg/dl. Erythrocytes contain higher content, of Zn (1.5 mg/dl) which is found in association, with the enzyme carbonic anhydrase., , Disease states, 1. Zinc deficiency is associated with growth, retardation, poor wound healing, anemia, loss of, appetite, loss of taste sensation, impaired, spermatogenesis etc. It is reported that Zn, deficiency in pregnant animals causes congenital, malformations of the fetus. Deficiency of Zn may, result in depression, dementia and other, psychiatric disorders. The neuropsychiatric, manifestations of chronic alcoholism may be, partly due to zinc deficiency.
Page 430 :
420, , BIOCHEMISTRY, , Acrodermatitis enteropathica is a rare, inherited metabolic disease of zinc deficiency, caused by a defect in the absorption of Zn from, the intestine., 2. Zinc toxicity is often observed in welders, due to inhalation of zinc oxide fumes. The, manifestations of Zn toxicity include nausea,, gastric ulcer, pancreatitis, anemia and excessive, salivation., , MOLYBDENUM, Molybdenum is a constituent of the enzymes, xanthine oxidase, aldehyde oxidase and sulfite, oxidase. Nitrite reductase (containing Mo) is a, plant enzyme, required for nitrogen fixation., The requirements of Mo are not clearly, known. However, it is widely distributed in, the natural foods. Dietary Mo is effectively, (60%-70%) absorbed by the small intestine., Some workers have reported that Mo, decreases the mobilization and utilization of, copper in the body., , Molybdenosis is a rare disorder caused by, excessive consumption of Mo. Its manifestations, include impairment in growth, diarrhea and, anemia. Intestinal absorption of copper is, diminished., , COBALT, Cobalt is only important as a constituent of, vitamin B12. Cobalt content of vitamin B12 is, about 4% by weight. The functions of cobalt are, the same as that of vitamin B12 (Chapter 7)., Administration of cobalt stimulates the, production of the hormone erythropoietin, which, promotes erythropoiesis., Prolonged administration of cobalt is toxic as, it results in polycythemia (increased RBC in, blood)., , FLUORINE, Fluoride is mostly found in bones and teeth., The beneficial effects of fluoride in trace, amounts are overshadowed by its harmful effects, caused by excess consumption., , Biochemical functions, 1. Fluoride prevents the development of, dental caries. It forms a protective layer of acid, resistant fluoroapatite with hydroxyapatite of the, enamel and prevents the tooth decay by bacterial, acids. Further, fluoride inhibits the bacterial, enzymes and reduces the production of acids., 2. Fluoride is necessary for the proper, development of bones., 3. It inhibits the activities of certain enzymes., Sodium fluoride inhibits enolase (of glycolysis), while fluoroacetate inhibits aconitase (of citric, acid cycle)., , Dietary requirements and sources, An intake of less than 2 ppm of fluoride will, meet the daily requirements. Drinking water is, the main source., , Disease states, 1. Dental caries : It is clearly established that, drinking water containing less than 0.5 ppm of, fluoride is associated with the development of, dental caries in children (Refer Chapter 13)., 2. Fluorosis : Excessive intake of fluoride is, harmful to the body. An intake above 2 ppm, (particularly > 5 ppm) in children causes, mottling of enamel and discoloration of teeth., The teeth are weak and become rough with, characteristic brown or yellow patches on their, surface. These manifestations are collectively, referred to as dental fluorosis., An intake of fluoride above 20 ppm is toxic, and causes pathological changes in the bones., Hypercalcification, increasing the density of the, bones of limbs, pelvis and spine, is a, characteristic feature. Even the ligaments of spine
Page 431 :
421, , Chapter 18 : MINERAL METABOLISM, , and collagen of bones get calcified. Neurological, disturbances are also commonly observed. The, manifestations described here constitute skeletal, fluorosis. In the advanced stages, the individuals, are crippled and cannot perform their daily, routine work due to stiff joints. This condition of, advanced fluorosis is referred to as genu valgum., The fluoride content of water in some parts of, Andhra Pradesh, Punjab and Karnataka is quite, high. Fluorosis is prevalent in these regions,, causing concern to government and health, officials., 3. Fluoridation of water and use of fluoride, tooth-pastes : In order to prevent the dental, caries in children, some advanced countries like, USA have started fluoridation of water. Further,, the consumer markets till recently were flooded, , with fluoride toothpastes. There is some, rethinking on these aspects due to the toxic, effects of excess fluoride., , SELENIUM, Selenium was originally identified as an, element that causes toxicity to animals (alkali, disease) in some parts of USA, containing large, amounts of Se in the soil. Later work, however,, has shown that Se in smaller amounts is, biologically important., , Biochemical functions, 1. Selenium, along with vitamin E, prevents, the development of hepatic necrosis and, muscular dystrophy., , ☞ Serum calcium level is increased (normal 9–11 mg/dl) in hyperparathyroidism. This, condition is also associated with elevated urinary excretion of Ca and P, often leading, to stone formation., , ☞ Tetany, caused by a drastic reduction in serum Ca, is characterized by neuromuscular, irritability and convulsions., , ☞ Rickets is due to defective calcification of bones. This may be caused by deficiency of, Ca and P or vitamin D or both., , ☞ Osteoporosis is the bone disorder of the elderly, characterized by demineralization, resulting in a progressive loss of bone mass. It is the major cause of bone fractures and, disability in the old people., , ☞ Decreased levels of serum Na (hyponatremia) is observed in diarrhea and vomiting,, besides Addison’s disease, while increased serum Na (hypernatremia) is found in, Cushing’s syndrome., , ☞ Iron deficiency anemia is the most prevalent nutritional disorder worldover. It is most, commonly observed in pregnant and lactating women., , ☞ Wilson’s disease is due to an abnormal copper metabolism. It is characterized by, abnormal deposition of copper in liver and brain, besides the low levels of plasma, copper and ceruloplasmin., , ☞ Endemic goitre, due to dietary iodine deficiency, is very common. Consumption of, iodized salt is advocated to overcome this problem., , ☞ Fluorosis is caused by an excessive intake of fluoride. The manifestations include, mottling of enamel and discoloration of teeth. In the advanced stages, hypercalcification, of limb bones and ligaments of spine get calcified, ultimately crippling the individual.
Page 432 :
422, 2. Se is involved in maintaining structural, integrity of biological membranes., 3. Se as selenocysteine is an essential, component of the enzyme glutathione, peroxidase. This enzyme protects the cells, against the damage caused by H2O2. It appears, from recent studies that selenocysteine is directly, incorporated during protein biosynthesis., Therefore, selenocysteine is considered as a, separate (21st) amino acid., 4. Se prevents lipid peroxidation and protects, the cells against the free radicals, including, superoxide (O–2)., , BIOCHEMISTRY, , are associated with increased risk of cardiovascular disease, and various cancers., , Toxicity, Selenosis is the toxicity due to very excessive, intake of Se. The manifestations of selenosis, include weight loss, emotional disturbances,, diarrhea, hair loss and garlic odor in breath. The, compound dimethyl selenide is responsible for, the garlic odor., , CHROMIUM, , 5. Se protects animals from carcinogenic, chemicals. However, the precise role of Se in, humans with regard to cancer prevention is not, clearly identified., , The total human body contains about 6 mg, chromium. The Cr content of blood is about 20, mg/dl. Cr performs several biochemical, functions., , 6. Se binds with certain heavy metals (Hg,, Cd) and protects the body from their toxic, effects., , 1. In association with insulin, Cr promotes, the utilization of glucose. Cr is a component of, a protein namely chromodulin which facilitates, the binding of insulin to cell receptor sites., , 7. A selenium containing enzyme 5v-deiodinase converts thyroxine (T4) to triiodothyronine in the thyroid gland., 8. Thioredoxin reductase, involved in purine, nucleotide metabolism, is also a selenoprotein., , Requirements and sources, A daily intake of 50-200 mg of Se has been, recommended for adults. The good sources of Se, are organ meats (liver, kidney) and sea foods., , Disese states, Deficiency : Se deficiency in animals leads to, muscular dystrophy, pancreatic fibrosis and, reproductive disorders. In humans, Keshan, disease, an endemic cardiomyopathy (in China), is attributed to the deficiency of Se. Epidemiological studies reveal that low serum Se levels, , 2. Cr lowers the total serum cholesterol level., 3. It is involved in lipoprotein metabolism., Cr decreases serum low density lipoproteins, (LDL) and increases high density lipoproteins, (HDL) and, thus, promotes health., 4. It is believed that Cr participates in the, transport of amino acids into the cells (heart and, liver)., The dietary requirement of Cr is not known. It, is estimated that an adult man consumes about, 10 to 100 mg/day. The good sources of Cr, include brewer’s yeast, grains, cereals, cheese, and meat., Chromium deficiency causes disturbances in, carbohydrate, lipid and protein metabolisms., Excessive intake of Cr results in toxicity, leading, to liver and kidney damage.
Page 433 :
Chapter 18 : MINERAL METABOLISM, , 1. The minerals or inorganic elements are required for normal growth and maintenance, of the body. They are classified as principal elements and trace elements. There are, seven principal elements—Ca, P, Mg, Na, K, Cl and S. The trace elements include Fe,, Cu, I, Zn, Mn, Mo, Co, F, Se and Cr., 2. Calcium is required for the development of bones and teeth, muscle contraction, blood, coagulation, nerve transmission etc. Absorption of Ca from the duodenum is promoted, by vitamin D, PTH and acidity while it is inhibited by phytate, oxalate, free fatty acids, and fiber. The normal level of serum Ca (9-11 mg/dl) is controlled by an interplay of, PTH, calcitriol and calcitonin., 3. Serum Ca level is elevated in hyperparathyroidism and diminished in hypoparathyroidism. Hypocalcemia causes tetany, the symptoms of which include neuromuscular, irritability, spasm and convulsions., 4. Phosphorus, besides being essential for the development of bones and teeth, is a, constituent of high energy phosphate compounds (ATP, GTP) and nucleotide coenzymes, (NAD+, NADP+)., 5. Sodium, potassium and chlorine are involved in the regulation of acid-base equilibrium,, fluid balance and osmotic pressure in the body. Sodium is the principal extracellular, cation (serum level 135-145 mEq/l), while potassium is the chief intracellular cation, (serum level 3.5-5.0 mEq/l)., 6. Iron is mainly required for O2 transport and cellular respiration. Absorption of iron is, promoted by ascorbic acid, cysteine, acidity and small peptides while it is inhibited by, phytate, oxalate and high phosphate., 7. Iron (Fe3+) is transported in the plasma in a bound form to transferrin. It is stored as, ferritin in liver, spleen and bone marrow. Iron deficiency anemia causes microcytic, hypochromic anemia. Excessive consumption of iron results in hemosiderosis which is, due to the tissue deposition of hemosiderin., 8. Copper is an essential constituent of several enzymes (e.g catalase, cytochrome oxidase,, tyrosinase). Ceruloplasmin is a copper containing protein required for the transport of, iron (Fe3+) in the plasma. Wilson’s disease is an abnormality in copper metabolism,, characterized by the deposition of copper in liver, brain and kidney., 9. Iodine is important as a component of thyroid hormones (T4 and T3) while cobalt is a, constituent of vitamin B12. Zinc is necessary for the storage and secretion of insulin, and maintenance of normal vitamin A levels in serum, besides being a component of, several enzymes (e.g. carbonic anhydrase, alcohol dehydrogenase)., 10. Fluorine in trace amounts (<2 ppm) prevents dental caries while its higher intake, leads to fluorosis. Selenium is assigned an antioxidant role as it protects the cells from, free radicals. Chromium promotes the utilization of glucose and reduces serum, cholesterol., , 423
Page 434 :
424, , BIOCHEMISTRY, , I. Essay questions, 1. Write briefly on the trace elements and their metabolism in the body., 2. Discuss the biochemical functions, dietary requirements, sources and absorption of calcium., 3. Write an essay on the iron metabolism in the body., 4. Describe the metabolism of copper, zinc and manganese., 5. Write on the biochemical importance and disease states of fluorine and selenium., , II. Short notes, (a) Homeostasis of calcium, (b) Osteoporosis, (c) Phosphorus, (d) Sodium and chlorine,, (e) Potassium, (f) Factors affecting Fe absorption, (g) Hemosiderosis, (h) Wilson’s disease, (i) Iodine,, (j) Magnesium., , III. Fill in the blanks, 1. The normal concentration of serum calcium ______________., 2. The vitamin derived hormone that regulates calcium homeostasis ______________., 3. The inorganic element found in the structure of majority of high-energy compounds, ______________., 4. Several kinase enzymes require the mineral cofactor ______________., 5. The principal cation of extracellular fluid ______________., 6. The normal concentration of serum potassium ______________., 7. Iron is transported in the plasma in a bound form to a protein ______________., 8. The copper containing protein involved for the conversion of ferrous iron (Fe2+) to ferric iron, (Fe3+) in the plasma ______________., 9. The zinc containing protein in the saliva involved in taste sensation ______________., 10. The element involved in the protection of cells against the damage of H2O2 and other free, radicals ______________., , IV. Multiple choice questions, 11. The following substance(s) is(are) involved in the regulation of plasma calcium level, (a) Calcitriol (b) Parathyroid hormone (c) Calcitonin (d) All of them., 12. The following is a sulfur containing essential amino acid, (a) Methionine (b) Cysteine (c) Cystine (d) All of them., 13. Iron in the mucosal cells binds with the protein, (a) Transferrin (b) Ferritin (c) Ceruloplasmin (d) Hemosiderin., 14. The following element is involved in wound healing, (a) Calcium (b) Sodium (c) Zinc (d) Magnesium., 15. Pick up element that prevents the development of dental caries, (a) Fluorine (b) Calcium (c) Phosphorus (d) Sodium.
Page 435 :
CLINICAL BIOCHEMISTRY AND NUTRITION, 19, 1, ■, 20, 2, ■, 21, 3, ■, , Hormones, , 427, , Organ Function Tests, , 453, , Water, Electrolyte and, Acid-base Balance, , 468, , 4, 22 Tissue Proteins and, 5, Body Fluids, , ■, 6, 23, ■, , 487, 502, , Nutrition, , 7, , Section, , IV, IV
Page 436 :
“This page intentionally left blank"
Page 437 :
Section 4, , Clinical Biochemistry and Nutrition, , Chapter, , Hormones, , 19, , The hormones speak :, , “We are the chemical messengers of the body!, Diversified in our structure and function;, Act either directly or through messengers;, ‘Growth, health and welfare’ is our motto.”, , T, , he living body possesses a remarkable, communication system to coordinate its, biological functions. This is achieved by two, distinctly organized functional systems., 1. The nervous system coordinates the body, functions through the transmission of electrochemical impulses., , 2. The endocrine system acts through a wide, range of chemical messengers known as, hormones., , Hormones are conventionally defined as, organic substances, produced in small amounts, by specific tissues (endocrine glands), secreted, into the blood stream to control the, metabolic and biological activities in the target, cells. Hormones may be regarded as the, chemical, messengers, involved, in, the, transmission of information from one tissue to, another and from cell to cell. The major, endocrine organs in human body are depicted in, Fig.19.1)., , CLASSIFICATION OF HORMONES, Hormones may be classified in many ways, based on their characteristics and functions. Two, types of classification are discussed here, , I. Based on the chemical nature, The hormones can be categorized into three, groups considering their chemical nature., 1. Protein or peptide hormones e.g. insulin,, glucagon, antidiuretic hormone, oxytocin., 2. Steroid hormones e.g. glucocorticoids,, mineralocorticoids, sex hormones., 3. Amino acid derivatives e.g. epinephrine,, norepinephrine, thyroxine (T4), triiodothyronine, (T3)., , II. Based on the mechanism of, action, Hormones are classified into two broad, groups (I and II) based on the location of the, , 427
Page 438 :
428, , BIOCHEMISTRY, , Pineal gland, Pituitary gland, , Thyroid gland, , Pancreas, Adrenal gland, , Testis (male), , Ovary, (female), , stimulate the release of certain molecules,, namely the second messengers which, in, turn, perform the biochemical functions., Thus, hormones themselves are the first, messengers., Group II hormones are subdivided into three, categories based on the chemical nature of the, second messengers., (a) The second messenger is cAMP e.g., ACTH, FSH, LH, PTH, glucagon,, calcitonin., (b) The second messenger is phosphatidylinositol/calcium e.g. TRH, GnRH, gastrin,, CCK., (c) The second messenger is unknown e.g., growth hormone, insulin, oxytocin,, prolactin., The principal human hormones, their classification based on the mechanism of action, and, major functions are given in Table 19.1., , Mechanism of action of, group I hormones, , Fig. 19.1 : Diagrammatic representation of, major endocrine glands., , receptors to which they bind and the signals, used to mediate their action., 1. Group I hormones : These hormones bind, to intracellular receptors to form receptorhormone, complexes, (the, intracellular, messengers) through which their biochemical, functions are mediated. Group I hormones, are lipophilic in nature and are mostly, derivatives of cholesterol (exception—T3 and, T4). e.g. estrogens, androgens, glucocorticoids,, calcitriol., 2. Group II hormones : These hormones bind, to cell surface (plasma membrane) receptors and, , These hormones are lipophilic in nature and, can easily pass across the plasma membrane., They act through the intracellular receptors, located either in the cytosol or the nucleus., The, hormone-receptor complex binds to, specific regions on the DNA called hormone, responsive element (HRE) and causes increased, expression of specific genes (Fig.19.2). It is, believed that the interaction of hormone, receptor complex with HRE promotes initiation, and, to a lesser extent, elongation and, termination of RNA synthesis (transcription). The, ultimate outcome is the production of specific, proteins (translation) in response to hormonal, action., , Mechanism of action of, group II hormones, These hormones are considered as the first, messengers. They exert their action through, mediatory molecules, collectively called second, messengers.
Page 439 :
429, , Chapter 19 : HORMONES, , TABLE 19.1 Principal human hormones—classification, (by mechanism of action), origin and major functions, , Hormone(s), , Origin, , Major Function(s), , Group I. HORMONES THAT BIND TO INTRACELLULAR RECEPTORS, Estrogens, , Ovaries and adrenal cortex Female sexual characteristics, menstrual cycle., , Progestins, , Ovaries and placenta, , Androgens, , Testes and adrenal cortex Male sexual characteristics, spermatogenesis., , Glucocorticoids, , Adrenal cortex, , Affect metabolisms, suppress immune system., , Mineralocorticoids, , Adrenal cortex, , Maintenance of salt and water balance., , Calcitriol (1, 25–DHCC), , Kidney (final form), , Promotes absorption of Ca2+ from intestine, kidney and bone., , Thyroid hormones (T3, T4), , Thyroid, , Promote general metabolic rate., , Involved in menstrual cycle and maintenance of pregnancy., , Group II. HORMONES THAT BIND TO CELL SURFACE RECEPTORS, A. The second messenger is cAMP, Adrenocorticotropic hormone (ACTH) Anterior pituitary, , Stimulates the release of adrenocorticosteroids., , Follicle stimulating hormone (FSH), , Anterior pituitary, , In females, stimulates ovulation and estrogen synthesis., In males, promotes spermatogenesis., , Luteinizing hormone (LH), , Anterior pituitary, , Stimulates synthesis of estrogens and progesterone and, causes ovulation. Promotes androgen synthesis by testes., , Chorionic gonadotropin (hCG), , Anterior pituitary, , Stimulates progesterone release from placenta., , Thyroid stimulating hormone (TSH), , Anterior pituitary, , Promotes the release of thyroid hormones (T3, T4)., , E-Endorphins and enkephalins, , Anterior pituitary, , Natural endogenous analgesics (pain relievers)., , Antidiuretic hormone (ADH), , Posterior pituitary (stored) Promotes water reabsorption by kidneys., , Glucagon, , Pancreas, , Increases blood glucose level, stimulates glycogenolysis, and lipolysis., , Parathyroid hormone (PTH), , Parathyroid, , Increases serum calcium, promotes Ca2+ release from bone., , Calcitonin, , Thyroid, , Lowers serum calcium. Decreases Ca2+ uptake by bone and kidney., , Epinephrine, , Adrenal medulla, , Increases heart rate and blood pressure. Promotes glycogenolysis in liver and muscle and lipolysis in adipose tissue., , Norepinephrine, , Adrenal medulla, , Stimulates lipolysis in adipose tissue., , B. The second messenger is phosphatidyl inositol/calcium, Thyrotropin-releasing hormone (TRH), , Hypothalamus, , Promotes TSH release., , Gonadotropin-releasing hormone (GnRH), , Hypothalamus, , Stimulates release of FSH and LH., , Gastrin, , Stomach, , Stimulates gastric HCI and pepsinogen secretion., , Cholecystokinin (CCK), , Intestine, , Stimulates contraction of gall bladder and secretion of pancreatic, enzymes., , C. The second messenger is unknown/unsettled, Growth hormone (GH), , Anterior pituitary, , Promotes growth of the body (bones and organs)., , Prolactin (PRL), , Anterior pituitary, , Growth of mammary glands and lactation., , Oxytocin, , Posterior pituitary (stored) Stimulates uterine contraction and milk ejection., , Insulin, , Pancreas, , Lowers blood glucose (hypoglycemic effect), promotes protein, synthesis and lipogenesis., , Somatomedins (insulin-like, growth factors, IGF-I, IGF-II), , Liver, , Growth related functions of GH are mediated., Stimulates growth of cartilage.
Page 440 :
430, , BIOCHEMISTRY, , cAMP—THE SECOND, MESSENGER, , Plasma membrane, H, , Cyclic AMP (cAMP, cyclic, adenosine 3c,5c-monophosphate), is a ubiquitous nucleotide. It, consists of adenine, ribose and a, phosphate (linked by 3c,5c, linkage). cAMP acts as a second, messenger for a majority of, polypeptide hormones., The membrane-bound enzyme, adenylate cyclase converts ATP to, cyclic AMP. cAMP is hydrolysed, by phosphodiesterase to 5c-AMP, (Fig.19.3)., , Adenylate cyclase, system, A series of events occur at the, membrane level that influence, the activity of adenylate cyclase, leading to the synthesis of cAMP., This process is mediated by, G-proteins, so designated due to, their ability to bind to guanine, nucleotides., , Action of cAMP—a, general view, , H, , H, , R, , H, , R, , R, , Cytosol, , Nucleus, , DNA, , Acceptor, Gene, Transcription, mRNA, , mRNA, Translation, Specific protein, , Biochemical, response, , Once, produced,, cAMP, performs its role as a second, Fig. 19.2 : Mechanism of action of steroid hormones (H–Hormone;, R–Receptor; HR–Hormone-receptor complex)., messenger in eliciting biochemical responses (Fig.19.4)., cAMP activates protein kinase A, (A stands for cAMP). This enzyme is a heterote- protein that ultimately causes the biochemical, tramer consisting of 2 regulatory subunits (R) and response., 2 catalytic subunits (C)., It should, however, be remembered that, cAMP binds to inactive protein kinase and cAMP does not act on all protein kinases. For, causes the dissociation of R and C subunits., instance, on protein kinase C (the second, messenger is diacylglycerol)., 4cAMP + R2C2 o� R2(4 cAMP) + 2C, (inactive), (inactive), (active), Dephosphorylation of proteins : A group of, The active subunit (C) catalyses phosphory- enzymes called protein phosphatases hydrolyse, lation of proteins (transfer of phosphate group to and remove the phosphate group added to, serine and threonine residues). It is the phospho- proteins.
Page 441 :
431, , Chapter 19 : HORMONES, , ATP, Adenylate, cyclase, PPi, , Mg, , 2+, , NH2, N, , N, , N, , N, 5c, , O, , O, , CH2, , –, , O P O H, , H, , H, H, , 3c, , O, , OH, , 3c,5c-Cyclic adenosine, monophosphate (cAMP), H2O, , hormonal action. In response to the stimuli of, central nervous system, hypothalamus liberates, certain releasing factors or hormones. These, factors stimulate or inhibit the release of, corresponding tropic hormones from the anterior, pituitary. Tropic hormones stimulate the target, endocrine tissues to secrete the hormones they, synthesize., The, relationship, between, hypothalamus and pituitary with endocrine, glands is illustrated in Fig.19.6. In general, the, hormonal system is under feedback control. For, instance, adrenocorticotropic hormone (ACTH), inhibits the release of corticotropin releasing, hormone (CRH)., , Phosphodiesterase, 5c-AMP, , Fig. 19.3 : Synthesis and degradation of cAMP., , Degradation of cAMP : cAMP undergoes, rapid hydrolysis, catalysed by the enzyme, phosphodiesterase to 5’ AMP which is inactive., Hence, the effect of cAMP will be shortlived, if the hormone stimulating adenylate cyclase, is removed. Caffeine and theophylline, (methylxanthine, derivatives), can, inhibit, phosphodiesterases and increase the intracellular, levels of cAMP., , HYPOTHALAMIC AND, PITUITARY HORMONES, The pituitary gland or hypophysis (weighing, about 1 g) is located below the hypothalamus, of the brain. It consists of two distinct parts—, the anterior pituitary (adenohypophysis) and the, posterior pitutitary (neurohypophysis) connected, by pars intermedia (Fig.19.5). The latter is, almost absent in humans, although found in, lower organisms., , Hypothalamus is a specialized center in the, brain that functions as a master coordinator of, , HYPOTHALAMIC HORMONES, Hypothalamus produces at least six releasing, factors or hormones., 1. Thyrotropin-releasing hormone (TRH) : It, is a tripeptide consisting of glutamate derivative, (pyroglutamate), histidine and proline. TRH, stimulates anterior pituitary to release thyroidstimulating hormone (TSH or thyrotropin) which,, in turn, stimulates the release of thyroid, hormones (T3 and T4)., 2. Corticotropin-releasing hormone (CRH) :, It stimulates anterior pituitary to release, adrenocorticotropic hormone (ACTH) which in, turn, acts on adrenal cortex to liberate, adrenocorticosteroids. CRH contains 41 amino, acids., 3. Gonadotropin-releasing hormone (GnRH) :, It is a decapeptide. GnRH stimulates anterior, pituitary to release gonadotropins, namely, luteinizing hormone (LH) and follicle stimulating, hormone (FSH)., 4. Growth hormone-releasing hormone, (GRH) with 44 amino acids stimulates the, release of growth hormone (GH or somatotropin), which promotes growth., 5. Growth, hormone, release-inhibiting, hormone (GRIH) : It contains 14 amino acids, and is also known as somatostatin. GRIH inhibits, the release of growth hormone from the anterior, pituitary.
Page 442 :
432, , BIOCHEMISTRY, , Hormone, , Receptor, Plasma membrane, , GTP regulatory protein, Adenylate, cyclase, , Cytosol, PPi, , ATP, Phosphodiesterase, AMP, , 4cAMP, ( ), , R, , C, , R, , C, , R2C2, R, C, , C, , R, R 2(4 cAMP), , ATP, , ADP, , Phosphoprotein, , Protein, Phosphatase, , Pi, , Ultimate, biochemical, response, , Fig. 19.4 : Overview of synthesis and action of cAMP (R2C2–cAMP dependent protein kinase A;, R2–Regulatory subunits; C2–Catalytic subunits; C–Active catalytic unit of R2C2)., , 6. Prolactin release-inhibiting hormone, (PRIH) : It is believed to be a dopamine and/or, a small peptide that inhibits the release of, prolactin (PRL) from anterior pituitary., , hormones that influence—either directly or, indirectly—a variety of biochemical processes in, the body. The hormones of adenohypophysis are, broadly classified into three categories., I. The growth hormone-prolactin group., , ANTERIOR PITUITARY HORMONES, Anterior pituitary or adenohypophysis is truly, the master endocrine organ. It produces several, , II. The glycoprotein hormones., III. The pro-opiomelanocortin, family., , peptide
Page 444 :
434, 1. Effects on growth : As is obvious from the, name, GH is essential for the growth. The, growth-related effects of GH are mediated, through insulin like growth factor I (IGF-I) which, is also known as somatomedin C (formerly, sulfation factor), produced by liver., 2. Effects on protein metabolism : Growth, hormone has an anabolic effect on protein, metabolism. It promotes the uptake of amino, acids into the tissues and increases the protein, synthesis. The overall effect of GH is a positive, nitrogen balance that leads to increase in body, weight., 3. Effects on carbohydrate metabolism :, Growth hormone is antagonistic to insulin and, causes hyperglycemia. GH increases gluconeogenesis, decreases glucose utilization, impairs, glycolysis and reduces the tissue uptake of, glucose., 4. Effects on lipid metabolism : Growth, hormone promotes lipolysis in the adipose tissue, and increases the circulatory levels of free fatty, acids and their oxidation. It increases, ketogenesis, particularly in diabetes., 5. Effects on mineral metabolism : Growth, hormone promotes bone mineralization and its, growth, as clearly observed in the growing, children., , Abnormalities of GH production, Deficiency of GH :, Impairment in the, secretion of growth hormone in the growing age, causes dwarfism. The other deficiency metabolic, effects are not that serious in nature., Overproduction of GH : Excessive production, of GH causes gigantism in children and, acromegaly in adults. This usually occurs in the, acidophil tumor of pituitary gland. Gigantism is, characterized by increased growth of long bones, and this is observed before the epiphyseal plates, close. Acromegaly occurs after epiphyseal, closure and is characterized by increase in the, size of hands, facial changes (enlarged nose,, protruding jaw), excessive hair, thickening of, skin etc., , BIOCHEMISTRY, , Prolactin, Prolactin (PRL) is also called lactogenic, hormone, luteotropic hormone, mammotropin or, luteotropin., Biochemical functions of PRL : Prolactin is, primarily concerned with the initiation and, maintenance of lactation in mammals. PRL, increases the levels of several enzymes involved, in carbohydrate and lipid metabolism. PRL, promotes, HMP, shunt,, increases, lipid, biosynthesis and stimulates lactose production in, mammary glands., Prolactin promotes the growth of corpus luteum, (hence also known as luteotropic hormone) and, stimulates the production of progesterone., , II. The glycoprotein hormones, The following four hormones are glycoprotein, in nature and possess certain structural, similarities, despite their functional diversity., 1. Thyroid stimulating hormone (TSH), 2. Follicle stimulating hormone (FSH), 3. Luteinizing hormone (LH), 4. Human chorionic gonadotropin (hCG)., The last three hormones (2-4) are collectively, referred to as gonadotropins due to their, involvement in the function of gonads. The, hormone hCG is produced by human placenta, and not by pituitary. However, due to its, structural resemblance with other hormones, it is, also considered here., 1. Thyroid stimulating hormone (TSH) : TSH, is a dimer (DE) glycoprotein with a molecular, weight of about 30,000., Regulation of TSH production : The release of, TSH from anterior pituitary is controlled by a, feedback mechanism. This involves the hormones, of thyroid gland (T3 and T4) and thyrotropinreleasing hormone (TRH) of hypothalamus., Functions of TSH : The biochemical effects of, TSH on thyroid gland are briefly discussed here., TSH binds with plasma membrane receptors and, stimulates adenylate cyclase with a consequent, increase in cAMP level. TSH, through the, mediation of cAMP, exerts the following effects.
Page 445 :
435, , Chapter 19 : HORMONES, , l, , l, , l, , Promotes the uptake of iodide (iodide pump), from the circulation by thyroid gland., , III. The pro-opiomelanocortin, (POMC) peptide family, , Enhances the conversion of iodide (I–) to, active iodide (I+), a process known as, organification., , This family consists of the hormones—, adrenocorticotropic, hormone, (ACTH),, lipotropin (LPH) and melanocyte stimulating, hormone (MSH) and several (about 24), neuromodulators such as endorphins and, enkephalins., , Increases the proteolysis of thyroglobulin to, release T3 and T4 into the circulation., , TSH increases the synthesis of proteins,, nucleic acids and phospholipids in thyroid, gland., Gonadotropins : The follicle-stimulating, hormone (FSH), luteinizing hormone (LH) and, human chorionic gonadotropin (hCG) are, commonly known as gonadotropins. All three, are glycoproteins., The release of FSH and LH from the anterior, pituitary is controlled by gonadotropin-releasing, hormone (GnRH) of hypothalamus., 2. Biochemical functions of FSH : In females,, FSH stimulates follicular growth, increases the, weight of the ovaries and enhances the, production of estrogens., In males, FSH stimulates testosterone, production, required for spermatogenesis., FSH also promotes growth of seminiferous, tubules., 3. Biochemical functions of LH : Luteinizing, hormone, stimulates, the, production, of, progesterone from corpus luteum cells in females, and testosterone from Leydig cells in males. LH, and FSH are collectively responsible for the, development and maintenance of secondary, sexual characters in males., 4. Human chorionic gonadotropin (hCG) :, hCG is a glycoprotein (mol. wt. 100,000),, produced by syncytiotrophoblast cells of, placenta. The structure of hCG closely resembles, that of LH., The levels of hCG in plasma and urine, increase, almost, immediately, after, the, implantation of fertilized ovum. The detection of, hCG in urine is conveniently used for the early, detection (within a week after missing the, menstrual cycle) of pregnancy., , The synthesis of POMC family. is very, interesting. All the members of POMC are, produced from a single gene of the anterior, and intermediate lobes of pituitary. It is, fascinating that a single polypeptide—proopiomelanocortin—is the precursor (approximately 285 amino acids) that contains multiple, hormones. The name pro-opiomelano-cortin is, derived since it is a prohormone to opioids,, melanocyte-stimulating hormone and corticotropin., Products of POMC : The pituitary, multihormone precursor is synthesized as preproopiomelanocortin from which POMC, is formed. The POMC consists of 3 peptide, groups., 1. ACTH that can give rise to D-MSH and, corticotropin like intermediate lobe peptide, (CLIP)., 2. E-Lipotropin (E-LPH) that can produce, J-LPH, E-MSH and E-endorphin. The latter yields, J- and D-endorphins., 3. An N-terminal peptide that forms J-MSH., The products obtained from POMC are, depicted in Fig.20.7. These products undergo, many modifications such as glycosylation,, acetylation etc., 1. Adrenocorticotropic hormone (ACTH) :, ACTH is a polypeptide with 39 amino acids and, a molecular weight of 4,500. This hormone is, primarily concerned with the growth and, functions of adrenal cortex., Regulation of ACTH production : The release, of ACTH from the anterior pituitary is under the, regulation of hypothalamic hormone, namely, corticotropin releasing hormone (CRH).
Page 446 :
436, Biochemical, ACTH, l, , l, , l, , BIOCHEMISTRY, , functions, , of, , ACTH, promotes, the, conversion of cholesterol to, pregnenolone in the adrenal, cortex., , ACTH, , E-LPH (93 A.As), , 1–39, , 42–134, , D-MSH, 1–13, , J-LPH, 42–101, E-MSH, 84–101, , It enhances RNA and protein, synthesis and thus promotes, adrenocortical growth., ACTH increases lipolysis by, activating lipase of adipose, tissue., , CLIP, 18–39, , E-Endorphin (31 A.As), 104–134, J-Endorphin (15 A.As), 104–118, D-Endorphin (14 A.As), 104–117, Enkephalin, 5, , Fig. 19.7 : The members of the pro-opiomelanocortin (POMC) family, , derived from POMC cleavage. (Numbers in blocks represent amino acids, Overproduction of ACTH :, in sequence; In the brackets are the number of amino acids–AAs;, Cushing’s syndrome is caused, (ACTH–Adrenocorticotropic hormone; LPH–Lipotropin;, by an excessive production of, MSH–Melanocyte-stimulating hormone; CLIP–Corticotropin like, intermediate lobe peptide)., ACTH which may be due, to a tumor. This syndrome, is characterized by hyperpigmentation and increased production of is derived from POMC (Fig.19.7). E-Lipotropin, adrenocorticosteroids. The associated symptoms has 31 amino acids while its modified products, include negative nitrogen balance, impaired D and J-endorphins have 15 and 14 amino acids,, glucose tolerance, hypertension, edema, muscle respectively. Methionine enkephalin (Tyr–Gly–, Gly–Phe–Met) and leucine enkephalin (Tyr-Glyatrophy etc., Gly-Phe-Leu) are the two important pentapeptide, 2. E-Lipotropin (E-LPH) : E-LPH is derived, derivatives of E-endorphin., from POMC and contains 93 carboxy terminal, amino acids. This polypeptide consists of, Biochemical actions : Endorphins and, J-LPH and E-endorphin from which E-MSH enkephalins are peptide neurotransmitters that, and J-endorphin are, respectively, formed. produce opiate-like effects on the central, J-Endorphin can be converted to D-endorphin nervous system, hence they are also known as, and then to enkephalins (Fig.19.7). E-LPH is opioid-peptides. They bind to the same receptors, found only in the pituitary and not in other as the morphine opiates and are believed to, tissues since it is rapidly degraded., control the endogenous pain perception., The biochemical functions of E-LPH, as Endorphins and enkephalins are more potent, such, are limited. It promotes lipolysis and (20-30 times) than morphine in their function as, increases the mobilization of fatty acids. The analgesics., , most important function of E-LPH is its precursor, role for the formation of E-endorphin and, enkephalins., , Endorphins and enkephalins : These are the, natural analgesics that control pain and, emotions. They were discovered after an, unexpected finding of opiate receptors in the, human brain., Synthesis : Endorphins and enkephalins, are produced from E-endorphin which, in turn,, , It is believed that the pain relief through, acupuncture and placebos is mediated through, opioid peptides., 3. Melanocyte-stimulating hormone (MSH) :, Three types of MSH (D, E and J) are present in, the precursor POMC molecule. In humans, J, MSH is important while in some animals D and, E are functional. The activity of J-MSH is, contained in the molecule J-LPH or its precursor, E-LPH (Fig.19.7).
Page 447 :
437, , Chapter 19 : HORMONES, , 1, , 9, , (A) Cys — Tyr — Ile — Gln — Asn — Cys — Pro — Leu — Gly, , S, , S, , 1, , 9, , (B) Cys — Tyr — Phe — Gln — Asn — Cys — Pro — Arg — Gly, , S, , S, , Fig. 19.8 : Structures of (A) Human oxytocin and, (B) Human antidiuretic hormone (ADH)., , The functions of MSH has been clearly, established in some animals. MSH promotes the, synthesis of skin pigment melanin (melanogenesis), and disperses melanin granules that ultimately, leads to darkening of the skin. In humans, MSH, does not appear to play any role in melanin, synthesis., , POSTERIOR PITUITARY HORMONES, Two hormones namely oxytocin and, antidiuretic hormone (ADH, vasopressin) are, produced by the posterior pituitary gland, (neurohypophysis)., Both, of, them, are, nonapeptides (9 amino acids). Their structures, are depicted in Fig.19.8., , Oxytocin, The release of oxytocin from posterior, pituitary gland is caused by the neural impulses, of nipple stimulation. The other stimuli, responsible for oxytocin release include vaginal, and uterine distention., , Biochemical functions, 1. Effect on uterus : Oxytocin causes the, contraction of pregnant uterus (smooth muscles), and induces labor., 2. Effect on milk ejection : In mammals,, oxytocin causes contraction of myoepithelial, cells (look like smooth muscle cells) of breast., This stimulates the squeezing effect, causing milk, ejection from the breast., 3. Oxytocin synthesized in the ovary appears, to inhibit the synthesis of steroids., , Antidiuretic hormone (ADH), The release of ADH (also called vasopressin), is mostly controlled by osmoreceptors (of hypothalamus) and baroreceptors (of heart). Any, increase in the osmolarity of plasma stimulates, ADH secretion., Biochemical functions : ADH is primarily, concerned with the regulation of water balance, in the body. It stimulates kidneys to retain water, and, thus, increases the blood pressure., In the absence of ADH, the urine output, would be around 20 l/day. ADH acts on the, distal convoluted tubules of kidneys and causes, water reabsorption with a result that the urine, output is around 0.5-1.5 l/day., Mechanism of action : ADH stimulates, adenylate cyclase causing production of cAMP., Water reabsorption is promoted by cAMP., Inhibitors of adenylate cyclase (e.g. calcium), inhibit the activity of ADH. This supports the, view that ADH action is mostly mediated, through cAMP., Diabetes insipidus : This disorder is characterized by the excretion of large volumes of, dilute urine (polyuria). It may be due to, insufficient levels of ADH or a defect in the, receptors of target cells., , THYROID HORMONES, Thyroid gland (weighs about 30 g in adults) is, located on either side of the trachea below the, larynx. It produces two principal hormones, (Fig.19.9)—thyroxine (T4; 3,5,3’,5’-tetraiodothyronine) and 3,5,3’-triiodothyronine (T3)—, which regulate the metabolic rate of the body., Thyroid gland also secretes calcitonin, a hormone, concerned with calcium homeostasis (discussed, under calcium metabolism, Chapter 18)., , Biosynthesis of thyroid hormones, Iodine is essential for the synthesis of thyroid, hormones. More than half of the body’s total, iodine content is found in the thyroid gland.
Page 448 :
438, , BIOCHEMISTRY, , I, , I, 3c, , 3, , HO, , CH2 CH COOH, , O, 5c, , I, , 5, , NH 2, I, 3, 5, 3c, 5c-Tetraiodothyronine (thyroxine, T4), , I, , I, , HO, , CH2 CH COOH, , O, , NH 2, , I, 3,5,3c-Triiodothyronine (T3), I, , I, , HO, , O, , CH2 CH COOH, NH 2, , I, , 3, 3c, 5c-Triiodothyronine (reverse T3, rT3), , Fig. 19.9 : Structures of thyroid hormones, (Refer Fig. 15.21 for their biosynthesis)., , Thyroglobulin and synthesis of T3 and T4 :, Thyroglobulin (mol. wt. 660,000) is a, glycoprotein and precursor for the synthesis of, T3 and T4. Thyroglobulin contains about 140, tyrosine residues which can serve as substrates, for iodine for the formation of thyroid hormones., Tyrosine (of thyroglobulin) is first iodinated at, position 3 to form monoiodotyrosine (MIT) and, then at position 5 to form diiodotyrosine (DIT)., Two molecules of DIT couple to form thyroxine, (T4). One molecule of MIT, when coupled with, one molecule of DIT, triiodothyronine (T3) is, produced. The mechanism of coupling is not, well understood. The details of synthesis of T3, and T4 are given under tyrosine metabolism, (Chapter 15). A diagrammatic representation is, depicted in Fig.19.10., As the process of iodination is completed,, each molecule of thyroglobulin contains about, 6-8 molecules of thyroxine (T4). The ratio of T3, to T4 in thyroglobulin is usually around 1 : 10., , Uptake of iodide : The uptake of iodide by, the thyroid gland occurs against a concentration, gradient (about 20 : 1). It is an energy requiring, process and is linked to the ATPase dependent, Na+-K+ pump. Iodide uptake is primarily, controlled by TSH. Antithyroid agents such as, thiocyanate and perchlorate inhibit iodide, transport., , Thyroglobulin containing T4 and T3 can be, stored for several months in the thyroid gland. It, is estimated that the stored thyroid hormones can, meet the body requirement for 1-3 months., , Formation of active iodine : The conversion, of iodide (I–) to active iodine (I+) is an essential, step for its incorporation into thyroid hormones., Thyroid is the only tissue that can oxidize I– to, a higher valence state I+. This reaction requires, H2O2 and is catalysed by the enzyme, thyroperoxidase (mol. wt. 60,000). An NADPH, dependent system supplies H2O2., , Thyroglobulin is digested by lysosomal, proteolytic enzymes in the thyroid gland. The, free, hormones, thyroxine, (90%), and, triiodothyronine (10%) are released into the, blood, a process stimulated by TSH. MIT and, DIT produced in the thyroid gland undergo, deiodination by the enzyme deiodinase and the, iodine thus liberated can be reutilized., , I–, , O2, , H2O2, +, NADP NADPH + H, , +, , Thyroperoxidase, I+, , H2 O, , TSH promotes the oxidation of iodide to, active iodine while the antithyroid drugs, (thiourea, thiouracil, methinazole) inhibit., , Storage and release of, thyroid hormones, , Transport of T4 and T3, Two specific binding proteins—thyroxine, binding globulin (TBG) and thyroxine binding, prealbumin (TBPA)—are responsible for the, transport of thyroid hormones. Both T4 and T3, are more predominantly bound to TBG. A small, fraction of free hormones are biologically active., T4 has a half-life of 4-7 days while T3 has about, one day.
Page 449 :
439, , Chapter 19 : HORMONES, , correlated to thyroid hormones and this, in turn,, with ATP utilization. Obesity in some individuals, is attributed to a decreased energy utilization and, heat production due to diminished Na+-K+, ATPase activity., , Tgb, , I+, , Iodination, DIT, MIT, , Tgb, Thioperoxidase, , Coupling, , I–, , Deiodination, (deiodinase), , Tgb, T4, , T3, , Proteolysis, MIT + T , T + A.As, 3 4, DIT, , To target tissues, , Fig. 19.10 : Biosynthesis of thyroid hormones—, diagrammatic representation [Note : Refer Fig. 15.21 for, synthesis with structures; Tgb–Thyroglobulin; I+–Active, iodine; T3–Triiodothyronine; T4–Thyroxine; MIT–Monoiodotyrosine; DIT–Diiodotyrosine;, A. As–Amino acids]., , Biochemical functions of, thyroid hormones, Triiodothyronine (T3) is about four times more, active in its biological functions than thyroxine, (T4). The following are the biochemical functions, attributed to thyroid hormones (T3 and T4)., 1. Influence on the metabolic rate : Thyroid, hormones stimulate the metabolic activities and, increases the oxygen consumption in most of the, tissues of the body (exception—brain, lungs,, testes and retina)., Na+-K+ ATP pump : This is an energy dependent process which consumes a major share of, cellular ATP. Na+-K+ ATPase activity is directly, , 2. Effect on protein synthesis : Thyroid, hormones act like steroid hormones in promoting, protein synthesis by acting at the transcriptional, level (activate DNA to produce RNA). Thyroid, hormones, thus, function as anabolic hormones, and cause positive nitrogen balance and promote, growth and development., 3. Influence on carbohydrate metabolism :, Thyroid hormones promote intestinal absorption, of glucose and its utilization. These hormones, increase gluconeogenesis and glycogenolysis,, with an overall effect of enhancing blood, glucose level (hyperglycemia)., 4. Effect on lipid metabolism : Lipid turnover, and utilization are stimulated by thyroid, hormones. Hypothyroidism is associated with, elevated plasma cholesterol levels which can be, reversed by thyroid hormone administration., , Regulation of T3 and T4 synthesis, The synthesis of thyroid hormones is, controlled by feedback regulation (Fig.19.11). T3, appears to be more actively involved than T4 in, the regulation process. The production of thyroid, stimulating hormone (TSH) by pituitary, and, thyrotropin releasing hormone (TRH) by, hypothalamus are inhibited by T3 and, to a lesser, degree, by T4. The increased synthesis of TSH, and TRH occurs in response to decreased, circulatory levels of T3 and T4. As already, discussed, the body has sufficient stores of, hormones to last for several weeks. Hence it, takes some months to observe thyroid functional, deficiency., , Metabolic fate of T3 and T4, Thyroid hormones undergo deiodination in, the peripheral tissues. The iodine liberated may, be reutilized by the thyroid. T3 and T4 may get, conjugated with glucuronic acid or sulfate in the, liver and excreted through bile. Thyroid, hormones are also subjected to deamination to
Page 450 :
440, , BIOCHEMISTRY, , produce tetraiodothyroacetic acid, (from T4) and triiodothyroacetic acid, (from T3) which may then undergo, conjugation and excretion., , Hypothalamus, , TRH, , Abnormalities of thyroid, function, Pituitary, , Among the endocrine glands,, thyroid is the most susceptible for, hypo- or hyperfunction., , TSH, , Three abnormalities associated, with thyroid functions are known., Goiter : Any abnormal increase, in the size of the thyroid gland is, known as goiter. Enlargement of, thyroid gland is mostly to, compensate the decreased synthesis, of thyroid hormones and is, associated with elevated TSH., Goiter is primarily due to a failure, in the autoregulation of T3 and T4, synthesis. This may be caused by, deficiency or excess of iodide., , Thyroid, gland, , T3 , T4, , Metabolic, , Protein, , Carbohydrate, , Utilization, , Maintenance, , Goitrogenic, substances, raten, synthesisn metabolismn, of lipidsn of H2O, electrolyte, balance, (goitrogens) : These are the, substances that interfere with the, Fig. 19.11 : Regulation of synthesis and functions of thyroid, hormones—an overview (TRH–Thyrotropin-stimulating hormone;, production of thyroid hormones., TSH–Thyroid stimulating hormone; T3–Triiodothyronine;, These include thiocyanates, nitrates, T4–Thyroxine;, –Promoting effect;, –Inhibitory effect)., and perchlorates and the drugs such, as thiourea, thiouracil, thiocarbamide, etc. Certain plant foods—cabbage, cauliflower and irritability, anxiety, rapid heart rate, loss of, turnip—contain goitrogenic factors (mostly thio- weight despite increased appetite, weakness,, diarrhea, sweating, sensitivity to heat and often, cyanates)., protrusion of eyeballs (exopthalmos)., Simple endemic goiter : This is due to iodine, deficiency in the diet. It is mostly found in the, Hyperthyroidism is caused by Grave’s disease, geographical regions away from sea coast where (particularly in the developed countries) or due, the water and soil are low in iodine content. to increased intake of thyroid hormones. Grave’s, Consumption of iodized salt is advocated to disease is due to elevated thyroid stimulating, overcome the problem of endemic goiter. In IgG also known as long acting thyroid stimulator, certain cases, administration of thyroid hormone (LATS) which activates TSH and, thereby,, is also employed., increases thyroid hormonal production., Hyperthyroidism : This is also known as, thyrotoxicosis, and, is, associated, with, overproduction, of, thyroid, hormones., Hyperthyroidism is characterized by increased, metabolic rate (higher BMR) nervousness,, , Thyrotoxicosis is diagnosed by scanning and/, or estimation of T3, T4 (both elevated) and TSH, (decreased) in plasma. The treatment includes, administration of antithyroid drugs. In severe, cases, thyroid gland is surgically removed.
Page 451 :
441, , Chapter 19 : HORMONES, , Hypothyroidism : This is due to an, impairment in the function of thyroid gland that, often causes decreased circulatory levels of T3, and T4. Disorders of pituitary or hypothalamus, also contribute to hypothyroidism. Women are, more susceptible than men. Hypothyroidism is, characterized by reduced BMR, slow heart rate,, weight gain, sluggish behaviour, constipation,, sensitivity to cold, dry skin etc., Hypothyroidism in children is associated with, physical and mental retardation, collectively, known as cretinism. Early diagnosis and proper, treatment are essential. Hypothyroidism in adult, causes myxoedema, characterized by bagginess, under the eyes, puffiness of face, slowness in, physical and mental activities., Thyroid hormonal administration is employed, to treat hypothyroidism., , Laboratory diagnosis of, thyroid function, Measurement of basal metabolic rate (BMR), was once used to reflect thyroid activity. The, estimation of serum protein bound iodine (PBI),, representing the circulating thyroid hormones,, was employed for a long time to assess thyroid, function. The normal serum PBI concentration is, 3-8 Pg/100 ml., Hypothyroidism is associated with decreased, PBI and hyperthyroidism with increased PBI., In recent years, more sensitive and reliable, tests have been developed to assess thyroid, activity. The concentration of free T3 and T4,, and TSH are measured (by RIA or ELISA) and, their serum normal concentrations are, Free triiodothyronine (T3) — 80–220 ng/dl, Free thyroxine (T4), , — 0.8–2.4 ng/dl, , Total thyroxine (T4), , — 5–12 Pg/dl, , Thyroid stimulating, hormone (TSH), , — <10 PU/ml, , Radioactive iodine uptake (RAIU) and, scanning of thyroid gland are also used for, diagnosis., , Zona glomerulosa, Zona fasciculata, Zona reticularis, Medulla, , Fig. 19.12 : Adrenal gland with zones (3) and medulla., , Thyroid activity and, serum cholesterol, Serum cholesterol level is increased in, hypothyroidism and decreased in hyperthyroidism., Unfortunately, cholesterol estimation will be of no, value in the assessment of thyroid function. This is, due to the fact that serum cholesterol level is, elevated in many other disorders (diabetes,, obstructive jaundice, nephrotic syndrome etc.)., However, cholesterol estimation may be utilized, for monitoring thyroid therapy., , HORMONES OF ADRENAL CORTEX, The adrenal glands are two small organs (each, weighing about 10 g), located above the kidneys., Each adrenal consists of two distinct tissues—an, outer cortex (with 3 zones) and inner medulla, (Fig.19.12)., As many as 50 steroid hormones (namely, adrenocorticosteroids), produced by adrenal, cortex, have been identified. However, only a, few of them possess biological activity., , Adrenocorticosteroids are classified into three, groups according to their dominant biological, action. However, there is some overlap in their, functions., 1. Glucocorticoids : These are 21-carbon, steroids, produced mostly by zona fasciculata., They affect glucose (hence the name), amino, acid and fat metabolism in a manner that is, opposite to the action of insulin. Cortisol (also, known as hydrocortisone) is the most important, glucocorticoid in humans. Corticosterone is, predominantly found in rats.
Page 452 :
442, , BIOCHEMISTRY, , 2. Mineralocorticoids : These are also 21carbon containing steroids produced by zona, glomerulosa. They regulate water and electrolyte, balance. Aldosterone is the most prominent, mineralocorticoid., 3. Androgens and estrogens : The innermost, adrenal cortex zona reticularis produces small, quantities of androgens (19-carbon) and, estrogens (18-carbon). These hormones affecting, sexual development and functions are mostly, produced by gonads. Dehydroepiandrosterone—, a precursor for androgens—is synthesized in, adrenal cortex., , Synthesis of adrenocorticosteroids, Cholesterol, elimination of, pregnenolone., precursor for, hormones., , undergoes cleavage with an, a 6-carbon fragment to form, Pregnenolone is the common, the synthesis of all steroid, , Conversion of cholesterol to pregnenolone is, catalysed by cytochrome P450 side chain cleavage, enzyme. This reaction is promoted by ACTH., The enzymes—hydroxylases, dehydrogenases/, isomerases, and, lyases, associated, with, mitochondria or endoplasmic reticulum—are, responsible for the synthesis of steroid hormones., The metabolic pathway for the formation of major, adrenocorticosteroids is given in Fig.19.13., , Biochemical functions of, adrenocorticosteroids, 1. Glucocorticoid hormones : The important, glucocorticoids are—cortisol, cortisone and, corticosterone. They bring about several, biochemical functions in the body., (a) Effects on carbohydrate metabolism :, Glucocorticoids promote the synthesis of, glucose (gluconeogenesis). This is brought, about by increasing the substrates, (particularly amino acids) and enhancing, the synthesis of phosphoenolpyruvate, carboxykinase, the rate limiting enzyme, in gluconeogenesis., The overall influence of glucocorticoids, on carbohydrate metabolism is to increase, , blood, glucose, concentration., The, biological actions of glucocorticoids, generally oppose that of insulin., (b) Effects on lipid metabolism : Glucocorticoids increase the circulating free fatty, acids. This is caused by two mechanisms., (i) Increased breakdown of storage triacylglycerol (lipolysis) in adipose tissue., (ii) Reduced utilization of plasma free fatty, acids for the synthesis of triacylglycerols., (c) Effects on protein and nucleic acid, metabolism : Glucocortiocoids exhibit, both catabolic and anabolic effects on, protein and nucleic acid metabolism., They, promote, transcription, (RNA, synthesis) and protein biosynthesis in, liver., These, anabolic, effects, of, glucocorticoids are caused by the, stimulation of specific genes., Glucocorticoids (particularly at high, concentration) cause catabolic effects in, extrahepatic tissues (e.g. muscle, adipose, tissue, bone etc.). This results in enhanced, degradation of proteins., (d) Effects on water and electrolyte metabolism : The influence of glucocorticoids, on water metabolism is mediated through, antidiuretic hormone (ADH). Deficiency, of glucocorticoids causes increased, production of ADH. ADH decreases, glomerular filtration rate causing water, retention in the body., (e) Effects on the immune system : Glucocorticoids (particularly cortisol), in high, doses, suppress the host immune, response. The steroid hormones act at, different levels—damaging lymphocytes,, impairment of antibody synthesis,, suppression of inflammatory response etc., (f) Other physiological effects of glucocorticoids : Glucocorticoids are involved in, several physiological functions., (i) Stimulate the fight and flight response, (to face sudden emergencies) of, catecholamines.
Page 454 :
444, , BIOCHEMISTRY, , (ii) Increase the production of gastric HCI, and pepsinogen., (iii) Inhibit the bone formation, hence the, subjects are at a risk for osteoporosis., Mechanism of action of glucocorticoids :, Glucocorticoids bind to specific receptors on the, target cells and bring about the action. These, hormones mostly act at the transcription level, and control the protein synthesis., 2. Mineralocorticoid hormones : The most, active and potent mineralocorticoid is, aldosterone. It promotes Na+ reabsorption at the, distal convoluted tubules of kidney. Na+, retention is accompanied by corresponding, excretion of K+, H+ and NH4+ ions., Regulation of aldosterone synthesis : The, production of aldosterone is regulated by, different mechanisms. These include reninangiotensin, potassium, sodium and ACTH., Mechanism of aldosterone action :, Aldosterone acts like other steroid hormones. It, binds with specific receptors on the target tissue, and promotes transcription and translation., Metabolism of adrenocorticosteroids : The, steroid hormones are metabolized in the liver, and excreted in urine as conjugates of, glucuronides or sulfates., The urine contains mainly two steroids—, 17-hydroxysteroids and 17-ketosteroids—derived, from the metabolism of glucocorticoids and, mineralocorticoids., Androgens, synthesized, by gonads also contribute to the formation of, 17-ketosteroids., Urinary 17-ketosteroids estimated in the, laboratory are expressed in terms of, dehydroepiandrosterone and their normal, excretion is in the range of 0.2–2.0 mg/day., , Abnormalities of adrenocortical, function, Addison’s, disease, adrenocortical function, disease. This disorder, decreased blood glucose, , :, Impairment, in, results in Addison’s, is characterized by, level (hypoglycemia),, , loss of weight, loss of appetite (anorexia), muscle, weakness, impaired cardiac function, low blood, pressure, decreased Na+ and increased K+ level, in serum, increased susceptibility to stress etc., Cushing’s syndrome : Hyperfunction of, adrenal cortex may be due to long term, pharmacological use of steroids or tumor of, adrenal cortex or tumor of pituitary. Cushing’s, syndrome is characterized by hyperglycemia, (due to increased gluconeogenesis), fatigue,, muscle wasting, edema, osteoporosis, negative, nitrogen balance, hypertension, moon-face etc., , Assessment of adrenocortical, function, The adrenocortical function can be assessed, by measuring plasma cortisol (5-15 Pg/dl at 9.00, AM), plasma ACTH, urinary 17-ketosteroids etc., , HORMONES OF ADRENAL MEDULLA, Adrenal medulla is an extension of, sympathetic nervous system. It produces two, important hormones—epinephrine (formerly, adrenaline) and norepinephrine (formerly, noradrenaline). Both these hormones are, catecholamines since they are amine derivatives, of catechol nucleus (dihydroxylated phenyl ring)., Epinephrine is a methyl derivative of, norepinephrine., Dopamine, is, another, catecholamine, produced as an intermediate, during, the, synthesis, of, epinephrine., Norepinephrine and dopamine are important, neurotransmitters in the brain and autonomic, nervous system. The structures of the three, catecholamines are given in Fig.19.14., , Synthesis of catecholamines, The amino acid tyrosine is the precursor for, the synthesis of catecholamines. The pathway is, described under tyrosine metabolism (Chapter, 15, Fig.15.22). Catecholamines are produced in, response to fight, fright and flight. These include, the emergencies like shock, cold, fatigue,, emotional conditions like anger etc.
Page 455 :
445, , Chapter 19 : HORMONES, , Biochemical functions, of catecholamines, Catecholamines, cause, diversified biochemical effects, on the body. The ultimate goal, of their action is to mobilize, energy resources and prepare the, individuals to meet emergencies, (e.g. shock, cold, low blood, glucose etc.)., , HO, , HO, , HO, , HO, , CH2 CH2 NH2, , Catechol, , Dopamine, HO, , HO, HO, , CH CH2 NH2, , HO, , CH2 CH2 NH, OH, , OH, Norepinephrine, , CH3, , Epinephrine, , 1. Effects on carbohydrate, Fig. 19.14 : Catecholamines (dopamine, norepinephrine and, metabolism : Epinephrine and, epinephrine) produced by adrenal medulla, norepinephrine, in, general, (Refer Fig. 15.22 for biosynthesis)., increase the degradation of, glycogen, (glycogenolysis),, synthesis of glucose (gluconeogenesis) and transferase (COMT) and monoamine oxidase, (MAO), found in many tissues act on, decrease glycogen formation (glycogenesis)., The overall effect of catecholamines is to catecholamines. The metabolic products, elevate blood glucose levels and make it metanephrine and vanillylmandelic acid (VMA), available for the brain and other tissues to meet are excreted in urine., the emergencies., 2. Effects on lipid metabolism : Both, epinephrine and norepinephrine enhance the, breakdown of triacylglycerols (lipolysis) in, adipose tissue. This causes increase in the free, fatty acids in the circulation which are effectively, utilized by the heart and muscle as fuel source., The metabolic effects of catecholamines are, mostly related to the increase in adenylate, cyclase activity causing elevation in cyclic AMP, levels (refer carbohydrate and lipid metabolisms, for more details)., 3. Effects on physiological functions : In, general, catecholamines (most predominantly, epinephrine) increase cardiac output, blood, pressure and oxygen consumption. They cause, smooth muscle relaxation in bronchi, gastrointestinal tract and the blood vessels supplying, skeletal muscle. On the other hand,, catecholamines stimulate smooth muscle, contraction of the blood vessels supplying skin, and kidney. Platelet aggregation is inhibited by, catecholamines., , Metabolism of catecholamines, Catecholamines are rapidly inactivated and, metabolized. The enzymes—catechol-O methyl-, , Abnormalities of, catecholamine production, Pheochromocytomas : These are the tumors, of adrenal medulla. The diagnosis of, pheochromocytoma is possible only when there, is an excessive production of epinephrine and, norepinephrine that causes severe hypertension., In the individuals affected by this disorder, the, ratio of norepinephrine, to epinephrine is, increased. The measurement of urinary VMA, (normal <8 mg/day) is helpful in the diagnosis of, pheochromocytomas., , HORMONES OF GONADS, The gonads (testes in males, ovaries in, females) perform closely related dual functions., 1. Synthesize sex hormones;, 2. Produce germ cells., The steroid sex hormones are responsible for, growth,, development,, maintenance, and, regulation of reproductive system. Sex hormones, are essentially required for the development of, germ cells.
Page 456 :
446, The sex hormones are categorized into three, groups, 1. Androgens or male sex hormones which, are C-19 steroids., 2. Estrogens or female sex hormones which, are C-18 steroids. Ring A of steroid nucleus is, phenolic in nature and is devoid of C-19 methyl, group., 3. Progesterone is a C-21 steroid produced, during the luteal phase of menstrual cycle and, also during pregnancy., , BIOCHEMISTRY, , l, , Spermatogenesis., , l, , Male pattern of aggressive behavior., , 2. Biochemical functions : Many specific, biochemical effects of androgens that ultimately, influence the physiological functions stated, above are identified. Androgens are anabolic in, nature., l, , ANDROGENS, The male sex hormones or androgens are, produced by the Leydig cells of the testes and to, a minor extent by the adrenal glands in both the, sexes. Ovaries also produce small amounts of, androgens., , l, , l, , Biosynthesis of androgens, Cholesterol is the precursor for the synthesis, of androgens. It is first converted to, pregnenolone which then forms androstenedione, by two pathways—either through progesterone, or through 17-hydroxypregnenolone (Fig.19.15)., Testosterone is produced from androstenedione., The production of androgens is under the control, of LH and FSH., Active form of androgen : The primary, product of testes is testosterone. However, the, active hormone in many tissues is not, testosterone but its metabolite dihydrotestosterone (DHT). Testosterone, on reduction, by the enzyme 5 D-reductase, forms DHT. This, conversion mostly occurs in the peripheral, tissues. Some workers consider testosterone as a, prohormone and dihydrotestosterone, the more, potent form as the hormone., , Physiological and biochemical, functions of androgens, 1. Sex-related physiological functions : The, androgens, primarily DHT and testosterone,, influence :, l, , l, , Effects on protein metabolism : Androgens, promote RNA synthesis (transcription) and, protein synthesis (translation). Androgens, cause positive nitrogen balance and increase, the muscle mass., Effects on carbohydrate and fat metabolisms :, Androgens increase glycolysis fatty acid, synthesis and citric acid cycle., Effects on mineral metabolism : Androgens, promote mineral deposition and bone growth, before the closure of epiphyseal cartilage., , ESTROGENS, Estrogens, are, predominantly, ovarian, hormones, systhesized by the follicles and, corpus luteum of ovary. These hormones are, responsible for maintenance of menstrual cycle, and reproductive process in women., , Synthesis of estrogens, Estrogen synthesis occurs from the precursor, cholesterol (Fig.19.15). Estrogens are produced, by aromatization (formation of aromatic ring) of, androgens. The ovary produces estradiol (E2) and, estrone (E1) while the placenta synthesizes these, two steroid hormones and estriol (E3). The, synthesis of estrogens is under the control of LH, and FSH., , Physiological and biochemical, functions of estrogens, 1. Sex-related physiological functions : The, estrogens are primarily concerned with, Growth, development and maintenance of, female reproductive organs., , Growth, development and maintenance of, male reproductive organs., , l, , Sexual differentiation and secondary sexual, characteristics., , l, , Maintenance of menstrual cycles., , l, , Development of female sexual characteristics.
Page 457 :
447, , Chapter 19 : HORMONES, , Cholesterol, , Pregnenolone, , Progesterone, 17-D-Hydroxyprogesterone, , 17-D-Hydroxyprogesterone, Dehydroepiandrosterone, , Androstenedione, Aromatase, , Dehydrogenase/, Isomerase, , O, , OH, , 5 D-Reductase, , A, , A, O, , HO, , OH, , O, , Estrone (E1), , H, 5 D-Dihydrotestosterone, (DHT), , Testosterone, , 16 D-Hydroxylase, , Aromatase, , OH, , OH, OH, , A, HO, , A, HO, , Estriol (E3), , 17 E-Estradiol (E2), , Fig. 19.15 : Biosynthesis of steroid sex hormones from cholesterol, (Note : Male and female sex hormones are given together)., , 2. Biochemical functions : Estrogens are, involved in many metabolic functions., l, , Lipogenic effect : Estrogens increase lipogenesis in adipose tissue and, for this reason,, women have relatively more fat (about 5%), than men., , l, , Hypocholesterolemic effect : Estrogens lower, the plasma total cholesterol. The LDL fraction, of lipoproteins is decreased while the HDL, fraction is increased. This explains the, low incidence of atherosclerosis and coronary, heart diseases in the women during reproductive age.
Page 458 :
448, , l, , l, , l, , BIOCHEMISTRY, , Anabolic effect : Estrogens in general promote, transcription and translation. The synthesis of, many proteins in liver is elevated e.g., transferrin, ceruloplasmin., Effect on bone growth : Estrogens like, androgens promote calcification and bone, growth. It is believed that decalcification, of bone in the postmenopausal women, leading to osteoporosis is due to lack of, estrogens., Effect on transhydrogenase : Transhydrogenase is an enzyme activated by estrogen. It, is capable of transferring reducing equivalents, from NADPH to NAD+. The NADH so formed, can be oxidized. It is explained that in the, women after menopause, due to deficiency of, , estrogens, the transhydrogenase activity is low., This results in the diversion of NADPH, towards lipogenesis—causing obesity., , PROGESTERONE, Progesterone is synthesized and secreted by, corpus luteum and placenta. Progesterone, as, such, is an intermediate in the formation, of steroid hormones from cholesterol (See, Fig.20.13). LH controls the production of, progesterone., , Biochemical functions of, progesterone, 1. Progesterone is essentially required for the, implantation of fertilized ovum and maintenance, of pregnancy., , + Growth hormone deficiency causes dwarfism while its excessive production results in, gigantism (in children) or acromegaly (in adults)., , + Identification of hCG in urine is employed for the early detection of pregnancy., + Cushing’s syndrome is due to overproduction of ACTH that results in the increased, synthesis of adrenocorticosteroids. The symptoms of this syndrome include hypertension,, edema and negative nitrogen balance., , + Endorphins and enkephalins are the natural pain-killers in the brain. It is believed that, the pain relief through acupuncture and placebos is mediated through these compounds., , + Deficiency of ADH causes diabetes insipidus, a disorder characterized by excretion of, large volumes of dilute urine (polyuria)., , + Thyroid hormones directly influence Na+ – K+ ATP pump which consumes a major share, of cellular ATP. Obesity in some individuals is attributed to decreased energy utilization, (heat production) due to diminished Na+ – K+ ATPase activity., , + Catecholamines are produced in response to fight, fright and flight. The ultimate goal, of catecholamine function is to mobilize energy resources and prepare the individual to, meet emergencies such as shock, cold, fatigue, anger etc., , + Pheochromocytomas are the tumors of adrenal medulla, characterized by excessive, production of epinephrine and norepinephrine, associated with severe hypertension., , + Sex hormones are primarily responsible for growth, development, maintenance and, regulation of reproductive system., , + The low incidence of atherosclerosis and coronary heart disease in the women during, reproductive age is due to estrogens.
Page 459 :
449, , Hormone level, , Chapter 19 : HORMONES, , 2. Luteal phase : After the ovulation occurs,, the ruptured follicles form corpus luteum and, start producing progesterone and estradiol. The, predominant hormone of luteal phase is progesterone which prepares the endometrium of, uterus for implantation of the fertilized ovum., LH maintains the corpus for a few days. In the, absence of implantation, the corpus luteum, regresses and sheds endometrium causing, menstruation. And another new cycle begins., , LH surge, Follicular, phase, , Luteal, phase, Progesterone, , Estradiol, , LH, , FSH, , 0, , 4, , 8, , 12 14 16, , Menstruation, , 20, 24, Days, , 28, , Ovulation, , Fig. 19.16 : Hormonal pattern in women during, mestrual cycle (FSH–Follicle stimulating hormone;, LH–Luteinizing hormone)., , 2. It promotes the growth of glandular tissue, in uterus and mammary gland., 3. Progesterone increases the body temperature by 0.5–1.5 F°. The exact mechanism of this, thermogenic effect is not clearly known. The, measurement of temperature was used as an, indicator for ovulation., , THE MENSTRUAL CYCLE, The occurrence of menstrual cycle is a good, example of coordination among the hormonal, functions. In humans, the menstrual cycle is, under the control of FSH, LH, estrogens and, progesterone. The cycle normally varies between, 25 and 35 days in length, with a mean of 28, days. The menstrual cycle can be divided into, two phases—follicular phase and luteal phase, (Fig.19.16)., 1. Follicular phase : Follicular stimulating, hormone (FSH) causes the development and, maturation of ovarian follicles. As the follicle, enlarges, estradiol progessively rises and reaches, its peak value 24 hours before LH and FSH attain, their respective maximum levels. LH surge or, peak initiates ovulation-release of ovum from the, ruptured follicles. The levels of progesterone are, low during follicular phase, , The luteal phase is always fixed, with 14 ± 2, days in length. The observed variations in the, length of menstrual cycle are due to changes in, the follicular phase. In case of implantation of, the, fertilized, ovum,, human, chorionic, gonadotropin (hCG) is produced by the cells of, implanted early embryo. hCG stimulates corpus, luteum to synthesize progesterone. This, continues till the plancenta starts making high, quantities of progesterone., , Menopause, The menstrual cycles which begin in the, women after puberty, continue till the age of, 45-50 years. The cycles cease around this age, which coincides with the loss of ovarian, function. The progesterone and estrogen levels, are very low in these women. However, the, concentration of LH and FSH are elevated due to, lack of feedback inhibition by estrogens., Post-menopausal women are susceptible to, two complications associated with insufficient, levels of sex hormones., 1. Atrophy of secondary sex tissues : Mainly, the epithelial tissue of vagina and lower urinary, tract., 2. Osteoporosis : Decreased density of bones, and increased susceptibility to fractures., , GASTROINTESTINAL, (OR GUT) HORMONES, The digestion and absorption of nutrients, (Chapter 8) is a complicated process which is, regulated by the autonomic nervous system. This, occurs in association with peptide hormones of, gastrointestinal tract (GIT).
Page 460 :
450, The specialized cells lining the GIT are, responsible for the production of GIT hormones., Hence GIT may be considered as the largest, mass of cells that secrete hormones. A large, number of GIT hormones have been identified., However, only four GIT hormones have been, well characterised., 1. Gastrin : This hormone contains 17 amino, acids and is produced by gastric mucosa. It, stimulates the secretion of gastric HCI and, pepsinogen (proenzyme of pepsin). The release, of gastrin is stimulated by vagus nerve of, stomach and partially digested proteins. HCI and, certain other hormones inhibit gastrin release., 2. Secretin : It is a 27-amino acid containing, polypeptide and resembles glucagon in many, ways. Secretin is synthesized by the mucosa of, the upper small intestine. It is released in, response to the presence of HCI in chyme in the, duodenum which is passed on from the stomach., Secretin stimulates pancreatic cells to produce, bicarbonate (HCO–3) in order to neutralize HCI., 3. Cholecystokinin (CCK) : It contains 33, amino acids and is produced by the upper part, of small intestine. The secretion of CCK is, stimulated by the products of protein and lipid, digestion, namely peptides, amino acids, monoor diacylglycerols, fatty acids and glycerol., Cholecystokinin stimulates the contraction of, gall bladder and increases the flow of bile into, duodenum. It also promotes the secretion of, digestive enzymes and HCO–3 from pancreas., 4. Gastric inhibitory peptide (GIP) : It, contains 43 amino acids and is produced by, duodenal mucosa. The release of GIP is, stimulated by the presence of glucose in the gut., The most important function of GIP is to, stimulate the release of insulin from pancreas., This is evident from the fact that the plasma, insulin level is elevated much before the increase, in blood glucose. GIP also inhibits gastric HCI, secretion, gastric motility and its emptying., , BIOCHEMISTRY, , GIT hormones show certain structural, relations and may be considered under two, families., (i) Gastrin family : Some of the C-terminal, amino acids are identical. This family, includes gastrin and CCK., (ii) Secretin family : Secretin, GIP and, glucagon are structurally related, hence, may be considered under this family., Besides the hormones described above,, several other hormones (in hundreds!) from the, GIT have been identified. These hormones are, often known as candidate hormones, since their, biological functions are yet to be precisely, identified. The candidate hormones include, vasoactive intestinal peptide (VIP), motilin,, enteroglucagon, substance P, neurotensin,, somatostatin and enkephalins., , Mechanism of action, of GIT hormones, Many of the GIT hormones have receptor sites, specific for their action. At least two distinct, mechanisms have been identified through which, these hormones act., 1. Production of cAMP through the activation, of adenylate cyclase e.g. secretin, VIP etc., 2. Stimulation of intracellular Ca2+ usually, mediated, through, the, metabolism, of, phosphatidylinositol e.g. gastrin , CCK., Both these mechanisms ultimately influence, the enzyme secretions/other biological effects., , Other hormones, Besides the hormones discussed above, there, are a few other important hormones which are, not referred to in this chapter. Insulin and, glucagon are described under diabetes mellitus, (Chapter 36) while parathyroid hormone and, calcitonin are discussed under calcium, metabolism (Chapter 18) These hormones are, not given here to avoid repetition.
Page 461 :
Chapter 19 : HORMONES, , 1. Hormones are the organic substances, produced in minute quantities by specific tissues, (endocrine glands) and secreted into the blood stream to control the biological activities, in the target cells. They may be regarded as the chemical massengers involved in the, regulation and coordination of body functions., 2. Hormones are classified based on their chemical nature or mechanism of action., Chemically, they may be proteins or peptides (insulin, oxytocin), steroids, (glucocorticoids, sex hormones) and amino acid derivatives (epinephrine, thyroxine). By, virtue of the function, group I hormones bind to the intracellular receptors (estrogens,, calcitriol), while group II hormones (ACTH, LH) bind to the cell surface receptors and, act through the second messengers., 3. Cyclic AMP (cAMP) is an intracellular second messenger for a majority of polypeptide, hormones. Membrane bound adenylate cyclase enzyme, through the mediation of G, proteins, is responsible for the synthesis of cAMP. cAMP acts through protein kinases, that phosphorylate specific proteins which, in turn, cause the ultimate biochemical, response. Phosphatidylinositol/calcium system also functions as a second messenger for, certain hormones (TRH, gastrin)., 4. Hypothalamus is the master coordinator of hormonal action as it liberates certain, releasing factors or hormones (TRH, CRH, GRH, GRIH) that stimulate or inhibit the, corresponding trophic hormones from the anterior pituitary., 5. Anterior pituitary gland is the master endocrine organ that produces several hormones, which influence either directly or indirectly (through the mediation of other endocrine, organs) a variety of biochemical processes in the body. For instance, growth hormone, is directly involved in growth promoting process while TSH, FSH and ACTH,, respectively influence thyroid gland, gonads and adrenal cortex to synthesize hormones., 6. Thyroid gland produces two principal hormones—thyroxine (T4) and triiodothyronine, (T3)—which are primarily concerned with the regulation of the metabolic activity of the, body. Goiter is a disorder caused by enlargement of thyroid gland and is mainly due, to iodine deficiency in the diet., 7. Adrenal cortex synthesizes glucocorticoids (e.g. cortisol) that influence glucose, amino, acid and fat metabolism, and mineralocorticoids (e.g. aldosterone) that regulate water, and electrolyte balance. Androgens and estrogens (sex hormones) in small quantities, are also synthesized by the adrenal cortex., 8. Adrenal medulla produces two important hormones—epinephrine and norepinephrine, (catecholamines). They influence diversified biochemical functions with an ultimate goal, to mobilize energy resources and prepare the individual to meet emergencies (shock,, anger, fatigue etc.), 9. The steroid sex hormones, primarily androgens in males and estrogens in females, are, respectively synthesized by the testes and ovaries. These hormones are responsible for, growth, development, maintenance and regulation of reproductive system in either sex., 10. Several gastrointestinal hormones (e.g. gastrin, secretin) have been identified that are, closely involved in the regulation of digestion and absorption of foodstuffs., , 451
Page 462 :
452, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the role of second messengers in hormonal action., 2. Write an account of the anterior pituitary hormones., 3. Discuss in detail the synthesis and biochemical functions of thyroid hormones., 4. Describe the hormones of adrenal cortex with special reference to glucocorticoids., 5. Write briefly on the synthesis and biochemical functions of sex hormones., , II. Short notes, (a) ‘G’-Proteins, (b) Inositol triphosphate, (c) Hypothalamic hormones, (d) ACTH, (e) Goiter,, (f) Epinephrine, (g) Cortisol, (h) Gastrin, (i) ADH, (j) Aldosterone., , III. Fill in the blanks, 1. The enzyme that catalyses the formation of cAMP from ATP is _______________., 2. The inorganic ion that can act as a second messenger for certain hormones is _______________., 3. The endocrine organ responsible for the synthesis of trophic hormones is _______________., 4. The compounds that produce opiate-like effects on the central nervous system are, _________________., 5. The enzyme that converts iodide (I–) to active iodine (I+) _______________., 6. The most predominant mineralocorticoid synthesized by adrenal cortex _______________., 7. The major urinary excretory product of catecholamines _______________., 8. The male sex hormone, testosterone, is converted to a more active form, namely, _______________., 9. The precursor for the synthesis of steroid hormones _______________., 10. The gastrointestinal hormone that increases the flow of bile from the gall bladder, _______________., , IV. Multiple choice questions, 11. Impairment in the synthesis of dopamine by the brain is a major causative factor for the disorder, (a) Parkinson’s disease (b) Addison’s disease (c) Cushing’s syndrome (d) Goiter., 12. One of the following hormones is an amino acid derivative, (a) Epinephrine (b) Norepinephrine (c) Thyroxine (d) All of them., 13. The most active mineralocorticoid hormone is, (a) Cortisol (b) Aldosterone (c) 11-Deoxycorticosterone (d) Corticosterone., 14. Name the hormone, predominantly produced in response to fight, fright and flight, (a) Thyroxine (b) Aldosterone (c) Epinephrine (d) ADH., 15. The hormone essentially required for the implantation of fertilized ovum and maintenance of, pregnancy, (a) Progesterone (b) Estrogen (c) Cortisol (d) Prolactin.
Page 463 :
Section 4, , Clinical Biochemistry and Nutrition, , Chapter, , Organ Function Tests, , 20, , The liver speaks :, , “Master organ I am, for the body’s metabolism !, Damage to my cells causes malfunction;, Raising serum bilirubin and certain enzymes markedly,, Tested in lab for my functional measurement.”, , E, , ach organ of the body has to perform its, biochemical functions to keep the body, as a, whole, in a healthy state. This is possible only, when the cells of the organ are intact in structure, and function. Any abnormality in the tissue,, caused by exogenous or endogenous factors, will, seriously impair the organ function which, in, turn, influences the health of the organism., Based on the functional capabilities of the, organs, specific biochemical investigations have, been developed in the laboratory, to assess their, function. In this chapter, the biochemical, investigations to assess the functioning of liver,, kidney, stomach and pancreas are discussed. The, tests to evaluate the function of endocrine organs, are discussed elsewhere (Chapter 19)., , Major functions of liver, 1. Metabolic functions : Liver actively, participates in carbohydrate, lipid, protein,, mineral and vitamin metabolisms., 2. Excretory functions : Bile pigments, bile, salts and cholesterol are excreted in the bile into, intestine., 3. Protective functions and detoxification :, Kupffer cells of liver perform phagocytosis to, eliminate foreign compounds. Ammonia is, detoxified to urea. Liver is responsible for the, metabolism of xenobiotics (detoxification)., , LIVER FUNCTION TESTS, , 4. Hematological, functions, :, Liver, participates in the formation of blood, (particularly in the embryo), synthesis of plasma, proteins (including blood clotting factors) and, destruction of erythrocytes., , Liver performs several diversified functions. It, is the central organ of body’s metabolism., , 5. Storage functions : Glycogen, vitamins A,, D and B12 and trace element iron are stored in, liver., , 453
Page 464 :
454, , BIOCHEMISTRY, , Causes of liver damage, Hepatocellular damage may occur due to, viruses (hepatitis A virus, hepatitis B virus), toxins, (carbon tetrachloride, aflatoxin), alcohol,, hepatocellular carcinoma, autoimmune hepatitis, etc., , TABLE 20.1 A list of liver (hepatic) functions, and the common markers in plasma for the, impaired function, Hepatic function, , Common plasma/serum, marker(s) for impaired function, , Heme catabolism, , nBilirubin, , Tests to assess liver function, , Enzymes, , The liver function tests (LFT) are the, biochemical investigations to assess the capacity, of the liver to carry out any of the functions it, performs. LFT will help to detect the, abnormalities and the extent of liver damage., , nAlanine transaminase, nAspartate transaminase, nJ-Glutamyltranspeptidase, , Protein synthesis, , pAlbumin, nProthrombin time, , Protein catabolism, , Two important facts should be borne in mind, while carrying out LFT., , nUrea, nAmmonia, , Lipid metabolism, , nCholesterol, nTriglycerides, , Drug metabolism, , nHalf-lives of drugs, , Bile acid metabolism, , nBile acids, , 1. Liver is a large-size factory of safety., Therefore, it can perform many of its functions, almost normally, despite the damage., 2. Selection of the right test is important in, LFT. This is due to the fact that since liver, participates in several functions, the function that, is measured in LFT may not be the one that is, adversely affected., The major liver function tests may be, classified as follows, 1. Tests based on excretory function—, Measurement of bile pigments, bile salts,, bromosulphthalein., , functions are listed in Table 20.1. The most, important markers namely, bilirubin, enzymes,, albumin, prothrombin time and drug metabolism, with special reference to jaundice and other liver, diseases are described., , BILIRUBIN, , 2. Tests based on serum enzymes derived, from liver—Determination of transaminases,, alkaline phosphatase, 5’-nucleotidase, J-glutamyltranspeptidase., , Bilirubin is a bile pigment, and is the excretory, end product of heme degradation. It is conjugated, in the liver to form bilirubin diglucuronide, and, excreted in bile. The details of bilirubin, metabolism are discussed elsewhere (Chapter 10)., , 3. Tests based on metabolic capacity—, Galactose tolerance, antipyrine clearance., , Serum bilirubin, , 4. Tests based on synthetic functions—, Prothrombin time, serum albumin., 5. Tests based on detoxification—Hippuric, acid synthesis., This above list, contains the most important, biochemical investigations to assess LFT. Among, these, the commonly used tests are described in, the following pages., , Markers of liver function, The important liver functions and the, common plasma/serum markers for the impaired, , The normal concentration of serum bilirubin, is in the range of 0.2-1.0 mg/dl. Of this, the, conjugated bilirubin (diglucuronide 75%;, monoglucuronide 25%) is 0.2-0.4 mg/dl, while, the unconjugated bilirubin is 0.2-0.6 mg/dl., , Icterus index, This is a simple test to measure the yellow, colour of serum due to bilirubin. It is rather, crude and almost outdated. However, it is often, useful for a rapid assessment of neonatal, jaundice.
Page 465 :
455, , Chapter 20 : ORGAN FUNCTION TESTS, , van den Bergh reaction, This is a specific reaction to identify the, increase in serum bilirubin (above the reference, level). Normal serum gives a negative van den, Bergh reaction., Mechanism of the reaction : van den Bergh, reagent is a mixture of equal volumes of, sulfanilic acid (in dilute HCl) and sodium nitrite., The principle of the reaction is that diazotised, sulfanilic acid (in the above mixture) reacts with, bilirubin to form a purple coloured azobilirubin., Direct and indirect reactions : Bilirubin as, such is insoluble in water while the conjugated, bilirubin is soluble. van den Bergh reagent reacts, with conjugated bilirubin and gives a purple, colour immediately (normally within 30, seconds). This is referred to as a direct positive, van den Bergh reaction. Addition of methanol, (or alcohol) dissolves the unconjugated bilirubin, which then gives the van den Bergh reaction, (normally within 30 minutes) positive and this is, referred to as indirect positive. If the serum, contains both unconjugated and conjugated, bilirubin in high concentration, the purple colour, is produced immediately (direct positive) which, is further intensified by the addition of alcohol, (indirect positive). This type of reaction is known, as biphasic., van den Bergh reaction and jaundice : This, reaction is highly useful in understanding the, nature of jaundice. This is due to the fact that the, type of jaundice is characterized by increased, serum concentration of unconjugated bilirubin, (hemolytic), conjugated bilirubin (obstructive) or, both of them (hepatic). Therefore, the response, of van den Bergh reaction can differentiate the, jaundice as follows, Indirect positive — Hemolytic jaundice, Direct positive, , — Obstructive jaundice, , Biphasic, , — Hepatic jaundice., , Bilirubin in urine, The conjugated bilirubin, being water soluble,, is excreted in urine. This is in contrast to, unconjugated bilirubin which is not excreted., , Bilirubin in urine can be detected by Fouchet’s, test or Gmelin’s test., , Bromosulphthalein (BSP) test, Bromosulphthalein is a dye used to assess the, excretory function of liver. It is a non-toxic, compound and almost exclusively excreted by, the liver (through bile). BSP is administered, intravenously (5 mg/kg body weight) and its, serum concentration is measured at 45 min and, at 2 hrs. In normal individuals, less than 5% of, the dye is retained at the end of 45 min. Any, impairment in liver function causes an increased, retention of the dye. This test is quite sensitive, to assess liver abnormality with particular, reference to excretory function., , SERUM ENZYMES, DERIVED FROM LIVER, Liver cells contain several enzymes which, may be released into the circulation in liver, damage. Measurement of selected enzymes in, serum is often used to assess the liver function., It must, however, be noted that there is no single, enzyme that is absolutely specific to liver alone., Despite this fact, serum enzymes provide, valuable information for LFT. Some of these, enzymes are discussed hereunder., , Transaminases or aminotransferases, The activities of two enzymes—namely serum, glutamate pyruvate transaminase (SGPT; recently, called as alanine transaminase—ALT) and serum, glutamate oxaloacetate transaminase (SGOT;, recently known as aspartate transaminase—AST), —are widely used to assess the liver function., ALT is a cytoplasmic enzyme while AST is found, in both cytoplasm and mitochondria. The activity, of these enzymes is low in normal serum (ALT, 5-40 IU/l; AST 5-45 IU/l). Serum ALT and AST, are increased in liver damage. However, alanine, transaminase is more sensitive and reliable for, the assessment of LFT., The normal AST/ALT ratio is around 0.8. This, ratio is increased (>2) in myocardial infarction,, alcoholic hepatitis, and cirrhosis. AST/ALT, ratio is decreased (i.e. ALT higher) in acute, hepatocellular damage and cholestasis.
Page 466 :
456, , BIOCHEMISTRY, , Alkaline phosphatase, , Other enzymes, , Alkaline phosphatase (ALP) is mainly derived, from bone and liver (the cells lining the bile, canaliculi). A rise in serum ALP (normal 3-13 KA, units/dl), usually associated with elevated serum, bilirubin is an indicator of biliary obstruction, (obstructive/posthepatic jaundice). ALP is also, elevated in cirrhosis of liver and hepatic tumors., , Serum, isocitrate, dehydrogenase, and, isoenzymes of lactate dehydrogenase (LDH4 and, LDH5) are also useful in LFT., , Liver is not the sole source of alkaline, phosphatase. Therefore, its measurement has to, be carefully viewed (along with others) before, arriving at any conclusion. The liver and bone, isoenzymes of ALP can be separated by, electrophoresis., , J-Glutamyl transpeptidase, This is a microsomal enzyme widely, distributed in body tissues, including liver., Measurement of J-glutamyl transpeptidase (GGT), activity provides a sensitive index to asses liver, abnormality. The activity of this enzyme almost, parallels that of transaminases in hepatic, damage. Serum GGT is highly elevated (normal, 5-40 IU/l) in biliary obstruction and alcoholism., Further, several drugs (e.g. phenytoin) induce, (liver synthesis) and increase this enzyme in, circulation., , 5c-Nucleotidase, The serum activity of 5’-nucleotidase (normal, 2-15 U/l) is elevated in hepatobiliary disease and, this parallels ALP. The advantage with 5’-nucleotidase is that it is not altered in bone diseae (as, is the case with ALP)., , Enzyme combinations, Very often, a combination of serum enzyme, estimations (instead of a single one) is used for a, better understanding of liver functions. For, instance, a large increase in transaminases, (particularly ALT) relative to a small increase in, alkaline phosphatase indicates hepatocellular, damage. On the other hand, a small increase in, transaminases and a large increase of alkaline, phosphatase shows biliary obstruction., , JAUNDICE, Jaundice (French : jaune—yellow) is characterized by yellow coloration of sclera (of eyes), and skin. This is due to the elevated serum, bilirubin level, usually beyond 2 mg/dl (normal, < 1 mg/dl)., The metabolism of heme to produce bilirubin, and its conjugated derivatives and the types of, jaundice have already been described. The, reader must refer this (Chapter 10) now. The, biochemical changes and the related parameters, for the differential diagnosis of the three types of, jaundice (hemolytic, obstructive and hepatic) are, given in Table 20.2., In the Fig.20.1, the normal and abnormal, bilirubin metabolism (along with the associated, , TABLE 20.2 Biochemical changes for the differential diagnosis of three types of jaundice, Parameter, , Hemolytic jaundice, (preheptic jaundice), , Obstructive jaundice, (posthepatic jaundice), , Hepatic jaundice, (Intrahepatic jaundice), , Serum bilirubin, , Unconjugated bilirubin n, , Conjugated bilirubin n, , Both n, , van den Bergh reaction, , Indirect positive, , Direct positive, , Biphasic, , Serum enzymes, , ALT, AST and ALP o, , ALP nn, ALT and AST marginal n, , ALT and AST nn, ALP marginal n, , Bilirubin in urine, , Not excreted, , Excreted, , Excreted, , Urobilinogen in urine, , Excretion n, , o or p, , o or p, , ALT : Alanine transaminase; AST : Aspartate transaminase; ALP : Alkaline phosphatase; n : Increase; p : Decrease; o : Normal.
Page 467 :
457, , Chapter 20 : ORGAN FUNCTION TESTS, , (A), , (B), , Erythrocytes, , �Unconjugated bilirubin, Blood, , Unconjugated bilirubin, (complexed with albumin), , ��Conjugated, bilirubin, , ALT, AST, , Conjugated bilirubin, , ALT, AST, Liver, , ALP, , Block, , Conjugated bilirubin, (with glucuronate), , Intestine, Conjugated, bilirubin, Kidney, , Urobilin, , Urobilinogen, , Conjugated, bilirubin, Urobilinogen, , Urobilinogen, Urobilin, (stercobilin), , Urobilin, (stercobilin), Urine, , Feces, , Urine, , Feces, , Fig. 20.1 : Normal and abnormal bilirubin metabolism (A) Normal bilirubin metabolism (B) Alterations in bilirubin, metabolism along with enzymes in three types of jaundice (Note : Colours indicate major changes; Red–changes in, hemolytic jaundice; Green–changes in hepatic jaundice; Blue–changes in obstructive jaundice; Dotted lines indicate, minor pathways; ALT–Alanine transaminase; AST–Aspartate transaminase; ALP–Alkaline phosphatase)., , enzyme changes) are depicted. The major, changes in the 3 types of jaundice are listed, below, Hemolytic jaundice : Elevated serum, unconjugated bilirubin, and increased urinary, excretion of urobilinogen., Obstructive jaundice : Elevated serum, conjugated bilirubin and increased activities of, alkaline phosphatase (ALP), alanine transaminase, (ALT) and aspartate transaminase (AST)., Hepatic, jaundice, :, Elevated, serum, unconjugated and conjugated bilirubin, and, increased activities of ALT and AST., The pattern of rise in the serum alanine, transaminase, aspartate transaminase and, bilirubin in acute viral hepatitis is depicted in, , Fig.21.2. It may be noted that the transaminase, activities (more predominantly ALT) are elevated, much before the bilirubin starts increasing., , Galactose tolerance, Galactose is a monosaccharide, almost exclusively metabolized by the liver. The liver, function can be assessed by measuring the, utilization of galactose. This is referred to, galactose tolerance test. The subject is given, intravenous administration of galactose (about, 300 mg/kg body weight). Blood is drawn at 10, minute intervals for the next 2 hours and, galactose estimated. In the normal individuals,, the half-life of galactose is about 10-15 minutes., This is markedly elevated in hepatocellular, damage (infective hepatitis, cirrhosis).
Page 468 :
458, , BIOCHEMISTRY, , Serum bilirubin, , Enzyme activity, , Alanine, transaminase, , Aspartate, transaminase, Bilirubin, , 1, , 2, , 3, , 4, , 5, 10, Weeks, , 15, , 20, , Fig. 20.2 : Pattern of rise in serum enzymes and, bilirubin in viral hepatitis., , Serum albumin, Albumin is solely synthesized by the liver. It, has a half-life of about 20-25 days, therefore, it, is a good marker to assess chronic (and not, acute) liver damage. Low serum albumin is, commonly observed in patients with severe liver, damage. It must, however, be noted that the, serum albumin concentration is also decreased, due to other factors such as malnutrition., , Functional impairment of liver is frequently, associated with increased synthesis of globulins., Cirrhosis of the liver causes a reversal of, albumin/globulin ratio (A/G ratio). Serum, electrophoresis of proteins reveals increased, albumin and decreased J-globulin concentration., This, however, may not have much diagnostic, importance since several diseases are associated, with altered electrophoretic pattern of serum, proteins., , Prothrombin time, The liver synthesizes all the factors concerned, with blood clotting. A decrease in the, concentration of plasma clotting factors is found, in the impairment of liver function. This can be, assessed in the laboratory by measuring, , prothrombin time which is prolonged in patients, with liver damage, compared to normal. The, half-lives of clotting factors are relatively short, (5-72 hrs.), therefore, changes in prothrombin, time occur quickly. Hence, this test is useful to, assess acute as well as chronic liver damages;, besides its help in the prognosis., Vitamin K is required for the synthesis of, blood clotting factors II, VII, IX and X. Therefore,, vitamin K deficiency can also cause prolonged, prothrombin time which must be ruled out,, before drawing conclusions on the liver, functions. This is done by measuring, prothrombin time before and after administration, of vitamin K., , Hippuric acid synthesis, The liver is the major site for the metabolism, of xenobiotics (detoxification). Measurement of, hippuric acid synthesis is an ideal test for, assessing the detoxification function of liver., Hippuric acid is produced in the liver when, benzoic acid combines with glycine., About 6 g of sodium benzoate dissolved in, (about 250 ml) water, is orally given to the, subject, after a light breakfast (usually 2 hrs later), and after emptying the bladder. Urine collections, are made for the next 4 hours and the amount of, hippuric, acid, excreted, is, estimated., Theoretically, 6 g of sodium benzoate should, yield 7.5 g of hippuric acid. In the healthy, persons, about 60% of sodium benzoate, (equivalent to 4.5 g hippuric acid) is excreted in, urine. A reduction in hippuric acid excretion, (particularly < 3 g) indicates hepatic damage., , Choice of liver functions tests, The choice of biochemical tests to measure, liver functions mostly depends on the purpose, of the investigation. The clinical history of the, subject is often a guiding factor in this regard. A, single test in isolation may have a little, diagnostic value., Frequently, a combination of laboratory, investigations are employed in LFT. These, include serum bilirubin (conjugated and
Page 469 :
459, , Chapter 20 : ORGAN FUNCTION TESTS, , unconjugated), alanine transaminase,, aspartate, transaminase,, alkaline, phosphatase, J-glutamyl transpeptidase, and, proteins, (albumin, globulins)., , Proximal, convoluted tubule, Bowman’s capsule, Glomerulus, , Distal convoluted, tubule, , KIDNEY (RENAL), FUNCTION TESTS, The kidneys are the vital, organs of the body, performing, the following major functions., , Collecting duct, , 1. Maintenance of homeostasis : The kidneys are largely, responsible for the regulation, of water, electrolyte and, acid-base balance in the, body., , Loop of Henle, , Fig. 20.3 : Diagrammatic representation of a nephron., , 2. Excretion of metabolic waste products :, The end products of protein and nucleic acid, metabolism are eliminated from the body. These, include urea, creatinine, creatine, uric acid,, sulfate and phosphate., 3. Retention of substances vital to body : The, kidneys reabsorb and retain several substances, of biochemical importance in the body e.g., glucose, amino acids etc., 4. Hormonal functions : The kidneys also, function as endocrine organs by producing, hormones., l, , l, , l, , Erythropoietin, a peptide hormone, stimulates, hemoglobin synthesis and formation of, erythrocytes., 1,25-Dihydroxycholecalciferol (calcitriol) –, the biochemically active form of vitamin D –, is finally produced in the kidney. It regulates, calcium absorption from the gut., Renin, a proteolytic enzyme liberated by, kidney, stimulates the formation of angiotensin II which, in turn, leads to aldosterone, production. Angiotensin II and aldosterone are, the hormones involved in the regulation of, electrolyte balance., , The formation of urine, Nephron is the functional unit of kidney. Each, kidney is composed of approximately one, million nephrons. The structure of a nephron, as, depicted in Fig.20.3, consists of a Bowman’s, capsule (with blood capillaries), proximal, convoluted tubule (PCT), loop of Henle, distal, convoluted tubule (DCT) and collecting tubule., The blood supply to kidneys is relatively, large. About 1200 ml of blood (650 ml plasma), passes through the kidneys, every minute. From, this, about 120-125 ml is filtered per minute by, the kidneys and this is referred to as glomerular, filtration rate (GFR). With a normal GFR, (120-125 ml/min), the glomerular filtrate formed, in an adult is about 175-180 litres per day, out, of which only 1.5 litres is excreted as urine., Thus, more than 99% of the glomerular filtrate is, reabsorbed by the kidneys., The process of urine formation basically, involves two steps—glomerular filtration and, tubular reabsorption., 1. Glomerular filtration : This is a passive, process that results in the formation of, ultrafiltrate of blood. All the (unbound), constituents of plasma, with a molecular weight
Page 470 :
460, less than about 70,000, are passed into the, filtrate. Therefore, the glomerular filtrate is, almost similar in composition to plasma., 2. Tubular reabsorption : The renal tubules, (PCT, DCT and collecting tubules) retain water, and most of the soluble constituents of the, glomerular filtrate by reabsorption. This may, occur either by passive or active process. The, excreted urine has an entirely different, composition compared to glomerular filtrate, from which it is derived. The normal, composition of urine is given elsewhere (Refer, inside backcover)., , Renal threshold substances, There are certain substances in the blood, whose excretion in urine is dependent on their, concentration. Such substances are referred to as, renal threshold substances. At the normal, concentration in the blood, they are completely, reabsorbed by the kidneys, with a result that their, excretion in urine is almost negligible., The renal threshold of a substance is defined, as its concentration in blood (or plasma) beyond, which it is excreted into urine. The renal, threshold for glucose is 180 mg/dl; for ketone, bodies 3 mg/dl; for calcium 10 mg/dl and for, bicarbonate 30 mEq/l. While calculating the renal, threshold of a particular compound, it is assumed, that both the kidneys are optimally functioning,, without any abnormality. But this is not always, true—in which case the renal threshold is altered., For instance, renal glycosuria is associated with, reduced threshold for glucose due to its, diminished tubular reabsorption., The term tubular maximum (Tm) is used to, indicate the maximum capacity of the kidneys to, absorb a particular substance. For instance,, tubular maximum for glucose (TmG) is 350, mg/min., , Tests to assess renal function, In view of the important and sensitive, functions the kidney performs (described, already), it is essential that the abnormalities, (renal damages), if any, must be detected at the, earliest. Several tests are employed in the, , BIOCHEMISTRY, , laboratory to assess kidney (renal) function. It, must, however, be remembered that about twothirds of the renal tissue must be functionally, damaged to show any abnormality by these tests., The kidney function tests may be divided into, four groups., 1. Glomerular function tests : All the, clearance tests (inulin, creatinine, urea) are, included in this group., 2. Tubular function tests : Urine concentration or dilution test, urine acidfication test., 3. Analysis of blood/serum : Estimation of, blood urea, serum creatinine, protein and, electrolyte are often useful to assess renal, function., 4. Urine examination : Simple routine examination of urine for volume, pH, specific gravity,, osmolality and presence of certain abnormal, constituents (proteins, blood, ketone bodies,, glucose etc.) also helps, of course to a limited, degree, to assess kidney functioning., Some of the important renal function tests are, discussed in the following pages., , CLEARANCE TESTS, The clearance tests, measuring the glomerular, filtration rate (GFR) are the most useful in, assessing the renal function. The excretion of a, substance can be expressed quantitatively by, using the concept of clearance., Clearance, in general, is defined as the, volume of plasma that would be completely, cleared of a substance per minute. In other, words, clearance of a substance refers to the, milliliters of plasma which contains the amount, of that substance excreted by kidney per, minute. Clearance (C), expressed as ml/minute,, can be calculated by using the formula, U u V, C = ———, P, where U = Concentration of the substance in, urine., V = Volume of urine in ml excreted per, minute., P = Concentration of the substance in, plasma.
Page 471 :
461, , Chapter 20 : ORGAN FUNCTION TESTS, , Care should be taken to express the concentrations of plasma and urine in the same units, (mmol/l or mg/dl)., The clearance of a given substance is, determined by its mode of excretion. The, maximum rate at which the plasma can be, cleared of any substance is equal to the GFR., This can be easily calculated by measuring the, clearance of a plasma compound which is freely, filtered by the glomerulus and is neither, absorbed nor secreted in the tubule. Inulin (a, plant carbohydrate, composed of fructose units), and 51Cr-EDTA satisfy this criteria. Inulin is, intravenously administered to measure GFR., In practice, however, measurement of, clearance for the substances already present in, the blood is preferred. The two compounds,, namely creatinine and urea, are commonly, employed for this purpose. Creatinine clearance, (~145 ml/min) is marginally higher than the GFR, as it is secreted by the tubules. On the other, hand, urea clearance (~75 ml/min) is less than, the GFR, since it is partially reabsorbed by the, tubules., , Diodrast (diiodopyridone acetic acid) is used, as a contrast medium to take urinary tract X-rays., Diodrast and para amino hippuric acid (PAH), are peculiar substances as they are entirely, excreted by a single passage of blood through, the kidneys. It is partly filtered by the glomerulus, and mostly excreted by the tubules. PAH has a, clearance of about 700 ml/min (or 1,200 ml, if, expressed as blood). Thus clearance of PAH, represents the renal plasma flow., , Creatinine clearance test, Creatinine is an excretory product derived, from creatine phosphate (largely present in, muscle). The excretion of creatinine is rather, constant and is not influenced by body, metabolism or dietary factors. As already stated,, creatinine is filtered by the glomeruli and only, marginally secreted by the tubules. The value of, creatinine clearance is close to GFR, hence its, measurement is a sensitive and good approach, to assess the renal glomerular function., Creatinine clearance may be defined as the, , volume (ml) of plasma that would be completely, cleared of creatinine per minute., Procedure : In the traditional method,, creatinine content of a 24 hr urine collection, and the plasma concentration in this period are, estimated. The creatinine clearance (C) can be, calculated as follows :, U u V, C = ———, P, where U = Urine concentration of creatinine, V = Urine output in ml/min (24 hr urine, volume divided by 24 u 60), P = Plasma concentration of creatinine., As already stated, creatinine concentration in, urine and plasma should be expressed in the, same units (mg/dl or mmol/l)., Modified procedure : Instead of a 24 hr urine, collection, the procedure is modified to collect, urine for 1 hr, after giving water. The volume of, urine is recorded. Creatinine contents in plasma, and urine are estimated. The creatinine, clearance can be calculated by using the formula, referred above., Reference values : The normal range of, creatinine clearance is around 120-145 ml/min., These values are slightly lower in women. In, recent years, creatinine clearance is expressed in, terms of body surface area., Diagnostic importance : A decrease in, creatinine clearance value (< 75% normal), serves as sensitive indicator of a decreased GFR,, due to renal damage. This test is useful for an, early detection of impairment in kidney function,, often before the clinical manifestations are seen., , Urea clearance test, Urea is the end product of protein, metabolism. After being filtered by the glomeruli,, it is partially reabsorbed by the renal tubules., Hence, urea clearance is less than the GFR and,, further, it is influenced by the protein content of, the diet. For these reasons, urea clearance is not, as sensitive as creatinine clearance for assessing, renal function. Despite this fact, several, laboratories traditionally use this test.
Page 472 :
462, , BIOCHEMISTRY, , Urea clearance is defined as the volume (ml), of plasma that would be completely cleared of, urea per minute. It is calculated by the formula, , 8, , U u V, Cm = ———, P, = Maximum urea clearance, , U = Urea concentration, (mg/ml), , in, , urine, , V = Urine excreted per minute in ml, P = Urea concentration, (mg/ml)., , in, , 9, , plasma, , The above calculation is applicable if the, output of urine is more than 2 ml per minute., This is referred to as maximum urea clearance, and the normal value is around 75 ml/min., Standard urea clearance : It is observed that, the urea clearance drastically changes when the, volume of urine is less than 2 ml/min. This is, known as standard urea clearance (Cs) and the, normal value is around 54 ml/min. It is, calculated by a modified formula, , Serum creatinine (mg/dl), , where Cm, , 10, , 7, 6, 5, 4, 3, 2, 1, , 0, , 20, , 40 60, , 80 100 120 140 160, GFR (ml/min), , Fig. 20.4 : The relationship between glomerular filtration, rate (GFR) and serum creatinine concentration., , Diagnostic importance : A urea clearance, value below 75% of the normal is viewed, seriously, since it is an indicator of renal, damage. Blood urea level as such is found to, increase only when the clearance falls below, 50% normal. As already stated, creatinine, clearance is a better indicator of renal function., , Osmolality and specific gravity : The, osmolality of urine is variable. In normal, individuals, it may range from 500-1,200, milliosmoles/kg. The plasma osmolality is around, 300 milliosmoles/kg. The normal ratio of the, osmolality between urine and plasma is around, 2-4. It is found that the urine (without any, protein or high molecular weight substance) with, an osmolality of 800 mosm/kg has a specific, gravity of 1.020. Therefore, measurement of, urine osmolality will also help to assess tubular, function., , Urine concentration test, , Analysis of blood (or serum), , This is a test to assess the renal tubular, function. It is a simple test and involves the, accurate measurement of specific gravity which, depends on the concentration of solutes in urine., A specific gravity of 1.020 in the early morning, urine sample is considered to be normal., , Estimation of serum creatinine and blood, urea are often used to assess the overall kidney, function, although these tests are less sensitive, than the clearance tests. Serum creatinine is a, better indicator than urea in this regard., The diagnostic importance of urea and creatinine estimations are discussed elsewhere (Refer, Chapter 15)., , Cs, , Uu, , V, P, , Several, measures, are, employed, to, concentrate urine and measure the specific, gravity. These include overnight water, deprivation and administration of antidiuretic, hormone. If the specific gravity of urine is above, 1.020 for at least one of the samples collected,, the tubular function is considered to be normal., , The relationship between GFR and serum, creatinine levels is depicted in Fig.20.4. It is, observed that the GFR must fall to about 50% of, its normal value before a significant increase in, serum creatinine occurs. Therefore, a normal
Page 473 :
463, , Chapter 20 : ORGAN FUNCTION TESTS, , serum creatinine level does not necessarily mean, that all is well with the kidney. It is estimated, that a loss of 50% of the functions of nephrons, leads to (approximate) doubling of serum, creatinine concentration., , Lumen, , CO2 + H2O, , Cystatin C is a protein marker of kidney, function (serum reference range 0.8-1.2 Pg/dl),, and is more sensitive than creatinine. Even minor, changes in GFR in the early stages of chronic, kidney diseases are associated with increased, cystatin C., , Carbonic, anhydrase, , ATP H2CO3, +, , K, , +, , Urine examination, , H, , The routine urine examination is undoubtedly, a guiding factor for renal function. The volume, of urine excreted, its pH, specific gravity,, osmolality, the concentration of abnormal, constituents (such as proteins, ketone bodies,, glucose and blood) may help to have some, preliminary knowledge of kidney function. More, information on urine laboratory tests is given in, the appendix., , Choice of renal function tests, In general, the assessment of kidney function, starts with the routine urine examination,, followed by serum creatinine and/or blood urea, estimations and, finally, the specific tests to, measure the tubular and glomerular functions, (clearance tests)., , GASTRIC FUNCTION TESTS, The stomach is a major organ of digestion, and performs the following functions, 1. Stomach, foodstuffs., , is, , a, , reservoir, , of, , ingested, , 2. It has a great churning ability which, promotes digestion., 3. Stomach elaborates HCI and proteases, (pepsin) which are responsible for the initiation, of digestive process., 4. The products obtained in the stomach, (peptides, amino acids) stimulate the release of, pancreatic juice and bile., , Blood, , Parietal cell, , HCl, –, , Cl, , +, , K, , –, , +, , HCO3, , H, , ADP + Pi, , –, , Cl, , –, , HCO3, –, , Cl, , Fig. 20.5 : Mechanism of HCl secretion, ( —represents K+ activated ATPase)., , Secretion of gastric HCI, The parietal (oxyntic) cells of gastric glands, produce HCI. The pH in the gastric lumen is as, low as 0.8 (against the blood pH 7.4). Therefore,, the protons are transported against the, concentration gradient by an active process., A unique enzyme—namely K+ activated, ATPase—present in the parietal cells is, connected with the mechanism of HCI secretion, (Fig.20.5). The process involves an exchange of, H+ ions (of the parietal cells) for K+ ions (of the, lumen). This is coupled with the consumption of, energy, supplied by ATP. The H+ are, continuously generated in the parietal cells by, the dissociation of carbonic acid which, in turn,, is produced from CO2. The bicarbonate ions, (HCO3–), liberated from the carbonic acid, (H2CO3) dissociation, enter the blood in, exchange for Cl– ions. The latter diffuse into the, gastric lumen to form HCI. Gastrin—a peptide, hormone of gastrointestinal tract—stimulates HCI, secretion., Following a meal, there is a slight elevation, in the plasma bicarbonate concentration which, is linked to the gastric HCI secretion. This is, referred to as alkaline tide.
Page 474 :
464, , BIOCHEMISTRY, , TESTS TO ASSESS, GASTRIC FUNCTION, There are several tests for gastric function, evaluation, some of the important ones are, briefly discussed., , Fractional test meal (FTM), This is rather old and not used these days., Fractional test meal involves the collection of, stomach contents by Ryle’s tube in fasting. This, is followed by a gastric stimulation, giving a test, meal (rice gruel, black coffee etc.) The stomach, contents are aspirated by Ryle’s tube at different, time periods (usually every 15 min for 2 hrs.), The samples are analysed for free and total, acidity in the laboratory. The results are normally, represented by a graph., , Alcohol test meal, In this case, the test meal in the form of 100, ml of 7% alcohol is administered. The response, to alcohol test meal is more rapid, and the test, time can be reduced to 11/2 hour. Clear, specimens can be collected by this test, and the, free acidity levels are relatively higher compared, to FTM., , Pentagastrin stimulation test, Pentagastrin is a synthetic peptide which, stimulates the gastric secretion in a manner, similar to the natural gastrin. The test procedure, adapted is as follows, The stomach contents are aspirated by Ryle’s, tube in a fasting condition. This is referred to as, residual juice. The gastric juice elaborated for, the next one hour is collected and pooled which, represents the basal secretion. Pentagastrin (5, mg/kg body weight) is now given to stimulate, gastric secretion. The gastric juice is collected at, 15 minute intervals for one hour. This represents, the maximum secretion., Each sample of the gastric secretion collected, is measured for acidity by titrating the samples, with N/10 NaOH to pH 7.4. The end point may, be detected by an indicator (phenol red) or a pH, meter., , Basal acid output (BAO) refers to the acid, output (millimol per hour) under the basal, conditions i.e. basal secretion., Maximal acid output (MAO) represents the, acid output (millimol per hour) after the gastric, stimulation by pentagastrin i.e. maximum, secretion., , + The impairment in the functions of any organ in the body will adversely influence the, health of the organism. Organ function tests are the laboratory tools to biochemically, evaluate the working of a given organ., , + Acute viral hepatitis is associated with elevated alanine transaminase (predominantly),, aspartate transaminase and bilirubin., , + Increase in serum J-glutamyl transpeptidase is observed in biliary obstruction and, alcoholism., , + A combination of laboratory investigations—instead of a single one—are commonly, employed in assessing organ function. Kidney function can be accurately assessed by, clearance tests, measuring glomerular filtration rate. A reduction in clearance reflects, renal damage., , + Zollinger-Ellison syndrome, a tumor of gastrin secreting cells of the pancreas, is, associated with increased gastric HCI production.
Page 475 :
465, , Chapter 20 : ORGAN FUNCTION TESTS, , In normal individuals, the BAO is 4-10 mmol/, hr while the MAO is 20-50 mmol/hr., , Diagnex blue containing azure-A-resin is, employed in the tubeless gastric analysis., , Augmented histamine test meal, , Abnormalities of gastric function, , Histamine is a powerful stimulant of gastric, secretion. The basal gastric secretion is collected, for one hour. Histamine (0.04 mg/kg body, weight) is administered subcutaneously and the, gastric contents are aspirated for the next one, hour (at 15 minute intervals). The acid content is, measured in all these samples., , Increased gastric HCI secretion is found in, Zollinger-Ellison syndrome (a tumor of gastrin, secreting cells of the pancreas), chronic, duodenal ulcer, gastric cell hyperplasia,, excessive histamine production etc., A decrease in gastric HCI is observed in, gastritis, gastric carcinoma, pernicious anemia etc., , Insulin test meal, This is also known as Hollander’s test. It is, mainly done to assess the completeness of, vagotomy (vagal resection). Insulin (0.1 unit/kg, body weight) is administered intravenously, which causes hypoglycemia (blood glucose, about 40 mg/dl), usually within 30 minutes, in, normal persons., If the vagotomy operation is successful,, insulin administration does not cause any, increase in the acid output, compared to, the basal level. This test has to be, carefully perfomed, since hypoglycemia is, dangerous., , Tubeless gastric analysis, In the traditional methods of gastric analysis,, a tube is invariably passed into the stomach to, collect the gastric juice. This causes, inconvenience to the subject. Recently,, some tests involving tubeless gastric analysis, have been developed. Such tests, however, are, mostly useful for preliminary screening., The principle of tubeless gastric analysis, involves administration of a cation exchange, resin that gets quantitatively exchanged with the, H+ ions of the gastric juice. The resin is, then excreted into urine which can be estimated, for an indirect measure of gastric acidity, (concentration of H+ ions)., , OTHER ORGAN FUNCTION TESTS, PANCREATIC FUNCTION TESTS, The pancreas is a specialized organ with, exocrine and endocrine functions. The endocrine, functions are discussed under the topic diabetes, mellitus (Chapter 36)., The exocrine functions involve the synthesis, of pancreatic juice containing several enzymes, (for the digestion of foodstuffs) and bicarbonate., The major enzymes of pancreatic juice are, trypsin, chymotrypsin, elastase, carboxypeptidase, amylase and lipase., Pancreatic enzymes in serum : Serum, amylase and lipase measurements are commonly, employed to assess the pancreatic function. Both, these enzyme activities are elevated in acute, pancreatitis, obstruction in the intestine and/or, pancreatic duct., , THYROID FUNCTION TESTS, Thyroid gland produces two principal, hormones—thyroxine (T4) and triiodothyronine, which regulate the metabolic rate of the, body. The laboratory tests employed for the, diagnosis of thyroid function are described in the, Chapter 19 on hormones.
Page 476 :
466, , BIOCHEMISTRY, , 1. Specific laboratory biochemical investigations are employed to assess the functioning of, the organs such as liver, kidney, stomach and pancreas., 2. The liver function can be evaluated by the tests based on its excretory function (serum, bilirubin), serum enzymes (transaminases), metabolic capability (galactose tolerance test), and synthetic functions (prothrombin time)., 3. Serum bilirubin (normal < 1mg/dl) is derived from heme degradation. It is mostly (75%), found in the conjugated form. van den Bergh reaction is a specific test to identify the, increased serum bilirubin. Conjugated bilirubin gives a direct positive test while the, unconjugated bilirubin gives an indirect positive test., 4. The serum enzymes—namely alanine transaminase (ALT), aspartate transaminase, (AST), alkaline phosphatase (ALP) and J- glutamyltranspeptidase (GGT)—are frequently, used for LFT. Increase in the activities of these enzymes indicates an impairment in, liver function., 5. Jaundice is due to elevated serum bilirubin level (>2 mg/dl). The three types of jaundice, (hemolytic, obstructive and hepatic) can be differentially diagnosed by biochemical tests., Thus, unconjugated bilirubin (indirect positive) is increased in hemolytic jaundice,, conjugated bilirubin (direct positive) in obstructive jaundice and both of them (biphasic), are increased in hepatic jaundice., 6. Impaired galactose tolerance test, diminished serum albumin concentration and, prolonged prothrombin time are also associated with liver malfunction., 7. The renal (kidney) function is usually assessed by evaluating either the glomerular, (clearance tests) or tubular function (urine concentration test). This is often guided by, blood analysis (for urea, creatinine) and/or urine examination., 8. The clearance is defined as the volume of the plasma that would be completely cleared, of a substance per minute. Inulin clearance represents glomerular filtration rate (GFR)., Creatinine clearance and urea clearance tests are often used to assess renal function., A decrease in their clearance is an indication of renal damage., 9. Impairment in renal function is often associated with elevated concentration of blood, urea, serum creatinine, decrease in osmolality and specific gravity of urine (by urine, concentration test)., 10. The tests to evaluate gastric function include fractional test meal, pentagastrin, stimulation test, augmented histamine test and tubeless gastric analysis. Gastric HCI, secretion is elevated in chronic duodenal ulcer and gastric hyperplasia. Gastritis and, pernicious anemia are associated with decreased gastric HCI. Pancreatic function is, assessed by serum amylase and lipase. Both of them are elevated in acute pancreatitis.
Page 477 :
Chapter 20 : ORGAN FUNCTION TESTS, , 467, , I. Essay questions, 1. Write briefly on the different laboratory investigations employed to assess liver function., 2. Discuss the biochemical parameters for the differential diagnosis of jaundice., 3. Give an account of the serum enzymes derived from liver and their importance in LFT., 4. Describe the renal function tests., 5. Discuss the different laboratory investigations to evaluate gastric function., , II. Short notes, (a) Serum bilirubin, (b) van den Bergh reaction, (c) Galactose tolerance test, (d) Prothrombin time, as LFT, (e) Renal threshold substances, (f) Glomerular filtration rate, (g) Creatinine clearance,, (h) Standard urea clearance, (i) Urine concentration test, (j) Gastric function tests., , III. Fill in the blanks, 1. Bilirubin is the excretory end product of ___________., 2. The laboratory reaction most commonly employed to detect the elevation of serum bilirubin is, ___________., 3. The serum enzyme most predominantly elevated in viral hepatitis is ___________., 4. Obstructive jaundice is characterized by an increase in the serum enzyme ___________., 5. The excretory function of liver can be evaluated by using a dye ___________., 6. The renal threshold for glucose is ___________., 7. The exogenous substance used to measure glomerular filtration rate (GFR) is ___________., 8. Standard urea clearance is calculated when the volume of urine output is less than ___________., 9. Name the stomach tube used to aspirate gastric juice ___________., 10. Name the synthetic peptide used to stimulate gastric secretion for evaluation of gastric function, ___________., , IV. Multiple choice questions, 11. In hemolytic jaundice, van den Bergh reaction is, (a) Indirect positive (b) Direct positive (c) Biphasis (d) None of these., 12. The serum enzyme elevated in alcoholic cirrhosis of liver is, (a) Alanine transaminase (b) Aspartate transaminase (c) Alcohol dehydrogenase (d) J-Glutamyl, transpeptidase., 13. Bilirubin is not excreted in urine in, (a) Obstructive jaundice (b) Hepatic jaundice (c) Hemolytic jaundice (d) All three., 14. Urea clearance is less than GFR because it is, (a) Partially secreted by the renal tubules (b) Partially reabsorbed by the tubules (c) Only filtered, by glomeruli (d) None of these., 15. The serum enzyme used to evaluate pancreatic function is, (a) Alkaline phosphatase (b) Amylase (c) Aspartate transaminase (d) Lactate dehydrogenase.
Page 478 :
Section 4, , Clinical Biochemistry and Nutrition, , Chapter, , Water, Electrolyte and, Acid-base Balance, , 21, , 21, , The acid-base homeostasis speaks :, , “We maintain the blood pH at 7.4!, Regulated by buffers, lungs and kidneys;, Increased hydrogen ion causes acidosis;, Decreased hydrogen ion leads to alkalosis.”, , T, , he organism possesses tremendous capacity, to survive against odds and maintain, homeostasis. This is particularly true with regard, to water, electrolyte and acid-base status of the, body. These three are interrelated, hence they, are considered together for the discussion in this, chapter. Kidney actively participates in the, regulation of water, electrolyte and acid-base, balance. The general functions of kidney have, already been described (Chapter 20)., , WATER AND LIFE, Water is the solvent of life. Undoubtedly,, water is more important than any other single, compound to life. It is involved in several body, functions., , Functions of water, 1. Water provides the aqueous medium to, the organism which is essential for the various, biochemical reactions to occur., , 2. Water directly participates as a reactant in, several metabolic reactions., 3. It serves as a vehicle for transport of, solutes., 4. Water is closely associated with the, regulation of body temperature., , Distribution of water, Water is the major body constituent. An adult, human contains about 60% water (men 55-70%,, women 45-60%). The women and obese individuals have relatively less water which is due to, the higher content of stored fat in an anhydrous, form., A 70 kg normal man contains about 42 litres, of water. This is distributed in intracellular, (inside the cells 28l) and extracellular (outside, the cells 14l) compartments, respectively known, as intracellular fluid (ICF) and extracellular fluid, (ECF). The ECF is further divided into interstitial, fluid (10.5l) and plasma (3.5l). The distribution, of water in man is given in Table 21.1., , 468
Page 479 :
Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , of water from the body—urine, skin, lungs and, feces., , TABLE 21.1 Distribution of water in, an adult man, weighing 70 kg., Compartment, , % Body weight, , Volume (l), , Total, , 60, , 42, , Intracellular fluid (ICF), , 40, , 28, , Extracellular fluid (ECF), , 20, , 14, , Interstitial fluid, , 15, , 10.5, , 5, , 3.5, , Plasma, , 469, , WATER TURNOVER AND BALANCE, The body possesses tremendous capacity to, regulate its water content. In a healthy, individual, this is achieved by balancing the, daily water intake and water output., , Water intake, Water is supplied to the body by exogenous, and endogenous sources., Exogenous water : Ingested water and, beverages, water content of solid foodsconstitute the exogenous source of water. Water, intake is highly variable which may range from, 0.5-5 litres. It largely depends on the social, habits and climate. In general, people living in, hot climate drink more water. Ingestion of water, is mainly controlled by a thirst centre located in, the hypothalamus. Increase in the osmolality of, plasma causes increased water intake by, stimulating thirst centre., Endogenous water : The metabolic water, produced within the body is the endogenous, water. This water (300-350 ml/day) is derived, from the oxidation of foodstuffs. It is estimated, that 1 g each of carbohydrate, protein and fat,, respectively, yield 0.6 ml, 0.4 ml and 1.1 ml of, water. On an average, about 125 ml of water is, generated for 1,000 Cal consumed by the body., , Water output, Water losses from the body are variable., There are four distinct routes for the elimination, , Urine : This is the major route for water loss, from the body. In a healthy individual, the urine, output is about 1-2 l/day. Water loss through, kidneys although highly variable, is well regulated to meet the body demands—to get rid of, water or to retain. It should, however, be, remembered that man cannot completely shut, down urine production, despite there being no, water intake. This is due to the fact that some, amount of water (about 500 ml/day) is essential, as the medium to eliminate the waste products, from the body., Hormonal regulation of urine production : It, is indeed surprising to know that about 180 litres, of water is filtered by the glomeruli into the renal, tubules everyday. However, most of this is, reabsorbed and only 1-2 litres is excreted as, urine. Water excretion by the kidney is tightly, controlled by vasopressin also known as, antidiuretic hormone (ADH) of the posterior, pituitary gland. The secretion of ADH is, regulated by the osmotic pressure of plasma. An, increase in osmolality promotes ADH secretion, that leads to an increased water reabsorption, from the renal tubules (less urine output). On the, other hand, a decrease in osmolality suppresses, ADH secretion that results in reduced water, reabsorption from the renal tubules (more urine, output). Plasma osmolality is largely dependent, on the sodium concentration, hence sodium, indirectly controls the amount of water in the, body., , Diabetes insipidus is a disorder characterized, by the deficiency of ADH which results in an, increased loss of water from the body., Skin : Loss of water (450 ml/day) occurs, through the body surface by perspiration. This is, an unregulated process by the body which, mostly depends on the atmospheric temperature, and humidity. The loss is more in hot, climate. Fever causes increased water loss, through the skin. It is estimated that for every, 1°C rise in body temperature, about 15%, increase is observed in the loss of water (through, skin).
Page 480 :
470, , BIOCHEMISTRY, , Drinking water, and beverages, (1,500 ml), , Metabolic, water, (300 ml), , Water, intake, (2,500 ml), , Urine, Lungs, Feces, Skin, (1,500 ml) (450 ml) (400 ml) (150 ml), , Water, output, (2,500 ml), , Foodstuffs, (700 ml), , Body water, 42,000 ml, , Fig. 21.1 : Water balance in the body, represented by, daily intake and output (values are variable)., , Lungs : During respiration, some amount of, water (about 400 ml/day) is lost through the, expired air. The latter is saturated with water and, expelled from the body. In hot climates and/or, when the person is suffering from fever, the, water loss through lungs is increased., The loss of water by perspiration (via skin), and respiration (via lungs) is collectively referred, to as insensible water loss., Feces : Most of the water entering the, gastrointestinal tract is reabsorbed by the, intestine. About 150 ml/day is lost through feces, in a healthy individual. Fecal loss of water is, tremendously increased in diarrhea., A summary of the water intake and output in, the body is depicted in Fig.21.1. It may be noted, that water balance of the body is regulated, predominantly by controlling the urine output., This happens after an obligatory water loss via, skin, lungs and feces., The abnormalities associated with water, balance—dehydration and overhydration—will, be described, following a discussion on, electrolyte balance., , ELECTROLYTE BALANCE, Electrolytes are the compounds which readily, dissociate in solution and exist as ions i.e., positively and negatively charged particles. For, , instance, NaCl does not exist as such, but it, exists as cation (Na+) and anion (Cl–). The, concentration of electrolytes are expressed as, milliequivalents (mEq/l) rather than milligrams., A gram equivalent weight of a compound is, defined as its weight in grams that can combine, or displace 1 g of hydrogen. One gram, equivalent weight is equivalent to 1,000, milliequivalents., The following formula is employed to convert, the concentration mg/l to mEq/l., mg per litre u Valency, mEq/l = ———————————, Atomic weight, , Electrolyte composition, of body fluids, Electrolytes are well distributed in the body, fluids in order to maintain the osmotic, equilibrium and water balance. A comparison of, electrolytes present in extracellular (plasma) and, intracellular (muscle) fluids is given in, Table 21.2. The total concentration of cations, and anions in each body compartment (ECF or, ICF) is equal to maintain electrical neutrality., There is a marked difference in the concentration of electrolytes (cations and anions), between the extracellular and intracellular fluids., Na+ is the principal extracellular cation while, K+ is the intracellular cation. This difference in, the concentration is essential for the cell survival, which is maintained by Na+ – K+ pump (for, details, Refer Chapter 33). As regards anions,, CI– and HCO3– predominantly occur in, extracellular fluids, while HPO4–, proteins and, organic acids are found in the intracellular fluids., , Osmolarity and osmolality, of body fluids, There are two ways of expressing the concentration of molecules with regard to the osmotic, pressure., 1. Osmolarity : The number, millimoles) per liter of solution., , of moles (or, , 2. Osmolality : The number of moles (or, millimoles) per kg of solvent.
Page 481 :
471, , Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , TABLE 21.2 Composition of electrolytes in the body fluids (expressed as mEq/l), , Extracellular fluid (plasma), Cations, Na+, , Intracellular fluid (muscle), , Anions, 142, , K+, Ca2+, Mg2+, , Cl–, , Cations, 103, , K+, , 5, , HCO3–, , 27, , Na+, , 5, , HPO 24 –, SO 42 –, , 2, , Mg2+, , 1, , Ca2+, , Proteins, , 16, , 3, , Organic acids, 155, , 150, , HPO42–, , 140, , 10, , HCO 3–, , 10, , 40, , Cl–, , 2, , SO42–, , 5, , 2, , Proteins, , 6, , Osmolality of plasma, Osmolality is a measure of the solute particles, present in the fluid medium. The osmolality of, plasma is in the range of 285-295 milliosmoles/, kg (Table 21.3). Sodium and its associated anions, make the largest contribution (~90%) to plasma, osmolality. Osmolality is generally measured by, osmometer., For practical purposes, plasma osmolality can, be computed from the concentrations (mmol/l), of Na+, K+, urea and glucose as follows, 2(Na+) + 2(K+) + Urea + Glucose, The factor 2 is used for Na+ and K+ ions to, account for the associated anion concentration, (assuming complete ionization of the molecules)., Since plasma Na+ is the most predominant, contributor to osmolality, the above calculation, is further simplified as follows, Plasma osmolality = 2 u Plasma Na+, (mmol/l), , The above calculation holds good only if, plasma concentration of glucose and urea are in, the normal range. This calculation, however, will, , 40, , Organic acids, , 155, , If the solvent is pure water, there is almost no, difference between osmolarity and osmolality., However, for biological fluids (containing, molecules such as proteins), the osmolality is, more commonly used. This is about 6% greater, than osmolarity., , (mmol/kg), , Anions, , 202, , 5, 202, , not be valid in severe hyperproteinemia and, lipemia., , Osmolality of ECF and ICF, Movement of water across the biological, membranes is dependent on the osmotic, pressure differences between the intracellular, fluid (ICF) and extracellular fluid (ECF). In a, healthy state, the osmotic pressure of ECF,, mainly due to Na+ ions, is equal to the osmotic, pressure of ICF which is predominantly due to, , TABLE 21.3 Distribution of, constituents in plasma osmolality, , Constituent (solute), Sodium, Associated anions, Potassium, Associated anions, Calcium, Associated anions, Magnesium, Associated anions, , Osmolality (mosm/kg), 135, 135, 3.5, 3.5, 1.5, 1.5, 1.0, 1.0, , Urea, , 5.0, , Glucose, , 5.0, , Protein, Total, , 1.0, 293
Page 482 :
472, , BIOCHEMISTRY, , K+ ions. As such, there is no net passage of water, molecules in or out of the cells, due to this, osmotic equilibrium., , Adrenal, gland, , Angiotensin II, , Regulation of electrolyte balance, Electrolyte and water balance are regulated, together and the kidneys play a predominant role, in this regard. The regulation is mostly achieved, through the hormones aldosterone, ADH and, renin-angiotensin., Aldosterone : It is a mineralocorticoid, produced by adrenal cortex. Aldosterone, increases Na+ reabsorption by the renal tubules, at the expense of K+ and H+ ions. The net effect, is the retention of Na+ in the body., Antidiuretic hormone (ADH) : An increase in, the plasma osmolality (mostly due to Na+), stimulates hypothalamus to release ADH. ADH, effectively increases water reabsorption by renal, tubules., Renin-angiotensin : The secretion of, aldosterone is controlled by renin-angiotensin, system. Decrease in the blood pressure (due to a, fall in ECF volume) is sensed by juxtaglomerular, apparatus of the nephron which secrete renin., Renin acts on angiotensinogen to produce, angiotensin I. The latter is then converted to, angiotensin II which stimulates the release of, aldosterone., The relation between renin, angiotensin and, aldosterone in the regulation of Na+ balance is, depicted in Fig.21.2. Aldosterone and ADH, coordinate with each other to maintain the, normal fluid and electrolyte balance., Atrial natriuretic factor (ANF) : ANF or, atriopeptin is a 28-amino acids containing, peptide. It is produced in the atrium of heart in, response to increased blood volume, elevated, blood pressure and high salt intake. ANF acts on, kidneys to increase GFR, sodium excretion and, urine output. Thus ANF opposes the actions of, renin and aldosterone (which increase salt, retention and blood pressure)., , Angiotensin I, Na+, reabsorption, , Renin, , Aldosterone, , Angiotensinogen, , Fig. 21.2 : Hormonal regulation of, Na+ balance by the kidney., , the ECF is directly related to the osmotic effect of, these ions (Na+ and Cl–). Therefore, the amount, of Na+ in the ECF ultimately determines its, volume., , Dietary intake and, electrolyte balance, Generally, the consumption of a wellbalanced diet supplies the body requirement of, electrolytes. Humans do not possess the ability, to distinguish between the salt hunger and water, hunger. Thirst, however, may regulate electrolyte, intake also. In hot climates, the loss of electrolyte, is usually higher. Sometimes it may be necessary, to supplement drinking water with electrolytes., , Dehydration, Dehydration is a condition characterized by, water depletion in the body. It may be due to, insufficient intake or excessive water loss or, both. Dehydration is generally classified into two, types., 1. Due to loss of water alone., , Na+ concentration and ECF, , 2. Due to, electrolytes., , deprivation, , of, , water, , and, , It is important to realise that Na+ and its, anions (mainly Cl–) are confined to the, extracellular fluid. And the retention of water in, , Causes of dehydration : Dehydration may, occur as a result of diarrhea, vomiting, excessive, sweating, fluid loss in burns, adrenocortical
Page 483 :
Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , 473, , The salient features of dehydration are given, hereunder, , lumen. These ions collectively raise the osmotic, pressure and suck the water into lumen. This, results in diarrhea with a heavy loss of water, (5–10 liters/day). If not treated in time, the, victims of cholera will die due to dehydration, and loss of dissolved salts. Thus, cholera and, other forms of severe diarrhea are the major, killers of young children in many developing, countries., , 1. The volume of the extracellular fluid (e.g., plasma) is decreased with a concomitant rise in, electrolyte concentration and osmotic pressure., , Oral rehydration therapy (ORT) is commonly, used to treat cholera and other diarrheal, diseases., , dysfunction, kidney diseases (e.g. renal insufficiency), deficiency of ADH (diabetes insipidus), etc., Characteristic features of dehydration : There, are three degrees of dehydration—mild,, moderate and severe., , 2. Water is drawn from the intracellular fluid, that results in shrunken cells and disturbed, metabolism e.g. increased protein breakdown., 3. ADH secretion is increased. This causes, increased water retention in the body and, consequently urine volume is very low., 4. Plasma protein and blood urea concentrations are increased., 5. Water depletion is often accompanied by, a loss of electrolytes from the body (Na+, K+, etc.)., 6. The principal clinical symptoms of severe, dehydration include increased pulse rate, low, blood pressure, sunken eyeballs, decreased skin, turgor, lethargy, confusion and coma., Treatment : The treatment of choice for, dehydration is intake of plenty of water. In the, subjects who cannot take orally, water should be, administered intravenously in an isotonic, solution (usually 5% glucose). If the dehydration, is accompanied by loss of electrolytes, the same, should be administered by oral or intravenous, routes. This has to be done by carefully, monitoring the water and electrolyte status of the, body., , Osmotic imbalance and dehydration, in cholera, Cholera is transmitted through water and, foods, contaminated by the bacterium Vibrio, cholerae. This bacterium produces a toxin which, stimulates the intestinal cells to secrete various, ions (Cl–, Na+, K+, HCO3– etc.) into the intestinal, , Overhydration, Overhydration or water intoxication is caused, by excessive retention of water in the body. This, may occur due to excessive intake of large, volumes of salt free fluids, renal failure,, overproduction of ADH etc. Overhydration is, observed after major trauma or operation, lung, infections etc., Water intoxication is associated with dilution, of ECF and ICF with a decrease in osmolality., The clinical symptoms include headache,, lethargy and convulsions. The treatment, advocated is stoppage of water intake and, administration of hypertonic saline., , Water tank model, The distribution of body water (in the ECF, and ICF), dehydration and overhydration can be, better understood by a water tank model, (Fig.21.3). The tank has an inlet and outlet,, respectively, representing the water intake, (mostly oral) and water output (mainly urine) by, the body., Dehydration is caused when the water output, exceeds the intake. On the other hand,, overhydration is due to more water intake and, less output., , Metabolism of electrolytes, The body distribution, dietary intake, intestinal, absorption and biochemical functions of, individual electrolytes are discussed under the, section mineral metabolism (Chapter 18). The
Page 484 :
474, , BIOCHEMISTRY, , Inlet, , N, , N, , N, , ECF, , ECF, , ECF, , ICF, , Normal, , ICF, , ICF, , Outlet, , Dehydration, , Overhydration, , Fig. 21.3 : Water tank model representing body fluid compartments, (N–Normal level; ECF–Extracellular fluid; ICF–Intracellular fluid)., , electrolyte disorders, particularly hypernatremia, and hyponatremia (of sodium); hyperkalemia and, hypokalemia (of potassium) must also be referred., , Diuretics in the treatment of edema, and hypertension, Diuretics are the drugs that stimulate water, and sodium excretion, so that urine volume is, increased. The commonly used diuretics are, bendrofluazide, frusemide, spironolactone and, mannitol. Diuretics are important in the treatment, of edema, heart failure and hypertension., , ACID-BASE BALANCE, The normal pH of the blood is maintained in, the narrow range of 7.35-7.45, i.e. slightly, alkaline. The pH of intracellular fluid is rather, variable. Thus, for erythrocytes the pH is 7.2,, while for skeletal muscle, it may be as low as 6.0., Maintenance of blood pH is an important, homeostatic mechanism of the body. In normal, circumstances, the regulation is so effective that, the blood pH varies very little. Changes in blood, pH will alter the intracellular pH which, in turn,, influence the metabolism e.g. distortion in, protein structure, enzyme activity etc. It is, estimated that the blood pH compatible to life is, 6.8-7.8. (For a good understanding of acid-base, balance, adequate knowledge on acids, bases,, pH and buffers is essential (refer Chapter 40)., , Production of acids by the body, The metabolism of the body is accompanied, by an overall production of acids. These include, the volatile acids like carbonic acid (most, predominent, about 20,000 mEq/day) or nonvolatile acids (about 80 mEq/day) such as lactic, acid, sulfuric acid, phosphoric acid etc. Carbonic, acid is formed from the metabolic product CO2;, lactic acid is produced in anaerobic metabolism;, sulfuric acid is generated from proteins (sulfur, containing amino acids); phosphoric acid is, derived, from, organic, phosphates, (e.g., phospholipids). All these acids add up H+ ions, to the blood. A diet rich in animal proteins, results in more acid production by the body that, ultimately leads to the excretion of urine which, is profoundly acidic., , Production of bases by the body, The formation of basic compounds in the, body, in the normal circumstances, is negligible., Some amount of bicarbonate is generated from, the organic acids such as lactate and citrate. A, vegetarian diet has a tendency for a net, production of bases. This is due to the fact that, vegetarian diet produces salts of organic acids, such as sodium lactate which can utilize H+ ions, produced in the body. For this reason, a, vegetarian diet has an alkalizing effect on the, body. This is reflected by the excretion of neutral, or slightly alkaline urine by these subjects.
Page 485 :
475, , Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , MAINTENANCE OF BLOOD pH, The body has developed three lines of, defense to regulate the body’s acid-base balance, and maintain the blood pH (around 7.4)., , By taking the reciprocals and logarithms (for, logs, multiplication becomes addition)., log, , 1, [H� ], , log, , I. Blood buffers, II. Respiratory mechanism, , log, , 1, Ka, , 1, Ka, , � log, , The blood contains 3 buffer systems., 1. Bicarbonate buffer, 2. Phosphate buffer, 3. Protein buffer., 1. Bicarbonate buffer system : Sodium bicarbonate and carbonic acid (NaHCO3 – H2CO3), is the most predominant buffer system of the, extracellular fluid, particularly the plasma., Carbonic acid dissociates into hydrogen and, bicarbonate ions., H+ + HCO3–, , pKa � log, , >H @ >HCO @, >, , @, , The equation may be rewritten as follows, , > @, H�, , Ka, , >H CO @ ·, , >, , 2, , 3, , HCO–, , We know that pH = log, , 3, , @, , 1 ·, [H� ], , >H CO @, , pKa � log, , pH, , …… (4), , 3, , >Base@ ·, > Acid @, , …… (5), , It is evident from this equation that the pH is, dependent on ratio of the concentration of the, base to acid (HCO3– and H2CO3 in equation 4)., Blood pH and the ratio of HCO3– to, H2CO3 : The plasma bicarbonate (HCO3–), concentration is around 24 mmol/l (range 22-26, mmol/l). Carbonic acid is a solution of CO2 in, water. Its concentration is given by the product, of pCO2 (arterial partial pressure of CO2 = 40, mm Hg) and the solubility constant of CO2, (0.03)., Thus H2CO3 = 40 u 0.03 = 1.2 mmol/l., The Henderson-Hasselbalch equation for, bicarbonate buffer is, pKa, , >HCO @ ·, � log, –, 3, , >H CO @, 2, , …… (1), , (Ka = Dissociation constant of H2CO3)., , –, 3, , The above equation is valid for any buffer, pair. The general equation referred to as, Henderson-Hasselbalch equation for any buffer, is written as, , pH, , –, 3, , >HCO @ ·, 2, , By the law of mass action, at equilibrium, , H2CO3, , …… (3), , 3, , pKa, , pH, , A buffer may be defined as a solution of a, weak acid (HA) and its salt (BA) with a strong, base. The buffer resists the change in pH by the, addition of acid or alkali and the buffering, capacity is dependent on the absolute, concentration of salt and acid. It should be borne, in mind that the buffer cannot remove H+ ions, from the body. It temporarily acts as a shock, absorbant to reduce the free H+ ions. The H+, ions have to be ultimately eliminated by the, renal mechanism (described later)., , Ka, , >H CO @, , The equation 3 may now be written as, , I. Blood buffers, , �, , –, 3, , 2, , III. Renal mechanism., , H2CO3, , >HCO @, , 3, , Substituting the values (blood pH = 7.4;, pKa for H2CO3 = 6.1; HCO3– = 24 mmol/l;, H2CO3 = 1.2 mmol/l), in the above equation, 7.4 = 6.1 + log 24, , 1.2, , …… (2), , = 6.1 + log 20, = 6.1 + 1.3, = 7.4
Page 486 :
476, It is evident that at a blood pH 7.4, the ratio, of bicarbonate to carbonic acid is 20 : 1. Thus,, the bicarbonate concentration is much higher, (20 times) than carbonic acid in the blood. This, is referred to as alkali reserve and is responsible, for the effective buffering of H+ ions, generated, in the body. In normal circumstances, the, concentration of bicarbonate and carbonic acid, determines the pH of blood. Further, the, bicarbonate buffer system serves as an index to, understand the disturbances in the acid-base, balance of the body., 2. Phosphate buffer system : Sodium, dihydrogen phosphate and disodium hydrogen, phosphate (NaH2PO4 – Na2HPO4) constitute the, phosphate buffer. It is mostly an intracellular, buffer and is of less importance in plasma due to, its low concentration. With a pK of 6.8 (close to, blood pH 7.4), the phosphate buffer would have, been more effective, had it been present in high, concentration. It is estimated that the ratio of, base to acid for phosphate buffer is 4 compared, to 20 for bicarbonate buffer., 3. Protein buffer system : The plasma, proteins and hemoglobin together constitute the, protein buffer system of the blood. The buffering, capacity of proteins is dependent on the pK of, ionizable groups of amino acids. The imidazole, group of histidine (pK = 6.7) is the most effective, contributor of protein buffers. The plasma, proteins account for about 2% of the total, buffering capacity of the plasma., Hemoglobin of RBC is also an important, buffer. It mainly buffers the fixed acids, besides, being involved in the transport of gases (O2 and, CO2). More details on hemoglobin are given, under respiratory mechanism for regulation of pH., , II. Respiratory mechanism, for pH regulation, Respiratory system provides a rapid, mechanism for the maintenance of acid-base, balance. This is achieved by regulating the, concentration of carbonic acid (H2CO3) in the, blood i.e. the denominator in the bicarbonate, buffer system. The details of CO2 transport, and the role of hemoglobin in this process, , BIOCHEMISTRY, , are described elsewhere (Chapter 10, Refer, Fig.10.6)., The large volumes of CO2 produced by the, cellular metabolic activity endanger the acidbase equilibrium of the body. But in normal, circumstances, all of this CO2 is eliminated from, the body in the expired air via the lungs, as, summarized below., Carbonic anhydrase, , H2CO3, , CO2 + H2O ., , The rate of respiration (or the rate of removal, of CO2) is controlled by a respiratory centre,, located in the medulla of the brain. This centre is, highly sensitive to changes in the pH of blood., Any decrease in blood pH causes hyperventilation, to blow off CO2, thereby reducing the H2CO3, concentration. Simultaneously, the H+ ions are, eliminated as H2O., Respiratory control of blood pH is rapid but, only a short term regulatory process, since, hyperventilation cannot proceed for long., Hemoglobin as a buffer : Hemoglobin of, erythrocytes is also important in the respiratory, regulation of pH. At the tissue level, hemoglobin, binds to H+ ions and helps to transport CO2 as, HCO3– with a minimum change in pH (referred, to as isohydric transport). In the lungs, as, hemoglobin combines with O2, H+ ions are, removed which combine with HCO3– to form, H2CO3. The latter dissociates to release CO2 to, be exhaled (Refer Fig.10.6)., Generation of HCO3– by RBC : Due to lack of, aerobic metabolic pathways, RBC produce very, little CO2. The plasma CO2 diffuses into the RBC, along the concentration gradient where it, combines with water to form H2CO3. This, reaction is catalysed by carbonic anhydrase (also, called carbonate dehydratase). In the RBC,, H2CO3 dissociates to produce H+ and HCO3– ., The H+ ions are trapped and buffered by, hemoglobin. As the concentration of HCO3–, increases in the RBC, it diffuses into plasma, along with the concentration gradient, in, exchange for Cl– ions, to maintain electrical, neutrality., This, phenomenon,, referred, to as chloride shift, helps to generate HCO3–, (Fig.21.4).
Page 487 :
477, , Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , Plasma, , the renal regulation of pH which occurs by the, following mechanisms., , Erythrocyte, , 1. Excretion of H+ ions, CO2, , 2. Reabsorption of bicarbonate, , CO2 + H2O, , 3. Excretion of titratable acid, , CA, , 4. Excretion of ammonium ions., H2CO3, HHb, –, HCO3, –, , Cl, , –, HCO3, –, , +, , +H, , Hb, , Cl, , Fig. 21.4 : Generation of bicarbonate by the erythrocyte, (CA–Carbonic anhydrase; Hb–Hemoglobin)., , III. Renal mechanism for pH, regulation, The role of kidneys in the maintenance of, acid-base balance of the body (blood pH) is, highly significant. The renal mechanism tries to, provide a permanent solution to the acid-base, disturbances. This is in contrast to the temporary, buffering system and a short term respiratory, mechanism, described above., The kidneys regulate the blood pH by, maintaining the alkali reserve, besides excreting, or reabsorbing the acidic or basic substances, as, the situation demands., Urine pH normally lower than blood pH :, The pH of urine is normally acidic (~6.0). This, clearly indicates that the kidneys have, contributed to the acidification of urine, when it, is formed from the blood plasma (pH 7.4). In, other words, the H+ ions generated in the body, in the normal circumstances, are eliminated by, acidified urine. Hence the pH of urine is, normally acidic (~6.0), while that of blood is, alkaline (7.4). Urine pH, however, is variable, and may range between 4.5-9.5, depending on, the concentration of H+ ions., Carbonic anhydrase and renal regulation of, pH : The enzyme carbonic anhydrase (inhibited, by acetazolamide) is of central importance in, , 1. Excretion of H+ ions : Kidney is the only, route through which the H+ can be eliminated, from the body. H+ excretion occurs in the, proximal convoluted tubules (renal tubular cells), and is coupled with the regeneration of HCO3–., The process depicted in Fig.21.5, occurs as, follows., Carbonic anhydrase catalyses the production, of carbonic acid (H2CO3) from CO2 and H2O in, the renal tubular cell. H2CO3 then dissociates to, H+ and HCO3–. The H+ ions are secreted into the, tubular lumen in exchange for Na+. The Na+ in, association with HCO3– is reabsorbed into the, blood. This is an effective mechanism to, eliminate acids (H+) from the body with a, simultaneous generation of HCO3–. The latter, adds up to the alkali reserve of the body. The H+, combines with a non-carbonate base and is, excreted in urine., 2. Reabsorption of bicarbonate : This mechanism is primarily responsible to conserve the, blood HCO3–, with a simultaneous excretion of, H+ ions. The normal urine is almost free from, HCO3–. This is explained as follows (Fig.21.6)., , Blood, , Na, , +, , –, , HCO3, , Renal tubular cell, , Na, , +, , –, , Tubular lumen, , Na, +, , HCO3 + H, , H2CO3, , +, , H + + B–, , HB, , CA, , CO2 + H 2O, , Excreted, , Fig. 21.5 : Renal regulation of blood, pH–Excretion of H+ ions (CA–Carbonic anhydrase).
Page 488 :
478, , BIOCHEMISTRY, , Blood, +, , Na, , –, , HCO3, , Renal tubular cell, , Na, , +, , Na, , –, , +, , HCO3 + H, , H2CO3, CA, , H2O + CO2, , mechanism helps to maintain the steady state, and will not be effective for the elimination of, H+ or generation of new HCO3–., , Tubular lumen, + Plasma, , +, , H, , –, , HCO3, , (Filtered), , H2CO3, CA, , CO2 + H2O, , Fig. 21.6 : Renal regulation of blood pH–Reabsorption, of bicarbonate (CA–Carbonic anhydrase)., , Bicarbonate freely diffuses from the plasma, into the tubular lumen. Here HCO3– combines, with H+, secreted by tubular cells, to form, H2CO3. H2CO3 is then cleaved by carbonic, anhydrase (of tubular cell membrane) to form, CO2 and H2O. As the CO2 concentration builds, up in the lumen, it diffuses into the tubular cells, along the concentration gradient. In the tubular, cell, CO2 again combines with H2O to form, H2CO3 which then dissociates into H+ and, HCO3–. The H+ is secreted into the lumen in, exchange for Na+. The HCO3– is reabsorbed into, plasma in association with Na+. Reabsorption of, HCO3– is a cyclic process with the net excretion, of H+ or generation of new HCO3–. This is, because the H+ is derived from water. This, , Blood, , Renal tubular cell, , 3. Excretion of titratable acid : Titratable, acidity is a measure of acid excreted into urine, by the kidney. This can be estimated by titrating, urine back to the normal pH of blood (7.4). In, quantitative terms, titratable acidity refers to the, number of milliliters of N/10 NaOH required to, titrate 1 liter of urine to pH 7.4. Titratable acidity, reflects the H+ ions excreted into urine which, resulted in a fall of pH from 7.4 (that of blood)., The excreted H+ ions are actually buffered in the, urine by phosphate buffer as depicted in, Fig.21.7, and briefly described hereunder., As already discussed, H+ ion is secreted into, the tubular lumen in exchange for Na+ ion. This, Na+ is obtained from the base, disodium, hydrogen phosphate (Na2HPO4). The latter in, turn combines with H+ to produce the acid,, sodium dihydrogen phosphate (NaH2PO4), in, which form the major quantity of titratable acid, in urine is present. As the tubular fluid moves, down the renal tubules, more and more H+ ions, are added, resulting in the acidification of urine., This causes a fall in the pH of urine to as low as, 4.5. Any further fall in the pH will cause, depletion of Na+ ions., 4. Excretion of ammonium ions : This is, another mechanism to buffer H+ ions secreted, into the tubular fluid. The H+ ion combines with, , Tubular lumen, , Na 2HPO4, Na, , +, –, , HCO3, , Na, , +, –, , Na, +, , HCO3 + H, , H2CO3, , +, , pH 7.4, , NaHPO 4–, , +, , H, , NaH2PO 4, , CA, , CO2 + H2O, , Excreted, , Fig. 21.7 : Renal regulation of blood pH–Excretion of titratable acid, by phosphate buffer mechanism (CA–Carbonic anhydrase)., , pH 4.5
Page 489 :
479, , Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , Blood, , Renal tubular cell, , Tubular lumen, , Glutamine, Glutaminase, , NH3, , NH3, , aerobic metabolism may be exhaled via lungs,, or converted to HCO3– by erythrocytes and, kidneys to add up to the alkali reserve of the, body., , Buffers of intracellular fluids, , Glutamate, +, , Na, , –, , HCO3, , Na, , +, –, , Na, +, , +, , +, , HCO3 + H, , H, , +, , NH4, , H2CO3, , The regulation of pH within the cells is as, important as that discussed above for the, extracellular fluid. The H+ ions generated in the, cells are exchanged for Na+ and K+ ions. This is, particularly observed in skeletal muscle which, reduces the potential danger of H+ accumulation, in the cells., , CA, , CO2 + H2O, , Excreted, , Fig. 21.8 : Renal regulation of blood pH–Excretion of, ammonium ions (CA–Carbonic anhydrase)., , +, , NH3 to form ammonium ion (NH4 ). The renal, tubular cells deamidate glutamine to glutamate, and NH3. This reaction is catalysed by the, enzyme glutaminase. The NH3, liberated in this, reaction, diffuses into the tubular lumen where it, combines with H+ to form NH4+ (Fig.21.8)., Ammonium ions cannot diffuse back into tubular, cells and, therefore, are excreted into urine., NH4+, , is a major urine acid. It is estimated that, about half to two-thirds of body acid load is, eliminated in the form of NH4+ ions. For this, reason, renal regulation via NH4+ excretion is, very effective to eliminate large quantities of, acids produced in the body. This mechanism, becomes predominant particularly in acidosis., , Carbon dioxide—the central, molecule of pH regulation, As is observed from the foregoing discussion,, CO2 is of central importance in the acid-base, balance of the body. It has the ability to combine, with H2O to from H2CO3 which can dissociate, to HCO3– and H+. A summary of the interaction, between the lungs, erythrocytes and kidneys in, handling CO2 to maintain pH of the blood is, depicted in Fig.21.9. The CO2 generated by, , DISORDERS OF, ACID-BASE BALANCE, The body has developed an efficient system, for the maintenance of acid-base equilibrium, with a result that the pH of blood is almost, constant (7.4). The blood pH compatible to life, is 6.8-7.8, beyond which life cannot exist., For a better understanding of the disorders of, acid-base balance, the Henderson-Hasselbalch, equation must be frequently consulted., , pH, , pKa � log, , >HCO @, –, 3, , >H CO @, 2, , 3, , pH 7.4, , Metabolism, (CO2 generated), , CO2, (H2CO3), , Lungs, (CO2 exhaled), , HCO–3, Erythrocytes, (CO2 transported,, HCO3– generated), , Kidneys, –, (HCO3 generated, H+ lost), , Fig. 21.9 : Carbon dioxide—the central molecule of, blood pH regulation.
Page 490 :
480, , BIOCHEMISTRY, , It is evident from the above equation that the, blood pH (H+ ion concentration) is dependent, on the relative concentration (ratio) of, bicarbonate (HCO3–) and carbonic acid (H2CO3)., The acid-base disorders are mainly classified as, 1. Acidosis—a decline in blood pH, (a) Metabolic acidosis—due to a decrease, in bicarbonate., (b) Respiratory acidosis—due, increase in carbonic acid., , to, , an, , 2. Alkalosis—a rise in blood pH, (a) Metabolic alkalosis—due, increase in bicarbonate., (b) Respiratory, alkalosis—due, decrease in carbonic acid., , to, to, , an, a, , TABLE 21.4 Major clinical causes of, acid-base disorders, Metabolic acidosis, , Respiratory acidosis, , Diabetes mellitus, (ketoacidosis), , Severe asthma, Pneumonia, Cardiac arrest, , Renal failure, , Obstruction in airways, , Lactic acidosis, , Chest deformities, , Severe diarrhea, Renal tubular acidosis, , Depression of, respiratory center (by, drugs e.g. opiates), , Metabolic alkalosis, , Respiratory alkalosis, , Severe vomiting, , Hyperventilation, , Hypokalemia, , Anemia, High altitude, , Intravenous administration, of bicarbonate, , Salicylate poisoning, , The four acid-base disorders referred above, are primarily due to alterations in either, bicarbonate or carbonic acid. It may be observed, that the metabolic acid-base balance disorders, are caused by a direct alteration in bicarbonate, concentration while the respiratory disturbances, are due to a change in carbonic acid level (i.e., CO2). This type of classification is more, theoretical. In the actual clinical situations,, mixed type of disorders are common., , makes every attempt to restore the pH to normal, level (7.4). This is referred to as compensation, which may be partial or full. Sometimes the acidbase disorders may remain uncompensated., , The terms acidemia and alkalemia,, respectively, refer to an increase or a decrease in, [H+] ion concentration in blood. They are,, however, not commonly used., , The principal acid-base disturbances, along, with the blood concentration of HCO3– and, H2CO3, in acute and compensated states are, given in the Table. 21.5., , Clinical causes of, acid-base disorders, , For the acute metabolic disorders (due to, changes in HCO3–), respiratory compensation sets, in and regulates the H2CO3 (i.e. CO2) by hyperor hypoventilation. As regards acute respiratory, disorders (due to changes in H2CO3), the, renal compensation occurs to maintain the, HCO3– level, by increasing or decreasing its, excretion., , The most important clinical causes/disease, states that result in acid-base disorders are listed, in Table 21.4. Metabolic acidosis could occur, due to diabetes mellitus (ketoacidosis), lactic, acidosis, renal failure etc. Respiratory acidosis is, common in severe asthma and cardiac arrest., Vomiting and hypokalemia may result in, metabolic alkalosis while hyperventilation and, severe anemia may lead to respiratory alkalosis., , Compensation of, acid-base disorders, To counter the acid-base disturbances, the, body gears up its homeostatic mechanism and, , In the Table 21.6, a summary of the acid-base, disorders, with, primary, changes, and, compensatory mechanisms is given., , Anion gap, For a better understanding of acid-base, disorders, adequate knowledge of anion gap is, essential. The total concentration of cations and
Page 491 :
481, , Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , anions (expressed as mEq/l) is equal in the body, fluids. This is required to maintain electrical, neutrality., The commonly measured electrolytes in the, plasma are Na+, K+, Cl– and HCO3–. Na+ and K+, together constitute about 95% of the plasma, cations. Cl– and HCO3– are the major anions,, contributing to about 80% of the plasma anions., The remaining 20% of plasma anions (not, normally measured in the laboratory) include, proteins, phosphate, sulfate, urate and organic, acids., Anion gap is defined as the difference, between the total concentration of measured, cations (Na+ and K+) and that of measured anion, (Cl– and HCO3–). The anion gap (A–) in fact, represents the unmeasured anions in the plasma, which may be calculated as follows, by, substituting the normal concentration of, electrolytes (mEq/l)., + HCO3– + A–, , Na+ + K+, , =, , Cl–, , 136 +, , =, , 100 + 25, , =, , 15 mEq/l, , 4, –, , A, , + A–, , The anion gap in a healthy individual is, around 15 mEq/l (range 8-18 mEq/l). Acid-base, , disorders are often associated with alterations in, the anion gap., , Metabolic acidosis, The primary defect in metabolic acidosis is a, reduction in bicarbonate concentration which, leads to a fall in blood pH. The bicarbonate, concentration may be decreased due to its, utilization in buffering H+ ions, loss in urine or, gastrointestinal tract or failure to be regenerated., The most important cause of metabolic, acidosis is due to an excessive production of, organic acids which combine with NaHCO3– and, deplete the alkali reserve., NaHCO3– + Organic acids o Na salts of, organic acids + CO2, Metabolic acidosis is commonly seen in, severe uncontrolled diabetes mellitus which is, associated with excessive production of, acetoacetic acid and E-hydroxybutyric acid (both, are organic acids)., Anion gap and metabolic acidosis : Increased, production and accumulation of organic acids, causes an elevation in the anion gap. This type, of picture is seen in metabolic acidosis, associated with diabetes (ketoacidosis)., , TABLE 21.5 Acid-base disorders along with the concentrations of, bicarbonate (HCO3–) and carbonic acid (H2CO3) in plasma, , Blood pH, , [HCO3–], , [H2CO3], , Metabolic acidosis, Acute, Compensated (by n� ventilation), , p, � � or o, , p, p, , o, p, , Respiratory acidosis, Acute, Compensated (HCO3– retained by kidney), , p, � � or o, , o, n, , n, n, , Metabolic alkalosis, Acute, Compensated (by p ventilation), , n, � � or o, , n, , n, n, , o, n, , Respiratory alkalosis, Acute, Compensated (nHCO3– excretion by kidney), , n, � � or o, , o, p, , p, p, , Disorder, , n, , n, , n, , n, , n, , n� : Increased; p� : Decreased; o� : Normal; � � � : Marginally decreased; � � � : Marginally increased.
Page 492 :
482, , BIOCHEMISTRY, , TABLE 21.6 Acid-base disorders with primary changes and compensatory mechanisms, , Disorder, , Primary change, , Compensatory, mechanism, , Timescale for, compensation, , Metabolic acidosis, , Decreased plasma, bicarbonate, , Hyperventilation, (decrease in pCO2), , Minutes to hours, , Metabolic alkalosis, , Increased plasma bicarbonate, , Hypoventilation, (increase in pCO2), , Minutes to hours, , Respiratory acidosis, , Increased pCO2, , Elevation in plasma, bicarbonate; increase in, renal reabsorption of, bicarbonate, , Days, , Respiratory alkalosis, , Decreased pCO2, , Reduction in plasma, bicarbonate; decrease, in renal reabsorption, of bicarbonate, , Days, , Compensation of metabolic acidosis : The, acute metabolic acidosis is usually compensated, by hyperventilation of lungs. This leads to an, increased elimination of CO2 from the body, (hence H2CO3p). but respiratory compensation, is only short-lived. Renal compensation sets in, within 3-4 days and the H+ ions are excreted as, NH4+ ions., , Respiratory acidosis, The primary defect in respiratory acidosis is, due to a retention of CO2 (H2CO3n). There may, be several causes for respiratory acidosis which, include depression of the respiratory centre, (overdose of drugs), pulmonary disorders, (bronchopneumonia) and breathing air with high, content of CO2., The renal mechanism comes for the rescue to, compensate respiratory acidosis. More HCO3– is, generated and retained by the kidneys which, adds up to the alkali reserve of the body. The, +, excretion of titratable acidity and NH4 is, elevated in urine., , Metabolic alkalosis, The primary abnormality in metabolic, alkalosis is an increase in HCO3– concentration., This may occur due to excessive vomiting, (resulting in loss of H+) or an excessive intake of, , sodium bicarbonate for therapeutic purposes, (e.g. control of gastric acidity). Cushing’s, syndrome (hypersecretion of aldosterone) causes, increased retention of Na+ and loss of K+ from, the body. Metabolic alkalosis is commonly, associated, with, low, K+, concentration, +, (hypokalemia). In severe K deficiency, H+ ions, are retained inside the cells to replace missing, K+ ions. In the renal tubular cells, H+ ions are, exchanged (instead of K+) with the reabsorbed, Na+. Paradoxically, the patient excretes acid, urine despite alkalosis., The respiratory mechanism initiates the, compensation by hypoventilation to retain CO2, (hence H2CO3n). This is slowly taken over by, renal mechanism which excretes more HCO3–, and retains H+., , Respiratory alkalosis, The primary abnormality in respiratory, alkalosis is a decrease in H2CO3 concentration., This, may, occur, due, to, prolonged, hyperventilation resulting in increased exhalation, of CO2 by the lungs. Hyperventilation is, observed in conditions such as hysteria,, hypoxia, raised intracranial pressure, excessive, artificial ventilation and the action of certain, drugs (salicylate) that stimulate respiratory, centre.
Page 493 :
Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , The renal mechanism tries to compensate by, increasing the urinary excretion of HCO3–., , Mixed acid-base disorders, Sometimes, the patient may have two or more, acid-base disturbances occurring simultaneously., In such instances, both HCO3– and H2CO3 are, altered. In general, if the biochemical data (of, blood gas analysis) cannot be explained by a, specific acid-base disorder, it is assumed that a, mixed disturbance is occurring. Many a times,, compensatory mechanisms may lead to mixed, acid-base disorders., , 483, , plasma K+) or hypokalemia (low plasma K+) can, be life-threatening. The relevance of potassium, balance in certain acid-base disorders is, discussed briefly., , Acid-base disorders, and plasma potassium, , Potassium and diabetic ketoacidosis : The, hormone insulin increases K+ uptake by cells, (particularly from skeletal muscle). The patient of, severe uncontrolled diabetes (i.e. with metabolic, acidosis) is usually with hypokalemia. When, such a patient is given insulin, it stimulates K+, entry into cells. The result is that plasma K+ level, is further depleted. Hypokalemia affects heart, functioning, and is life threatening. Therefore, in, the treatment of diabetic ketoacidosis, potassium, has to be given (unless the patients have high, plasma K+ concentration)., , Plasma potassium concentration (normal, 3.5-5.0 mEq/l) is very important as it affects the, contractility of the heart. Hyperkalemia (high, , Potassium and alkalosis : Low plasma, concentration of K+ (hypokalemia) leads to an, increased excretion of hydrogen ions, and thus, , + Existence of life is unimaginable in the absence of water., + Kidneys play a predominant role in the regulation of water, electrolyte and acid-base balance., + Electrolyte and water balance regulation occurs through the involvement of hormones—, aldosterone, ADH and renin-angiotensin., , + Severe dehydration is characterized by low blood pressure, sunken eyeballs, lethargy,, confusion and coma., , + Sodium is the principal extracellular cation while K+ is intracellular. The maintenance, of the differential concentration of these electrolytes is essential for the survival of life, which is brought about by Na+-K+ pump., , + The body metabolism is accompanied by the production of acids such as carbonic acid,, sulfuric acid, phosphoric acid etc., , + Vegetarian diet has an alkalizing effect on the body. This is attributed to the formation of, organic acids such as sodium lactate which can deplete H+ ions by combining with them., , + The blood pH is maintained by blood buffers, respiratory and renal mechanisms., + Carbon dioxide is the central molecule of acid-base regulation., + Disturbances in acid-base regulation result in acidosis (decreased blood pH) or alkalosis, (raised blood pH)., , + Uncontrolled diabetes mellitus is associated with metabolic acidosis, commonly referred, to as ketoacidosis (due to the overproduction of ketone bodies).
Page 494 :
484, , BIOCHEMISTRY, , may cause metabolic alkalosis. Conversely,, metabolic alkalosis is associated with increased, renal excretion of K+., In view of the importance discussed above,, the measurement of plasma K+ concentration, assumes significance in the acid-base disorders., In cases of these disorders associated with, hypokalemia, potassium supplementation (with, carefull monitoring of plasma K+) needs to be, considered., , artery in the forearm, or (less commonly) from, the femoral artery in the leg is used. The, biochemical profile measured include pO2,, pCO2, and pH (H+ ion concentration). The, concentration of bicarbonate is calculated by, using Henderson-Hasselbalch equation. In fact,, the blood gas analysers employed in the, hospitals are designed to perform the various, calculations automatically and give the final, results. The reference ranges of blood gas, analysis are given in Table 21.7., , BLOOD GAS MEASUREMENT, The measurement of blood gas is an important, investigation in the laboratory service. In certain, conditions associated with respiratory failure, and/or acid-base disorders, blood gas (CO2 and, O2) measurement assumes significance. Based, on the results obtained and the severity of the, condition, oxygen treatment or artificial, ventilation is carried out., For blood gas analysis, a sample of arterial, blood collected from (most commonly) radial, , TABLE 21.7 Reference ranges of arterial, blood gas analysis, Parameter, , Concentration/value, , [H+], , 35–43 mmol/l, , pH, , 7.35–7.45, , pCO2, , 4.5–6.0 kPa, , pO2, , 10.5–13.5 kPa, , Bicarbonate*, , 24–30 mmol/l, , *Bicarbonate concentration is calculated from pH and pCO2 values.
Page 495 :
Chapter 21 : WATER, ELECTROLYTE AND ACID-BASE BALANCE, , 1. Water is the solvent of life and constitutes about 60% of the total body weight,, distributed in intracellular and extracellular fluids. The daily water intake (by drinking,, from foodstuffs and metabolic water) and output (loss via urine, skin, lungs and feces), maintain the body balance of water., 2. Electrolytes are distributed in the intracellular and extracellular fluids to maintain the, osmotic equilibrium and water balance, Na+ is the principal extracellular cation while, K+ is the intracellular cation. As regards anions, Cl – and HCO3– predominantly occur, in the extracellular fluids while HPO42–, proteins and organic acids are present in the, intracellular fluids., 3. The osmolality of plasma is about 285 milliosmoles/kg, which is predominantly, contributed by Na+ and its associated anions. Thus, for practical purposes, plasma, osmolality can be calculated from Na+ concentration (2 u Na+ in mmol/l)., 4. Water and electrolyte balance are usually regulated together and this is under the, control of hormones—aldosterone, antidiuretic hormone and renin., 5. Dehydration of the body may be due to insufficient water intake or its excessive loss, or both. Depletion of water in the ICF causes disturbance in metabolism. The, manifestations of severe dehydration include increased pulse rate, low blood pressure,, sunken eyeballs, decreased skin turgor, lethargy and coma., 6. The normal pH of blood is maintained in the narrow range of 7.35–7.45. The, metabolism of the body is accompanied by an overall production of acids. The body has, developed three lines of defense (blood buffers, respiratory and renal mechanisms) to, regulate the acid-base balance and maintain the blood pH., 7. Among the blood buffers, bicarbonate buffer (with a ratio of HCO3– to H2CO3 as 20 : 1), is the most important in regulating blood pH. Phosphate and protein buffer systems, also contribute in this regard. The respiratory system regulates the concentration of, carbonic acid by controlling the elimination of CO2 via lungs., 8. The renal (kidney) mechanism regulates blood pH by excreting H+ and NH4+ ions, besides the reabsorption of HCO3–. The pH of urine is normally acidic which indicates, that the kidneys have contributed to the acidification of urine., 9. The acid-base disorders are classified as acidosis (metabolic or respiratory) and alkalosis, (metabolic or respiratory), respectively, due to a rise or fall in blood pH. The metabolic, disturbances are associated with alterations in HCO3– concentration while the respiratory, disorders are due to changes in H2CO3 (i.e. CO2)., 10. Blood gas measurement includes the parameters pO2, pCO2, pH and bicarbonate, and, it is very important to evaluate and treat acid-base disorders., , 485
Page 496 :
486, , BIOCHEMISTRY, , I. Essay questions, 1. Describe the role of kidney in the regulation of blood pH., 2. Give an account of the water distribution and its balance in the body., 3. Campare the composition of electrolytes in the extracellular and intracellular fluids. Discuss the, regulation of electrolyte balance., 4. Describe the role of blood buffers in the acid-base balance., 5. Classify acid-base disorders and discuss them with compensatory mechanisms., , II. Short notes, (a) Dehydration, (b) Vasopressin and water balance, (c) Osmolality of plasma, (d) Acids produced, in the body, (e) Henderson-Hasselbalch equation, (f) Bicarbonate buffer, (g) Excretion of H+ by, kidney, (h) Titratable acidity, (i) Metabolic acidosis, (j) Anion gap., , III. Fill in the blanks, 1. The hormone controlling water excretion via kidneys is ____________., 2. The principal cation of extracellular fluid is ____________., 3. The normal osmolality of plasma is ____________., 4. Na+ reabsorption by renal tubules is increased by the hormone ____________., 5. The most predominant volatile acid generated in the body is ____________., 6. The most important buffer system regulating blood pH is ____________., 7. At a normal blood pH 7.4, the ratio of bicarbonate to carbonic acid is ____________., 8. The body acid load is predominantly eliminated in the form of ____________., 9. The primary defect in metabolic acidosis is a reduction in the plasma concentration of, ____________., 10. The respiratory alkalosis is primarily associated with a decrease in the plasma concentration of, ____________., , IV. Multiple choice questions, 11. The metabolic (endogenous) water is derived by the oxidation of, (a) Carbohydrate (b) Protein (c) Fats d) All of them., 12. The most predominant anion in the extracellular fluids, (a) Cl– (b) HCO3– (c) HPO42– (d) Protein., 13. The only route through which H+ ions are eliminated from the body, (a) Lungs (b) Stomach (c) Kidneys (d) None of them., 14. Name the amino acid from which ammonia is derived in the renal tubular cells which is finally, excreted as NH4+, (a) Asparagine (b) Glutamine (c) Glutamate (d) Aspartate., 15. The anion gap refers to the unmeasured plasma anion concentration (in the laboratory) and is, represented by, (a) Proteins and organic acids (b) Phosphate and sulfate (c) Urate (d) All of them.
Page 497 :
Section 4, , Clinical Biochemistry and Nutrition, , Chapter, , Tissue Proteins and Body Fluids, , 22, , The protein, collagen, speaks :, , Gly, , X, , Y, , Gly, , X, , Y, , Gly, , X, , Y, , “I am the most abundant protein in mammals;, Triple helical in structure, with distinct types;, Predominantly composed of glycine and proline;, I give strength, support and shape to tissues.”, , T, , he body possesses a vast number of proteins, designed with specific structures to perform, specialized functions. A selected few of the most, important proteins that are intimately connected, with the tissue structure and functions are briefly, described in this chapter. In addition, the body, fluids are also discussed., , of the total body protein. Collagen is the, predominant component of the connective, tissue, although its distribution varies in different, tissues. For instance, collagen forms 90% of the, organic matrix of bones, 85% of tendons, 70%, of skin, and 4% of liver., , Functions of collagen, CONNECTIVE TISSUE PROTEINS, The connective tissue or extracellular matrix, (ECM) refers to the complex material, surrounding the mammalian cells in tissues. The, major protein components of ECM include, collagen, elastin, fibrillin, fibronectin, laminin, and proteoglycans. Besides these proteins, the, structural proteins namely keratins are also, described., , 1. Being a major component of the, connective tissue, collagen gives strength,, support and shape to the tissues. The tensile, strength of collagen fiber is impressive. To break, a collagen fiber of 1 mm in diameter, a load of, 10–40 kg is needed! However, in diseased states, with altered collagen structure, the tensile, strength is reduced., , COLLAGEN, , 2. Collagen contributes to proper alignment, of cells, which in turn helps in cell proliferation,, and their differentiation to different tissues and, organs., , Collagen is the most abundant protein in, mammals, comprising approximately one-third, , 3. Collagen (that is exposed in blood vessels), contributes to thrombus formation., , 487
Page 498 :
488, , BIOCHEMISTRY, , Types of collagen, Fibril, , Collagen is not a single homogeneous, protein, but a group of structurally related, and genetically distinct proteins. In, humans, at least 19 different types of, collagens, composed of 30 distinct, polypeptide chains (encoded by separate, genes), have been identified. The types of, collagen are numbered (by Roman, numerals) as I, II…XIX. The different types, of collagen are suited to perform, specialized functions in tissues. For, instance, collagens type I and type II are, respectively found in skin and bone., , Structure of collagen, , Collagen molecule, 300 nm, , 1.4 nm Triple helix, , D-chain, (polypeptide), Gly, , X, , Y, , Gly, , X, , Y, , Gly, , X, , Y, , Amino acid sequence, , Fig. 22.1 : A diagrammatic representation of the structure of, collagen and fibril, (X and Y represent amino acids other than glycine), , In principle, all types of collagen are, triple helical structures. The triple helix may, occur throughout the molecule, or only a part of, the molecule., Type I mature collagen, containing about, 1000 amino acids (for each polypeptide chain), possesses triple helical structure throughout the, molecule. It is composed of three similar, polypeptide chains twisted around each other to, form a rod like molecule of 1.4 nm diameter,, and about 300 nm length (Fig.22.1). The amino, acid composition of collagen is unique., Approximately 1/3rd of the amino acids are, contributed by glycine i.e. every third amino, acid in collagen is glycine. Hence, the repetitive, amino acid sequence of collagen is represented, by (Gly-X-Y)n, where X and Y represent other, amino acids. Thus, collagen may be regarded as, a polymer of glycine-led tripeptide. Among the, other amino acids, proline and hydroxyproline, are present in large quantities (about 100, residues each). These two amino acids confer, rigidity to the collagen molecule., The triple helical structure of collagen is, stabilized by an extensive network of hydrogen, bonds, covalent cross-links, electrostatic and, hydrophobic interactions, and van der Waals, forces., The triple helical molecules of collagen, assemble and form elongated fibrils, and then rod, like fibers in the tissues. The fibril formation, , occurs by a quarter staggered alignment i.e. each, triple helix of collagen is displaced longitudinally, from its neighbour by about one-quarter of its, length (Fig.22.1)., The strength of the collagen fibers is, contributed by the covalent cross links formed, between lysine and hydroxylysine residues. The, degree of collagen cross-linking increases with, age. Thus, in older people, the collagen, containing tissues (e.g. skin, blood vessels), become less elastic and more stiff, contributing, to health complications., , Biosynthesis of collagen, Collagen synthesis occurs in fibroblasts, and, the cells related to them e.g. osteoblasts in, bones, chondroblasts in cartilage, odontoblasts, in teeth., Collagen is synthesized on the ribosomes in a, precursor form namely preprocollagen. This, contains a signal peptide which directs the, protein to reach the endoplasmic reticulum (ER)., In the ER, the signal peptide is cleaved to form, procollagen. The latter undergoes extensive posttranslational modifications (hydroxylation and, glycosylation) and disulfide bonds formation., The procollagen so formed is secreted into the, extracellular medium, and subjected to the, action of aminoproteinase and carboxyproteinase to remove the terminal amino acids.
Page 499 :
489, , Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , This is followed by a spontaneous assembly of, the polypeptide chains (with about 1000 amino, acids in each) to form triple helical structures of, collagen., , Abnormalities associated, with collagen, The biosynthesis of collagen is a complex, process, involving at least 30 genes (in humans),, and about 8 post-translational modifications., Expectedly, many inherited diseases due to gene, mutations, linked with collagen formation have, been identified. A few of them are listed below., l, , l, , l, , l, , Ehlers-Danlos syndrome–a group of inherited, disorders characterized by hyperextensibility, of skin, and abnormal tissue fragility., Alport syndrome–due to a defect in the, formation of type IV collagen fibres found in, the basement membrane of renal glomeruli., The patients exhibit hematuria and renal, diseases., Osteogenesis imperfecta–characterized by, abnormal fragility of bones due to decreased, formation of collagen., Epidermolysis bullosa–due to alteration in the, structure of type VII collagen. The victims, exhibit skin breaks and blisters formation even, for a minor trauma., , Scurvy : This is a disease due to the, deficiency of vitamin C (ascorbic acid). Although, not a genetic disease, scurvy is related to the, improper formation of collagen, hence referred, here (vitamin C is needed for the posttranslational modifications of collagen). Scurvy, is characterized by bleeding of gums, poor, wound healing and subcutaneous hemorrhages., Lathyrism : It is a disease of bone deformities, caused by the consumption of Kesari dal, (Lathyrus sativa) in some parts of India. The toxic, compound namely E-oxalyl aminoalanine, (BOAA), found in kesari dal, interferes with the, cross-linking of lysine amino acids in collagen., BOAA is found to inhibit enzyme lysyl oxidase., , ELASTIN, Elastin is another important (besides, collagens) connective tissue protein. It is mainly, , responsible for the extensibility and elasticity of, tissues. Elastin is found in large quantities in, lungs, arterial blood vessels, elastic ligments etc., Elastin is synthesized as tropoelastin which, undergoes, post-translational, modifications, (formation, of, hydroxyproline,, and, no, hydroxylysine). Compared to collagen, elastin, structure is simple—no triple helix, no repeat, sequence of (Gly-X-Y)n., , Abnormalities associated, with elastin, l, , l, , Williams syndrome is a genetic disease due to, impairment in elastin synthesis. The, connective tissue and central nervous system, are affected., Decreased synthesis of elastin is found in, aging of skin and pulmonary emphysema., , FIBRILLIN, Fibrillin is a structural component, myofibrils found in various tissues., , of, , Marfan syndrome is a genetic disorder due to, a mutation in the gene for fibrillin. It is, characterized by hyperextensibility of joints and, skeletal system. Consequently, the patients of, Marfan syndrome are tall, and have long digits., These patients may also have cardiovascular, complications. Some researchers believe that, Abraham Lincoln was a victim of Marfan, syndrome., , FIBRONECTIN, Fibronectin, a glycoprotein, is closely, involved in the interaction of cells with, extracellular matrix. It actively participates in, cell adhesion and cell migration. In general,, tumor cells are deficient in fibronectin which, results in the lack of adhesion among the tumor, cells that may often lead to metastasis., , LAMININ, The basal lamina of glomerular membrane (of, renal cells) contains laminin. In fact, laminin is, one of the first extracellular proteins synthesized, during embryogenesis. It is actively involved in
Page 500 :
490, neuronal growth and nerve regeneration. In, the patients of Alzheimer’s disease, high, concentrations of laminin are found., , BIOCHEMISTRY, , S, , S, , S, , S, , S, , S, , S, , S, , S, , S, , S, , S, , KERATINS, Keratins are structural proteins found in hair,, skin, nails, horns etc. The 3 polypeptides of, keratin form D-helical structure and are held, together by disulfide bonds. The toughness and, strength of keratin are directly related to the, number of disulfide bonds. Thus, the harder, keratin possesses more disulfide bonds. The, mechanical strength of the hair is attributed to, disulfide bonds., , Reduction, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , SH, , Curling, , Hair waving (curling), When the hair is exposed to moist heat, the, D-helices of D-keratin can be stretched. This, results in the formation of E-conformation from, D-helices. On cooling, the hair structure is, reverted back to D-conformation. This property, of D- and E-conformations of keratin is exploited, in hair waving or curling., The hair to be curled is first bent to, appropriate shape. By applying a reducing agent,, the disulfide bonds (of cystine) are converted to, sulfhydyl groups (cysteine). This results in the, uncoiling of D-helical structure. After some time,, th reducing agent is removed, and an oxidizing, agent is added. This allows the formation of, some new disulfide bonds between cysteine, residues (Fig.22.2). The hair is now washed and, cooled. The desired curls are formed on the hair, due to new disulfide bonds and altered D-helical, structure of keratin. It may however, be noted, that a permanent curling of hair is not possible., The new hair that grows will be the native, original hair only (without curls)., , PROTEOGLYCANS, Proteoglycans are conjugated proteins, containing glycosaminoglycans (GAGs). Several, proteoglycans with variations in core proteins, and GAGs are known e.g. syndecan, betaglycan,, aggrecan, fibromodulin. For more information on, the structure and functions of proteoglycans, Refer Chapter 2. GAGs, the components, of proteoglycans, are affected in a group of, , SH, , SH, SH, , SH, , SH, SH, , SH, , SH, , SH, SH, SH, , SH, Oxidation, , SH, , S, , S, , S, , S, SH, , S, , S, S, S, S, , S, , Fig. 22.2 : A diagrammatic representation of hair waving, with suitable alterations in keratin structure ( S S, corresponds to disulfide bonds of cystine; SH, indicates sulfhydryl groups of cysteine), , genetic disorders namely mucopolysaccharidoses, (Chapter 13)., , CONTRACTILE PROTEINS, The proteins that are involved in the, movement of body organs (e.g. muscle, heart,, lung) are regarded as contractile proteins. It is, worthwhile to understand the basic structure of, muscle before learning the contractile proteins., , STRUCTURE OF MUSCLE, Muscle is the single largest tissue of the, human body. Muscle constitutes about 20% of, body mass at birth, 40% in young adults and
Page 501 :
491, , Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , (A), Muscle, , Muscle fibre, , H band, , Z line, , Z line, , 1-2 Pm, , Myofibril, A band, , I, band, , Sarcomere, , ( B), , I band, , A band, , I band, , Thick filament, , Thin filament, Z band, , H band, 2300 nm, (Sarcomere), , 1500 nm, (Sarcomere), , Z band, , Extended form, , Contracted form, , Fig. 22.3 : (A) Structure of myofibril of a straited muscle (B) Arrangement of filaments of myofibril in extended and, contracted form. (Note : The length of sarcomere is reduced from 2300 nm to 1500 nm during contraction), , 30% in aged adults. Three types of muscles are, found in vertebrates-skeletal, cardiac and, smooth. The skeletal and cardiac muscles are, striated while the smooth muscles are nonstriated., The structure of striated muscle is represented, in Fig.22.3. It is composed of bundles of, multinucleated muscle fibre cells. Each cell is, surrounded by an electrically excitable plasma, , membrane, the sarcolemma. The muscle fibre, cells are long which may extend the entire length, of the muscle. The intracellular fluid of fibre cells, is the sarcoplasm (i.e. cytoplasm) into which the, myofibrils are embedded. The sarcoplasm is rich, in glycogen, ATP, creatine phosphate, and the, enzymes of glycolysis., When the myofibril is examined under, electron microscope, alternating dark bands
Page 502 :
492, , BIOCHEMISTRY, , G-actin, , 6-7 nm, F-actin, , Tropomyosin, , Troponin, 38.5 nm, , Assembled thin fliament, , Fig. 22.4 : A diagrammatic representation of the thin filament of a sarcomere., , (anisotropic or A bands) and light bands, (isotropic or I bands) are observed. The less, dense central region of A band is referred to as, H band (or H line). A narrow and dense Z line, bisects the I band. The region of the muscle fibre, between two Z lines is termed as sarcomere., Sarcomere is the functional unit of muscle., In the electron microscopy, it is further, observed that the myofibrils are composed of, thick and thin longitudinal filaments. The thick, filaments contain the protein myosin, and are, confined to A band. The thin filaments lie in the, I band, and can extend into A band (but not into, H line). These thin filaments contain the proteins, actin, tropomyosin and troponin., During the course of muscle contraction, the, thick and thin filaments slide over each other, (sliding filament model of muscle contraction)., Consequently, the H bands and I bands shorten., However, there is no change in the length of, thick and thin filaments. The length of sarcomere, which is around 2300 nm in an extended form, of myofibril is reduced to 1500 nm in a, contracted form (Fig.22.3B)., , MUSCLE PROTEINS, More than 20% of the muscle mass is, composed of proteins. This is largely contributed, by structural proteins namely actin, myosin, and, the actin cross-linking proteins, tropomyosin and, troponin. Muscle also contains other proteins –, myoglobin, collagen, enzymes etc. The term, sarcopenia is used to indicate the loss of skeletal, muscle mass with age., , ACTIN, Actin is a major constituent of thin filaments, of sarcomere. It exists in two forms – monomeric, G-actin (i.e. globular actin) and polymeric, F-actin (i.e. filament actin). G-actin constitutes, about 25% of the muscle proteins by weight. In, the presence of Mg2+ ions, G-actin polymerizes, to form an insoluble double helical F-actin with, a thickness of 6-7 nm (Fig.22.4)., , Tropomyosin and troponin : These two are, cross-linking proteins found in association with, actin. Although, minor in terms of mass, they are, important in terms of their function. Tropomyosin, composed of two chains, attaches to
Page 503 :
493, , Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , G, , Light, chains, , G, , G, , G, , G, , G, , HMM, sub-fragment I, , Papain, Heavy, meromyosin, (HMM), , HMM, sub-fragment II, , Trypsin, , Heavy, chains, , Light, meromyosin, (LMM), , Fig. 22.5 : A diagrammatic representation of myosin along with its digested, products by trypsin and papain (G-Globular head)., , F-actin in the grooves (Fig.22.4). Troponin, consists of three polypeptide chains – troponin T, (TpT binding to tropomyosin), tropinin I (TpI, that inhibits F-actin myosin interaction) and, troponin C (TpC, calcium binding polypeptide)., TpC is comparable to calmodulin., , MYOSINS, Myosins are actually a family of proteins with, about 15 members. The myosin that is, predominantly present in muscle is myosin II., In terms of quantity, myosin constitutes, approximately 55% of muscle protein, and is, found in thick filaments. Myosin is composed of, six polypeptide chains (hexamer). It contains one, pair of heavy (H) chains, and two pairs of light, (L) chains., Limited digestion of myosin with trypsin and, papain has helped to understand its structure and, function (Fig.22.5)., , Light and heavy meromyosins, When myosin is digested with trypsin, two, fragments namely light meromyosin (LMM) and, heavy meromyosin (HMM) are produced. Light, meromyosin represents the D-helical fibres of the, tail of myosin, and cannot bind to F-actin., Heavy meromyosin contains the fibrous and, globular portions of myosin. HMM inhibits, ATPase activity and binds to F-actin., On digestion by papain, heavy meromyosin, yields two sub-fragments S-1 and S-2 (HMM S-1,, HMM S-2). HMM S-2 fragment is fiber-like, does, not bind to F-actin and has no ATPase activity., On the other hand, HMM S-1 is globule-like,, binds to L-chains, and possesses ATPase activity., , MUSCLE CONTRACTION, An outline of the reactions involving muscle, contraction is depicted in Fig.22.6, and briefly, described in the next page.
Page 504 :
494, , BIOCHEMISTRY, , Actin, , Sources of ATP for, muscle contraction, , ATP-Myosin, H2O, , (5), , (1), , Actin-Myosin, ATP, , ATP, , ADP-PiMyosin, , ATP is a constant source of energy for muscle, contraction and relaxation cycle. ATP can be, generated from the following ways., l, , (4), (2), , Actin-Myosin, (3), ADP + Pi, , Actin, , Actin-Myosin, ADP-Pi, , Fig. 22.6 : Major biochemical events occurring during, a cycle of muscle contraction and relaxation (The, numbers 1-5 represent the steps in muscle contraction;, The zig zag rounds indicate high energy states)., , 1. During the relaxation phase of muscle, contraction, the S-1 head of myosin hydrolyses, ATP to ADP and Pi. This results in the formation, high energy ADP-Pi myosin complex., 2. On contraction, the muscle gets stimulated, (through the participation of actin, Ca2+,, troponin, tropomyosin etc.) to finally form actinmyosin-ADP-Pi complex., 3. The next step is the power stroke which, drives movement of actin filaments over myosin, filaments. This is followed by the release of ADP, and Pi, and a conformation change in myosin., The actin-myosin complex is in a low energy, state., 4. A fresh molecule of ATP now binds to, form actin-myosin ATP complex., 5. Actin is released, as myosin-ATP has low, affinity for actin. This step is crucial for, relaxation which is dependent on the binding of, ATP to actin-myosin complex., A fresh cycle of muscle contraction and, relaxation now commences with the hydrolysis, of ATP and the formation of ADP-Pi-myosin, complex. It has to be noted that it is ultimately, the ATP that is the immediate source of energy, for muscle contraction., , By substrate level phosphorylation of glycolysis, using glucose or glycogen., , l, , By oxidative phosphorylation., , l, , From creatine phosphate., , OTHER PROTEINS OF MUSCLE, There are a large number of other proteins, that are involved in the structure and functions, of muscle. These include titin, nebulin,, dystrophin, calcineurin and desmin. Titin is the, largest protein known. The gene coding for, dystrophin is the largest gene (2,300 bp)., , Muscular dystrophy, Muscular dystrophy is a hereditary disease in, which muscles progressively deteriorate. This is, caused by mutations in the gene (located on Xchromosome) coding for the protein dystrophin., , PROTEIN MISFOLDING, AND DISEASES, The process of protein folding is complex and, has been briefly described in Chapter 25., Sometimes, improperly folded proteins may be, formed (either spontaneous or by gene, mutations). Such misfolded proteins usually, get degraded within the cell. However, as the, individuals age, the misfolded proteins, accumulate and cause a number of diseases., Prion diseases and amyloidosis, two groups of, diseases due to protein misfolding are briefly, discussed., , Prion diseases, The term prion represents proteinous, infectious agents. Prion proteins (PrP) are the, altered forms of normal proteins. However, no, differences in the primary structure (i.e. amino, acid sequence) and post-translational modifications are observed.
Page 505 :
495, , Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , Certain, changes, in, three-dimensional, structure are seen in prion proteins. The major, alteration is the replacement of D-helices by, E-sheets in PrP. This confers resistance to, proteolytic digestion of prion proteins. PrP are, highly infectious, and can act as template to, convert non-infectious proteins (with D-helices), to infectious forms (Fig.22.7). This process, continues in an exponential manner to, accumulate a large number of prion proteins in, tissues., Prion proteins are implicated as causative, agents in the following diseases., l, , l, , l, , Transmissible spongiform encephalopathies, (TSEs) and Creutzfeldt Jacob disease in, humans., , D-Helix of a protein, (non-infectious), , Infectious prion, (with E-sheets), , Interaction, , Scrapie in sheep, Bovine spongiform encephalopathy (popularly, known as mad cow disease) in cattle., , Kuru is an interesting prion disease. It was, first reported in Papau New Guinea in the tribal, people who practice cannibalism (they eat the, brains of the dead people)., As of now, there is no treatment for prion, diseases. Transmissible spongiform encephalopathies are invariably fatal in humans., , Two molecules of infectious prions, (with E-sheets), , Amyloidosis, The term amyloids is used to refer to the, altered proteins (with E-sheets) that accumulate, in the body, particularly in the nervous system., Amyloids are formed by protein misfolding or, due to gene mutations. They are not infectious, agents as prion proteins. However, as the age, advances, amyloids accumulate, and they have, been implicated in many degenerating diseases., A total of at least 15 different proteins are, involved in amyloidosis., Alzheimer’s disease is a neurodegenerative, disorder, affecting about 5-10% of the people, above 60 years of age. It is characterized by, memory, loss,, confusion,, hallucinations,, personality changes with abnormal behaviour., As the disease progresses, the patient may enter, a vegetative state, and may die after 10 years, after the onset of the disease manifestations. The, , These two molecules separate and convert, another two non-infectious proteins to, infectious prions, , Exponentially increased infectious prions, , Fig. 22.7 : A model for the formation of infectious prions, (Red thick lines represent E-sheets in protein)., , accumulation of amyloids (in the form of, amyloid plaque) has been clearly demonstrated, in the patients of Alzheimer’s disease., A specific protein, namely E-amyloid which is, prone for self aggregation is believed to be, the causative agent of Alzheimer’s disease,
Page 506 :
496, , BIOCHEMISTRY, , TABLE 22.1 Composition of milk in different species, , Constituent, , Human, , Cow, , Buffalo, , Goat, , Water, , 87.6, , 87.2, , 83.5, , 87.0, , Total solids (g/dl), , 12.4, , 12.8, , 16.5, , 13.0, , Carbohydrates (g/dl), , 7.5, , 4.4, , 5.4, , 4.6, , Lipids (g/dl), , 3.8, , 3.8, , 6.5, , 3.5, , Proteins (g/dl), , 1.1, , 3.3, , 4.3, , 3.7, , Calcium (mg/dl), , 35, , Magnesium (mg/dl), , 150, , 2.2, , 160, , 175, , 13, , 10, , 8, , Phosphorus (mg/dl), , 16, , 100, , 100, , 70, , Sodium (mg/dl), , 15, , 60, , 60, , 50, , Potassium (mg/dl), , 55, , 140, , 130, , 85, , E-Amyloid is formed from a conformational, transformation of D-helix. Apolipoprotein E, promotes the conformational change of, D-amyloid to E-amyloid., , BODY FLUIDS, The specialized fluids of the body are milk,, cerebrospinal fluid, amniotic fluid, pleural fluid,, aqueous humor, sweat and tears. In a broader, perspective, blood and plasma also biological, fluids. Their biochemical importance is discussed, elsewhere (Hemoglobin, Chapter 10; Plasma, proteins, Chapter 9; Diagnostic enzymes,, Chapter 6; Acid-base balance, Chapter 21)., Urine is an excretory biological fluid. (Note :, Serum is prepared in the laboratory, hence in a, strict sense, not a natural biological fluid), , MILK, Milk is secreted by mammary glands. It is, almost a complete natural food. Colostrum, refers to the mother’s milk secreted during the, first few days after delivery. Milk is the only food, for the offsprings of mammals on their birth., , COMPOSITION OF MILK, The major constituents of milk in different, species—human, cow, buffalo and goat are given, , in Table 22.1. Water is the major constituent,, with a concentration in the range of 83–87%,, depending on the species. The remaining, 13–17% is made up of solids–carbohydrates,, lipids, proteins, minerals and vitamins., , Carbohydrates in milk, Milk contains the disaccharide lactose which, imparts sweetness. Human milk has a higher, concentration of lactose (7.5%) compared to, milk of other species. Thus, human milk is sweet, enough for the babies to relish. Milk sugar, (lactose) serves two major functions., 1. It provides galactose, a structural unit for, the growing infant., 2. In the intestine, it gets metabolized to, lactic acid which eliminates harmful bacteria., , Lipids in milk, The lipids in the milk are dispersed as small, globules. Milk fat is mainly composed of, triacylglycerols. Mono- and diacylglycerols are, also present in trace quantities. The fatty acids, found in milk (i.e. in TG) are mostly medium or, short chain, and saturated e.g. palmitic acid,, myristic acid, stearic acid, lauric acid and butyric, acid. Oleic acid, an unsaturated fatty acid, is, also present.
Page 507 :
497, , Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , Proteins in milk, The major milk proteins are casein (about, 80%) and lactalbumin. Small concentrations of, enzymes (proteases, lipase, lysozyme) and, immunoglobulins are also found., Milk casein (a phosphoprotein) is almost a, complete protein (next to egg albumin),, containing all the essential amino acids. It is, present in milk in the form of aggregates called, micelles. The white colour of milk is due to the, dispersion of calcium caseinate micelles., Whey proteins : If milk is acidified, casein, gets precipitated at isoelectric point (pH 4.7)., The supernatant fluid contains whey proteins, (20% of milk proteins). These include, lactalbumin, lactoglobulin and various enzymes., , Minerals in milk, Milk is rich in calcium, magnesium, phosphorus, sodium, potassium and chlorine., However, milk is a poor source of iron and, copper., , Vitamins in milk, Both fat soluble and water soluble vitamins, are found in good concentration in milk., However milk is deficient in vitamin C., , Calorific value of milk, Due to variability in the nutrient composition, (carbohydrates, fats and proteins), the calorific, value of milk from different species varies. Thus,, human milk can provide about 70 Cal/100 ml,, while for buffalo milk, it is around 95 Cal/100 ml., , CEREBROSPINAL FLUID (CSF), Cerebrospinal fluid is a clear, colourless, liquid formed within the cavities (ventricles) of, brain and around the spinal cord. CSF originates, in the choroid plexus (as an ultrafiltrate of, plasma) and returns to blood through arachnoid, villi. About 500 ml of CSF is formed everyday., However, at any given time, there is about, 120–150 ml CSF in the system. Further, CSF is, completely replaced about three times a day., , Functions of CSF, As the brain has no lymphatic system, CSF, drains into the ventricular system and moves into, , spaces surrounding the brain and spinal cord., The major functions of CSF are listed., l, , l, , l, , CSF serves as a hydraulic shock absorber. It, can diffuse the force from a hard blow to the, skull that might otherwise cause severe injury., It helps in the regulation of intracranial, pressure., It is believed that CSF influences the hunger, sensation and eating behaviours., , Collection of CSF, Cerebrospinal fluid is usually collected by a, spinal puncture for the purpose of biochemical, analysis. The puncture is performed in, the lumbar region, between the third and, fourth, or between the fourth and fifth lumbar, vertebrae., The sterile lumbar puncture (spinal tap) is, carried out in a side lying (lateral) position with, head fixed into the chest and knees. This position, helps to increase the space between the lumbar, vertebrae so that the needle can be inserted with, ease. A sitting position of the patient with head, flexed to chest can also be used for lumbar, puncture., , Composition of CSF in health and, disease, The normal composition of cerebrospinal fluid, is given in the Table 22.2. From the diagnostic, point of view, the total cell count of lymphocytes, (Reference : 0-5 u 106/1), protein concentration, (15–45 mg/dl) and glucose concentration (45–85, mg/dl) are important., In the Table 22.3, the major alterations in the, CSF in the disease states are given. The total cell, count and protein content are increased, while glucose concentration is reduced in, tuberculosis meningitis. In case of brain tumors,, there is no change in total cell count while the, protein concentration may be marginally, increased., The colour and appearance of CSF is, sometimes a guiding factor in the disease, diagnosis. For instance, CSF is opalescent and, slightly yellow coloured in tuberculosis, meningitis.
Page 508 :
498, , BIOCHEMISTRY, , Functions of amniotic fluid, TABLE 22.2 Normal composition of, cerebrospinal fluid, , l, , l, , Parameter, , Description/concentration, , Volume, , 90–150 ml, , Appearance, , Clear and colourless, , Specific gravity, , 1.006–1.008, , Osmolality, , 280–290 mOsm/kg, , Total cell count (lymphocytes), , 0–5 u 106/l, , pH, , 7.3–7.4, , Protein, , 15–45 mg/dl, , A/G ratio (albumin/globulin), , 8:1, , Glucose, , 45–85 mg/dl, , Chloride, , 118–130 mEq/l, , Calcium, , 2.1–2.7 mEq/l, , Sodium, , 145–155 mEq/l, , Potassium, , 2.0–3.5 mEq/l, , It provides physical protection to the fetus., Amniotic fluid is a medium for the exchange, of various chemicals., , Diagnostic importance of, amniotic fluid, The term amniocentesis is used for the, process by which amniotic fluid is collected for, analysis. The diagnostic importance of amniotic, fluid is given below., Assessment of fetal maturity : Fetal maturity, can be assessed by cytological staining of fat, cells, and estimation of creatinine concentration, (> 1.6 mg/dl indicates fetal maturity)., Lung maturity : The fetal lung maturity is, evaluated by measuring lecithin–sphingomyelin, (L/S) ratio. A L/S ratio of 2 : 1 or more indicates, lung maturity. If L/S ratio is less than 1.2 : 1, it, is better to delay the induced delivery until the, lung has become more mature., , AMNIOTIC FLUID, Amniotic fluid is a liquid produced by the, membranes and the fetus. It surrounds the fetus, throughout pregnancy. The volume of amniotic, fluid increases with the gestational age. Thus, the, volume increases from 30 ml (at 2 weeks of, gestation) to 350 ml (at 20 weeks), and thereafter, to 500–1000 ml. Amniotic fluid is almost clear, with some desquamated fetal cells and a little, lipid., , Diagnosis of congenital disorders : Amniotic, fluid analysis is useful for the prenatal diagnosis, of congenital disorders. Some of the important, ones are listed., l, , Chromosomal disorders such as Down’s, syndorme., , l, , Metabolic disorders e.g. cystic fibrosis., , l, , Sex-linked disorders e.g. hemophilia., , l, , Enzyme defects e.g. Tay-Sachs disease., , TABLE 22.3 Changes in cerebrospinal fluid in the disease states, , Disease, , Colour and, appearance, , Total cell, count, , Protein, , Glucose, , Normal, , Clear and colourless, , 0–5 u 106/l, , 15–45 mg/dl, , 45–85 mg/dl, , Tuberculosis meningitis, , Opalescent and slightly, yellow, , Increased, , Increased, , Relatively low, , Bacterial meningitis, , Opalescent and turbid, , Markedly increased, , Markedly increased, , Markedly decreased, , Brain tumour, , Clear and colourless, , No change, , Increased, , Low, , RBC and WBC, present, , Increased, , Almost normal, , Subarachnoid hemorrhage Slightly blood colour
Page 509 :
Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , Assessment of hemolytic diseases : Estimation, of bilirubin in amniotic fluid is useful to evaluate, the severity of hemolytic diseases., Measurement of D-fetoprotein : Increased, levels of D-fetoprotein (normal 15–40 Pg/ml, during gestation; at 40 weeks < 1.0 Pg/ml) are, associated with neural tube defects, fetal distress,, Turner syndrome. Elevated D-fetoprotein may, also indicate a possible death of the fetus., , PLEURAL FLUID, Pleural fluid is the filtrate of plasma, and is, present in a minimal quantity in pleural cavity., The amount of pleural fluid increases in disease, states due to pleural effusion., Pleural fluid may be transudate or exudate,, based on the composition. If the ratio of protein, content between pleural fluid and plasma is less, than 0.5, it is transudate. If this ratio is more than, 0.5, the pleural fluid is exudate., Transudate is fluid-like, with specific gravity, <1.015. It will not clot, and is not associated, with inflammation. Transudate is observed in, nephrotic syndrome (due to low osmotic, pressure of plasma), congestive cardiac failure, , 499, , (increased hydrostatic pressure) and obstruction, of lymph flow (chylous effusion)., Exudate is viscous in nature with specific, gravity >1.015. It may clot, and is associated, with inflammation or malignancy. Exudate, accumulates in various infections (tuberculosis,, pneumonia, rheumatoid arthritis) and some, cancers (e.g. lung cancer)., Biochemical analysis of exudate is useful in, certain disease states, l, Amylase activity increased in pancreatitis., l, Rheumatoid factor elevated in rheumatoid, arthritis., l, Carcinoembryonic antigen (CEA) increased in, malignancy., l, Triacylglycerols elevated in chylothorax., , AQUEOUS HUMOR, Aqueous humor is the fluid that fills, the anterior chamber of the eye. This fluid is, responsible for maintaining the intraocular, tension. Aqueous humor, secreted by the ciliary, body, enters the anterior chamber. Blockade in, the flow of aqueous humor causes glaucoma due, to increased intraocular pressure., , + Improper formation of collagen is associated with certain genetic diseases e.g. EhlersDanlos syndrome (abnormal tissue fragility), osteogenesis imperfecta (abnormal fragility, of bones)., , + Defective formation of collagen is observed in scurvy, caused by vitamin C deficiency., This results in bleeding of gums and poor wound healing., , + Hair waving (curling) through artificial means is possible with suitable alterations in the, structure of keratins., , + Muscular dystrophy occurs due a mutation in the gene coding for the protein, dystrophin., , + Protein misfolding results in prion diseases (e.g. mad cow disease) and amyloidosis, (Alzheimer’s disease)., , + Biochemical analysis of CSF is useful for the diagnosis of certain diseases – tuberculosis, meningitis (increased protein and decreased glucose concentrations)., , + Amniotic fluid is analysed to assess fetal maturity (creatinine concentration > 1.6 mg/, dl), lung maturity (lecithin–sphingomyelin ratio > 2 : 1) and for the prenatal diagnosis, of congenital disorders (e.g. hemophilia, Down’s syndrome).
Page 510 :
500, , BIOCHEMISTRY, , 1. The major proteins of connective tissue are collagen, elastin, fibrillin, laminin and, proteoglycans. Among these, collagen is the most abundant, constituting one-third of, the total body proteins., 2. Type I mature collagen is a triple helical structure i.e. contains three polypeptide chains, each with about 1000 amino acids. The repetitive amino acid sequence of collagen is, (Gly-X-Y)n. Glycine constitutes about 1/3 rd of the amino acids while X and Y represent, other amino acids., 3. Keratins are structural proteins found in hair, skin, nails and horns. The strength of the, keratins is directly related to the number of disulfide bonds., 4. Muscle is the single largest tissue of the human body (30–40% of body weight). It is, composed of fibre cells into which myofibrils are embedded. Each myofibril contains, alternating A and I bands. Sarcomere is the functional unit of muscle., 5. Actin, myosin, tropomyosin and troponin are the major contractile proteins found in, muscles. The muscle contraction and relaxation occur due to the active involvement of, these proteins. ATP is the immediate source of energy for muscle contraction., 6. Proper folding of proteins is essential for their structure. Misfolding of proteins results, in certain diseases e.g. mad cow disease, Alzheimer’s disease., 7. The specialized fluids of the body include milk, cerebrospinal fluid, amniotic fluid,, aqueous humor, sweat and tears., 8. Milk is almost a complete food with various nutrients—carbohydrates, lipids, proteins,, vitamins and minerals. However, milk is deficient in vitamin C, iron and copper., 9. Cerebrospinal fluid is an ultrafiltrate of plasma. In the disease, tuberculosis meningitis,, the total cell count and protein concentration are increased, while glucose concentration, is decreased in CSF., 10. Amniotic fluid is a liquid produced by the fetus. Its biochemical analysis is important, for the diagnostic purpose – assessment of fetal maturity, diagnosis of congenital, diseases.
Page 511 :
Chapter 22 : TISSUE PROTEINS AND BODY FLUIDS, , 501, , I. Essay questions, 1. Give an account of the structure and functions of collagen. Add a note on the abnormalities, associated with collagen., 2. Describe the muscle proteins, and muscle contraction., 3. Discuss the protein misfolding and various diseases related to it., 4. Give an account of the composition of milk., 5. Describe the functions and composition of cerebrospinal fluid. Add a note on the alterations in, CSF in diseased states., , II. Short notes, (a) Biosynthesis of collagen, (b) Collagen and scurvy, (c) Elastin, (d) Light and heavy meromyosins,, (e) Prion diseases, (f) Amyloidosis, (g) Hair waving, (h) Vitamins and minerals in milk, (i) Collection, of CSF, (j) Amniotic fluid., , III. Fill in the blanks, 1. The most abundant protein in mammals ____________., 2. The amino acid that contributes to one-third of the total number of amino acids in collagen, ____________., 3. The toxic compound that interferes with the cross-linking of lysine in collagen, causing, lathyrism ____________., 4. Marfan syndrome is a genetic disorder due to a mutation of the gene coding for ____________., 5. Name the carbohydrates associated with the structure of proteoglycans ____________., 6. The region of the muscle fibre between two Z lines is termed as ____________., 7. Name the major protein found in the structure of thin filaments of sarcomere ____________., 8. The white colour of milk is due to the dispersion of ____________., 9. Name the vitamin deficient in milk ____________., 10. The fetal lung maturity is evaluated by measuring ____________ ratio., , IV. Multiple choice questions, 11. The number of polypeptide chains present in collagen, (a) 1 (b) 2 (c) 3 d) 4., 12. The functional unit of muscle, (a) Fibre cell (b) Myofibril (c) H band d) Sarcomere., 13. The immediate source of energy for muscle contraction, (a) ATP (b) Creatine phosphate (c) GTP d) Phosphoenol pyruvate., 14. One of the following minerals is lacking in milk, (a) Calcium (b) Sodium (c) Iron d) Potassium., 15. One of the following biochemical parameters is increased in tuberculosis meningitis, (a) Glucose (b) Protein (c) Sodium d) Chloride.
Page 512 :
Section 4, , Clinical Biochemistry and Nutrition, , Chapter, , Nutrition, , 23, , The nutrition speaks :, , “Some eat to live,, And some live to eat!, My function is, To cater for all.”, , W, , hether a man eats for living or lives for, eating, food is his prime concern., Nutrition may be defined as the utilization of, food by living organisms. Biochemists have, largely contributed to the science of nutrition., , areas—ideal nutrition, undernutrition and, overnutrition. Ideal nutrition is the concern of, everyone. Undernutrition is the prime concern, of developing countries while overnutrition is a, serious concern of developed countries., , Nutrition significantly promotes man’s, development, his health and welfare. The subject, nutrition, perhaps, is the most controversial. This, is due to the fact that nutrition is concerned with, food, and everyone feels competent enough to, talk like an expert on nutrition. Further, high, public awareness and the controversial reports, by scientists also contribute to the controversy., , A sound knowledge of chemistry and metabolism of foodstuffs (carbohydrates, lipids, proteins,, vitamins and minerals) is an essential prerequisite, for a better understanding of nutrition. The reader, must, therefore, first refer these chapters. The, principles of nutrition with special reference to, energy demands, carbohydrates, fats, proteins,, recommended dietary/daily allowances (RDA),, balanced diet and nutritional disorders are, discussed in the following pages., , Methodology in nutrition : Most of the, existing knowledge on nutrition is originally, derived from animal experimentation. This is, despite the fact that there may exist several, differences in the biochemical composition, between man and animals! For instance, some, animals can synthesize ascorbic acid while man, cannot do so., Study of human nutrition : The study of, nutrition may be logically divided into three, , NUTRITION AND ENERGY SUPPLY, Food is the fuel source of the body. The, ingested food undergoes metabolism to liberate, energy required for the vital activities of the, body., , 502
Page 513 :
503, , Chapter 23 : NUTRITION, , TABLE 23.1 Calorific values of foodstuffs, , Foodstuff, , Energy value (Cal/g), In bomb calorimeter In the body, , Carbohydrate, , 4.1, , 4, , Fat, , 9.4, , 9, , Protein, , 5.4, , 4, , Alcohol, , 7.1, , 7, , Respiratory quotient of foodstuffs, The respiratory quotient (R. Q.) is the ratio of, the volume of CO2 produced to the volume of, O2 utilized in the oxidation of foodstuffs., , Energy content of foods, The calorific value (energy content) of a food, is calculated from the heat released by the total, combustion of food in a calorimeter., Unit of heat : Calorie is the unit of heat. One, calorie represents the amount of heat required to, rise the temperature of one gram of water by 1°C, (i.e. from 15° to 16°C). A calorie is too small a, unit. Therefore, it is more conveniently expressed, as kilocalories (1,000 times calorie) which is, represented by kcal or simply Cal (with capital ‘C’)., The joule is also a unit of energy used in, some countries. The relationship between, calories and joules (J) is, 1 Cal (1 kcal) = 4.128 KJ, The joule, nutritionists., , is, , less, , commonly, , It must be noted that the nutrients, namely, vitamins and minerals, have no calorific value,, although they are involved in several important, body functions, including the generation of, energy from carbohydrates, fats and proteins., , Carbohydrates : The carbohydrates are, completely oxidized and their R. Q. is close, to 1, as represented below for glucose., C6H12O6 + 6O2 ±A 6CO2 + 6H2O, R. Q. for carbohydrate =, , Fats : Fats have relatively lower R.Q. since, they have a low oxygen content. For this reason,, fats require more O2 for oxidation. The R.Q., for the oxidation of the fat, tristearin is given, below., 2 C57H110O6 + 16 3O2 ±A 114 CO2 + 110 H2O, R. Q. for fat =, , used, , by, , Calorie value of foods : The energy values of, the three principal foodstuffs—carbohydrate, fat, and protein—measured in a bomb calorimeter, and in the body are given in the Table 23.1. The, carbohydrates and fats are completely oxidized, (to CO2 and H2O) in the body; hence their fuel, values, measured in the bomb calorimeter or in, the body, are almost the same. Proteins,, however, are not completely burnt in the body, as they are converted to products such as urea,, creatinine and ammonia, and excreted. Due to, this reason, calorific value of protein in the body, is less than that obtained in a bomb calorimeter., The energy values of carbohydrates, fats and, proteins (when utilized in the body) respectively,, are 4, 9 and 4 Cal/g., , Alcohol is a recent addition to the calorie, (7 Cal/g) contribution, as it is a significant dietary, component for some people., , CO2, 6, =, =1., O2, 6, , CO2, O2, , =, , 114, = 0. 7., 163, , Proteins : The chemical nature of proteins is, highly variable, and this cannot be represented, by any specific formula. By indirect, measurements, the R.Q. of protein is found to be, around 0.8., Mixed diet : The R. Q. of the diet consumed, is dependent of the relative composition of, carbohydrates, fats and proteins. For a normally, ingested diet, it is around 0.8., , UTILIZATION OF ENERGY IN MAN, Man consumes energy to meet the fuel, demands of the three ongoing processess in the, body., 1. Basal metabolic rate, 2. Specific dynamic action, 3. Physical activity.
Page 514 :
504, Besides the above three, additional energy, supply is needed during growth, pregnancy and, lactation., , BASAL METABOLIC RATE, Basal metabolism or basal metabolic rate, (BMR) is defined as the minimum amount of, energy required by the body to maintain life at, complete physical and mental rest in the postabsorptive state (i.e. 12 hours after the last meal)., It may be noted that resting metabolic rate, (RMR) is in recent use for BMR., Under the basal conditions, although the, body appears to be at total rest, several, functions within the body continuously occur., These include working of heart and other, organs,, conduction, of, nerve, impulse,, reabsorption by renal tubules, gastrointestinal, motility and ion transport across membranes, (Na+-K+ pump consumes about 50% of basal, energy)., , Measurement of BMR, Preparation of the subject : For the, measurement of BMR the subject should be, awake, at complete physical and mental rest, in, a post-absorptive state and in a comfortable, surrounding (at 25˚C)., Measurement : The BMR is determined either, by the apparatus of Benedict and Roth (closed, circuit device) or by the Douglas bag method, (open circuit device). The former is more, frequently used., By Benedict-Roth method, the volume of O2, consumed (recorded on a graph paper) by the, subject for a period of 2-6 minutes under basal, conditions is determined. Let this be A liters for, 6 minutes. The standard calorific value for one, liter O2 consumed is 4.825 Cal., Heat produced in 6 min = 4.825 u A, Heat produced in one hour = 4.825A u 10, Units of BMR : BMR is expressed as Calories, per square meter of body surface area per hour, i.e. Cal/sq.m/hr., , BIOCHEMISTRY, , For the calculation of body surface area, the, simple formula devised by Du Bois and Du Bois, is used., A = H0.725 u W0.425 u 71.84, where, , A = Surface area in cm2, H = Height in cm, W = Weight in kg., , To convert the surface area into square meters, (m2), divide the above value (cm2) by 10,000., Nomograms of body surface area (directly in m2), from heights and weights are readily available in, literature., Normal values of BMR : For an adult man, 35–38 Cal/sq. m/hr; for an adult woman 32-35, Cal/sq.m/hr. A BMR value between –15% and, +20% is considered as normal., Some authors continue to represent BMR as, Cal/day. For an adult man BMR is around 1,600, Cal/day, while for an adult woman around, 1,400 Cal/day. This is particularly important for, easily calculating energy requirements per day., , Factors affecting BMR, 1. Surface area : The BMR is directly proportional to the surface area. Surface area is related, to weight and height., 2. Sex : Men have marginally higher (about, 5%) BMR than women. This is due to the higher, proportion of lean muscle mass in men., 3. Age : In infants and growing children, with, lean muscle mass, the BMR is higher. In adults,, BMR decreases at the rate of about 2% per, decade of life., 4. Physical activity : BMR is increased in, persons (notably athletes) with regular exercise., This is mostly due to increase in body surface, area., 5. Hormones : Thyroid hormones (T3 and T4), have a stimulatory effect on the metabolism of, the body and, therefore, BMR. Thus, BMR is, raised in hyperthyroidism and reduced in, hypothyroidism. In fact, the measurement of, BMR was earlier used to assess thyroid function.
Page 515 :
Chapter 23 : NUTRITION, , The other hormones such as epinephrine,, cortisol, growth hormone and sex hormones, increase BMR., 6. Environment : In cold climates, the BMR is, higher compared to warm climates., 7. Starvation : During the periods of, starvation, the energy intake has an inverse, relation with BMR, a decrease up to 50% has, been reported. This may be an adaptation by the, body., 8. Fever : Fever causes an increase in BMR., An elevation by more than 10% in BMR is, observed for every 1˚C rise in body temperature., 9. Disease states : BMR is elevated in various, infections, leukemias, polycythemia, cardiac, failure, hypertension etc. In Addison’s disease, (adrenal insufficiency), BMR is marginally, lowered., 10. Racial variations : The BMR of Eskimos is, much higher. The BMR of Oriental women living, in USA is about 10% less than the average BMR, of American women., , Significance of BMR, BMR is important to calculate the calorie, requirement of an individual and planning of, diets. Determination of BMR is useful for the, assessment, of, thyroid, function., In, hypothyroidism, BMR is lowered (by about, – 40%) while in hyperthyroidism it is elevated, (by about + 70%). Starvation and certain disease, conditions also influence BMR (described, above)., , SPECIFIC DYNAMIC ACTION, The phenomenon of the extra heat, production by the body, over and above the, calculated caloric value, when a given food is, metabolized by the body, is known as specific, dynamic action (SDA). It is also known as, calorigenic action or thermogenic action or, thermic action (effect) of food., SDA for different foods : For a food, containing 25 g of protein, the heat production, from the caloric value is 100 Cal (25 u 4 Cal)., , 505, However, when 25 g protein is utilized by the, body, 130 Cal of heat is liberated. The extra 30, Cal is the SDA of protein. Likewise, consumption, of 100 Cal of fat results in 113 Cal and 100 Cal, of carbohydrate in 105 Cal, when metabolized, in the body. SDA for protein, fat and, carbohydrate are 30%, 13% and 5%,, respectively. Thus, proteins possess the highest, SDA while carbohydrates have the lowest., SDA for mixed diet : For a mixed diet, the, SDA is not an additive value of different foods, but it is much less. The presence of fats and, carbohydrates reduces the SDA of proteins. Fats, are most efficient in reducing SDA of foodstuffs., For a regularly consumed mixed diet, the SDA is, around 10%., Significance of SDA : For the utilization of, foods by the body, certain amount of energy is, consumed from the body stores. This is actually, an expenditure by the body for the utilization of, foodstuffs. It is the highest for proteins (30%) and, lowest for carbohydrates (5%) and for a mixed, diet around 10%. It is, therefore, essential that, an additional 10% calories should be added to, the total energy needs (of the body) towards, SDA. And the diet should be planned,, accordingly. (SDA is quite comparable to the, handling charges levied by a bank for an, outstation cheque)., The higher SDA for protein indicates that it is, not a good source of energy. Fat is the best, source of energy due to its lowering effect on, SDA. However, excessive utilization of fat leads, to ketosis., Mechanism of SDA : The exact cause of SDA, is not known. It is generally believed that SDA of, foods is due to the energy required for digestion,, absorption, transport, metabolism and storage of, foods in the body., Intravenous administration of amino acids or, the oral ingestion of proteins gives the same, SDA. This shows that the SDA of proteins is not, due to their digestion and absorption., Hepatectomy abolishes SDA, thereby indicating, that SDA is closely connected with the metabolic, functions of liver. The SDA of proteins is, primarily to meet the energy requirements for
Page 516 :
506, , BIOCHEMISTRY, , TABLE 23.2 Type of physical, activity and energy expenditure, (over and above BMR, about 65 Cal/hr)., , Physical activity, , Energy requirement, (Cal/hr), , Sitting (quietly), , 25, , Standing (quietly), , 30, , Writing/eating/reading, , 30, , Car driving, , 60, , Typing, , 75, , Household work (dish washing), , 80, , Walking (slow), , 130, , Sexual intercourse, , 140, , Cycling (slow), , 150, , Running (moderate), , 500, , Swimming, , 600, , Walking upstairs, , 800, , deamination, synthesis of urea, biosynthesis of, proteins, synthesis of triacylglycerol (from carbon, skeleton of amino acids). It has been, demonstrated that certain amino acids, (phenylalanine, glycine and alanine) increase the, SDA. It is a common experience that, consumption of a protein rich diet makes us feel, warm and comfortable in cold weather. This is, due to the high SDA of proteins., The SDA of carbohydrates is attributed to the, energy expenditure for the conversion of glucose, to glycogen., As regards fat, the SDA may be due to its, storage, mobilization and oxidation., , PHYSICAL ACTIVITY OF THE BODY, The physical activity of the individual is, highly variable. The amount of energy needed, for this depends mainly on the duration and, intensity of muscular activity. The expenditure of, energy for the various physical activities has, been calculated (Table 23.2)., For the sake of convenience, the individuals, are grouped into four categories with regard to, , their physical activity and the requirement of, energy., Light work, — 30–40% of BMR, (teachers, office workers, doctors), Moderate work, (housewives, students), , — 40–50% of BMR, , Heavy work, — 50–60% of BMR, (agricultural labourers, miners), Very heavy work, — 60–100% of BMR, (construction workers, rickshaw pullers), , Energy requirements of man, As already stated, the three factors—basal, metabolic rate, specific dynamic action and, physical activity—determine the energy needed, by the body. In an individual with light work,, about 60% of the calories are spent towards, BMR, about 30% for physical activity and about, 10% to take care of the SDA., The daily requirement of energy is rather, variable which depends on the BMR (in turn, depends on age, sex, body size etc.) and physical, activity. As per some rough calculation, caloric, requirements of adults per day (Cal/day) are in, the following ranges., Light work, , — 2,200–2,500, , Moderate work, , — 2,500–2,900, , Heavy work, , — 2,900–3,500, , Very heavy work — 3,500–4,000, , NUTRITIONAL IMPORTANCE OF, CARBOHYDRATES, Dietary carbohydrates are the chief source of, energy. They contribute to 60-70% of total, caloric requirement of the body. Incidentally,, carbohydrate rich foods cost less., Carbohydrates are the most abundant dietary, constituents, despite the fact that they are not, essential nutrients to the body. From the, nutritional point of view, carbohydrates are, grouped into 2 categories.
Page 517 :
507, , Chapter 23 : NUTRITION, , 1. Carbohydrates utilized by the body—, starch, glycogen, sucrose, lactose, glucose,, fructose etc., 2. Carbohydrates not utilized (not digested), by the body—cellulose, hemicellulose, pectin,, gums etc., Among the carbohydrates utilized by the, body, starch is the most abundant. The, consumption of starch has distinct advantages, due to its bland taste, satiety value and slow, digestion and absorption. Sucrose (the table, sugar), due to its sweetness, can be consumed to, a limited extent. Excessive intake of sucrose, causes dental caries, and an increase in, plasma lipid levels is associated with many, health complications., , Functions of carbohydrates, 1. Major sources of energy : Carbohydrates, are the principal source of energy, Yhplying, 60–80% of the caloric requirements of the body., 2. Protein sparing action : Proteins perform a, specialized function of body building and, growth. The wasteful expenditure of proteins to, meet the energy needs of the body should be, curtailed. Carbohydrates come to the rescue and, spare the proteins from being misused for caloric, purpose., 3. Absolute requirement by brain : The brain, and other parts of central nervous system are, dependent on glucose for energy. Prolonged, hypoglycemia may lead to irreversible brain, damage., 4. Required for the oxidation of fat : Acetyl, CoA is the product formed in fatty acid, oxidation. For its further oxidation via citric acid, cycle, acetyl CoA combines with oxaloacetate,, the latter is predominantly derived from, carbohydrates. It may therefore be stated ‘Fat, burns in a fuel of carbohydrate’., 5. Synthesis of pentoses : Pentoses (e.g., ribose) are the constituents of several compounds, in the body e.g. nucleic acids (DNA, RNA),, coenzymes (NAD+, FAD). These pentoses are, produced in carbohydrate metabolism., 6. Synthesis of fat : Excess consumption of, carbohydrates leads to the formation of fat which, is stored in the adipose tissue., , 7. Importance of non-digestible carbohydrates : These are the carbohydrates not, utilized by the body. Yet, they are important, since they improve bowel motility, prevent, constipation, lower cholesterol absorption and, improve glucose tolerance (details discussed, later)., , High fructose corn syrups (HFCS), HFCS, are, produced, from, glucose, by employing enzymatic processes that, convert glucose into fructose. HFCS contain, approximately equal amount of glucose and, fructose. They are commonly used as substitutes, for sucrose in beverages, including soft drinks,, and processed foods., The composition and metabolism of HFCS and, sucrose are similar except that HFCS is ingected, as a mixture of monosaccharides. Further, most, studies have shown that there is no significant, difference between sucrose and HFCS with regard, to post-prandial glucose and insulin response., , Glycemic index, There are variations in the increase and fall of, blood glucose levels after the ingestion of, different carbohydrate containing foods. These, quantitative differences are assayed by glycemic, index which measures the time course of postprandial glucose concentrations from a graph., Glycemic index may be defined as the area, under the blood glucose curve after the ingestion, of a food compared with the area under the, blood glucose curve after taking the same, amount of carbohydrate as glucose. It is, expressed as percentage., , Glycemic index =, , Area under the blood, glucose curve after, ingestion of test meal, , u 100, , Area under the curve after, ingestion of glucose, , A graphic representation of high and low, glycemic indices is depicted in Fig. 23.1., The glycemic index of a complex, carbohydrate (i.e. starch) is lower than a refined, carbohydrate (i.e. glucose). This is explained on, the basis of slow digestion and absorption of
Page 518 :
508, , BIOCHEMISTRY, , Blood glucose, concentration (mg/dl), , High glycemic, index, , FIBER IN NUTRITION, , 150, 100, 50, , Low glycemic, index, , 30, , 60, , 90, , 120, , Time (in minutes) after, ingestion of food, , Fig. 23.1 : The glycemic index curve after the, ingestion of two different foods., , complex carbohydrates. Further, the glycemic, index of carbohydrate is usually lower when it is, combined with protein, fat or fiber. The glycemic, index of some selected foods is given in, Table 23.3., The food item like ice cream has relatively, lower glycemic index. This may be explained on, the basis of high fat content which lowers the, glucose absorption., The nutritional importance of glycemic index, is controversial. This is due to the fact that the, foods with low glycemic index need not be, good for health. However, low glycemic index, foods usually have higher satiety value, (creating a sense of stomachfulness), and thus, may be helpful in limiting the caloric intake., Nutritionists are of the opinion that foods, with high fiber content and low glycemic index, (e.g. whole grains, fruits, vegetables) should be, preferred for consumption., , Sources of carbohydrates, Carbohydrates are abundant in several, naturally occurring foods. These include table, sugar (99%), cereals (60–80%), pulses (50–60%),, roots and tubers (20–40%) and bread (50–60%)., , Requirement of carbohydrates, In a well balanced diet, at least 40% of the, caloric needs of the body should be met from, carbohydrates., , The complex carbohydrates that are not, digested by the human enzymes are collectively, referred to as dietary fiber. Soluble fibers, mostly, found in fruits and legumes, dissolve in water, and form gels (e.g., pectins, gums, mucilages)., Insoluble fibers, present in vegetables and grains,, adsorb water and swell up (e.g. cellulose, hemicellulose, lignin). Certain fibers (e.g. pectins,, gums) are digestible by intestinal bacterial, ezymes. It may be stated that once regarded as a, nutritional waste, a lot of importance is now, given to dietary fiber in human health., , Beneficial effects of fiber, 1. Prevents constipation : Fiber can absorb, 10–15 times its own weight of water, by drawing, fluid into the lumen of the intestine. This, increases bowel motility, and prevents, constipation, besides decreasing the risk of, hemorrhoids and diverticulosis., 2. Eliminates bacterial toxins : Fiber also, adsorbs toxic compounds produced by intestinal, bacteria and helps in their easier expulsion., 3. Decreases GIT cancers : The lower, incidence of cancers of gastrointestinal tract (e.g., colon and rectum) in vegetarians compared to, non-vegetarians is attributed to dietary fiber., TABLE 23.3 Glycemic index of some, selected foods, Food item, , Glycemic index, , Glucose, , 100, , Carrots, , 90–95, , Honey, , 80–90, , Bread, rice, , 70–80, , Banana, potato, , 60–70, , Sweet potato, , 50–60, , Oranges, apples, , 40–45, , Ice cream, milk, , 35–40, , Fructose, , 20–25, , Soy beans, , 15–20
Page 519 :
509, , Chapter 23 : NUTRITION, , 4. Improves glucose tolerance : Fiber, improves glucose tolerance by the body. This is, mainly done by a diminished rate of glucose, absorption from the intestine., 5. Reduces plasma cholesterol level : Fiber, decreases the absorption of dietary cholesterol, from the intestine. Further, fiber binds with the, bile salts and reduces their enterohepatic, circulation. Thus, degradation of cholesterol to, bile salts and its disposal from the body is, increased., , 15-50% of the body energy requirements., Phospholipids and cholesterol (from animal, sources) are also important in nutrition. The, nutritional and biochemical functions of fat,, phospholipids and cholesterol have already been, discussed in detail and the reader must, invariably refer them now (Chapters 3 and 14)., , Major nutritional functions of lipids, Dietary lipids have two major nutritive, functions., , 6. Satiety value : Dietary fiber adds to the, weight of the foodstuff ingested and gives a, sensation of stomachfullness, giving satiety, without consumption of excess calories., , 1. Supply triacylglycerols that normally, constitute about 90% of dietary lipids which is a, concentrated source of fuel to the body., , Adverse affects of fiber, , 2. Provide essential fatty acids and fat soluble, vitamins (A, D, E and K)., , Some of the food fads went to the extent of, ingesting huge quantities of rice bran to achieve, all the benefits of fiber. This led to several, complications. In general, the harmful effects are, mostly observed in people consuming large, quantities of dietary fiber., 1. Digestion and absorption of protein are, adversely affected., 2. The intestinal absorption of certain, minerals (e.g. Ca, P, Mg) is decreased., 3. Intestinal bacteria ferment some fibers,, causing flatulence and often discomfort., , Drinking plenty of water along with fiber is, advocated to reduce adverse effects of fiber., , Sources of dietary fiber, Fruits, leafy vegetables, vegetables, whole, wheat legumes, rice bran etc. are rich sources of, fiber. The ideal way to increase fiber intake is to, reduce intake of refined carbohydrates, besides, eating vegetables, fresh fruits and whole grains., In general, vegetarians consume more fiber than, non-vegetarians. An average daily intake of, about 30 g fiber is recommended., , ESSENTIAL FATTY ACIDS, The unsaturated fatty acids which the body, cannot synthesize and, therefore, must be, consumed in the diet are referred to as essential, fatty acids (EFA)., The fatty acids—linoleic and linolenic acid—, cannot be synthesized by humans. In a strict, sense, only these two are essential fatty acids., Arachidonic acid can be synthesized from, linoleic acid in some animal species, including, man. However, the conversion efficiency of, linoleic acid to arachidonic acid is not clearly, known in man. And for this reason, some, nutritionists recommend that it is better to, include some amount of arachidonic acid also, in the diet., , Functions of EFA, 1. Essential fatty acids are the structural, components of biological membranes., 2. Participate in the transport and utilization, of cholesterol., 3. Prevent fat accumulation in the liver., , NUTRITIONAL IMPORTANCE, OF LIPIDS, Triacylglycerols (fats and oils) are the, concentrated dietary source of fuel, contributing, , 4. Required, prostaglandins., , for, , the, , synthesis, , of, , 5. Maintain proper growth and reproduction, of the organisms.
Page 520 :
510, , BIOCHEMISTRY, , Deficiency of EFA, , CHOLESTEROL IN NUTRITION, , The EFA deficiency in humans is, characterized by a scaly dermatitis on the, posterior and lateral parts of limbs and buttocks., This condition is referred to as phrynoderma or, toad skin., , Animal foods are the only dietary source of, cholesterol. However, the role of dietary, cholesterol on plasma cholesterol is less, important than the amount and types of fatty, acids consumed., , EFA content of foods, , REQUIREMENT OF DIETARY FAT, , The essential fatty acids, more frequently, called polyunsaturated fatty acids (PUFA), are, predominantly present in vegetable oils and fish, oils. The rich vegetable sources include, sunflower oil, cotton seed oil, corn oil, soyabean, oil etc. The fat of animal origin (exception—fish),, contain less PUFA e.g. butter, fat of meat, pork., , Dietary intake of EFA, Nutritionists recommend that at least 30% of, the dietary fat should contain PUFA. Very high, intake of PUFA (i.e. totally replacing saturated, fatty acids) may not be advisable. This is due to, the fact that excess PUFA, unless accompanied, by antioxidants (vitamin E, carotenes), is believed, to be injurious to the cells due to the, overproduction of free radicals., , Z-3 and Z-6 fatty acids, These are long chain PUFA with double bond, beginning at 3rd (Z-3) and 6th (Z-6) position, from the methyl end. Z-3 fatty acids (e.g., linolenic acid, docosahexaenoic acid (DHA),, eicosapentaenoic acid (EPA) and Z-6 fatty acids, (e.g. arachidonic acid) are known to reduce, serum cholesterol and triacylglycerols, thereby, decrease the tendency for thrombosis, lower, blood presure and reduce the risk of CHD. In, recent years, Z-3 fatty acids are included in, infant formulas to promote brain development., , Consumption of dietary fats and oils is, considered in terms of their contribution towards, the energy needs of the body. There is a wide, variation in fat intake. It is much higher (up to, 50% of daily calories) in affluent societies, compared to the poorer sections of the people, (about 15% of calories). The recommended fat, intake is around 20–30% of the daily calorie, requirement, containing about 50% of PUFA., , NUTRITIONAL IMPORTANCE, OF PROTEINS, Proteins have been traditionally regarded as, ‘body-building foods’. However, 10-15% of the, total body energy is derived from proteins. As far, as possible, carbohydrates spare proteins and, make the latter available for body-building, process. The functions carried out by proteins in, a living cell are innumerable, a few of them are, listed hereunder., , Functions of proteins, 1. Proteins are the fundamental basis of cell, structure and its function., 2. All the enzymes, several hormones,, immunoglobulins, etc., are proteins., , TRANS FATTY ACIDS (TFA), , 3. Proteins are involved in the maintenance, of osmotic pressure, clotting of blood, muscle, contraction etc., , TFA possess double bonds and are formed, during partial hydrolysis of vegetable oils. TFA, are widely used in food industry due to long, shelf-life. They increase LDL and decrease HDL,, and thus promote altherogenesis and heart, diseases. Therefore, TFA should be avoided in, the diet, as far as possible., , 4. During starvation, proteins (amino acids), serve as the major suppliers of energy. It may be, noted that the structural proteins themselves, serve as ‘storage proteins’ to meet the emergency, energy needs of the body. This is in contrast to, lipids and carbohydrates which have storage, forms.
Page 521 :
511, , Chapter 23 : NUTRITION, , Body protein, , Essential amino acids, , The nutritional importance of proteins is, Catabolism, Anabolism, based on the content of essential amino acids, (Chapter 4)., There are ten essential amino, Dietary protein Input Amino acid Output Excretion as, acids—arginine, valine, histidine, isoleucine, (Nitrogen in; I), +, pool, urea + NH4, leucine, lysine, methionine, phenylalanine,, (Nitrogen out; U), Feces, Skin, tryptophan and threonine (code to recall—AV, (F), (S), HILL MP TT). Of these two—namely arginine, Fig. 23.2 : Overview of nitrogen balance, and, histidine—are, semi-essential., The, (At equilibrium N input = N output;, requirement of 8 essential amino acids per kg, For positive N balance, N input > N output;, body weight per day is given in Table 23.4., for negative N balance N input < N output)., Cysteine and tyrosine can respectively spare the, requirement of methionine and phenylalanine., Thus, an individual is said to be in a nitrogen, balance if the intake and output of nitrogen are, NITROGEN BALANCE, Dietary protein is almost an exclusive source the same (Fig. 23.2). There are two other, of nitrogen to the body. Thus, nitrogen balance situations—a positive and a negative nitrogen, truly represents the protein (16% of which is balance., nitrogen) utilization and its loss from the body., Nitrogen balance is determined by comparing, the intake of nitrogen (chiefly by proteins) and, the excretion of nitrogen (mostly undigested, protein in feces; urea and ammonia in urine). A, normal healthy adult is in a nitrogen equilibrium, since the daily dietary intake (I) is equal to the, loss through urine (U), feces (F) and sweat (S)., I = U + F + S, The term fudge factor (approximately 3g) is, used to represent nitrogen lost in feces, sweat, and nails etc., TABLE 23.4 Requirements of essential amino acids, , Amino acid, , Requirement, (mg/kg body weight/day), , Valine, , 14, , Isoleucine, , 12, , Leucine, , 16, , Lysine, , 12, , Methionine*, , 10, , Phenylalanine*, , 16, , Tryptophan, , 3, , Threonine, , 8, , * Cysteine, , and tyrosine can, respectively, spare (partly) the, requirement of methionine and phenylalanine., , Positive nitrogen balance : This is a state in, which the nitrogen intake is higher than the, output. Some amount of nitrogen is retained in, the body causing a net increase in the body, protein. Positive nitrogen balance is observed in, growing children, pregnant women or during, recovery after serious illness., Negative nitrogen balance : This is a situation, in which the nitrogen output is higher than the, input. The result is that some amount of nitrogen, is lost from the body depleting the body protein., Prolonged negative nitrogen balance may even, lead to death. This is sometimes observed in, children suffering from kwashiorkor or, marasmus., Negative nitrogen balance may occur due to, inadequate dietary intake of protein (deficiency, of even a single essential amino acid) or, destruction of tissues or serious illness. In all, these cases, the body adapts itself and increases, the breakdown of tissue proteins causing loss of, nitrogen from the body., , Other factors influencing, nitrogen balance, Besides the major factors discussed above, (growth, pregnancy, protein deficiency, injury,, illness) several other factors influence nitrogen, balance.
Page 522 :
512, , BIOCHEMISTRY, , Hormones : Growth hormone and insulin promote positive nitrogen balance while corticosteroids result in negative nitrogen balance., , feces and urine samples. Biological value can be, calculated by the following formula, N absorbed – N lost in metabolism, , Disease states : Cancer and uncontrolled, diabetes cause negative nitrogen balance., , BV, , ASSESSMENT OF NUTRITIVE, VALUE OF PROTEINS, , BV =, , Knowledge on the quantity of dietary protein, alone is not sufficient to evaluate the nutritional, importance of proteins. This is in contrast to, dietary carbohydrates and lipids. The quality of, the proteins which depends on the composition, of essential amino acids is more important., Several laboratory methods are in use to assess, the nutritive value of proteins. Of these, four, methods—protein efficiency ratio, biological, value, net protein utilization and chemical, score—are discussed briefly., , where In = Nitrogen ingested, , Protein efficiency ratio (PER), This test consists of feeding weaning (21 day, old) albino rats with a 10% test protein diet and, recording the gain in body weight for a period of, 4 weeks. PER is represented by gain in the, weight of rats per gram protein ingested., PER, , Gain in body weight (g), Protein ingested (g), , ·, , The PER for egg protein is 4.5; for milk protein, 3.0; for rice protein 2.2., , Biological value (BV), The biological value of protein is defined as, the percentage of absorbed nitrogen retained by, the body., BV, , Nitrogen retained, Nitrogen absorbed, , N absorbed, , >I, , n, , @, , – F n – F c – Un – Uc, In – F n – F c, , For the measurement of BV, the experimental, animals, namely weaning albino rats are chosen., They are first fed with a protein-free diet for 10, days. Then they are kept on a 10% protein diet, to be tested for BV. Urine and feces are collected, for both the periods i.e. protein-free diet and, protein diet. Nitrogen is estimated in the diet,, , u100, , Fn = Nitrogen in feces (on protein diet), Fc = Nitrogen in feces (on protein-free diet), Un = Nitrogen in urine (on protein diet), Uc = Nitrogen in urine (on protein-free diet), For the calculation of BV of proteins,, experiments can be done even in human, subjects. The BV for different protein sources is, given in Table 23.5., The biological value provides a reasonably, good index for the nutritive value of proteins., But unfortunately this method has an inherent, drawback. It cannot take into account the, nitrogen that might be lost during the digestion, process. For instance, if the ingested nitrogen is, 100 mg, absorbed is 10 mg and retained is 8 mg,, the BV 8/10 u 100 = 80. This figure is erroneous,, since the major part of the protein (90 mg) did, not enter the body at all for utilization., , Net protein utilization (NPU), NPU is a better nutritional index than, biological value, since it takes into account the, digestibility factor. The experimental procedure, for NPU is similar to that of BV. Net protein, utilization can be calculated as, NPU, , u 100, , u 100, , Nitrogen retained, Nitrogen ingested, , u100, , Chemical score, This is based on the chemical analysis of the, protein for the composition of essential amino, acids which is then compared with a reference, protein (usually egg protein). The chemical score, is defined as the ratio between the quantitity of, the most limiting essential amino acid in the test
Page 523 :
513, , Chapter 23 : NUTRITION, , protein to the quantity of the same amino acid in, the egg protein, expressed as percentage., Chemical score, =, , mg of the limiting amino acid / g test protein, mg of the same amino acid / g egg protein, , u 100, , The chemical score of egg protein, for any, one of the essential amino acids, is taken as 100, and the rest of the proteins are compared., In the Table 23.5, the four methods employed, (PER, BV, NPU and chemical score) for the, assessment of nutritive value of proteins are, compared with regard to the different sources of, dietary proteins. Although there are certain, variations, anyone of these methods provides, sufficient information on the nutritive value of, proteins., , Mutual supplementation of proteins., As is observed from the Table 23.5, the, animal proteins are superior in their nutritive, value compared to the proteins of vegetable, origin. Further, some of the essential amino acids, are limiting in certain foods. For instance, rice, and wheat proteins are limiting in lysine and, threonine while the protein of Bengal gram is, limited in sulfur-containing amino acids, (methionine and cystine)., It is fortunate that humans (worldover) have, the habit of consuming a mixed diet, with, , different foods, simultaneously. This helps to, overcome the deficiency of certain essential, amino acids in one food by being supplemented, from the others. This phenomenon is referred, to as mutual supplementation. For instance,, an Indian diet with cereals (wheat, rice) is taken, along with pulses (dal). The limitation of lysine, and threonine in cereal proteins is overcome by, their supplementation from dal proteins., Simultaneously, the limitation of sulfurcontaining amino acids in dal is also, compensated by the cereals which are rich in, them., The nutritive value of protein of a particular, food can be enhanced by appropriate, combination with other foods. Due to the, consumption of mixed diets, dietary deficiency, of essential amino acids is most uncommon., Further, the principle of mixed diet takes care to, supply adequate quantities of essential amino, acids to the people subsisting on pure vegetarian, diets. It has to be remembered that the effect of, mutual supplementation in proteins is best, observed with the same meal (or at least on the, same day)., , Requirement of proteins, The requirement of protein is dependent on, its nutritive value, caloric intake and, physiological, states, (growth,, pregnancy,, , TABLE 23.5 Nutritive value of food proteins, assessed by PER, BV, NPU and chemical score, , Source of protein, , PER, , BV, , NPU, , Chemical score, , 4.5, , 94, , 90, , 100, , Milk, , 3.0, , 84, , 75, , 65, , S-Containing amino acids, , Fish, , 3.0, , 85, , 70, , 60, , Tryptophan, , Egg, , Limiting amino acid(s), Nil, , Meat, , 2.7, , 75, , 76, , 70, , S-Containing amino acids, , Rice, , 2.2, , 68, , 60, , 60, , Lysine, threonine, , Wheat, , 1.5, , 58, , 47, , 42, , Lysine, threonine, , Bengal gram, , 1.7, , 58, , 47, , 45, , S-Containing amino acids, , Red gram, , 1.5, , 57, , 46, , 45, , S-Containing amino acids, , Groundnut, , 1.7, , 55, , 45, , 44, , Lysine, threonine, S-amino acids, , Soyabean, , 2.1, , 65, , 55, , 55, , S-Containing amino acids, , PER–Protein efficiency ratio; BV–Biological value; NPU–Net protein utilization; S–Sulfur.
Page 524 :
514, , BIOCHEMISTRY, , lactation) of the individual. For an adult, 0.8-1.0, g protein/kg body weight/day is adequate. The, requirement should be nearly double for growing, children, pregnant and lactating women., , Dietary sources of proteins, The protein content of foods is variable,, cereals have 6-12%; pulses 18-22%; meat 1825%, egg 10-14%; milk 3-4% and leafy, vegetables 1-2%. In general, the animal proteins, are superior than vegetable proteins as the, dietary source., , NUTRITIONAL IMPORTANCE OF, VITAMINS AND MINERALS, The nutritional aspects including metabolism,, biochemical functions, dietary sources, requirements and associated disorders for vitamins, (Chapter 7) and for minerals (Chapter 18) have, already been discussed in much detail., , RECOMMENDED DIETARY, ALLOWANCES (RDA), The recommended dietary/daily allowances, (RDA) represents the quantities of the nutrients, to be provided in the diet daily for maintaining, good health and physical efficiency of the body., It must be remembered that RDA is not the, minimum amount to just meet the body needs,, but allowance is given for a safe margin., , Factors affecting RDA, 1. Sex : The RDA for men is about 20%, higher than that for women. Iron is an exception, as the requirement is greater in menstruating, women. Additional requirements (20-30% above, normal) are needed for pregnant and lactating, women., 2. Age : In general, the nutrient requirement, is much higher in the growing age. For instance,, the protein requirement for a growing child is, about 2 g/kg body wt/day compared to 1 g/kg, body wt/day for adults., , RDA an for adult man, The details of RDA for each of the nutrients in, relation to age, sex and physiological status is, described in the respective chapters. For a quick, recapitulation, the RDA of macronutrients, (carbohydrate, fat and protein) and selected, micronutrients (vitamins and minerals) for an, adult man weighing 70 kg are given in, Table 23.6., , BALANCED DIET, After discussing the nutritional aspects of, dietary ingredients and their RDA, it is, worthwhile to formulate a diet for man. A, , TABLE 23.6 Recommended dietary allowance, (RDA) of important nutrients for, an adult man, weighing 70 kg., , Nutrient(s), , RDA, , Carbohydrates, , 400 g, , Fats, , 70 g, , Proteins, , 56 g*, , Essential fatty acids, Vitamin A, Vitamin D, Vitamin E, , 4 g, 1,000 Pg **, 5 Pg***, 10 Pg, , Vitamin K, , 70 Pg, , Ascorbic acid, , 60 mg, , Thiamine, Riboflavin, Niacin, Pyridoxine, Folic acid, Cobalamin, , 1.5 mg, 2 mg, 20 mg, 2 mg, 150 Pg, 2 Pg, , Calcium, , 800 mg, , Phosphorus, , 800 mg, , Iron, , 10 mg, , * 0.8 g/kg body weight/day; ** Retinol equivalents;, ***As cholecalciferol
Page 525 :
515, , Chapter 23 : NUTRITION, , balanced diet or prudent diet is defined as the, diet which contains different types of foods,, possessing the nutrients—carbohydrates, fats,, proteins, vitamins and minerals—in a proportion, to meet the requirements of the body. A, balanced diet invariably supplies a little more of, each nutrient than the minimum requirement to, withstand the short duration of leanness and, keep the body in a state of good health., The basic composition of balanced diet is, highly variable, as it differs from country to, country, depending on the availability of, foods. Social and cultural habits, besides the, economic status, age, sex and physical activity, of the individual largely influence the intake of, diet., The Nutrition Expert Group, constituted by, the Indian Council of Medical Research has, recommended the composition of balanced diets, for Indians. This is done taking into account the, commonly available foods in India. The, composition of balanced diet (vegetarian and, non-vegetarian), for an adult man is given, Table 23.7., The Indian balanced diet is composed of, cereals (rice, wheat, jowar), pulses, vegetables,, roots and tubers, fruits, milk and milk products,, , fats and oils, sugar and groundnuts. Meat, fish, and eggs are present in the non-vegetarian diets., In case of vegetarians, an additional intake of, milk and pulses is recommended. The nutritional, composition of the most commonly consumed, Indian foods given in the Appendix VII. The, nutritional aspects of milk are given in the, Chapter 22., , Balanced diet in developed, countries, Some people in developed countries (e.g., U.S.A) consume excessive quantities of certain, nutrients. It is recommended that such people, have to reduce the intake of total calories, total, fat, saturated fatty acids, cholesterol, refined, sugars and salt. The U.S. Government, recommends a daily intake of less than 30% fat, against the present 40–50% towards calories., , NUTRITIONAL DISORDERS, While the people of developing countries, suffer from undernutrition, overnutrition is the, major concern of the developed countries. Some, of the important nutritional diseases are, discussed hereunder., , TABLE 23.7 Balanced diet for an adult man*, Sedentary work, Vegetarian Non-vegetarian, (g), (g), , Cereals, Pulses, , 400, , 400, , Moderate work, Vegetarian, Non-vegetarian, (g), (g), , 475, , 475, , Heavy work, Vegetarian Non-vegetarian, (g), (g), , 650, , 650, , 70, , 55, , 80, , 65, , 80, , 65, , 100, , 100, , 125, , 125, , 125, , 125, , Other vegetables, , 75, , 75, , 75, , 75, , 100, , 100, , Roots and tubers, , 75, , 75, , 100, , 100, , 100, , 100, , Fruits, , 30, , 30, , 30, , 30, , 30, , 30, , 200, , 100, , 200, , 100, , 200, , 100, , 35, , 40, , 40, , 40, , 50, , 50, , Green leafy vegetables, , Milk, Fats and oils, Meat and fish, , 30, , 30, , 30, , Eggs, , 30, , 30, , 30, , Sugar and jaggery, Groundnuts, , 30, , 30, , 40, , 40, , 55, , 55, , 50, , 50, , *Formulations based on the recommended dietary (daily) allowances (RDA) of the Indian Council of Medical Research (1989)
Page 526 :
516, , BIOCHEMISTRY, , Protein–energy malnutrition, , Marasmus, , Protein-energy malnutrition (PEM)—sometimes called protein-calorie malnutrition, (PCM)—is the most common nutritional disorder, of the developing countries. PEM is widely, prevalent in the infants and pre-school children., Kwashiorkor and marasmus are the two extreme, forms of protein-energy malnutrition., , Marasmus literally means ‘to waste’. It, mainly occurs in children under one year, age. Marasmus is predominantly due to, the deficiency of calories. This is usually, observed in children given watery gruels, (of cereals) to supplement the mother’s breast, milk., , Kwashiorkor, The term kwashiorkor was introduced by, Cicely Williams (1933) to a nutritional disease, affecting the people of Gold Coast (modern, Ghane) in Africa. Kwashiorkor literally means, sickness of the deposed child i.e. a disease the, child gets when the next baby is born., Occurrence and causes : Kwashiorkor is, predominantly found in children between 1-5, years of age. This is primarily due to insufficient, intake of proteins, as the diet of a weaning child, mainly consists of carbohydrates., Clinical symptoms : The major clinical, manifestations of kwashiorkor include stunted, growth, edema (particularly on legs and hands),, diarrhea, discoloration of hair and skin, anemia,, apathy and moonface., Biochemical manifestations : Kwashiorkor is, associated with a decreased plasma albumin, concentration (< 2 g/dl against normal 3–4.5, g/dl), fatty liver, deficiency of K+ due to diarrhea., Edema occurs due to lack of adequate plasma, proteins to maintain water distribution between, blood and tissues. Disturbances in the, metabolism of carbohydrate, protein and fat are, also observed. Several vitamin deficiencies, occur. Plasma retinol binding protein (RBP) is, reduced. The immunological response of the, child to infection is very low., Treatment : Ingestion of protein-rich foods or, the dietary combinations to provide about 3–4 g, of protein/kg body weight/day will control, kwashiorkor. The treatment can be monitored by, measuring plasma albumin concentration,, disappearance of edema and gain in body, weight., , The symptoms of marasmus include growth, retardation, muscle wasting (emaciation), anemia, and weakness. A marasmic child does not show, edema or decreased concentration of plasma, albumin. This is a major difference to distinguish, marasmus from kwashiorkor. In the Table 23.8,, a comparison between kwashiorkor and, marasmus is given., , Signs comparable to marasmus in, advanced cancer and AIDS, The patients of certain chronic diseases like, cancer and AIDS are fequently undernourished,, resulting in a codition called cachexia. This is, mainly due to the loss of body proteins as a, result of hypermetabolism, particularly increased, basal metabolic rate. Further, increased, metabolisms leading to thermogenesis is also, observed in cancer and AIDS., , Nutritional anemias, Anemia, is, characterized, by, lower, concentration of hemoglobin (reference 14–16, g/dl) with a reduced ability to transport oxygen., Nutritional anemias are classified based on the, size of erythrocytes., l, , l, , l, , Microcytic anemia—most common, with, reduced RBC size. Occurs due to the, deficiency of iron, copper and pyridoxine., Macrocytic anemia—RBC are large and, immature. Mostly due to the deficiency of, folic acid and vitamin B12., Normocytic anemia—Size of the RBC is, normal, but their quantity in blood is, low. Mostly found in protein-energy, malnutrition.
Page 527 :
517, , Chapter 23 : NUTRITION, , TABLE 23.8 Comparison between kwashiorkor and marasmus, Clinical/biochemical parameter, , Kwashiorkor, , Marasmus, , Age of onset, , Pre-school children (1-5 yr), , Weaned infants (< 1 yr), , Main nutritional cause, , Low protein intake, , Low calorie intake, , Body weight, , 60–80% of normal, , Less than 60% of normal, , Growth, , Mild retardation, , Severe retardation, , Oedema, , Present, , Absent, , Facial appearance, , Moon face, , Like old man’s face, , Abdomen, , Protruding, , Shrunken, , Skin, , Dermatitis, , Dry and atrophic, , Muscles, , Undergo wasting, , Weak and atrophic, , Subcutaneous fat, , Present, , Absent, , Vitamin deficiencies, , Present, , Present, , Serum albumin, , 0.5–2 g/dl, , 2–3 g/dl, , Serum cortisol, , Normal or decreased, , Increased, , Fasting blood glucose, , Decreased, , Decreased, , Serum K+, , Decreased, , Normal, , OTHER NUTRITIONAL DISORDERS, There are several other nutritional disorders, which have been discussed elesewhere. These, include obesity, body mass index and, atherosclerosis (Chapter 14); vitamin deficiency, disorders—xerophthalmia, rickets, beri-beri,, pellagra, scurvy and pernicious anemia (Chapter, 7); goiter and other disorders of minerals, (Chapter 18). The biochemical ramifications of, starvation are discussed along with the, integration of metabolism (Chapter 16)., , THERAPEUTIC DIETS, Diet therapy in disease states is a part of, nutrition. Therapeutic diets are usually not, palatable. However, they possess high or low, amounts of specific nutrients to meet the body, , demands as per the situation. Selected examples, of therapeutic diets are listed, l, l, l, , l, , l, , Liquid diets — for post-operative patients, Low sodium diets — for hypertensive people, Low fat diet — for patients of malabsorption, syndrome, Low protein diet — for patients of hepatic, encephalopathy, renal failure, High fiber diet — for patients of constipation,, CHD, diabetes mellits, , Atkins diet, In Atkins diet, fat and protein are high, and, carbohydrate very low (<50g/day; <10% of a, 2000 Cal/day). Atkins diet is advocated for, weight loss programmes of obese people. High, fat diet reduces appetite, and thus food intake., However, long term human consumption of, Atkins diet is controversial.
Page 528 :
518, , BIOCHEMISTRY, , NUTRITIONAL STATUS AND, CLINICAL PRACTICE, , TABLE 23.9 Drug and nutrient interactions, Drug, , The nutritional status of an individual is, important in clinical practice. The dietary, requirements of nutrients are variable, and are, mostly related to age and sex, and physiological, status. Some examples are listed, l, Infants and young children have increased, needs of protein, iron and calcium., l, During teenage, high calcium and magnesium, are recommended., l, In pregnancy, and lactation, the requirements, of iron, calcium, magnesium, folic acid and, vitamin B6 and B12 are increased., l, Elderly people have to take more of vitaminsB6 and B12, folic acid and vitamin D, and, minerals chromium, zinc etc. However,, vitamin A intake should be restricted in the, elderly to avoid toxicity., In general, illness and metabolic stress, increase the nutritional demands. For instances,, , Risk of nutrient deficiencies, , Oral contraceptives, , Vitamin B6, vitamin B12, folic acid, , Diuretics, , Potassium, zinc, , Anticonvulsants, , Folic acid, vitamin D, vitamin K, , Isoniazid, , Vitamin B6, , Corticosteroids, , Vitamin D, calcium,, potassium, zinc, , Alcohol, , Thiamine, vitamin B6, folic acid, , liver and kidney diseases reduce the formation, of active vitamin D (calcitriol), and storage and, utilization of vitamins—folic acid, vitamin B12, and vitamin D., , Drug and nutrient interactions, Many drugs are known to lead to potential, nutrient deficiencies (Table 23.9). For instance,, oral contraceptives may result in deficiency, manifestations of vitamin B6, B12 and folic acid., , + Most of the information on human nutrition is based on the research carried out in, experimental animals., , + The body at total rest (physical and mental) requires energy to meet the basal requirements such as working of heart, conduction of nerve impulse, membrane transport etc., , + Carbohydrates are the most abundant dietary constituents despite the fact that they are, not essential nutrients to the body., , + Adequate intake of dietary fiber prevents constipation, eliminates bacterial toxins,, reduces GIT cancers, improves glucose tolerance and reduces plasma cholesterol., , + In general, vegetable oils are good sources for essential fatty acids while animal proteins, are superior for the supply of essential amino acids., , + The biological value (BV) of protein represents the percentage of absorbed nitrogen, retained in the body. The BV for egg protein is 94 while that for rice is 68., , + The recommended dietary allowance (RDA) of nutrients depends on the sex and age,, besides pregnancy and lactation in the women., , + The habit of consuming mixed diet by man is largely responsible to enhance the nutritive, value of foods, besides preventing several nutritional deficiencies (e.g. amino acids)., , + Kwashiorkor and marasmus, the two extreme forms of protein-energy malnutrition in, infants and pre-school children, are highly prevalent in developing countries.
Page 529 :
519, , Chapter 23 : NUTRITION, , NUTRIGENOMICS, Nutrigenomics, a new field of nutrition,, relates to 3 distinct areas of nutrient-gene, interactions, 1. Nutritional, genetics, involves, the, individual genetic differences and their influence, on the nutrient intake., 2. Nutritional epigenetics describes the, nutrient induced changes in DNA, such as DNA, , methylation, histone post-translational changes, etc., 3. Nutritional transcriptomics involves the, effects of nutrients on gene expression. The role, vitamins A and D on nuclear receptors, and in, turn on gene expression is well known., Nutrigenomics may soon revolutionize the, clinical and nutritional practice, and result in, individualized RDA for disease prevention and, treatment., , 1. The calorific values of carbohydrates, fats and proteins respectively are 4, 9 and 4, Cal/g. These three nutrients (macronutrients) supply energy to the body to meet, the requirements of basal metabolic rate, specific dynamic action and physical, activity., 2. Basal metabolic rate (BMR) represents the minimum amount of energy required by the, body to maintain life at complete physical and mental rest, in the post-absorptive state., The normal BMR for an adult man is 35-38 Cal/m2 body surface/hr., 3. Specific dynamic action (SDA) is the extra heat produced by the body over and above, the calculated calorific value of foodstuff. It is higher for proteins (30%), lower for, carbohydrates (5%), and for a mixed diet, it is around 10%., 4. Carbohydrates are the major source of body fuel supplying about 40-70% of body, calories. The non-digested carbohydrates (cellulose, pectin), referred to as fiber, prevent, constipation, improve glucose tolerance and reduce plasma cholesterol., 5. Lipids are the concentrated source of energy. They also provide essential fatty acids, (linoleic and linolenic acids) and fat-soluble vitamins (A, D, E and K)., 6. Proteins are the body building foods that supply essential amino acids, besides meeting, the body energy requirement partly (10-15%)., 7. Several methods are employed to assess the nutritive value of proteins. These include, protein efficiency ratio, biological value, net protein utilization and chemical score., 8. The recommended dietary allowance (RDA) represents the quantities of nutrients to be, provided daily in the diet for maintaining good health and physical efficiency. The RDA, for protein is 1g/kg body weight/day., 9. A balanced diet is the diet which contains different types of foods with the nutrients,, namely carbohydrates, fats, proteins, vitamins and minerals, in a proportion to meet, the body requirements., 10. Protein-energy malnutrition (PEM) is the most common nutritional disorder in the, developing countries. Kwashiorkor is primarily due to inadequate protein intake while, marasmus is mainly caused by calorie deficiency.
Page 530 :
520, , BIOCHEMISTRY, , I. Essay questions, 1., 2., 3., 4., 5., , Define BMR. Discuss the factors affecting BMR., Describe the different methods employed for the nutritional evaluation of proteins., Define a balanced diet. Formulate a diet for a medical student., Discuss the protein-energy malnutrition with special reference to kwashiorkor., Give an account of the recommended dietary allowance (RDA) for macro- and micronutrients., , II. Short notes, (a) Essential amino acids, (b) Mutual supplementation of proteins, (c) Caloric value of foods,, (d) Specific dynamic action, (e) Energy requirements of man, (f) Fiber in nutrition, (g) Kwashiorkor,, (h) Limiting amino acids, (i) Nitrogen balance, (j) Biological value of proteins., , III. Fill in the blanks, 1. One calorie of energy is equivalent to ______________ Joules (KJ)., 2. The endocrine organ most predominantly associated with BMR is ______________., 3. The non-digestible carbohydrates are collectively known as ______________., 4. The major source of energy to the body is supplied by ______________., 5. The nutritional assessment method used to know the most limiting essential amino acid in, relation to a standard protein is ______________., 6. The daily normal requirement of protein in an adult is ______________., 7. The percentage of absorbed nitrogen retained in the body represents ______________., 8. The proteins of Bengal gram are limiting in the amino acids ______________., 9. The nutrient required in greater amounts in menstruating women compared to men is _______., 10. The biochemical parameter often used as an index for monitoring the recovery from kwashiorkor, is _______., , IV. Multiple choice questions, 11. The specific dynamic action (SDA) is the greatest for the following foodstuff, (a) Protein (b) Carbohydrate (c) Fat (d) Vitamins., 12. The reference protein for the calculation of chemical score, (a) Meat protein (b) Fish protein (c) Milk protein (d) Egg protein., 13. The essential amino acid limiting in rice, (a) Methionine (b) Tryptophan (c) Lysine (d) Histidine., 14. A continuous supply of energy to the body is necessary to meet the requirements of, (a) Basal metabolic rate (b) Specific dynamic action (c) Physical activity (d) All of them., 15. One of the following is the most important essential fatty acid in the diet, (a) Linoleic acid (b) Arachidonic acid (c) Oleic acid (d) Palmitic acid.
Page 531 :
“This page intentionally left blank"
Page 532 :
MOLECUL, MOLECULAR, AR BIOL, BIOLOGY, OGY AND BIOTECHNOL, BIOTECHNOLOGY, OGY, 24, ■, 25, ■, 26, ■, 27, ■, , DNA-Replication,, Recombination, and Repair, , 523, , Transcription and Translation 542, Regulation of Gene, Expression, , 566, , Recombinant DNA and, Biotechnology, , 578, , Section, , V
Page 533 :
Section 5, , Molecular Biology and Biotechnology, , Chapter, , DNA–Replication,, Recombination, and Repair, , 24, , The hereditary molecule, DNA speaks :, , “I replicate and recombinate,, To permit the cells to proliferate,, Environmental insults try to damage me,, But I protect myself with adequate repairs”, , D, , eoxyribonucleic, acid, (DNA), is, a, macromolecule that carries genetic, information from generation to generation. It is, responsible to preserve the identity of the species, over millions of years. DNA may be regarded as, a reserve bank of genetic information or a, memory bank., A single mammalian fetal cell contains only a, few picograms (10–12 g) of DNA. It is surprising, that this little quantity of DNA stores information, that will determine the differentiation and every, function of an adult animal., , Why did DNA evolve as genetic, material?, RNA molecules, in principle, can perform the, cellular functions that are carried out by DNA., In fact, many viruses contain RNA as the genetic, material. Chemically, DNA is more stable than, RNA. Hence, during the course of evolution,, DNA is preferred as a more suitable molecule, for long-term repository of genetic information., , DNA, , Transcription, , RNA, , Translation, , Protein, , Replication, , Fig. 24.1 : The central dogma of life., , The central dogma of life, The biological information flows from DNA, to RNA, and from there to proteins. This is the, central dogma of life (Fig.24.1). It is ultimately, the DNA that controls every function of the cell, through protein synthesis., As the carrier of genetic information, DNA in, a cell must be duplicated (replicated),, maintained and passed down accurately to the, daughter cells. Three distinct processes are, designed for this purpose. The ‘three Rs’ of, DNA-replication, recombination, and repair, are, dealt with in this chapter. There are certain, common features between the three Rs., , 523
Page 534 :
524, l, , l, , l, , BIOCHEMISTRY, , They act on the same substrate (DNA)., They are primarily concerned with the making, and breaking of phosphodiester bonds (the, backbone of DNA structure)., Enzymes used in the three processes are, mostly similar/comparable., , REPLICATION OF DNA, DNA is the genetic material. When the cell, divides, the daughter cells receive an identical, copy of genetic information from the parent cell., Replication is a process in which DNA copies, itself to produce identical daughter molecules, of DNA. Replication is carried out with high, fidelity which is essential for the survival of the, species. Synthesis of a new DNA molecule is a, complex process involving a series of steps., The salient features of replication in, prokaryotes are described first. This is followed, by some recent information on the eukaryotic, replication., , Daughter DNA, , Parent DNA, , Daughter DNA, , Fig. 24.2 : DNA replication—semiconservative., , REPLICATION IN PROKARYOTES, , These sites mostly consist of a short sequence of, A-T base pairs. A specific protein called dna A, (20-50 monomers) binds with the site of origin, for replication. This causes the double-stranded, DNA to separate., , Replication is semiconservative, , Replication bubbles, , The parent DNA has two strands complementary to each other. Both the strands undergo, simultaneous replication to produce two, daughter molecules. Each one of the newly, synthesized DNA has one-half of the parental, DNA (one strand from original) and one-half of, new DNA (Fig.24.2). This type of replication is, known as semiconservative since half of the, original DNA is conserved in the daughter DNA., The first experimental evidence for the, semiconservative DNA replication was provided, by Meselson and Stahl (1958)., , The two complementary strands of DNA, separate at the site of replication to form a, bubble. Multiple replication bubbles are formed, in eukaryotic DNA molecules, which is essential, for a rapid replication process (Fig.24.3)., , Initiation of replication, The initiation of DNA synthesis occurs at a, site called origin of replication. In case of, prokaryotes, there is a single site whereas in, eukaryotes, there are multiple sites of origin., , RNA primer, For the synthesis of new DNA, a short, fragment of RNA (about 5-50 nucleotides,, variable with species) is required as a primer., The enzyme primase (a specific RNA, polymerase) in association with single-stranded, binding proteins forms a complex called, primosome, and produces RNA primers. A, constant synthesis and supply of RNA primers, should occur on the lagging strand of DNA. This, is in contrast to the leading strand which has, almost a single RNA primer.
Page 535 :
Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , 525, , proteins. They possess no enzyme activity. SSB, proteins bind only to single-stranded DNA, (separated by helicases), keep the two strands, separate and provide the template for new DNA, synthesis. It is believed that SSB proteins also, protect the single-stranded DNA degradation by, nucleases., , DNA synthesis catalysed, by DNA polymerase III, +, , Fig. 24.3 : Schematic representation of multiple, replication bubbles in DNA replication., , DNA synthesis is semidiscontinuous, and bidirectional, The replication of DNA occurs in 5’ to 3’, direction, simultaneously, on both the strands of, DNA. On one strand, the leading (continuous or, forward) strand—the DNA synthesis is, continuous. On the other strand, the lagging, (discontinuous or retrograde) strand—the, synthesis of DNA is discontinuous. Short pieces, of DNA (15-250 nucleotides) are produced on, the lagging strand., In the replication bubble, the DNA synthesis, occurs in both the directions (bidirectional) from, the point of origin., , Replication fork and DNA synthesis, , The synthesis of a new DNA strand, catalysed, by DNA polymerase III, occurs in 5’o3’, direction. This is antiparallel to the parent, template DNA strand. The presence of all the, four deoxyribonucleoside triphosphates (dATP,, dGTP, dCTP and dTTP) is an essential, prerequisite for replication to take place., The synthesis of two new DNA strands,, simultaneously, takes place in the opposite, direction—one is in a direction (5’o3’) towards, the replication fork which is continuous, the other, in a direction (5’o3’) away from the replication, fork which is discontinuous (Fig.24.4)., The incoming deoxyribonucleotides are, added one after another, to 3’ end of the growing, DNA chain (Fig.24.5). A molecule of pyrophosphate (PPi) is removed with the addition of, each nucleotide. The template DNA strand (the, parent) determines the base sequence of the, newly synthesized complementary DNA., , Polarity problem, , DNA helicases : These enzymes bind to both, the DNA strands at the replication fork., Helicases move along the DNA helix and, separate the strands. Their function is, comparable with a zip opener. Helicases are, dependent on ATP for energy supply., , The DNA strand (leading strand) with its, 3c-end (3c-OH) oriented towards the fork can be, elongated by sequential addition of new, nucleotides. The other DNA strand (lagging, strand) with 5c-end presents some problem, as, there is no DNA polymerase enzyme (in any, organism) that can catalyse the addition of, nucleotides to the 5c end (i.e. 3co5c direction) of, the growing chain. This problem however is, solved by synthesizing this strand as a series of, small fragments. These pieces are made in the, normal 5co3c direction, and later joined, together., , Single-stranded DNA binding (SSB) proteins :, These are also known as DNA helix-destabilizing, , Okazaki pieces : The small fragments of the, discontinuously synthesized DNA are called, , The separation of the two strands of parent, DNA results in the formation of a replication, fork. The active synthesis of DNA occurs in this, region. The replication fork moves along the, parent DNA as the daughter DNA molecules are, synthesized.
Page 536 :
526, Okazaki pieces. These are, produced on the lagging, strand of the parent DNA., Okazaki pieces are later, joined, to, form, a, continuous strand of DNA., DNA polymerase I and, DNA ligase are responsible, for this process (details, given later)., , Proof-reading, function of, DNA polymerase III, Fidelity of replication is, the most important for the, very existence of an, organism., Besides, its, 5’o3’ directed catalytic, function, DNA polymerase, III also has a proof-reading, activity. It checks the, incoming nucleotides and, allows only the correctly, matched, bases, (i.e., complementary bases) to, be added to the growing, DNA strand. Further, DNA, polymerase, edits, its, mistakes (if any) and, removes, the, wrongly, placed nucleotide bases., , BIOCHEMISTRY, , dna A protein, 3c, , 5c, 3c, , 5c, Native DNA, dna A protein, 3c, , 5c, , 5c, , 3c, Replication bubble, , 3c, 5c, , Leading, strand, , Lagging, strand, 5c, , 5c, , 3c, , 3c, , Lagging, strand, , Leading, strand, Origin of replication, , Leading 3c, strand, 5c, , RNA primer, DNA polymerase III, , Newly, synthesized, DNA, , Replacement of, RNA primer by DNA, , 5c, 5c, 3c, DNA helicase, , SSB, , The synthesis of new, DNA strand continues till it Lagging 3c, strand, Okazaki pieces, 5c, is in close proximity to, RNA primer. Now the, Replication fork, DNA polymerase I comes, Fig. 24.4 : Overview of DNA replication process, into picture. It removes the, (SSB–Single-stranded binding proteins)., RNA primer and takes its, position. DNA polymerase, I catalyses the synthesis (5’o3’ direction) of a the DNA synthesized by DNA polymerase III and, fragment of DNA that replaces RNA primer the small fragments of DNA produced by DNA, (Fig.24.6)., polymerase I. This process—nick sealing-requires, The enzyme DNA ligase catalyses the, formation of a phosphodiester linkage between, , energy, provided by the breakdown of ATP to, AMP and PPi.
Page 537 :
527, , Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , Complementary strand, , DNA template, , 3c, , 5c, , P, , P, , P, , P, , P, , P, , P, , P, , P, , P, , C, , T, , G, , A, , A, , C, , T, , G, , A, , T, , G, , A, , C, , U, , T, , G, , A, , C, , T, , A, , P, , P, , P, , P, , P, , P, , P, , OH, , P, , P, , P, , 5c, , OH 3c, , P, P, RNA primer, , P, , Growing DNA, , Entering dATP, , Fig. 24.5 : DNA replication with a growing complementary strand., , Another enzyme—DNA polymerase II—has, been isolated. It participates in the DNA repair, process., , Supercoils and DNA topoisomerases, As the double helix of DNA separates from, one side and replication proceeds, supercoils are, formed at the other side. The formation of, supercoils can be better understood by, comparing DNA helix with two twisted ropes, tied at one end. Hold the ropes at the tied end, in a fixed position. And let your friend pull the, ropes apart from the other side. The formation of, supercoils is clearly observed., Type I DNA topoisomerase cuts the single, DNA strand (nuclease activity) to overcome the, problem of supercoils and then reseals the strand, (ligase activity). Type II DNA topoisomerase (also, known as DNA gyrase) cuts both strands and, reseals them to overcome the problem of, supercoils. DNA topoisomerases are targeted by, drugs (campthoterin for topoisomerase I, and, amsacrime and etoposide for topoisomerase I,, and amsacrime and etoposide for topoisomerase, II) in the treatment of cancers., , REPLICATION IN EUKARYOTES, Replication of DNA in eukaryotes closely, resembles that of prokaryotes. Certain differences,, however, exist. Multiple origins of replication is, a characteristic feature of eukaryotic cell. Further,, , at least five distinct DNA polymerases are known, in eukaryotes. Greek letters are used to number, these enzymes., 1. DNA polymerase D is responsible for the, synthesis of RNA primer for both the leading and, lagging strands of DNA., 2. DNA polymerase E is involved in the, repair of DNA. Its function is comparable with, DNA polymerase I found in prokaryotes., 3. DNA polymerase J participates in the, replication of mitochondrial DNA., 4. DNA polymerase G is responsible for the, replication on the leading strand of DNA. It also, possesses proof-reading activity., 3c, 5c, 5c, , 5c, , 5c Newly synthesized DNA, 3c DNA template, , Excised, RNA primer, 3c, , 3c, , DNA polymerase I, 5c, 3c, Nick sealed, by DNA ligase, , 3c, 5c, , 5c Daughter DNA, 3c Parent DNA, , Fig. 24.6 : Overview of the action of DNA, polymerase I and DNA ligase.
Page 538 :
528, 5. DNA polymerase H is involved in DNA, synthesis on the lagging strand and proof-reading, function., The differences in the DNA replication, between bacteria and human cells, attributed to, the enzymes, are successfully used in, antibacterial therapy to target pathogen (bacterial), replication and spare the host (human) cells., , PROCESS OF REPLICATION, IN EUKARYOTES, The replication on the leading (continuous), strand of DNA is rather simple, involving DNA, polymerase G and a sliding clamp called, proliferating cell nuclear antigen (PCNA). PCNA, is so named as it was first detected as an antigen, in the nuclei of replicating cells. PCNA forms a, ring around DNA to which DNA polymerase G, binds. Formation of this ring also requires another, factor namely replication factor C (RFC)., The replication on the lagging (discontinuous), strand in eukaryotes is more complex when, compared to prokaryotes or even the leading, strand of eukaryotes. This is depicted in, Fig.24.7, and briefly described hereunder., The parental strands of DNA are separated by, the enzyme helicase. A single-stranded DNA, binding protein called replication protein A, (RPA) binds to the exposed single-stranded, template. This strand has been opened up by the, replication fork (a previously formed Okazaki, fragment with an RNA primer is also shown in, Fig.24.4)., The enzyme primase forms a complex with, DNA polymerase D which initiates the synthesis, of Okazaki fragments. The primase activity of, pol D-primase complex is capable of producing, 10-bp RNA primer. The enzyme activity is then, switched from primase to DNA polymerase D, which elongates the primer by the addition of, 20–30 deoxyribonucleotides. Thus, by the action, of pol D-primase complex, a short stretch of, DNA attached to RNA is formed. And now the, complex dissociates from the DNA., The next step is the binding of replication, factor C (RFC) to the elongated primer (short, RNA-DNA). RFC serves as a clamp loader, and, , BIOCHEMISTRY, , catalyses the assembly of proliferating cell, nuclear antigen (PCNA) molecules. The DNA, polymerase G binds to the sliding clamp and, elongates the Okazaki fragment to a final length, of about 150–200 bp. By this elongation, the, replication complex approaches the RNA primer, of the previous Okazaki fragment., The RNA primer removal is carried out by a, pair of enzymes namely RNase H and flap, endonuclease I (FENI). This gap created by RNA, removal is filled by continued elongation of the, new Okazaki fragment (carried out by, polymerase G, described above). The small nick, that remains is finally sealed by DNA ligase., Eukaryotic DNA is tightly bound to histones, (basic proteins) to form nucleosomes which, in, turn, organize into chromosomes. During the, course of replication, the chromosomes are, relaxed and the nucleosomes get loosened. The, DNA strands separate for replication, and the, parental histones associate with one of the, parental strands. As the synthesis of new DNA, strand proceeds, histones are also produced, simultaneously, on the parent strand. At the end, of replication, of the two daughter chromosomal, DNAs formed, one contains the parental histones, while the other has the newly synthesized, histones., , INHIBITORS OF DNA REPLICATION, Bacteria contain a specific type II, topoisomerase namely gyrase. This enzyme cuts, and reseals the circular DNA (of bacteria), and, thus overcomes the problem of supercoils., Bacterial gyrase is inhibited by the antibiotics, ciprofloxacin, novobiocin and nalidixic acid., These are widely used as antibacterial agents, since they can effectively block the replication, of DNA and multiplication of cells. These, antibacterial agents have almost no effect on, human enzymes., Certain compounds that inhibit human, topoisomerases are used as anticancer agents, e.g. adriamycin, etoposide, doxorubicin. The, nucleotide analogs that inhibit DNA replication, are also used as anticancer drugs e.g., 6-mercaptopurine, 5-fluorouracil.
Page 540 :
530, , BIOCHEMISTRY, , CELL CYCLE AND, DNA REPLICATION, , Detection of, damaged, DNA, , G2, , The cell cycle consists of four distinct phases, in higher organisms—mitotic, G1, S and G2, phases (Fig.24.8). When the cell is not growing,, it exists in a dormant or undividing phase (G0)., G1 phase is characterized by active protein, synthesis., Replication of DNA occurs only once in, S-phase and the chromosomes get doubled i.e., diploid genome gets converted into tetraploid., The entire process of new DNA synthesis, takes place in about 8–10 hours, and a large, number of DNA polymerases (500–1,000) are, simultaneously involved in this process. It is, believed that methylation of DNA serves as a, marker to inhibit replication., The G2 phase is characterized by enlargement, of cytoplasm and this is followed by the, actual cell division that occurs in the mitotic, phase., , Cyclins and cell cycle, Cyclins are a group of proteins that are closely, associated with the transition of one phase of, cell cycle to another, hence they are so named., The most important cyclins are cyclin A, B,, D and E. The concentrations of cyclins increase, or decrease during the course of cell cycle., These cyclins act on cyclin-dependent kinases, (CDKs) that phosphorylate certain substances, essential for the transition of one cycle to, another., , Cell cycle check points, As depicted in Fig.24.8, there occurs a, continuous monitoring of the cell cycle with, respect to DNA replication, chromosome, segregation and integrity. If any damage to DNA, is detected either in G1 or G2 phase of the cycle,, or if there is a formation of defective spindle (i.e., incomplete chromosomal segregation), the cell, cycle will not progress until appropriately, corrected. If it is not possible to repair the, damage done, the cells undergo apoptosis, (programmed cell death)., , Detection of, incomplete, replication, , S phase, , M, G1, , Detection of, improper, spindle, G0, , Damaged DNA, detected, , Fig. 24.8 : The cell cycle of a mammalian cell, (M–Mitotic phase; G1–Gap1 phase; G0–Dormant phase;, S phase – Period of replication; G2–Gap 2 phase)., , Cancer and cell cycle, Cancer represents an excessive division of, cells. In cancer, a large quantity of cells are in, mitosis and most of them in S-phase., Majority of the drugs used for cancer therapy, are designed to block DNA replication or inhibit, the enzymes that participate in replication, (directly or indirectly). Methotrexate (inhibits, dihydrofolate reductase) and 5-fluorouracil, (inhibits thymidylate synthase) block nucleotide, synthesis., In recent years, topoisomerase inhibitors are, being used. They block the unwinding of, parental DNA strands and prevent replication., , TELOMERES AND TELOMERASE, There are certain difficulties in the replication, of linear DNAs (or chromosomes) of eukaryotic, cells. The leading strand of DNA can be, completely synthesized to the very end of its, template. This is not possible wih the lagging, strand, since the removal of the primer, RNA leaves a small gap which cannot be, filled (Fig.24.9A). Consequently, the daughter, chromosomes will have shortened DNA, molecules. This becomes significant after several, cell, cycles, involving, replication, of, chromosomes. The result is that over a period of, time, the chromosomes may lose certain, essential genes and the cell dies. This is, however, avoided to a large extent.
Page 542 :
532, , BIOCHEMISTRY, , TELOMERE IN SENESCENCE, AND CANCER, , ABCDEFGHIJKLKNOP, +, , Telomerase is highly active in the early, embryo, and after birth it is active in the, reproductive and stem cells. Stem cells divide, continuously throughout the lifetime of an, organism to produce new cells. These cells in, turn are responsible to tissues and organs in the, functional state e.g. hematopoietic stem cells of, bone marrow., Many biologists link the process of telomere, shortening with cell senescence (i.e. cell death)., This is mainly based on the observations made, in the in vitro mammalian cell cultures., However, some researchers question this relation, between telomere shortening and senescence., Cancerous cells are able to divide, continuously. There is a strong evidence to, suggest that the absence of senescence in cancer, cells is linked to the activation of the enzyme, telomerase. Thus, telomere length is maintained, throughout multiple cell divisions. It is however,, not clear whether telomerase activation is a, cause or an effect of cancer. There is however,, evidence to suggest that telomerase activation is, in fact the cause of certain cancers e.g., dyskeratosis congenita due to a mutation in the, gene responsible for the RNA component of, telomerase., The enzyme telomerase is an attractive target, for cancer chemotherapy. The drugs have, been designed to inactivate telomerase, and, consequently induce senescence in the cancer, cells. This in turn prevents the rapid cell, proliferation., , RECOMBINATION, Recombination, basically, involves, the, exchange of genetic information. There are, mainly two types of recombinations., 1. Homologous recombination : This is also, called as general recombination, and occurs, between, identical, or, nearly, identical, chromosomes (DNA sequences). The best, example is the recombination between the, paternal and maternal chromosomal pairs, (Fig.24.10)., , abcdefghijklmnop, Parental chromosomes, Homologous, recombination, , ABCDEFGHIjklmnop, +, abcdefghiJKLMNOP, Chromosomes with, DNA from both parents, , Fig. 24.10 : A diagrammatic representation of, homologous recombination., , 2. Non-homologous recombination : This is, regarded as illegitimate recombination and does, not require any special homologous sequences., Transposition is a good example of nonhomologous recombination. Random integration, of outside genes into mammalian chromosomes, is another example., , HOMOLOGOUS RECOMBINATION, It is a known fact that the chromosomes are, not passed on intact from generation to, generation. Instead, they are inherited from both, the parents. This is possible due to homologous, recombination. Three models have been put, forth to explain homologous recombinations., l, , Holliday model, , l, , Meselson-Radding model, , l, , Double-strand break model., , Holliday model, Holliday model (proposed by Holliday in, 1964) is the simplest among the homologous, recombination models. It is depicted in, Fig.24.11, and briefly explained in the next, page., The two homologous chromosomes come, closer, get properly aligned, and form single-
Page 543 :
533, , Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , strand breaks. This results in two aligned DNA, duplexes. Now the strands of each duplex partly, unwind and invade in the opposite direction to, form a two strands cross between the DNA, molecules., , A, , B, +, , a, b, Two homologous DNA molecules, with single-strand breaks, , There occurs simultaneous unwinding and, rewinding of the duplexes in such a way that, there is no net change in the amount of base, pairing, but the position of crossover moves. This, phenomenon referred to as branch migration,, results in the formation of heteroduplex DNA., The enzyme DNA ligase seals the nick. The two, DNA duplexes (4 strands of DNA), joined by a, single crossover point can rotate to create a fourstanded Holliday junction. Now the DNA, molecules are subjected to symmetrical cuts in, either of the two directions, and the cut ends are, resealed by ligase., , A, , B, , a, b, Cross DNA strands, A, , B, , a, b, Heteroduplex sealed by DNA ligase, , The DNA exchange is determined by the, direction of the cuts, which could be horizontal, or vertical. If the corss strands are cut, horizontally (cut 1), the flanking genes (or, markers, i.e. AB/ab) remain intact, and no, recombination occurs. On the other hand, if the, parental strands are cut vertically (cut 2), the, flanking genes get exchanged (i.e. Ab/aB) due to, recombination., , Cut 2, B, , A, Cut 1, , a, , b, , Four strands held together and cut, , B, , A, , NON-HOMOLOGOUS, RECOMBINATION, , Cut 1, , The recombination process without any, special homologous sequences of DNA is, regarded as non-homologous recombination., , a, , l, ca e, rti as, ve lig, 2- by, ut, C led, a, se, , Transposition, Transposition, primarily, involves, the, movement of specific pieces of DNA in the, genome. The mobile segments of DNA are called, transposons or transposable elements. They, were first discovered by Barbara McClintock (in, 1950) in maize, and their significance was, ignored for about two decades by other workers., Transposons are mobile and can move almost, to any place in the target chromosome. There, are two modes of transposition. One that, involves an RNA intermediate, and the other, which does not involve RNA intermediate., , b, , Cut 2, Holliday intermediate (molecule rotated), al, nt se, o, iz a, or lig, -h by, 1, ut d, C ale, se, A, b, A, , B, , +, , a, b, Recombined daughter, DNA strands, , +, , a, B, Recombined daughter, DNA strands, , Fig. 24.11 : Holliday model for homologous, recombination (Note : Heteroduplex regions, are shown in dotted boxes).
Page 544 :
534, , BIOCHEMISTRY, , elements (SINEs) are repeats of DNA sequences, which are present in about 500,000 copies per, haploid human genome e.g. Alu sequences., , Transposon, , Transcription, RNA, Reverse, transcription, DNA, Re-integration, , Transposon copy, (retrotransposon), , Fig. 24.12 : A diagrammatic representation of, retrotransposition., , Retrotransposition : Transposition involving, RNA intermediate represents retrotransposition, (Fig.24.12). By the normal process of, transcription, a copy of RNA formed from a, transposon (also called as retrotransposon). Then, by the enzyme reverse transcriptase, DNA is, copied from the RNA. The newly formed DNA, which is a copy of the transposon gets integrated, into the genome. This integration may occur, randomly on the same chromosome or, on a, different chromosome. As a result of the retrotransposition, there are now two copies of the, transposon, at different points on the genome., DNA transposition : Some transposons are, capable of direct transposition of DNA to DNA., This may occur either by replicative transposition, or conservative transposition (Fig.24.13). Both, the mechanisms require enzymes that are mostly, coded by the genes within the transposons., DNA transposition is less common than, retrotransposition in case of eukaryotes. However,, in case of prokaryotes, DNA transposons are more, important than RNA transposons., , Long interspersed elements (LINEs) are also, repeated DNA sequences and are present in, about 50,000 copies in the human genome e.g., L1 elements., Some of the diseases caused by mutations are, due to insertion of transposons into genes., , DAMAGE AND REPAIR OF DNA, Being the carrier of genetic information, the, cellular DNA must be replicated (duplicated),, maintained, and passed down to the daughter, cells accurately. In general, the accuracy of, replication is extremely high. However, there do, occur replication errors. It is estimated that, approximately one error is introduced per billion, base pairs during each cycle of replication. The, cells do posses the capability to repair damages, done to DNA to a large extent., , Consequences of DNA damage, Despite an efficient repair system for the, damaged DNA, replication errors do accumulate, that ultimately result in mutations. The human, body possesses 1014 nucleated cells, each with, 3 u 109 base pairs of DNA. It is estimated that, about 1016 cell divisions occur in a lifetime. If, 10–10 mutations per base pair per cell generation, escape repair, this results in about one mutation, per 106 base pairs in genome., , Replicative, Conservative, , Significance of transposition, It is now widely accepted that a large fraction, of the human genome has resulted due to the, accumulation of transposons. Short interspersed, , Fig. 24.13 : A diagrammatic representation of DNA, transposition (coloured blocks represent transposons).
Page 545 :
535, , Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , Besides the possible errors in replication, the, DNA is constantly subjected to attack by both, physical and chemical agents. These include, radiation, free radicals, chemicals etc., which, also result in mutations., It is fortunate that a great majority of the, mutations probably occur in the DNA that, does not encode proteins, and consequently will, not have any serious impact on the organism., This is not, however, all the time true, since, mutations do occur in the coding regions of, DNA also. There are situations in which the, change in a single base pair in the human, genome can cause a serious disease e.g. sicklecell anemia., , TYPES OF DNA DAMAGES, , TABLE 24.1 Major types of DNA damages, , Category, Single-base alteration, , Depurination, Base alkylation, Insertion or deletion of, nucleotides, Incorporation of base analogue, Two-base alteration, , UV light induced pyrimidine, dimer alteration (T–T), , Chain breaks, , Oxidative free radical formation, Ionizing radiation, , Cross-linkage, , The damages done to DNA by physical,, chemical and environmental agents may be, broadly classified into four categories with, different types (Table 24.1)., The DNA damage may occur due to singlebase alterations (e.g. depurination, deamination),, two-base alterations (e.g. pyrimidine dimer), chain breaks (e.g. ionizing radiation) and crosslinkages (e.g. between bases). Some selected, DNA damages are briefly described., The occurrence of spontaneous deamination, bases in aqueous solution at 37°C is well known., Cytosine gets deaminated to form uracil while, adenine forms hypoxanthine., Spontaneous depurination, due to cleavage of, glycosyl bonds (that connect purines to the, backbone) also occurs. It is estimated that, 2000–10,000 purines may be lost per, mammalian cell in 24 hours. The depurinated, sites are called as abasic sites. Originally, they, were detected in purines, and called apurinic, sites (AP sites) which represent lack of purine., Now, the term AP sites is generally used to, represent any base lacking in DNA., The production of reactive oxygen species is, often associated with alteration of bases e.g., formation of 8-hydroxy guanine. Free radical, formation and oxidative damage to DNA, increases with advancement of age., , Types, Deamination, (CoU; Aohypoxanthine), , Between bases in the same or, opposite strands, Between the DNA and protein, molecules, , Ultraviolet radiations result in the formation, of covalent links between adjacent pyrimidines, along the DNA strand to form pyrimidine, dimers. DNA chain breaks can be caused by, ionizing radiations (e.g. X-rays)., , MUTATIONS, The genetic macromolecule DNA is highly, stable with regard to its base composition and, sequence. However, DNA is not totally exempt, from gradual change., Mutation refers to a change in the DNA, structure of a gene. The substances (chemicals), which can induce mutations are collectively, known as mutagens., The changes that occur in DNA on mutation, are reflected in replication, transcription and, translation., , Types of mutations, Mutations are mainly of two major types—, point mutations, frameshift mutations (Fig.24.14)., 1. Point mutations : The replacement of one, base pair by another results in point mutation., They are of two sub-types.
Page 546 :
536, , BIOCHEMISTRY, , (a) Transitions : In this case, a purine (or a, pyrimidine) is replaced by another., (b) Transversions : These are characterized, by replacement of a purine by a, pyrimidine or vice versa., 2. Frameshift mutations : These occur when, one or more base pairs are inserted in or deleted, from the DNA, respectively, causing insertion or, deletion mutations., , (A), , —C—G—A—G—, , Transition, , —G—C—T—C—, , —C—G—G—G—, —G—C—C—C—, , —C—G—A—G— Transversion —C—G—T—G—, —G—C—T—C—, , —G—C—A—C—, , (B), , —C—G—A—T—G—, , Consequences of point mutations, The change in a single base sequence in, point mutation may cause one of the following, (Fig.24.15)., 1. Silent mutation : The codon (of mRNA), containing the changed base may code for the, same amino acid. For instance, UCA codes for, serine and change in the third base (UCU) still, codes for serine. This is due to degeneracy of the, genetic code. Therefore, there are no detectable, effects in silent mutation., 2. Missense mutation : In this case, the, changed base may code for a different amino, acid. For example, UCA codes for serine while, ACA codes for threonine. The mistaken (or, , t, , er, , —C—G—A—G—, , Ins, , ion, , —G—C—T—C— D, el, et, io, n, , —G—C—T—A—C—, , —C—G—C—, —G—C—G—, , Fig. 24.14 : An illustration of mutations (A)-Point, mutations; (B)-Frameshift mutations., , missense) amino acid may be acceptable,, partially acceptable or unacceptable with regard, to the function of protein molecule. Sickle-cell, anemia is a classical example of missense, mutation., , + A few micrograms (10–12 g) of DNA in a fetal cell stores the genetic information that, will determine the differentiation and every function of an adult animal. This is the, marvel of molecular biology., , + Topoisomerase inhibitors (e.g. adriamycin, etoposide) are useful to prevent DNA, replication, and thus uncontrolled cell proliferation in cancer. These compounds block, the unwinding of DNA strands., , + The progressive shortening of telomeres (DNA sequences at chromosomal ends) is, prevented by telomerase. This enzyme is an attractive target for cancer therapy., , + Mutations may sometimes result in serious diseases. e.g. sickle-cell anemia, cancer., + Xeroderma pigmentosum is a rare disease characterized by photosensitivity and risk for, skin cancer. This is due to a defect in the nucleotide excision repair of the damaged, DNA (caused by UV rays)., , + Hereditary nonpolyposis colon cancer is a common inherited cancer, and is due to a, faulty mismatch repair of defective DNA.
Page 547 :
537, , Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , in the reading of codons, translation continues., The result is that the protein synthesized will, have several altered amino acids and/or, prematurely terminated protein., , UCU, (codon for Ser), Silent, mutation, UCA, (codon for Ser), se, en n, iss tatio, M u, m, , A CA, (codon for Thr), , Mutations and cancer, , No, m nse, ut n, at se, ion, , UAA, (termination codon), , Fig. 24.15 : An illustration of point mutations, (represented by a codon of mRNA)., , 3. Nonsense mutation : Sometimes, the, codon with the altered base may become a, termination (or nonsense) codon. For instance,, change in the second base of serine codon, (UCA) may result in UAA. The altered codon, acts as a stop signal and causes termination of, protein synthesis, at that point., , Mutations are permanent alterations in DNA, structure, which have been implicated in the, etiopathogenesis of cancer., , REPAIR OF DNA, As already stated, damage to DNA caused by, replication errors or mutations may have serious, consequences. The cell possesses an inbuilt, system to repair the damaged DNA. This may, be achieved by four distinct mechanisms, (Table 24.2)., 1. Base excision-repair, 2. Nucleotide excision-repair, 3. Mismatch repair, 4. Double-strand break repair., , Consequences of frameshift, mutations, , Base excision-repair, , The insertion or deletion of a base in a, gene results in an altered reading frame of the, mRNA (hence the name frameshift). The, machinery of mRNA (containing codons) does, not recognize that a base was missing or a new, base was added. Since there are no punctuations, , The bases cytosine, adenine and guanine can, undergo spontaneous depurination to respectively, form uracil, hypoxanthine and xanthine. These, altered bases do not exist in the normal DNA,, and therefore need to be removed. This is carried, out by base excision repair (Fig.24.16)., , TABLE 24.2 Major mechanisms of DNA repair, , Mechanism, , Damage to DNA, , DNA repair, , Base excision-repair, , Damage to a single base due to, spontaneous alteration or by, chemical or radiation means., , Removal of the base by N–glycosylase;, abasic sugar removal, replacement., , Nucleotide excision-repair, , Damage to a segment of DNA by, spontaneous, chemical or radiation, means., , Removal of the DNA fragment (ï 30 nt, length) and replacement., , Mismatch repair, , Damage due to copying errors, (1-5 base unpaired loops)., , Removal of the strand (by exonuclease, digestion) and replacement., , Double-strand break repair, , Damage caused by ionizing radiations,, free radicals, chemotherapy etc., , Unwinding, alignment and ligation.
Page 548 :
538, , BIOCHEMISTRY, , TCCT, AGGA, Normal DNA, , TCUT, AGGA, Defective DNA, , U, , Uracil DNA, glycosylase, TCXT, , damaged part. An excision nuclease (exinuclease), cuts the DNA on either side (upstream and, downstream) of the damaged DNA. This, defective piece is degraded. The gap created by, the nucleotide excision is filled up by DNA, polymerase which gets ligated by DNA ligase, (Fig.24.17)., , Xeroderma pigmentosum (XP) is a rare, autosomal recessive disease. The affected, patients are photosensitive and susceptible to, skin cancers. It is now recognized that XP is due, to a defect in the nucleotide excision repair of, the damaged DNA., , AGGA, , Mismatch repair, Endonucleases, , AGGA, , Despite high accuracy in replication, defects, do occur when the DNA is copied. For instance,, cytosine (instead of thymine) could be, incorporated opposite to adenine. Mismatch, , DNA polymerase, DNA ligase, TCCT, , 3c, , 5c, , 5c, , 3c, , AGGA, , Defect recognition, and unwinding, , Fig. 24.16 : A diagrammatic representation of, base excision-repair of DNA., , A defective DNA in which cytosine is, deaminated to uracil is acted upon by the, enzyme uracil DNA glycosylase. This results in, the removal of the defective base uracil. An, endonuclease cuts the backbone of DNA strand, near the defect and removes a few bases. The, gap so created is filled up by the action of repair, DNA polymerase and DNA ligase., , Cutting at two sites, to remove defective, oligonucleotide, , Nucleotide excision-repair, , Degradation of, defective DNA, , The DNA damage due to ultraviolet light,, ionizing radiation and other environmental, factors often results in the modification of, certain bases, strand breaks, cross-linkages etc., Nucleotide excision-repair is ideally suited for, such large-scale defects in DNA. After the, identification of the defective piece of the DNA,, the DNA double helix is unwound to expose the, , Resynthesis and, religation, 3c, , 5c, , 5c, , 3c, , Fig. 24.17 : A diagrammatic representation of, nucleotide excision-repair of DNA.
Page 549 :
539, , Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , repair corrects a single mismatch base pair e.g., C to A, instead of T to A., The template strand of the DNA exists in a, methylated form, while the newly synthesized, strand is not methylated. This difference allows, the recognition of the new strands. The enzyme, GATC endonuclease cuts the strand at, an adjacent methylated GATC sequence, (Fig.24.18). This is followed by an exonuclease, digestion of the defective strand, and thus its, removal. A new DNA strand is now synthesized, to replace the damaged one., , 3c, , CH3, , CH3, , 5c, , 5c, 3c, , Single strand, cut by GATC endonuclease, CH3, , CH3, , Exonuclease, , Hereditary nonpolyposis colon cancer, (HNPCC) is one of the most common inherited, cancers. This cancer is now linked with faulty, mismatch repair of defective DNA., , DNA polymerase, CH3, , CH3, , Double-strand break repair, Double-strand breaks (DSBs) in DNA are, dangerous. They result in genetic recombination, which may lead to chromosomal translocation,, broken chromosomes, and finally cell death., DSBs can be repaired by homologous, recombination or non-homologous end joining., Homologous recombination occurs in yeasts, while in mammals, non-homologous and joining, dominates., , DEFECTS IN DNA REPAIR, AND CANCER, Cancer develops when certain genes, that regulate normal cell division fail or are, , Ligase, 3c, 5c, , CH3, , CH3, , 5c, 3c, , Fig. 24.18 : A diagrammatic representation of, mismatch repair of DNA., , altered. Defects in the genes encoding proteins, involved in nucleotide-excision repair, mismatch, repair and recombinational repair are linked to, human cancers. For instance, as already referred, above, HNPCC is due to a defect in mismatch, repair.
Page 550 :
540, , BIOCHEMISTRY, , 1. The central dogma of life revolves around the flow of information from DNA to RNA,, and from there to proteins., 2. Replication is a process in which DNA copies itself to produce identical daughter, molecules of DNA. DNA replication is semiconservative, bidirectional and occurs by, the formation of bubbles and forks., 3. Prokaryotic DNA syntheis is catalysed by the enzyme DNA polymerase III. This enzyme, possesses proof-reading activity and edits the mistakes that might occur during, nucleotide incorporation., 4. Replication in eukaryotes (particularly on the lagging strand) is more complex and, involves several factors e.g. replication protein A, replication factor C, flap, endonuclease., 5. Telomeres (repeat TTAGGG sequences) are the special structures that prevent the, continuous loss of DNA at the end of the chromosome during the course of replication., 6. Recombination involves the exchange of genetic information through the exchange of, DNA. Transposition refers to the movement of specific pieces of DNA (called, transposons) in the genome., 7. Damage to DNA may be due to single base alteration, two-base alteration, chain breaks, and cross linkages. The cells possess an inbuilt system to repair the damaged DNA, , I. Essay questions, 1., 2., 3., 4., 5., , Describe the replication of DNA., Give an account of recombination of DNA., Discuss different types of DNA damages, and the repair mechanisms., What are mutations? Describe different types, and consequences of mutations., Give an account of telomeres and their role in senescence and cancer., , II. Short notes, (a) Replication fork, (b) Okazaki pieces, (c) RNA primer, (d) DNA topoisomerases, (e) Inhibitors of, DNA replication, (f) Telomerase, (g) Holliday model of DNA recombination, (h) Transposition,, (i) Frameshift mutations, (j) Missense mutation, (k) Mismatch repair, (l) Xeroderma pigmentosum., , III. Fill in the blanks, 1. DNA strands for replication process are separated by the enzyme ______________., 2. The small fragments of DNA produced during replication are called ______________., 3. During the course of DNA replication, the proof-reading function is carried out by the enzyme, ______________.
Page 551 :
Chapter 24 : DNA–REPLICATION, RECOMBINATION, AND REPAIR, , 541, , 4. The problem of supercoils in DNA replication is overcome by a group of enzymes, namely, ______________., 5. The proteins that are associated with the transition of one phase of cell cycle to another, ______________., 6. Name the DNA sequence that prevents the continuous loss of DNA at the end of the, chromosome during the course of replication ______________., 7. The mobile segments of DNA are called ______________., 8. Any change in the DNA sequence of a gene is commonly referred to as ______________., 9. Sickle-cell anemia is a good example of ______________ mutation., 10. One common example of inherited cancer with faulty mismatch repair of defective DNA, ______________., , IV. Multiple choice questions, 11. The chemical nature of the primer required for the synthesis of DNA, (a) DNA (b) Histone (c) RNA (d) hnRNA., 12. The enzyme responsible for the synthesis of RNA primer in eukaryotes, (a) DNA polymerase D (b) DNA polymerase E (c) DNA polymerase� J (d) Topisomerases., 13. The repeat sequence of nucleotides in telomeres, (a) TTGGGA (b) TTAGGG (c) GGGATT (d) TTGAGG., 14. The DNA damage caused by deamination is an example of, (a) Single-base alteration (b) Two-base alteration (c) Chain breaks (d) Cross linkage., 15. The mutation involving the replacement of one purine by another, (a) Frameshift mutation (b) Transition (c) Transversion (d) None of the above.
Page 552 :
Section 5, , Molecular Biology and Biotechnology, , Chapter, , Transcription and Translation, , 25, fMet, 3c end, , A—C—C, , The genetic code speaks :, , 5c end, , Complementary, binding, , Anticodon, , UAC, 5c, , AUG, , 3cmRNA, , “Triplet base sequence of messenger RNA, I am;, Universal, specific, non-overlapping, degenerate, in character;, Faithfully work under the dictates of DNA :, To execute my master’s orders for protein synthesis.”, , Codon, , T, , he conventional concept of central dogma of, life which in essence is “DNA makes RNA, makes protein” is an oversimplification of, molecular biology. With the advances in cell, biology and rapid developments in bioinformatics, the terms genome, transcriptome, and proteome are in current use to represent, the central dogma of life (Fig.25.1)., , Replication, , Replication, , DNA, , GENOME, , Transcription, RNA, Translation, PROTEIN, , GENOME, The total DNA (genetic information), contained in an organism or a cell is regarded as, the genome. Thus, the genome is the storehouse, of biological information. It includes the, chromosomes in the nucleus and the DNA in, mitochondria, and chloroplasts., Genomics : The study of the structure and, function of genome is genomics. The term, functional genomics is used to represent the, gene expression and relationship of genes with, gene products. Structural genomics refers to the, structural motifs and complete protein structures., Comparative genomics involves the study of, , Conventional concept, (pre-bioinformatics era), , Transcription, TRANSCRIPTOME, Translation, PROTEOME, Current concept, (bioinformatics era), , Fig. 25.1 : The central dogma of life (or molecular, biology) represented in the form of conventional, and current concepts., , comparative gene function and phylogeny., Metagenomics refers to the study of genomes of, whole communities of microscopic life, (microorganisms, viruses)., , TRANSCRIPTOME, The RNA copies of the active protein, coding genes represent transcriptome. Thus,, transcriptome is the initial product of gene, , 542
Page 553 :
543, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , expression, proteins., , which, , directs, , the, , synthesis, , of, , Transcriptomics : The study of transcriptome, that involves all the RNA molecules made by a, cell, tissue or an organism is transcriptomics., , PROTEOME, The cell’s repertoire (repository/storehouse) of, proteins with their nature and biological, functions is regarded as proteome. Thus,, proteome represents the entire range of proteins, and their biological functions in a cell., , D, E, , D, Ec, , Core enzyme, , Sigma factor, , Holoenzyme, , Fig. 25.2 : RNA polymerase of E. coli., , Proteomics : The study of the proteome., Metabolomics : The use of genome sequence, analysis for determining the capability of a cell,, tissue or an organism to synthesize small, molecules (metabolites) is metabolomics., Whether the central dogma of life is, represented in the conventional or more recent, form, replication, transcription and translation, are the key or core processes that ultimately, control life. Replication of DNA has been, described in Chapter 24, while transcription and, translation are discussed in this chapter., , TRANSCRIPTION, Transcription is a process in which ribonucleic acid (RNA) is synthesized from DNA., The word gene refers to the functional unit of, the DNA that can be transcribed. Thus, the, genetic information stored in DNA is expressed, through RNA. For this purpose, one of the two, strands of DNA serves as a template (non-coding, strand or antisense strand) and produces, working copies of RNA molecules. The other, DNA strand which does not participate in, transcription is referred to as coding strand or, sense strand or non-template strand. (Coding, strand commonly used since with the exception, of T for U, primary mRNA contains codons with, the same base sequence)., , Transcription is selective, The entire molecule of DNA is not expressed, in transcription. RNAs are synthesized only for, , some selected regions of DNA. For certain other, regions of DNA, there may not be any, transcription at all. The exact reason for the, selective transcription is not known. This may be, due to some inbuilt signals in the DNA, molecule., The product formed in transcription is referred, to as primary transcript. Most often, the primary, RNA transcripts are inactive. They undergo, certain alterations (splicing, terminal additions,, base modifications etc.) commonly known as, post-transcriptional modifications, to produce, functionally active RNA molecules., There exist certain differences in the, transcription between prokaryotes and eukaryotes., The RNA synthesis in prokaryotes is given in some, detail. This is followed by a brief discussion on, eukaryotic transcription., , TRANSCRIPTION IN PROKARYOTES, A single enzyme—DNA dependent RNA, polymerase or simply RNA polymerase—, synthesizes all the RNAs in prokaryotes. RNA, polymerase of E. coli is a complex holoenzyme, (mol wt. 465 kDa) with five polypeptide, subunits—2D, 1E and 1E’ and one sigma(s) factor, (Fig.25.2). The enzyme without sigma factor is, referred to as core enzyme (D2EE’)., An overview of RNA synthesis is depicted in, Fig.25.3. Transcription involves three different, stages—initiation, elongation and termination, (Fig.25.4).
Page 554 :
544, , BIOCHEMISTRY, , Coding strand, 3c, , 5c, 3c, , 5c, 5c, , RNA, , Template, strand, , RNA polymerase, , Fig. 25.3 : An overview of transcription., , Initiation, The binding of the enzyme RNA polymerase, to DNA is the prerequisite for the transcription to, start. The specific region on the DNA where the, enzyme binds is known as promoter region., There are two base sequences on the coding, DNA strand which the sigma factor of RNA, polymerase can recognize for initiation of, transcription (Fig.25.5)., 1. Pribnow box (TATA box) : This consists of, 6 nucleotide bases (TATAAT), located on the left, side about 10 bases away (upstream) from the, starting point of transcription., 2. The ‘–35’ sequence : This is the second, recognition site in the promoter region of DNA., It contains a base sequence TTGACA, which is, located about 35 bases (upstream, hence –35), away on the left side from the site of, transcription start., , Elongation, As the holoenzyme, RNA polymerase, recognizes the promoter region, the sigma factor, is released and transcription proceeds. RNA is, synthesized from 5’ end to 3’ end (5’o3’), antiparallel to the DNA template. RNA, polymerase utilizes ribonucleotide triphosphates, (ATP, GTP, CTP and UTP) for the formation of, RNA. For the addition of each nucleotide to the, growing chain, a pyrophosphate moiety is, released., The sequence of nucleotide bases in the, mRNA is complementary to the template DNA, strand. It is however, identical to that of coding, strand except that RNA contains U in place of T, in DNA (Fig.25.6)., RNA, polymerase, differs, from, DNA, polymerase in two aspects. No primer is required, , for RNA polymerase and, further, this enzyme, does not possess endo- or exonuclease activity., Due to lack of the latter function (proof-reading, activity), RNA polymerase has no ability to repair, the mistakes in the RNA synthesized. This is in, contrast to DNA replication which is carried out, with high fidelity. It is, however, fortunate that, mistakes in RNA synthesis are less dangerous,, since they are not transmitted to the daughter, cells., The double helical structure of DNA unwinds, as the transcription goes on, resulting in, supercoils. The problem of supercoils is, overcome by topoisomerases (more details in, Chapter 24)., , Termination, The process of transcription stops by, termination signals. Two types of termination are, identified., 1. Rho (U) dependent termination : A specific, protein, named U factor, binds to the growing, RNA (and not to RNA polymerase) or weakly to, DNA, and in the bound state it acts as ATPase, and terminates transcription and releases RNA., The U factor is also responsible for the, dissociation of RNA polymerase from DNA., 2. Rho (U) independent termination : The, termination in this case is brought about by the, formation of hairpins of newly synthesized RNA., This occurs due to the presence of palindromes., A palindrome is a word that reads alike forward, and backward e.g. madam, rotor. The presence, of palindromes in the base sequence of DNA, template (same when read in opposite direction), in the termination region is known. As a result of, this, the newly synthesized RNA folds to form, hairpins (due to complementary base pairing), that cause termination of transcription.
Page 556 :
546, , BIOCHEMISTRY, , Coding, strand, , Pribnow, box, TATAAT, , – 35, Sequence, TTGACA, , 5c, , 3c, , Template 3c, strand, , 5c, –35 bases, , –10 bases, , Coding region of gene, , Start of, transcription, , Fig. 25.5 : Promoter regions of DNA in prokaryotes., , DNA, , 5c, , A, , T, , G C A, , T, , G G C A, , 3c Coding strand, , 3c, , T, , A, , C G T, , A, , C C G T, , 5c Template strand, , A, , U G C A, , RNA, , 5c, , U G G C A, , 3c, , Fig. 25.6 : Transcription—Complementary base pair relationship., , TRANSCRIPTION IN EUKARYOTES, RNA synthesis in eukaryotes is a much more, complicated process than the transcription, described above for prokaryotes. As such, all the, details of eukaryotic transcription (particularly, about termination) are not clearly known. The, salient features of available information are given, hereunder., , RNA polymerases, The nuclei of eukaryotic cells possess three, distinct RNA polymerases (Fig.25.7)., 1. RNA polymerase I is responsible for the, synthesis of precursors for the large ribosomal, RNAs., , 2. RNA polymerase II synthesizes the, precursors for mRNAs and small nuclear RNAs., 3. RNA polymerase III participates in the, formation of tRNAs and small ribosomal RNAs., Besides the three RNA polymerases found in, the nucleus, there also exists a mitochondrial, RNA polymerase in eukaryotes. The latter, resembles prokaryotic RNA polymerase in, structure and function., , Promoter sites, In eukaryotes, a sequence of DNA bases—, which is almost identical to pribnow box of, prokaryotes—is identified (Fig.25.8). This, sequence, known as Hogness box (or TATA box),, , 5c, , 3c, , 3c, , 5c, RNA polymerase I, , RNA polymerase II, , RNA polymerase III, , 3c, 5c, 5c, , Ribosomal, RNAs, , 3c, , Messenger, RNA, , Transfer RNA, , Fig. 25.7 : An overview of transcription in eukaryotes., , DNA
Page 557 :
547, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , CAAT box, Coding, strand, , 5c, , Template, strand, , 3c, , GGCCAATC, , Hogness, box, ATATAA, , 3c, 5c, , –70 bases, , –25 bases, , Coding region of gene, , Start of, transcription, , Fig. 25.8 : Promoter regions of DNA in eukaryotes., , is located on the left about 25 nucleotides away, (upstream) from the starting site of mRNA, synthesis. There also exists another site of, recognition between 70 and 80 nucleotides, upstream from the start of transcription. This, second site is referred to as CAAT box. One of, these two sites (or sometimes both) helps RNA, polymerase II to recognize the requisite, sequence on DNA for transcription., , Initiation of transcription, The molecular events required for the, initiation of transcription in eukaryotes are, complex, and broadly involve three stages., 1. Chromatin containing the promoter, sequence made accessible to the transcription, machinery., 2. Binding of transcription factors (TFs) to, DNA sequences in the promoter region., 3. Stimulation of transcription by enhancers., A large number of transcription factors, interact with eukaryotic promoter regions. In, humans, about six transcription factors have, been identified (TFIID, TFIIA, TFIIB, TFIIF, TFIIE,, TFIIH). It is postulated that the TFs bind to each, other, and in turn to the enzyme RNA, polymerase., , Enhancer can increase gene expression by, about 100 fold. This is made possible by binding, of enhancers to transcription factors to form, activators. It is believed that the chromatin forms, a loop that allows the promoter and enhancer, to be close together in space to facilitate, transcription., , Heterogeneous nuclear, RNA (hnRNA), The primary mRNA transcript produced by, RNA polymerase II in eukaryotes is often referred, to as heterogeneous nuclear RNA (hnRNA). This, is then processed to produce mRNA needed for, protein synthesis., , POST-TRANSCRIPTIONAL, MODIFICATIONS, The RNAs produced during transcription are, called primary transcripts. They undergo many, alterations—terminal base additions, base, modifications, splicing etc., which are, collectively referred to as post-transcriptional, modifications. This process is required to convert, the RNAs into the active forms. A group of, enzymes, namely ribonucleases, are responsible, for the processing of tRNAs and rRNAs of both, prokaryotes and eukaryotes., The prokaryotic mRNA synthesized in transcription is almost similar to the functional mRNA., In contrast, eukaryotic mRNA (i.e. hnRNA), undergoes extensive post-transcriptional changes., An outline of the post-transcriptional, modifications is given in Fig.25.9, and some, highlights are described., , Messenger RNA, The primary transcript of mRNA is the hnRNA, in eukaryotes, which is subjected to many, changes before functional mRNA is produced., 1. The 5’ capping : The 5’ end of mRNA is, capped with 7-methylguanosine by an unusual
Page 558 :
548, , BIOCHEMISTRY, , hnRNA (preRNA), , End, modifications, , Cap, , Poly(A) tail, , Splicing, , Introns, removed, , Cutting, , Chemical, modifications, , Cut pieces, , New chemical groups added, , Fig. 25.9 : An outline of post-transcriptional modifications of RNA (hnRNA-Heterogeneous nuclear RNA)., , 5’o5’, triphosphate, linkage., S-Adenosylmethionine is the donor of methyl group. This, cap is required for translation, besides stabilizing, the structure of mRNA., 2. Poly-A tail : A large number of eukaryotic, mRNAs possess an adenine nucleotide chain at, the 3’-end. This poly-A tail, as such, is not, produced during transcription. It is later added, to stabilize mRNA. However, poly-A chain gets, reduced as the mRNA enters cytosol., 3. Introns and their removal : Introns are the, intervening nucleotide sequences in mRNA, which do not code for proteins. On the, other hand, exons of mRNA possess genetic, code and are responsible for protein, synthesis. The splicing and excision of introns is, illustrated in Fig.25.10. The removal of introns is, promoted by small nuclear ribonucleoprotein particles (snRNPs). snRNPs (pronounced, as snurps) in turn, are formed by the, association of small nuclear RNA (snRNA) with, proteins., The term spliceosome is used to represent, the snRNP association with hnRNA at the, exon-intron junction., Post-transcriptional modifications of mRNA, occur in the nucleus. The mature RNA then, enters the cytosol to perform its function, (translation)., , A diagrammatic representation of the, relationship between eukaryotic chromosomal, DNA and mRNA is depicted in Fig.25.11., , Different mRNAs produced, by alternate splicing, Alternate patterns of hnRNA splicing result in, different mRNA molecules which can produce, , mRNA, Exon 1, , Intron, , Exon 2, , ATP, ADP + Pi, , SnRNPs, , Exon 1, Exon 2, , SnRNPs, +, Exon 1, , Exon 2, , Excised, intron, , Fig. 25.10 : Formation of mature RNA from eukaryotic, mRNA (SnRNPs–Small nuclear, ribonucleoprotein particles).
Page 559 :
549, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , 1.5 u 108 bp, , Chromosome, , 1.5 u 106 bp, , Gene cluster, (~16 genes), , conversion of CAA codon in mRNA (of, apoprotein B gene) to UAA by the enzyme, cytidine deaminase. As a result, originating from, the same gene, the liver synthesizes a 100-kDa, protein (apoB 100) while the intestinal cells, synthesize 48-kDa protein (apoB 48). This, happens due to formation of a termination codon, (UAA) from CAA in RNA editing., , Transfer RNA, , One gene (with 8 exons and, 7 introns), , 2.5 u 104 bp, , 8 u 103 nt, Primary transcript, , mRNA, , 2 u 103 nt, , Fig. 25.11 : A diagrammatic representation of the, relationship between eukaryotic chromosomal DNA and, mRNA (bp–Base pair; nt–Nucleotides)., , different proteins. Alternate splicing results in, mRNA heterogeneity. In fact, the processing of, hnRNA molecules becomes a site for the, regulation of gene expression., Faulty splicing can cause diseases : Splicing of, hnRNA has to be performed with precision to, produce functional mRNA. Faulty splicing may, result in diseases. A good example is one type of, E-thalassemia in humans. This is due to a, mutation that results in a nucleotide change at an, exon-intron junction. The result is a diminished, or lack of synthesis of E-chain of hemoglobin,, and consequently the disease E-thalassemia., , mRNA editing, The sequence in the DNA determines the, coding sequence in mRNA, and finally the, amino acid sequence in the protein. However,, in recent years, changes in the coding, information by editing of mRNA have been, reported. It is estimated that about 0.01% of the, mRNAs undergoes editing. One example is the, , All the tRNAs of prokaryotes and eukaryotes, undergo post-transcriptional modification. These, include trimming, converting the existing bases, into unusual ones, and addition of CCA, nucleotides to 3’ terminal end of tRNAs., , Ribosomal RNA, The preribosomal RNAs originally synthesized, are converted to ribosomal RNAs by a series of, post-transcriptional changes., , Inhibitors of transcription, The synthesis of RNA is inhibited by certain, antibiotics and toxins., Actinomycin D : This is also known as, dactinomycin. It is synthesized by Streptomyces., Actinomycin D binds with DNA template strand, and blocks the movement of RNA polymerase., This was the very first antibiotic used for the, treatment of tumors., Rifampin : It is an antibiotic widely used for, the treatment of tuberculosis and leprosy., Rifampin binds with the E-subunit of prokaryotic, RNA polymerase and inhibits its activity., D-Amanitin : It is a toxin produced by, mushroom, Amanita phalloides. This mushroom, is delicious in taste but poisonous due to the, toxin D-amanitin which tightly binds with RNA, polymerase II of eukaryotes and inhibits, transcription., , CELLULAR RNA CONTENTS, A typical bacterium normally contains, 0.05-0.10 pg of RNA which contributes to about, 6% of the total weight. A mammalian cell, being, larger in size, contains 20–30 pg RNA, and this
Page 560 :
550, , BIOCHEMISTRY, , Total RNA, , Coding RNA, (4% of total), , Non-coding RNA, (96% of total), , hnRNA, pre-rRNA, , pre-tRNA, , snRNA, , snoRNA, , scRNA, , mRNA, rRNA, , tRNA, , Fig. 25.12 : A diagrammatic representation of RNA content of a cell (Note : RNAs represented in, black are found in all organisms; RNAs in colour and exclusively present in eukaryotes only;, hnRNA–Heterogeneous nuclear RNA; rRNA–Ribosomal RNA : tRNA–Transfer RNA;, snRNA–Small nuclear RNA; snoRNA–Small nucleolar RNA; scRNA–Small cytoplasmic RNA)., , represents only 1% of the cell weight. Transcriptome, representing the RNA derived from, protein coding genes actually constitutes only, 4%, while the remaining 96% is the non-coding, RNA (Fig.25.12). The different non-coding RNAs, are ribosomal RNA, transfer RNA, small nuclear, RNA, small nucleolar RNA and small cytoplasmic, RNA. The functions of different RNAs are, described in Chapter 5 (Refer Table 5.3)., , REVERSE TRANSCRIPTION, Some of the viruses—known as retroviruses—, possess RNA as the genetic material. These, viruses cause cancers in animals, hence known, as oncogenic. They are actually found in the, transformed cells of the tumors., The, enzyme, RNA, dependent, DNA, polymerase —or simply reverse transcriptase—, is responsible for the formation of DNA from, RNA (Fig.25.13). This DNA is complementary, (cDNA) to viral RNA and can be transmitted into, host DNA., Synthesis of cDNA from mRNA : As already, described, the DNA expresses the genetic, information in the form of RNA. And the mRNA, determines the amino acid sequence in a, protein. The mRNA can serve as a template for, synthesis of double-stranded complementary, , DNA (cDNA) by using the enzyme reverse, transcriptase. This cDNA can be used as a probe, to identify the sequence of DNA in genes., , TRANSLATION, The genetic information stored in DNA is, passed on to RNA (through transcription), and, ultimately expressed in the language of proteins., The biosynthesis of a protein or a polypeptide in, a living cell is referred to as translation. The term, translation is used to represent the biochemical, translation of four-letter language information, from nucleic acids (DNA and then RNA) to 20, letter language of proteins. The sequence of, amino acids in the protein synthesized is, determined by the nucleotide base sequence of, mRNA., , 3c, 5c, , 5c Viral RNA, Primer, Reverse, transcriptase, , 3c, 5c, , 5c RNA, 3c DNA, , Fig. 25.13 : Reverse transcription of RNA virus.
Page 561 :
551, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , Variability of cells in translation, There are wide variations in the cells with, respect to the quality and quantity of proteins, synthesized. This largely depends on the need, and ability of the cells. Erythrocytes (red blood, cells) lack the machinery for translation, and, therefore cannot synthesize proteins., In general, the growing and dividing cells, produce larger quantities of proteins. Some of, the cells continuously synthesize proteins for, export. For instance, liver cells produce albumin, and blood clotting factors for export into the, blood for circulation. The normal liver cells are, very rich in the protein biosynthetic machinery,, and thus the liver may be regarded as the protein, factory in the human body., , GENETIC CODE, The three nucleotide (triplet) base sequences, in mRNA that act as code words for amino acids, in protein constitute the genetic code or simply, codons. The genetic code is regarded as a, dictionary of nucleotide bases (A, G, C and U ), that determines the sequence of amino acids in, proteins., The codons consist of the four nucleotide, bases, the purines—adenine (A) and guanine (G),, and the pyrimidines—cytosine (C) and uracil (U)., These four bases produce 64 different, combinations (43) of three base codons, as, depicted in Table 25.1. The nucleotide sequence, of the codon on mRNA is written from the 5’end to 3’ end. Sixty one codons code for the 20, amino acids found in protein., The three codons UAA, UAG and UGA do, not code for amino acids. They act as stop, signals in protein synthesis. These three codons, are collectively known as termination codons or, non-sense codons. The codons UAG, UAA and, UGA are often referred to, respectively, as, amber, ochre and opal codons., The codons AUG—and, sometimes, GUG—, are the chain initiating codons., , Other characteristics of, genetic code, The genetic code is universal, specific, nonoverlapping and degenerate., , 1. Universality : The same codons are used, to code for the same amino acids in all the living, organisms. Thus, the genetic code has been, conserved during the course of evolution. Hence, genetic code is appropriately regarded as, universal. There are, however, a few exceptions., For instance, AUA is the codon for methionine, in mitochondria. The same codon (AUA) codes, for isoleucine in cytoplasm. With some, exceptions noted, the genetic code is universal., 2. Specificity : A particular codon always, codes for the same amino acid, hence the, genetic code is highly specific or unambiguous, e.g. UGG is the codon for tryptophan., 3. Non-overlapping : The genetic code is read, from a fixed point as a continuous base sequence., It is non-overlapping, commaless and without any, punctuations. For instance, UUUCUUAGAGGG, is read as UUU/CUU/AGA/GGG. Addition or, deletion of one or two bases will radically change, the message sequence in mRNA. And the protein, synthesized from such mRNA will be totally, different. This is encountered in frameshift, mutations which cause an alteration in the, reading frame of mRNA., 4. Degenerate : Most of the amino acids have, more than one codon. The codon is degenerate, or redundant, since there are 61 codons, available to code for only 20 amino acids. For, instance, glycine has four codons. The codons, that designate the same amino acid are called, synonyms. Most of the synonyms differ only in, the third (3c end) base of the codon., The Wobble hypothesis explains codon, degeneracy (described later)., , Codon-anticodon recognition, The codon of the mRNA is recognized by the, anticodon of tRNA (Fig.25.14). They pair with, each other in antiparallel direction (5c o 3c of, mRNA with 3c o 5c of tRNA). The usual, conventional complementary base pairing, (A U, C G) occurs between the first two, bases of codon and the last two bases, of anticodon. The third base of the codon is, rather lenient or flexible with regard to
Page 563 :
553, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , fMet, , PROTEIN BIOSYNTHESIS, , 3c end A C C, 5c end, , The protein synthesis which involves the, translation of nucleotide base sequence of, mRNA into the language of amino acid sequence, may be divided into the following stages for the, convenience of understanding., I. Requirement of the components, II. Activation of amino acids, , Anticodon, , Complementary, binding, , III. Protein synthesis proper, UAC, , 5c, , AUG, , IV. Chaperones and protein folding, 3c mRNA, , Codon, , Fig. 25.14 : Complementary binding of codon, (of mRNA) and anticodon (of tRNA)., , Mutations and genetic code, Mutations result in the change of nucleotide, sequences in the DNA, and consequently in the, RNA. The different types of mutations are, described in Chapter 24. The ultimate effect of, mutations is on the translation through the, alterations in codons. Some of the mutations are, harmful., The occurrence of the disease sickle-cell, anemia due to a single base alteration, (CTC o CAC in DNA, and GAG o GUG in, RNA) is a classical example of the seriousness of, mutations. The result is that glutamate at the 6th, position of E-chain of hemoglobin is replaced by, valine. This happens since the altered codon, GUG of mRNA codes for valine instead of, glutamate (coded by GAG in normal people)., , Frameshift mutations are caused by deletion, or insertion of nucleotides in the DNA that, generate altered mRNAs. As the reading frame of, mRNA is continuous, the codons are read in, continuation, and amino acids are added. This, results in proteins that may contain several, altered amino acids, or sometimes the protein, synthesis may be terminated prematurely., , V. Post-translational modifications., , I. REQUIREMENT OF THE, COMPONENTS, The protein synthesis may be considered as a, biochemical factory operating on the ribosomes., As a factory is dependent on the supply of raw, materials to give a final product, the protein, synthesis also requires many components., 1. Amino acids : Proteins are polymers of, amino acids. Of the 20 amino acids found in, protein structure, half of them (10) can be, synthesized by man. About 10 essential amino, acids have to be provided through the diet., Protein synthesis can occur only when all the, amino acids needed for a particular protein are, available. If there is a deficiency in the dietary, supply of any one of the essential amino acids,, the translation stops. It is, therefore, necessary, that a regular dietary supply of essential amino, acids, in sufficient quantities, is maintained, as it, is a prerequisite for protein synthesis., As regards prokaryotes, there is no, requirement of amino acids, since all the 20 are, synthesized from the inorganic components., 2. Ribosomes : The functionally active ribosomes are the centres or factories for protein, synthesis. Ribosomes may also be considered as, workbenches of translation. Ribosomes are huge, complex structures (70S for prokaryotes and 80S, for eukaryotes) of proteins and ribosomal RNAs., Each ribosome consists of two subunits—one big, and one small. The functional ribosome has two
Page 564 :
554, , BIOCHEMISTRY, , Completely synthesized protein, , Beginning of, protein synthesis, , NH2, , H2N, , NH2, , NH2, , 5c, , 3c mRNA, , Fig. 25.15 : A polyribosome in protein synthesis., , sites—A site and P site. Each site covers both the, subunits. A site is for binding of aminoacyl tRNA, and P site is for binding peptidyl tRNA, during, the course of translation. Some authors consider, A site as acceptor site, and P site as donor site., In case of eukaryotes, there is another site called, exit site or E site. Thus, eukaryotes contain three, sites (A, P and E) on the ribosomes., The ribosomes are located in the cytosomal, fraction of the cell. They are found in association, with rough endoplasmic reticulum (RER) to form, clusters RER—ribosomes, where the protein, synthesis occurs. The term polyribosome, (polysome) is used when several ribosomes, simultaneously translate on a single mRNA, (Fig.25.15)., 3. Messenger RNA (mRNA) : The specific, information required for the synthesis of a given, protein is present on the mRNA. The DNA has, passed on the genetic information in the form of, codons to mRNA to translate into a protein, sequence., 4. Transfer RNAs (tRNAs) : They carry the, amino acids, and hand them over to the growing, peptide chain. The amino acid is covalently, bound to tRNA at the 3’-end. Each tRNA has a, three nucleotide base sequence—the anticodon,, which is responsible to recognize the codon, (complementary bases) of mRNA for protein, synthesis., In man, there are about 50 different tRNAs, whereas in bacteria around 40 tRNAs are found., Some amino acids (particularly those with, multiple codons) have more than one tRNA., , 5. Energy sources : Both ATP and GTP are, required for the supply of energy in protein, synthesis. Some of the reactions involve the, breakdown of ATP or GTP, respectively, to AMP, and GMP with the liberation of pyrophosphate., Each one of these reactions consumes two high, energy phosphates (equivalent to 2 ATP)., 6. Protein factors : The process of translation, involves a number of protein factors. These are, needed for initiation, elongation and termination, of protein synthesis. The protein factors are, more complex in eukaryotes compared to, prokaryotes., , II. ACTIVATION OF AMINO ACIDS, Amino acids are activated and attached to, tRNAs in a two step reaction. A group of, enzymes—namely aminoacyl tRNA synthetases—, are required for this process. These enzymes are, highly specific for the amino acid and the, corresponding tRNA., The amino acid is first attached to the enzyme, utilizing ATP to form enzyme-AMP-amino acid, complex. The amino acid is then transferred to, the 3’ end of the tRNA to form aminoacyl tRNA, (Fig.25.16)., , III. PROTEIN SYNTHESIS PROPER, The protein or polypeptide synthesis occurs, on the ribosomes (rather polyribosomes). The, mRNA is read in the 5co3c direction and the, polypeptide synthesis proceeds from N-terminal, end to C-terminal end. Translation is directional, and collinear with mRNA.
Page 565 :
555, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , Amino acid, , ATP, Aminoacyl-tRNA, synthetase ( E ), , PPi, E -AMP- Amino acid, AA, 3c, A, C, C, , A, C, C, , 5c, , E, tRNA, , AMP, Aminoacyl, tRNA, , Fig. 25.16 : Formation of aminoacyl tRNA (AA–Amino acid; E–Enzyme)., , The prokaryotic mRNAs are polycistronic,, since a single mRNA has many coding regions, that code for different polypeptides. In contrast,, eukaryotic mRNA is monocistronic, since it, codes for a single polypeptide., In case of prokaryotes, translation commences, before the transcription of the gene is completed., Thus, simultaneous transcription and translation, are possible. This is not so in case of eukaryotic, organisms since transcription occurs in the, nucleus whereas translation takes place in the, cytosol. Further, the primary transcript (hnRNA), formed from DNA has to undergo several, modifications to generate functional mRNA., Protein synthesis is comparatively simple in, case of prokaryotes compared to eukaryotes., Further, many steps in eukaryotic translation, were not understood for quite sometime. For, these reasons, majority of the textbooks earlier, used to describe translation in prokaryotes in, detail, and give most important and relevant, information for eukaryotic translation. With the, advances in molecular biology, the process of, , protein biosynthesis in eukaryotes is better, understood now., , Translation in eukaryotes is briefly described, here, along with some relevant features of, prokaryotic protein biosynthesis. Translation, proper is divided into three stages—initiation,, elongation and termination (as it is done for, transcription)., , INITIATION OF TRANSLATION, The initiation of translation in eukaryotes is, complex, involving at least ten eukaryotic, initiation factors (eIFs). Some of the eIFs contain, multiple (3-8) subunits. The process of translation, initiation can be divided into four steps, (Fig.25.17)., 1. Ribosomal dissociation., 2. Formation of 43S preinitiation complex., 3. Formation of 48S initiation complex., 4. Formation of 80S initiation complex.
Page 567 :
557, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , Ribosomal dissociation, , Formation of 80S initiation complex, , The 80S ribosome dissociates to form 40S and, 60S subunits. Two initiating factors namely eIF3 and eIF-1A bind to the newly formed 40S, subunit, and thereby block its reassociation with, 60S subunit. For this reason, some workers name, eIF-3 as anti-association factor., , 48S initiation complex binds to 60S ribosomal, subunit to form 80S initiation complex. The, binding involves the hydrolysis of GTP (bound to, eIF-2). This step is facilitated by the involvement, of eIF-5., , Formation of 43S preinitiation, complex, A ternary complex containing met-tRNAi and, eIF-2 bound to GTP attaches to 40S ribosomal, subunit to form 43S preinitiation complex. The, presence of eIF-3 and eIF-1A stabilizes this, complex (Note : Met-tRNA is specifically, involved in binding to the initiation condon, AUGs; hence the superscripti is used in mettRNAi)., , Formation of 48S initiation complex, The binding of mRNA to 43S preinitiation, complex results in the formation of 48S initiation, complex through the intermediate 43S initiation, complex. This, however, involves certain, interactions between some of the eIFs and, activation of mRNA., eIF-4F complex is formed by the association, of eIF-4G, eIF-4A with eIF-4E. The so formed, eIF-4F (referred to as cap binding protein) binds, to the cap of mRNA. Then elF-4A and elF-4B, bind to mRNA and reduce its complex structure., This mRNA is then transferred to 43S complex., For the appropriate association of 43S, preinitiation complex with mRNA, energy has to, be supplied by ATP., Recognition of initiation codon : The, ribosomal initiation complex scans the mRNA, for the identification of appropriate initiation, codon. 5c-AUG is the initiation codon and its, recognition is facilitated by a specific sequence, of nucleotides surrounding it. This marker, sequence for the identification of AUG is called, as Kozak consensus sequences. In case of, prokaryotes the recognition sequence of, initiation codon is referred to as Shine- Dalgarno, sequence., , As the 80S complex is formed, the initiation, factors bound to 48S initiation complex are, released, and recycled. The activation of eIF-2, requires eIF-2B (also called as guanine, nucleotide exchange factor) and GTP. The, activated eIF-2 (i.e. bound to GTP) requires eIF2C to form the ternary complex., Regulation of initiation, , The eIF-4F, a complex formed by the, assembly of three initiation factors controls, initiation, and thus the translation process. eIF4E, a component of eIF-4F is primarily, responsible for the recognition of mRNA cap., And this step is the rate-limiting in translation., eIF-2 which is involved in the formation of, 43S preinitiation complex also controls protein, biosynthesis to some extent., , Initiation of translation, in prokaryotes, The formation of translation initiation, complex in prokaryotes is less complicated, compared to eukaryotes. The 30S ribosomal, subunit is bound to initiation factor 3 (IF-3) and, attached to ternary complex of IF-2, formyl mettRNA and GTP. Another initiation factor namely, IF-I also participates in the formation of, preinitiation complex. The recognition of, initiation codon AUG is done through ShineDalgarno sequence. A 50S ribosome unit is now, bound with the 30S unit to produce 70S, initiation complex in prokaryotes., , ELONGATION OF TRANSLATION, Ribosomes elongate the polypeptide chain by, a sequential addition of amino acids. The amino, acid sequence is determined by the order of the, codons in the specific mRNA. Elongation, a, cyclic process involving certain elongation
Page 568 :
558, factors (EFs), may be divided into three steps, (Fig.25.18)., , BIOCHEMISTRY, , 1. Binding of aminoacyl t-RNA to A-site., , In case of prokaryotes, the elongation factors, are different, and they are EF-Tu, EF-Ts (in place, of of EF-1a) and EF-G (instead of EF-2)., , 2. Peptide bond formation., , Incorporation of amino acids, , 3. Translocation., , Binding of aminoacyl—tRNA to, A-site, The 80S initiation complex contains mettRNAi in the P-site, and the A-site is free. Another, aminoacyl-tRNA is placed in the A-site. This, requires proper codon recognition on the mRNA, and the involvement of elongation factor 1a, (EF-Ia) and supply of energy by GTP. As the, aminoacyl-tRNA is placed in the A-site, EF-1D, and GDP are recycled to bring another, aminoacyl-tRNA., , Peptide bond formation, The enzyme peptidyltransferase catalyses the, formation of peptide bond (Fig.25.19). The, activity of this enzyme lies on 28S RNA of 60S, ribosomal subunit. It is therefore the rRNA (and, not protein) referred to as ribozyme that, catalyses the peptide bond formation. As the, amino acid in the aminoacyl-tRNA is already, activated, no additional energy is required for, peptide bond formation., The net result of peptide bond formation is, the attachment of the growing peptide chain to, the tRNA in the A-site., , Translocation, As the peptide bond formation occurs, the, ribosome moves to the next codon of the mRNA, (towards, 3c-end)., This, process, called, translocation, basically involves the movement, of growing peptide chain from A-site to P-site., Translocation requires EF-2 and GTP. GTP gets, hydrolysed and supplies energy to move mRNA., EF-2 and GTP complex recycles for, translocation., In recent years, another site namely exit site, (E-site) has been identified in eukaryotes. The, deacylated tRNA moves into the E-site, from, where it leaves the ribosome., , It is estimated that about six amino acids per, second are incorporated during the course of, elongation of translation in eukaryotes. In case, of prokaryotes, as many as 20 amino acids can, be incorporated per second. Thus the process of, protein/polypeptide synthesis in translation, occurs with great speed and accuracy., , TERMINATION OF TRANSLATION, Termination is a simple process when, compared to initiation and elongation. After, several cycles of elongation, incorporating amino, acids and the formation of the specific protein/, polypeptide molecule, one of the stop or, termination signals (UAA, UAG and UCA), terminates the growing polypeptide. The, termination codons which act as stop signals do, not have specific tRNAs to bind. As the, termination codon occupies the ribosomal, A-site, the release factor namely eRF recognizes, the stop signal. eRF-GTP complex, in association, with the enzyme peptidyltransferase, cleaves the, peptide bond between the polypeptide and the, tRNA occupying P-site. In this reaction, a water, molecule, instead of an amino acid is added., This hydrolysis releases the protein and tRNA, from the P-site. The 80S ribosome dissociates to, form 40S and 60S subunits which are recycled., The mRNA is also released., , INHIBITORS OF PROTEIN, SYNTHESIS, Translation is a complex process and it has, become a favourite target for inhibition by, antibiotics. Antibiotics are the substances, produced by bacteria or fungi which inhibit the, growth of other organisms. Majority of the, antibiotics interfere with the bacterial protein, synthesis and are harmless to higher organisms., This is due to the fact that the process of, translation sufficiently differs between prokaryotes, and eukaryotes. The action of a few important, antibiotics on translation is described next.
Page 570 :
560, , BIOCHEMISTRY, , AA1, AA1 AA 2, HN, , AA 2, , R3 C H, C O, , P-Site, , HN2, , HN2, , R3 C H, , R4 C H, , C O, , C O, , O, , O, , Peptide, bond, , HN, A-Site, , R4 C H, C O, , Peptidyltransferase, , O, , H2 O, , 3c mRNA, , 5c, , 5c, , 3c mRNA, , Ribosome, , Fig. 25.19 : Formation of peptide bond in translation (P–site — Peptidyl tRNA site; A–site — Aminoacyl tRNA site)., , Streptomycin : Initiation of protein synthesis, is inhibited by streptomycin. It causes misreading, of mRNA and interferes with the normal pairing, between codons and anticodons., Tetracycline : It inhibits the binding of, aminoacyl tRNA to the ribosomal complex. In, fact, tetracycline can also block eukaryotic, protein synthesis. This, however, does not, happen since eukaryotic cell membrane is not, permeable to this drug., Puromycin : This has a structural resemblance, to aminoacyl tRNA. Puromycin enters the A site, and gets incorporated into the growing peptide, chain and causes its release. This antibiotic, prevents protein synthesis in both prokaryotes, and eukaryotes., Chloramphenicol : It acts as a competitive, inhibitor of the enzyme peptidyltransferase and, thus interferes with elongation of peptide chain., Erythromycin : It inhibits translocation by, binding with 50S subunit of bacterial ribosome., Diphtheria toxin : It prevents translocation in, eukaryotic protein synthesis by inactivating, elongation factor eEF2., , IV. CHAPERONES AND, PROTEIN FOLDING, The three dimensional conformation of, proteins is important for their biological, functions. Some of the proteins can, spontaneously generate the correct functionally, active conformation e.g. denatured pancreatic, ribonuclease. However, a vast majority, of proteins can attain correct conformation,, only through the assistance of certain, proteins referred to as chaperones. Chaperones, are heat shock proteins (originally discovered, in response to heat shock). They facilitate, and, favour, the, interactions, on, the, polypeptide surfaces to finally give the, specific, conformation, of, a, protein., Chaperones, can, reversibly, bind, to, hydrophobic regions of unfolded proteins and, folding intermediates. They can stabilize, intermediates,, prevent, formation, of, incorrect intermediates, and also prevent, undesirable interactions with other proteins. All, these activities of chaperones help the protein to, attain compact and biologically active, conformation.
Page 571 :
561, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , Types of chaperones, Chaperones are categorized into two major, groups, 1. Hsp70 system : This mainly consists of, Hsp70 (70-kDa heat shock protein) and Hsp40, (40-kDa Hsp). These proteins can bind, individually to the substrate (protein) and help in, the correct formation of protein folding., 2. Chaperonin system : This is a large, oligomeric assembly which forms a structure into, which the folded proteins are inserted. The, chaperonin system mainly has Hsp60 and Hsp10, i.e. 60 kDa Hsp and 10 kDa Hsp. Chaperonins, are required at a later part of the protein folding, process, and often work in association with, Hsp70 system., , Protein misfolding and diseases, The failure of a protein to fold properly, generally leads to its rapid degradation. Cystic, fibrosis (CF) is a common autosomal recessive, disease. Some cases of CF with mutations that, result in altered protein (cystic fibrosis, transmembrane conductance regulator or in, short CFTR) have been reported. Mutated CFTR, cannot fold properly, besides not being able to, , get glycosylated or transported. Therefore, CFTR, gets degraded., Certain neurological diseases which are due, to cellular accumulation of aggregates of, misfolded proteins or their partially degraded, products have been identified. The term prions, (proteinous infectious agents) is used to, collectively represent them., Prions exhibit the characteristics of viral or, microbial pathogens and have been implicated, in many diseases. e.g. mad cow disease,, Creutzfeldt-Jacob disease, Alzheimer’s disease,, Huntington’s disease (Refer Chapter 22)., , V. POST-TRANSLATIONAL, MODIFICATIONS OF PROTEINS, The proteins synthesized in translation are, as, such, not functional. Many changes take place, in the polypeptides after the initiation of their, synthesis or, most frequently, after the protein, synthesis is completed. These modifications, include protein folding (described already),, trimming by proteolytic degradation, intein, splicing and covalent changes which are, collectively, known, as, post-translational, modifications (Fig.25.20)., , + Faulty splicing of hnRNA may result in certain diseaes e.g. E-thalassemia., + Inhibitors of transcription are used as therapeutic agents. Thus, actinomycin D was the, first antibiotic used in the treatment of tumors. Rifampin is employed to treat, tuberculosis and leprosy., , + Retroviruses (RNA is the genetic material) are oncogenic i.e. cause cancers., + Several antibiotics selectively block bacterial translation, and thus inhibit their growth, e.g. streptomycin, tetracycline, puromycin., , + Protein misfolding often results in the formation of prions (proteinous infectious, agents) which have been implicated in many diseases e.g. mad cow disese, Alzheimer’s, disease., , + Lebers’ hereditary optic neuropathy is caused by mutation in mtDNA in males. The, victims become blind due to loss of central vision as a result of neuroretinal, degeneration.
Page 572 :
562, , BIOCHEMISTRY, , Polypeptide, , Folding, , Proteolytic, cleavage, , Pieces of polypeptide, , Intein, splicing, , Chemical (covalent), modifications, , Position of the, removed intein, , New chemical groups, , Tertiary structure, , Fig. 25.20 : An outline of post-translational modifications of proteins., , Proteolytic degradation, Many proteins are synthesized as the, precursors which are much bigger in size than, the functional proteins. Some portions of, precursor molecules are removed by proteolysis, to liberate active proteins. This process is, commonly referred to as trimming. The, formation of insulin from preproinsulin,, conversion of zymogens (inactive digestive, enzymes e.g. trypsinogen) to the active enzymes, are some examples of trimming., , Intein splicing, Inteins are intervening sequences in certain, proteins. These are comparable to introns in, mRNAs. Inteins have to be removed, and exteins, ligated in the appropriate order for the protein to, become active., , Covalent modifications, The proteins synthesized in translation are, subjected to many covalent changes. By these, modifications in the amino acids, the proteins, may be converted to active form or inactive, form., Selected, examples, of, covalent, modifications are described below., 1. Phosphorylation : The hydroxyl group, containing amino acids of proteins, namely, serine, threonine and tyrosine are subjected to, phosphorylation. The phosphorylation may either, increase or decrease the activity of the proteins. A, , group of enzymes called protein kinases catalyse, phosphorylation while protein phosphatases are, responsible for dephosphorylation (removal of, phosphate group). Many enzymes that undergo, phosphorylation or dephosphorylation are known, in metabolisms (e.g. glycogen synthase)., 2. Hydroxylation : During the formation of, collagen, the amino acids proline and lysine are, respectively converted to hydroxyproline and, hydroxylysine. This hydroxylation occurs in the, endoplasmic reticulum and requires vitamin C., 3. Glycosylation : The attachment of carbohydrate moiety is essential for some proteins to, perform, their, functions., The, complex, carbohydrate moiety is attached to the amino, acids, serine and threonine (O-linked) or to, asparagine (N-linked), leading to the synthesis of, glycoproteins., Vitamin K dependent carboxylation of, glutamic acid residues in certain clotting factors, is also a post-translational modification., In the Table 25.2, selected examples of posttranslational modification of proteins through, their amino acids are given., , PROTEIN TARGETING, The eukaryotic proteins (tens of thousands), are distributed between the cytosol, plasma, membrane and a number of cellular organelles
Page 573 :
563, , Chapter 25 : TRANSCRIPTION AND TRANSLATION, , TABLE 25.2 Selected examples of posttranslational modifications of proteins, through their amino acids, , Amino acid, Amino-terminal, amino acid, Carboxy terminal, amino acid, Arginine, Aspartic acid, Cysteine (—SH), , Glutamic acid, Histidine, Lysine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, , Post-translational, modification(s), Glycosylation, acetylation,, myristoylation, formylation., Methylation, ADP-ribosylation, Methylation, Phosphorylation, hydroxylation, Cystine (—S—S—) formation,, selenocysteine formation,, glycosylation., Methylation, J-carboxylation., Methylation, phosphorylation., Acetylation, methylation,, hydroxylation, biotinylation., Sulfoxide formation., Glycosylation, hydroxylation., Hydroxylation, glycosylation., Phosphorylation, glycosylation., Phosphorylation, methylation, glycosylation., Hydroxylation., Hydroxylation, phosphorylation,, sulfonylation, iodination., , (nucleus, mitochondria, endoplasmic reticulum, etc.). At the appropriate places, they perform, their functions., The proteins, synthesized in translation, have, to reach their destination to exhibit their, biological activities. This is carried out by a, process called protein targeting or protein, sorting or protein localization. The proteins, move from one compartment to another by, multiple mechanisms., The protein transport from the endoplasmic, reticulum through the Golgi apparatus, and, beyond uses carrier vesicles. It may be, however,, noted that only the correctly folded proteins are, recognized as the cargo for transport. Protein, targeting and post-translational modifications, occur in a well coordinated manner., , Certain glycoproteins are targeted to reach, lysosomes, as the lysosomal proteins can, recognize the glycosidic compounds e.g., N-acetylglucosamine phosphate., For the transport of secretory proteins, a, special mechanism is operative. A signal peptide, containing 15–35 amino acids, located at the, amino terminal end of the secretory proteins, facilitates the transport., , Protein targeting to mitochondria, Most of the proteins of mitochondria are, synthesized in the cytosol, and their transport to, mitochondria is a complex process. Majority of, the proteins are synthesized as larger preproteins, with N-terminal presequences for the entry of, these proteins into mitochondria. The transport, of unfolded proteins is often facilitated by, chaperones., One protein namely mitochondrial matrix, targeting signal, involved in protein targeting has, been identified. This protein can recognize, mitochondrial receptor and transport certain, proteins from cytosol to mitochondria. This is an, energy-dependent process., , Protein targeting to, other organelles, Specific signals for the transport of proteins to, organelles such as nuclei and peroxisomes have, been identified., The smaller proteins can easily pass through, nuclear pores. However, for larger proteins,, nuclear localization signals are needed to, facilitate their entry into nucleus., , MITOCHONDRIAL DNA,, TRANSCRIPTION AND TRANSLATION, The mitochondrial DNA (mtDNA) has, structural and functional resemblances with, prokaryotic DNA. This fact supports the view, that mitochondria are derivatives of prokaryotes., mtDNA is circular in nature and contains about, 16,000 nucleotide bases.
Page 574 :
564, , BIOCHEMISTRY, , A vast majority of structural and functional, proteins of the mitochondria are synthesized in, the cytosol, under the influence of nuclear DNA., However, certain proteins (around 13), most of, them being the components of electron transport, chain, are synthesized in the mitochondria (e.g., cytochrome b of complex III, two subunits of, ATP synthase). Transcription takes place in the, mitochondria leading to the synthesis of mRNAs,, tRNAs and rRNAs. Two types of rRNA and about, 22 species of tRNA have been so far identified., Transcription is followed by translation resulting, in protein synthesis., The mitochondria of the sperm cell do not, enter the ovum during fertilization, therefore,, , mtDNA is inherited from the mother., Mitochondrial DNA is subjected to high rate of, mutations (about 10 times more than nuclear, DNA) that causes inherited defects in oxidative, phosphorylation. The best known among them, are certain mitochondrial myopathies and, Leber’s hereditary optic neuropathy. The latter is, mostly found in males and is characterized by, blindness due to loss of central vision as a result, of neuroretinal degeneration. Leber’s hereditary, optic neuropathy is a consequence of single, base mutation in mtDNA. Due to this, the amino, acid histidine, in place of arginine, is, incorporated into the enzyme NADH coenzyme, Q reductase., , 1. Transcription is the process in which RNA is synthesized from DNA, which is carried, out in 3 stages–initiation, elongation and termination., 2. In case of prokaryotes, a single enzyme synthesizes all the RNAs. In eukaryotes, RNA, polymerase I, II and III respectively catalyse the formation of rRNAs, mRNAs and, tRNAs., 3. The primary mRNA transcript (i.e. hnRNA) undergoes post-transcriptional modifications, e.g. base modifications, splicing etc., 4. Reverse transcription is the process of synthesizing DNA from RNA by the enzyme, reverse transcriptase., 5. Biosynthesis of a protein or a polypeptide is known as translation. The amino acid, sequence of a protein is determined by the triplet nucleoside base sequences of mRNA,, arranged as codons., 6. The genetic code (codons)–composed of A, G, C and U–is universal, specific, nonoverlapping and degenerate. Of the 64 codons, three (UAA, UAG, UGA) are termination, codons while the rest code for amino acids., 7. Ribosomes are the factories of protein biosynthesis. Translation involves activation of, amino acids, protein synthesis proper (initiation, elongation and termination), protein, folding and post-translational modifications., 8. The post-translational modifications include proteolytic degradation, intein splicing and, covalent modifications (phosphorylation, hydroxylation, glycosylation etc.). These, modifications are required to make the proteins biologically active., 9. The proteins synthesized in translation reach the destination to exhibit their biological, activity. This is carried out by a process called protein targeting or protein sorting., 10. The mitochondria possess independent DNA with the machinery for transcription and, translation. However, only a few proteins (around 13) are actually synthesized in the, mitochondria.
Page 575 :
Chapter 25 : TRANSCRIPTION AND TRANSLATION, , 565, , I. Essay questions, 1. Give an account of transcription. Compare the RNA synthesis between prokaryotes and, eukaryotes., 2. Describe protein biosynthesis (translation)., 3. Discuss the inhibitors of transcription and translation., 4. Give an account of post-transcriptional and post-translational modifications., 5. What is genetic code? Describe the characteristics of genetic code. Add a note on the effects, of mutations on genetic code., , II. Short notes, (a) Genome, (b) Heterogeneous nuclear RNA (hnRNA), (c) Eukaryotic RNA polymerases, (d) Introns, and exons, (e) Reverse transcription, (f) Wobble hypothesis, (g) Anticodon, (h) Shine-Dalgarno, sequence, (i) Peptidyltransferase, (j) Chaperones, (k) Protein targeting., , III. Fill in the blanks, 1. The total DNA (genetic information) contained in an organism (or a cell) is referred to as, , ______________., 2. The primary transcript produced by RNA polymerase II is eukaryotes ______________., 3. The intervening nucleotide sequences in mRNA that do not code for proteins ______________., 4. The synthesis of complementary DNA (cDNA) from mRNA is catalysed by the enzyme, ______________., 5. A single tRNA is capable of recognizing more than one codon, and this phenomenon is referred, to as ______________., 6. The factories for protein biosynthesis are ______________., 7. The enzyme peptidyltransferase calalyses the formation of peptide bond during translation. The, chemical nature of this enzyme is ______________., 8. The proteins that facilitate the formation of specific conformation of proteins are, ______________., 9. The common term used for the diseases due to misfolding of proteins _______., 10. The process of delivery of proteins in a cell to the site their biological activity is _______., , IV. Multiple choice questions, 11. The codon(s) that terminate(s) protein biosynthesis, (a) UAA (b) UAG (c) UGA (d) All of them., 12. The nitrogenous base that is never found in the genetic code, (a) Adenine (b) Guanine (c) Thymine (d) Cytosine., 13. The total DNA (genetic information) contained in a living cell (or organism) is regarded as, (a) Genome (b) Transciptome (c) Proteome (d) Gene., 14. The enzyme responsible for the synthesis of mRNAs in eukaryotic cells, (a) RNA polymerase I (b) RNA polymerase II (c) RNA polymerase III (d) RNA polymerase D., 15. Mitochondrial DNA is inherited from, (a) Mother only (b) Father only (c) Both of them (d) Either mother or father.
Page 576 :
Section 5, , Molecular Biology and Biotechnology, , Chapter, , Regulation of Gene Expression, , 26, , The genes speak :, , “Functional units of DNA, we are;, Ultimate for all cellular activities;, Tailored to express as per tissue demands;, Mystery of our molecular actions await unfolding.”, , D, , NA, the chemical vehicle of heredity, is, composed of functional units, namely, genes. The term genome refers to the total, genetic information contained in a cell. The, bacterium Escherichia coli contains about 4,400, genes present on a single chromosome. The, genome of humans is more complex, with 23, pairs of (diploid) chromosomes containing, 6 billion (6 u 109) base pairs of DNA, with, an estimated 30,000–40,000 genes. At any, given time, only a fraction of the genome is, expressed., The living cells possess a remarkable property, to adapt to changes in the environment by, regulating the gene expression. For instance,, insulin is synthesized by specialized cells of, pancreas and not by cells of other organs (say, kidney, liver), although the nuclei of all the cells, of the body contain the insulin genes. Molecular, regulatory mechanisms facilitate the expression, of insulin gene in pancreas, while preventing its, expression in other cells., , GENE REGULATION—GENERAL, The regulation of the expression of genes is, absolutely, essential, for, the, growth,, development, differentiation and the very, existence of an organism. There are two types of, gene regulation-positive and negative., 1. Positive regulation : The gene regulation, is said to be positive when its expression is, increased by a regulatory element (positive, regulator)., 2. Negative regulation : A decrease in the, gene expression due to the presence of a, regulatory element (negative regulator) is referred, to as negative regulation., It may be noted here that double negative, effect on gene regulation results in a positive, phenomenon., , Constitutive and inducible genes, The genes are generally considered under two, categories., , 566
Page 577 :
567, , Chapter 26 : REGULATION OF GENE EXPRESSION, , 1. Constitutive, genes :, The, products, (proteins) of these genes are required all the time, in a cell. Therefore, the constitutive genes (or, housekeeping genes) are expressed at more or, less constant rate in almost all the cells and,, further, they are not subjected to regulation e.g., the enzymes of citric acid cycle., 2. Inducible genes : The concentration of the, proteins synthesized by inducible genes is, regulated by various molecular signals. An, inducer increases the expression of these genes, while a repressor decreases, e.g. tryptophan, pyrrolase of liver is induced by tryptophan., The term pseudogenes is used to represent, DNA sequences that have significant homology, to a functional gene, but they cannot express, due to mutations. Thus pseudogenes are nonfunctional. However, they significantly increase, the size of the eukaryotic genome without any, contribution to the expression of genes., , One cistron-one subunit concept, The chemical product of a gene expression is, a protein which may be an enzyme. It was, originally believed that each gene codes for a, specific enzyme, leading to the popular concept,, one gene-one enzyme. This however, is not, necessarily valid due to the fact that several, enzymes (or proteins) are composed of two or, more nonidentical subunits (polypeptide chains)., The cistron is the smallest unit of genetic, expression. It is the fragment of DNA coding for, the subunit of a protein molecule. The original, concept of one gene-one enzyme is replaced by, one cistron-one subunit., , Models to study gene expression, Elucidation of the regulation of gene, expression in prokaryotes has largely helped to, understand the principles of the flow of, information from genes to mRNA to synthesize, specific proteins. Some important features of, prokaryotic gene expression are described first., This is followed by a brief account of eukaryotic, gene expression., , THE OPERON CONCEPT, The operon is the coordinated unit of genetic, expression in bacteria. The concept of operon, was introduced by Jacob and Monod in 1961, (Nobel Prize 1965), based on their observations, on the regulation of lactose metabolism in, E. coli. This is popularly known as lac operon., , LACTOSE (LAC) OPERON, Structure of lac operon, The lac operon (Fig.26.1) consists of a, regulatory gene (I; I for inhibition), operator gene, (O) and three structural genes (Z, Y, A). Besides, these genes, there is a promoter site (P), next to, the operator gene, where the enzyme RNA, polymerase binds. The structural genes Z, Y, and A respectively, code for the enzymes, E-galactosidase, galactoside permease and, galactoside acetylase. E-Galactosidase hydrolyses, lactose (E-galactoside) to galactose and glucose, while permease is responsible for the transport of, lactose into the cell. The function of acetylase, (coded by A gene) remains a mystery., The structural genes Z, Y and A transcribe, into a single large mRNA with 3 independent, translation units for the synthesis of 3 distinct, enzymes. An mRNA coding for more than one, protein is known as polycistronic mRNA., Prokaryotic organisms contain a large number of, polycistronic mRNAs., , Repression of lac operon, The regulatory gene (I) is constitutive. It is, expressed at a constant rate leading to the, synthesis of lac repressor. Lac repressor is a, tetrameric (4 subunits) regulatory protein (total, mol. wt. 150,000) which specifically binds to, the operator gene (O). This prevents the binding, of the enzyme RNA polymerase to the promoter, site (P), thereby blocking the transcription of, structural genes (Z, Y and A). This is what, happens in the absence of lactose in E. coli. The, repressor molecule acts as a negative regulator, of gene expression.
Page 578 :
568, , BIOCHEMISTRY, , Regulatory, gene, , Promoter, site, , (A), , I, , P, , (B), , I, , P, , Operator, gene, O, , Structural genes, , Z, , Y, , A, , Z, , Y, , A, , Z, , Y, , A, , mRNA, , Repressor, tetramer, , Repressor, subunits, CAP-cAMP, , (C), , I, , P, RNAP, , Polycistronic mRNA, , mRNA, , E-Galactosidase Permease, , Acetylase, , Lactose, , Inactive, repressor, , Fig. 26.1 : Model of lactose operon in E.coli (A) Structure of lac operon (B) Repression of lac operon, (C) Derepression of lac operon. (CAP—cAMP–catabolite gene activator, protein bound to cAMP; RNAP–RNA polymerase)., , Derepression of lac operon, In the presence of lactose (inducer) in the, medium, a small amount of it can enter the, E. coli cells. The repressor molecules have a high, affinity for lactose. The lactose molecules bind, and induce a conformational change in the, repressor. The result is that the repressor gets, , inactivated and, therefore, cannot bind to the, operator gene (O). The RNA polymerase attaches, to the DNA at the promoter site and transcription, proceeds, leading to the formation of, polycistronic mRNA (for genes Z, Y and A) and,, finally, the 3 enzymes. Thus, lactose induces the, synthesis of the three enzymes E-galactosidase,
Page 579 :
569, , Chapter 26 : REGULATION OF GENE EXPRESSION, , galactoside permease and galactoside acetylase., Lactose acts by inactivating the repressor, molecules, hence this process is known as, derepression of lac operon., Gratuitous inducers : There are certain, structural analogs of lactose which can induce, the lac operon but are not the substrates for the, enzyme E-galactosidase. Such substances are, known as gratuitous inducers. Isopropylthiogalactoside (IPTG) is a gratuitous inducer,, extensively used for the study of lac operon., The catabolite gene activator protein : The, cells of E. coli utilize glucose in preference to, lactose; when both of them are present in the, medium. After the depletion of glucose in the, medium, utilization of lactose starts. This, indicates that glucose somehow interferes with, the induction of lac operon. This is explained as, follows., The attachment of RNA polymerase to the, promoter site requires the presence of a, catabolite gene activator protein (CAP) bound to, cyclic AMP (Fig.26.2). The presence of glucose, lowers the intracellular concentration of cAMP, by inactivating the enzyme adenylyl cyclase, responsible for the synthesis of cAMP. Due to the, diminished levels of cAMP, the formation of CAPcAMP is low. Therefore, the binding of RNA, polymerase to DNA (due to the absence of CAPcAMP) and the transcription are almost negligible, in the presence of glucose. Thus, glucose, interferes with the expression of lac operon by, depleting cAMP levels. Addition of exogenous, cAMP is found to initiate the transcription of, many inducible operons, including lac operon., , Glucose, , brane, , Mem, , Out, side, Insi, de, , Glucose, , Adenylyl cyclase, , ATP, , cAMP ( ), , CAP, ( ), CAP–cAMP (, , I, , P, , ), , O, Z, lac operon, , Y, , A, , Polycistronic mRNA, , Fig. 26.2 : Control of lac operon by catabolite gene, activator protein (CAP) and the role of glucose., , contain tryptophan. If tryptophan is not present, in the medium in adequate quantity, the, bacterial cell has to make it, as it is required for, the growth of the bacteria., The tryptophan operon of E. coli is depicted, in Fig.26.3. This operon contains five structural, genes (trpE, trpD, trpC, trpB, trpA), and the, regulatory elements—primary promoter (trpP),, operator (trpO), attenuator (trpa), secondary, internal promoter (TrpP2), and terminator (trpt)., The five structural genes of tryptophan operon, code for three enzymes (two enzymes contain, two different subunits) required for the synthesis, of tryptophan from chorismate., , It is now clear that the presence of CAP-cAMP, is essential for the transcription of structural, genes of lac operon. Thus, CAP-cAMP acts as a, positive regulator for the gene expression. It is,, therefore, evident that lac operon is subjected to, both positive (by repressor, described above) and, negative regulation., , The tryptophan repressor is always turned on,, unless it is repressed by a specific molecule called, corepressor. Thus lactose operon (described, already) is inducible, whereas tryptophan operon, is repressible. The tryptophan operon is said to be, derepressed when it is actively transcribed., , TRYPTOPHAN OPERON, , Tryptophan acts as a corepressor to shut down, the synthesis of enzymes from tryptophan, operon. This is brought out in association with a, specific protein, namely tryptophan repressor., , Tryptophan is an aromatic amino acid, and is, required for the synthesis of all proteins that, , Tryptophan operon regulation, by a repressor
Page 581 :
571, , Chapter 26 : REGULATION OF GENE EXPRESSION, , GENE EXPRESSION, IN EUKARYOTES, Each cell of the higher organism contains the, entire genome. As in prokaryotes, gene, expression in eukaryotes is regulated to provide, the appropriate response to biological needs., This may occur in the following ways, l, , l, , l, , Expression of certain genes (housekeeping, genes) in most of the cells., Activation of selected genes upon demand., Permanent inactivation of several genes in all, but a few types., , In case of prokaryotic cells, most of the DNA, is organized into genes which can be, transcribed. In contrast, in mammals, very little, of the total DNA is organized into genes and, their associated regulatory sequences. The, function of the bulk of the extra DNA is not, known., Eukaryotic gene expression and its regulation, are highly complex. Some of the important, aspects are briefly described., , CHROMATIN SRUCTURE, AND GENE EXPRESSION, The DNA in higher organisms is extensively, folded and packed to form protein-DNA, complex called chromatin. The structural, organization of DNA in the form of chromatin, plays an important role in eukaryotic gene, expression. In fact, chromatin structure, provides an additional level of control of gene, expression., A selected list of genes (represented by the, products) along with the respective chromosomes, on which they are located is given in Table 26.1., In general, the genes that are transcribed, within a particular cell are less condensed and, more open in structure. This is in contrast to, genes that are not transcribed which form highly, condensed chromatin., , Histone acetylation and deacetylation, Eukaryotic DNA segments are wrapped, around histone proteins to form nucleosome., , TABLE 26.1 A selected list of genes, (represented by the products) along with, respective chromosomes, Genes, , Chromosome, number, , Alkaline phosphatase, , 1, , Apolipoprotein B, , 2, , Transferrin, , 3, , Alcohol dehydrogenase, , 4, , HMG CoA reductase, , 5, , Steroid 21-hydroxylase, , 6, , Arginase, , 7, , Carbonic anhydrase, , 8, , Interferon, , 9, , Parathyroid hormone, , 11, , Glyceraldehyde 3-phosphate dehydrogenase, , 12, , Adenosine deaminase, , 13, , D1-Antitrypsin, , 14, , Cytochrome P450, , 15, , Hemoglobin D-chain, , 16, , Growth hormone, , 17, , Prealbumin, , 18, , Creatine phosphokinase (M chain), , 19, , Adenosine deaminase, , 20, , Superoxide dismutase, , 21, , Immunoglobulin (O chain), , 22, , Glucose 6-phosphate dehydrogenase, , X, , Steroid sulfatase, , Y, , Acetylation or deacetylation of histones is an, important factor in determining the gene, expression. In general, acetylation of histones, leads to activation of gene expression while, deacetylation reverses the effect., Acetylation predominantly occurs on the, lysine residues in the amino terminal ends of, histones. This modification in histones reduces, the positive charges of terminal ends (tails), and, decreases their binding affinity to negatively, charged DNA. Consequently, nucleosome, structure is disrupted to allow transcription.
Page 582 :
572, , Methylation of DNA, and inactivation of genes, Cytosine in the sequence CG of DNA gets, methylated to form 5c-methylcytosine. A major, portion of CG sequences (about 20%) in human, DNA exists in methylated form. In general,, methylation leads to loss of transcriptional, activity, and thus inactivation of genes. This, occurs due to binding of methylcytosine binding, proteins to methylated DNA. As a result,, methylated DNA is not exposed and bound to, transcription factors. It is interesting to note that, methylation of DNA correlates with deacetylation, of histones. This provides a double means for, repression of genes., The activation and normal expression of, genes, and gene inactivation by DNA, methylation are depicted in Fig.26.4., , BIOCHEMISTRY, , In the illustration given in the Fig.26.5, gene, I is activated by a combination of activators 1, 2, and 3. Gene II is more effectively activated by, the combined action of 1, 3 and 4. Activator 4, is not in direct contact with DNA, but it forms a, bridge between activators 1 and 3, and activates, gene II. As regards gene III, it gets inactivated by, a combination of 1, 5 and 3. In this case, protein, 5 interferes with the binding of protein 2 with, the DNA, and inactivates the gene., , MOTIFS IN PROTEINS, AND GENE EXPRESSION, A motif literally means a dominant element., Certain motifs in proteins mediate the binding of, regulatory proteins (transcription factors) to, (A), , Transcription, start site, , ENHANCERS AND TISSUE-SPECIFIC, GENE EXPRESSION, , Polymerase II, binding site, DNA binding proteins, , Enhancers (or activators) are DNA elements, that facilitate or enhance gene expression. The, enhancers provide binding sites for specific, proteins that regulate transcription. They, facilitate binding of the transcription complex to, promoter regions., Some of the enhancers possess the ability to, promote transcription in a tissue-specific manner., For instance, gene expression in lymphoid cells, for the production immunoglobulins (Ig) is, promoted by the enhancer associated with Ig, genes between J and C regions., Transgenic animals are frequently used for the, study of tissue-specific expression. The available, evidence from various studies indicates that the, tissue-specific gene expression is largely, mediated through the involvement of enhancers., , Gene activation and expression, (B), , DNA methylation, CH3 CH3 CH3, , CH3 CH3 CH3 CH3, , Methylcytosine, binding proteins, CH3 CH3 CH3, , CH3 CH3 CH3 CH3, , COMBINATION OF DNA ELEMENTS, AND PROTEINS IN GENE EXPRESSION, Gene expression in mammals is a, complicated process with several environmental, stimuli on a single gene. The ultimate response, of the gene which may be positive or negative is, brought out by the association of DNA elements, and proteins., , Gene inactivation and no expression, , Fig. 26.4 : Methylation of DNA and inactivation of genes, (A) Gene activation in the absence of DNA methylation, (B) Gene inactivation due to methylation, (, , represent CG sequences).
Page 583 :
573, , Chapter 26 : REGULATION OF GENE EXPRESSION, , 3, , 2, Gene I, , 1, (A), , Gene activated, 1, (B), , 4, , 3, Gene II, , Gene activated, 2, 1, (C), , 5, , A great majority of specific protein-DNA, interactions are brought out by four unique, motifs—helix-turn-helix (HTH), zinc finger,, leucine zipper, helix-loop-helix (HLH)., These amino acid motifs bind with high, affinity to the specific site and low affinity to, other parts of DNA. The motif-DNA interactions, are maintained by hydrogen bonds and van der, Waals forces., , Helix-turn-helix motif, 3, , Gene III, , Gene inactivated, , Fig. 26.5 : A diagrammatic representation of the, association of DNA elements and proteins in gene, regulation. A, B and C represent genes I, II and III, (1…5 represent proteins)., , DNA. The specific control of transcription occurs, by the binding of regulatory proteins with high, affinity to the correct regions of DNA., , The helix-turn-helix (HTH) motif is about 20, amino acids which represents a small part of a, large protein. HTH is the domain part of the, protein which specifically interacts with the, DNA (Fig.26.6A). Examples of helix-turn-helix, motif proteins include lactose repressor, and, cyclic AMP catabolite activator protein (CAP) of, E. coli, and several developmentally important, transcription factors in mammals., , Zinc finger motif, Sometime ago, it was recognized that the, transcription factor TFIIIA requires zinc for its, , + Regulation of gene expression to adapt to the changes in the environment is a, remarkable property of living cells e.g. synthesis of insulin by E-cells of pancreas and, nowhere else., , + The growth, development and differentiation of an organism involves complex, mechanisms which ultimately depend on gene regulation., , + The house-keeping genes or constitutive genes are expressed at almost a constant rate, in the cells, and they are not usually subjected to regulations e.g. enzymes of Krebs, cycle., , + The malignant cells develop drug resistance to long term administration of, methotrexate. This occurs by amplification of the genes coding for dihydrofolate, reductase., , + The human body has the capability to produce around 10 billion antigen-specific, immunoglobulins. This is achieved by a process called gene rearrangement., , + Knowledge on the gene expression and its regulation helps in the understanding and, control of several diseases, including cancer.
Page 584 :
574, , BIOCHEMISTRY, , The zinc fingers bind to the major groove of, DNA, and lie on the face of the DNA. This, binding makes a contact with 5 bp of DNA. The, steroid hormone receptor transcription factors, use zinc finger motifs to bind to DNA., , Leucine zipper motif, (A) Helix-turn-helix, C, , C, , C, , Zn, C, , H, Zn, , C, , (B) Zinc finger (Cys-Cys), , C, , H, , Zinc finger (Cys-His), , The basic regions of leucine zipper (bZIP), proteins are rich is the amino acid leucine. There, occurs a periodic repeat of leucine residues at, every seventh position. This type of repeat, structure allows two identical monomers or, heterodimers to zip together and form a dimeric, complex. This protein-protein complex associates, and interacts with DNA (Fig.26.6C). Good, examples of leucine zipper proteins are the, enhancer binding proteins (EBP)—fos and jun., , Helix-loop-helix motif, Two amphipathic (literally means a feeling of, closeness) D-helical segments of proteins can, form helix-loop-helix motif and bind to DNA., The dimeric form of the protein actually binds to, DNA (Fig.26.6D)., (C) Leucine zipper, , GENE REGULATION, IN EUKARYOTES, The important features of eukaryotic gene, expression along with the regulatory aspects are, described in the preceeding pages. Besides, transcription, eukaryotic cells also employ, variety of other mechanisms to regulate gene, expression. The most important ones are listed, below, and briefly described next., , (D) Helix-loop-helix, , Fig. 26.6 : A diagrammatic representation of common, motifs in proteins interacting with DNA., , activity. On analysis, it was revealed that each, TFIIIA contains zinc ions as a repeating, coordinated complex. This complex is formed by, the closely spaced amino acids cysteine and, cysteine, followed by a histidine—histidine pair., In some instances, His-His is replaced by a, second Cys-Cys pair (Fig.26.6B)., , 1. Gene amplification, 2. Gene rearrangement, 3. Processing of RNA, 4. Alternate mRNA splicing, 5. Transport of mRNA from nucleus to cytoplasm, 6. Degradation of mRNA., , Gene amplification, In this mechanism, the expression of a gene is, increased several fold. This is commonly, observed during the developmental stages of, eukaryotic organisms. For instance, in fruit fly
Page 585 :
575, , Chapter 26 : REGULATION OF GENE EXPRESSION, , estimated that the human body can produce, about 10 billion (1010) antibodies in response to, antigen stimulations. The molecular mechanism, of this antibody diversity was not understood for, long. It is now explained on the basis of gene, rearrangement or transposition of genes or, somatic recombination of DNA., , Fig. 26.7 : A diagrammatic representation of, gene amplification (the genes are depicted in, blue and red colours)., , (Drosophila), the amplification of genes coding, for egg shell proteins is observed during the, course of oogenesis. The amplification of the, gene (DNA) can be observed under electron, microscope (Fig.26.7)., The occurrence of gene amplification has also, been reported in humans. Methotrexate is an, anticancer drug which inhibits the enzyme, dihydrofolate reductase. The malignant cells, develop drug resistance to long term, administration of methotrexate by amplifying the, genes coding for dihydrofolate reductase., , Gene rearrangment, The body possesses an enormous capacity to, synthesize a wide range of antibodies. It is, , V (500), , J (6), , The structure of a typical immunoglobulin, molecule consists of two light (L) and two heavy, (H) chains. Each one of these chains (L or H), contains an N-terminal variable (V) and, C-terminal constant (C) regions (Refer Fig.9.3)., The V regions of immunoglobulins are, responsible for the recognition of antigens. The, phenomenon of gene rearrangement can be, understood from the mechanism of the synthesis, of light chains of immunoglobulins (Fig.26.8)., Each light chain can be synthesized by three, distinct DNA segments, namely the variable (VL),, the joining (JL) and the constant (CL). The, mammalian genome contains about 500 VL, segments, 6 JL segments and 20 CL segments., During the course of differentiation of, B-lymphocytes, one VL segment (out of the 500), is brought closer to JL and CL segments. This, occurs on the same chromosome. For the sake of, illustration, 100th VL, 3rd JL and 10th CL segments, are rearranged in Fig.26.8. The rearranged DNA, (with VL, JL and CL fragments) is then transcribed, to produce a single mRNA for the synthesis of a, specific light chain of the antibody. By, , C (20), Original DNA, , Rearranged DNA, V100, , J3 C10, Primary transcript, , mRNA, , Protein, (light chain of Ig), , Fig. 26.8 : A diagrammatic representation of gene rearrangement for the synthesis of light chain of immunoglobulin.
Page 586 :
576, , BIOCHEMISTRY, , innumerable combinations of VL, JL and CL, segments, the body’s immune system can, generate, millions, of, antigen, specific, immunoglobulin molecules., The formation of heavy (H) chains of, immunoglobulins also occurs by rearrangement, of 4 distinct genes—variable (VH), diversity (D),, joining (JH) and constant (CH)., , Cap— 5c–NCS, , Coding region, , 3c–NCS —A—A—(A)n, , Stem loop, structure, Stem loop, structure, , AU, rich region, , Fig. 26.9 : A diagrammatic representation of a typical, eukaryotic mRNA. (NCS-Non-coding sequences), , Processing of RNA, The RNA synthesized in transcription, undergoes modifications resulting in a functional, RNA. The changes include intron-exon splicing,, polyadenylation etc. (Chapter 25)., , Alternate mRNA splicing, Eukaryotic cells are capable of carrying out, alternate mRNA processing to control gene, expression. Different mRNAs can be produced, by alternate splicing which code for different, proteins (for more details, Refer Chapter 25)., , Degradation of mRNA, The expression of genes is indirectly, influenced by the stability of mRNA. Certain, hormones regulate the synthesis and degradation, of some mRNAs. For instance, estradiol prolongs, the half-life of vitellogenin mRNA from a few, hours to about 200 hours., It appears that the ends of mRNA molecules, determine the stability of mRNA. A typical, eukaryotic mRNA has 5c-non-coding sequences, (5c-NCS), a coding region and a 3c-NCS. All the, mRNAs are capped at the 5c end, and most of, them have a polyadenylate sequence at the, 3c end (Fig.26.9). The 5c cap and poly (A) tail, protect the mRNA against the attack by, exonuclease. Further, stem-loop structures in, NCS regions, and AU rich regions in the 3c NCS, also provide stability to mRNA., , EPIGENETIC REGULATION, OF GENE EXPRESSION, The term epigenetics is used to describe the, changes in the characteristics of a cell or an, organism that are not due to changes in the, nucleotide sequence of the DNA., , Epigenetics regulates gene expression by, modulating chromatin structure via histone, modification or modification of DNA via, methylation. For instance, acetylation of histones, leads to activation of gene expression, while, DNA methylation is associated with a reduction, in gene expression., The pattern of covalent modifications in, histones that provide a tunable means of, regulating gene expression is referred to as, histone code., Although there is no change in DNA, sequence, epigenetics is heritable due to, coordination between histone modification and, methylation of DNA., , Epigenetic therapy of cancers, Hypermethylation of DNA in some parts of, tumor suppressor genes is found in certain, cancers. The enzyme DNA methyltransferase, (DNMT) responsible for DNA methylation is, targeted for cancer therapy. Many inhibitors of, DNMT (e.g. 5-azacytidine) have been approved, by FDA, and are in use for the treatment of, leukemia., Likewise, inhibitors of histone deacetylase, (HDAC) are also employed in the epigenetic, therapy cancers. HDAC inhibitors stimulate, tumor suppressor gene expression by allowing, acetylation of histones in chromatin structure., FDA has aproved the use of vorinostat for the, treatment of T-cell lymphoma., Epigenetic therapy of cancer by employing, various inhibitors (individually or in combination), holds a great promise.
Page 587 :
Chapter 26 : REGULATION OF GENE EXPRESSION, , 577, , 1. DNA, the chemical vehicle of heredity, is composed of genes. The regulation of gene, expression is absolutely essential for the growth, development and differentiation of an, organism. A positive regulation increases gene expression while a negative regulation, decreases., 2. The operon is the coordinated unit of gene expression. The lac operon of E. coli consists, of regulatory genes and structural genes. The lac repressor binds to the DNA and halts, the process of transcription of structural genes. However, the presence of lactose, inactivates the repressor (derepression) leading to the expression of structural genes., 3. Tryptophan operon is regulated by a repressor. Tryptophan repressor binds to, tryptophan, and then to trp operator gene to turn off the transcription., 4. Eukaryotic gene expression and its regulation are highly complex. Acetylation of, histones leads to gene expression while deacetylation reverses the effect. In general,, methylation of DNA results in the inactivation of genes., 5. The protein-DNA interactions, brought out by motifs (helix-turn-helix, zinc finger,, leucine zipper, helix-loop-helix), are involved in the control of gene expression., 6. Eukaryotic cells have developed several mechanisms to regulate gene expression. These, include gene amplification, gene rearrangement, and processing, transport and, degradation of DNA., , I. Essay questions, 1. Describe lactose (lac) operon., 2. Write briefly on the gene expression and its regulation in eukaryotes., , II. Short notes, (a) One cistron-one subunit concept, (b) Catabolite gene activator protein, (c) Gene inactivation by, DNA methylation, (d) Zinc finger motif, (e) Gene amplification., , III. Fill in the blanks, 1. The number of genes found in human genome ______________., 2. The earlier concept of one gene-one enzyme is replaced by ______________., 3. The chromatin in higher organisms is chemically composed of ______________., , IV. Multiple choice questions, 4. The structural ‘Z’ gene of lactose (lac) operon is responsible for the synthesis of the enzyme(s), (a) E-Galactosidase (b) Permease (c) Acetylase (d) All of them., 5. Methylation of DNA results in, (a) Activation of genes (b) Inactivation of genes (c) No effect on genes (d) Inactivation of protein, motifs., 6. The specific control of transcription involves the following motif(s), (a) Helix-turn-helix (b) Zinc finger (c) Leucine zipper (d) All of them.
Page 588 :
Section 5, , Molecular Biology and Biotechnology, , Chapter, , Recombinant DNA and, Biotechnology, , 27, , The recombinant DNA speaks :, , “I am the hybridized DNA molecule;, Created by cutting and sealing;, When introduced into host cells;, I multiply and code for desired proteins.”, , T, , he term biotechnology represents a fusion or, an alliance between biology and technology., Frankly speaking, biotechnology is a newly, discovered discipline for age-old practices e.g., preparation of wine, beer, curd, bread. These, natural processes are regarded as old or, traditional biotechnology., The new or modern biotechnology embraces, all the genetic manipulations, cell fusion, techniques, and improvements made in the old, biotechnological processes. The biotechnology, with particular reference to recombinant DNA in, human health and disease is briefly described in, this chapter., Genetic engineering primarily involves the, manipulation of genetic material (DNA) to, achieve the desired goal in a pre-determined, way. Some other terms are also in common use, to describe genetic engineering., l, , Gene manipulation, , l, , Recombinant DNA (rDNA) technology, , l, , Gene cloning (molecular cloning), , l, , Genetic modifications, , l, , New genetics., , Brief history of recombinant, DNA technology, The present day DNA technology has its roots, in the experiments performed by Boyer and, Cohen in 1973. In their experiments, they, successfully recombined two plasmids (pSC 101, and pSC 102) and cloned the new plasmid in, E.coli. In the later experiments the genes of a frog, could be successfully transplanted, and expressed, in E.coli. This made the real beginning of modern, rDNA technology and laid foundations for the, present day molecular biotechnology., Some biotechnologists who admire BoyerCohen experiments divide the subject into two, chronological categories., 1. BBC-biotechnology, Cohen., , Before, , Boyer, , and, , 2. ABC-biotechnology, Cohen., , After, , Boyer, , and, , 578
Page 589 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , 579, , 1. Generation of DNA fragments and, selection of the desired piece of DNA (e.g. a, human gene)., Plasmid DNA, Donor DNA, , Restriction, endonuclease, , Restriction, endonuclease, , 2. Insertion of the selected DNA into a cloning, vector (e.g. a plasmid) to create a recombinant, DNA or chimeric DNA (Chimera is a monster in, Greek mythology that has a lion’s head, a goat’s, body and a serpent’s tail. This may be, comparable to Narasimha in Indian mythology)., 3. Introduction of the recombinant vectors, into host cells (e.g. bacteria)., , Desired DNA piece, Cut plasmid DNA, Ligase, , 4. Multiplication and selection of clones, containing the recombinant molecules., 5. Expression of the gene to produce the, desired product., Recombinant DNA technology with special, reference to the following aspects is described, , Recombinant DNA, Introduce into, host cells, , Multiplication, Selection of clones, , Protein, encoded by, cloned gene, , Fig. 27.1 : The basic principle of recombinant DNA, technology., , Recombinant DNA technology is a vast field., The basic principles and techniques of rDNA, technology along with the most important, applications are briefly described in this chapter., , BASIC PRINCIPLES, OF rDNA TECHNOLOGY, There are many diverse and complex, techniques involved in gene manipulation., However, the basic principles of recombinant, DNA technology are reasonably simple, and, broadly involve the following stages (Fig.27.1)., , l, , Molecular tools of genetic engineering., , l, , Host cells-the factories of cloning., , l, , Vectors-the cloning vehicles., , l, , Methods of gene transfer., , l, , Gene cloning strategies., , MOLECULAR TOOLS OF, GENETIC ENGINEERING, The term genetic engineer may be appropriate, for an individual who is involved in genetic, manipulations. The genetic engineer’s toolkit or, molecular tools namely the enzymes most, commonly, used, in, recombinant, DNA, experiments are briefly described., , Restriction endonucleases—, DNA cutting enzymes, Restriction endonucleases are one of the most, important groups of enzymes for the, manipulation of DNA. These are the bacterial, enzymes that can cut/split DNA (from any, source) at specific sites. They were first, discovered in E.coli restricting the replication of, bacteriophages, by cutting the viral DNA (The, host E.coli DNA is protected from cleavage by, addition of methyl groups). Thus, the enzymes, that restrict the viral replication are known as, restriction enzymes or restriction endonucleases.
Page 590 :
580, , BIOCHEMISTRY, , Nomenclature : Restriction endonucleases are, named by a standard procedure, with particular, reference to the bacteria from which they are, isolated. The first letter (in italics) of the enzymes, indicates the genus name, followed by the first, two letters (also in italics) of the species, then, comes the strain of the organism and finally a, Roman numeral indicating the order of discovery., A couple of examples are given below., , EcoRI is from Escherichia (E) coli (co), strain, Ry13 (R), and first endonuclease (I) to be, discovered. HindIII is from Haemophilus (H), influenzae (in), strain Rd (d) and, the third, endonucleases (III) to be discovered., Recognition sequences : Recognition sequence, is the site where the DNA is cut by a restriction, endonuclease. Restriction endonucleases can, , specifically recognize DNA with a particular, sequence of 4-8 nucleotides and cleave., Cleavage patterns : Majority of restriction, endonucleases (particularly type II) cut DNA at, defined sites within recognition sequence., A selected list of enzymes, recognition, sequences, and their products formed is given in, Table 27.1., The cut DNA fragments by restriction, endonucleases may have mostly sticky ends, (cohesive ends) or blunt ends, as given in, Table 27.1. DNA fragments with sticky ends, are particularly useful for recombinant DNA, experiments. This is because the single-stranded, sticky DNA ends can easily pair with any, other DNA fragment having complementary sticky, ends., , TABLE 27.1 Some restriction enzymes with sources, recognition sequences and the products formed, , Recognition sequence, , EcoRI, (Escherichia coli), , 5c˛˛G–A–A–T–T–C˛˛3c, 3c˛˛C–T–T–A–A–G˛˛5c, , ", , ˛˛G, C–T–T–A–A, , ", , 5c˛˛G–G–A–T–C–C˛˛3c, 3c˛˛C–C–T–A–G–G˛˛5c, , G–A–T–C–C˛˛, G˛˛, ˛˛G, ˛˛C–C–T–A–G, , *C–C˛˛, , ", , 5c˛˛G–G–C–C˛˛3c, 3c˛˛C–C–G–G˛˛5c, , ", , HaeIII, (Haemophilus aegyptius), , A–A–T–T–C˛˛, G˛˛, , ", , BamHI, (Bacillus amyloliquefaciens), , Products, , ", , Enzyme (source), , ", , 5c˛˛A–A–G–C–T–T˛˛3c, 3c˛˛T–T–C–G–A–A˛˛5c, , ", , HindIII, (Haemophilus influenzae), , A–G–C–T–T˛˛, A˛˛, ˛˛A, ˛˛T–T–C–G–A, , ", , 5c˛˛G–C–G–G–C–C–G–C˛˛3c, , (Nocardia otitidis), , 3c˛˛C–G–C–C–G–G–C–G˛˛5c, , ", , NotI, , (Note : Scissors indicate the sites of cleavage., , G–G˛˛, , *, , ˛˛ G–G, ˛˛ C–C, , G–G–C–C–G–C˛˛, C–G˛˛, ˛˛G–C, ˛˛C–G–C–C–G–G˛˛, , *The products are with blunt ends while for the rest, the products are with sticky ends).
Page 591 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , 5c P P P P P 3c, 5c P P P P P 3c, O, H, B B B B B B DNA ligase B B B B B B, B B B B B B, 3c P P P P P 5c, , H 2O, , 581, , Many enzymes are used in the recombinant, DNA technology/genetic engineering. A selected, list of these enzymes and the reactions catalysed, by them is given in Table 27.2., , B B B B B B, 3c P P P P P 5c, , Fig. 27.2 : Action of DNA ligase in the formation of, phosphodiester bond (B-base)., , DNA ligases—DNA joining enzymes, The cut DNA fragments are covalently joined, together by DNA ligases. These enzymes were, originally isolated from viruses. They also occur, in E.coli and eukaryotic cells. DNA ligases actively participate in cellular DNA repair process., The action of DNA ligases is absolutely, required to permanently hold DNA pieces. This, is so since the hydrogen bonds formed between, the complementary bases (of DNA strands) are, not strong enough to hold the strands together., DNA ligase joins (seals) the DNA fragments by, forming a phosphodiester bond between, the phosphate group of 5’-carbon of one, deoxyribose with the hydroxyl group of, 3’-carbon of another deoxyribose (Fig.27.2)., , HOST CELLS —, THE FACTORIES OF CLONING, The hosts are the living systems or cells in, which the carrier of recombinant DNA molecule, or vector can be propagated. There are different, types of host cells-prokaryotic (bacteria) and, eukaryotic (fungi, animals and plants). Some, examples of host cells used in genetic, engineering are given in Table 27.3., Host cells, besides effectively incorporating, the vector’s genetic material, must be, conveniently cultivated in the laboratory to, collect the products. In general, microorganisms, are preferred as host cells, since they multiply, faster compared to cells of higher organisms, (plants or animals)., , Prokaryotic hosts, Escherichia coli : The bacterium, Escherichia, coli was the first organism used in the DNA, technology experiments and continues to be the, host of choice by many workers., , TABLE 27.2 The most commonly used enzymes in recombinant DNA technology/genetic engineering, , Enzyme, Alkaline phosphatase, Bal 31 nuclease, DNA ligase, DNA polymerase I, DNase I, Exonuclease III, O exonuclease, Polynucleotide kinase, Restriction enzymes, Reverse transcriptase, RNase A, RNase H, Taq DNA polymerase, SI nuclease, Terminal transferase, , Use/reaction, Removes phosphate groups from 5c-ends of double/single-stranded DNA (or RNA)., For the progressive shortening of DNA., Joins DNA molecules by forming phosphodiester linkages between DNA segments., Synthesizes DNA complementary to a DNA template., Produces single-stranded nicks in DNA., Removes nucleotides from 3c-end of DNA., Removes nucleotides from 5c-end of DNA., Transfers phosphate from ATP to 5c-OH ends of DNA or RNA., Cut double-stranded DNA with a specific recognition site., Synthesizes DNA from RNA., Cleaves and digests RNA (and not DNA)., Cleaves and digests the RNA strand of RNA-DNA heteroduplex., Used in polymerase chain reaction, Degrades single-stranded DNA and RNA., Adds nucleotides to the 3c-ends of DNA or RNA. Useful in homopolymer tailing.
Page 592 :
582, , BIOCHEMISTRY, , TABLE 27.3 Some examples of host cells used, in genetic engineering, , Group, , Examples, , Prokaryotic, Bacteria, , Eukaryotic, Fungi, Animals, , Plants, , Escherichia coli, Bacillus subtilis, Streptomyces sp, Saccharomyces cerevisiae, Aspergillus nidulans, Insect cells, Oocytes, Mammalian cells, Whole organisms, Protoplasts, Intact cells, Whole plants, , The major drawback however, is that E.coli, (or even other prokaryotic organisms) cannot, perform post-translational modifications., , Bacillus subtilis : Bacillus subtilis is a rod, shaped non-pathogenic bacterium. It has been, used as a host in industry for the production of, enzymes, antibiotics, insecticides etc. Some, workers consider B.subtilis as an alternative to, E.coli., , Eukaryotic hosts, Eukaryotic organisms are preferred to produce, human proteins since these hosts with complex, structure (with distinct organelles) are more, suitable to synthesize complex proteins. The, most commonly used eukaryotic organism is the, yeast, Saccharomyces cerevisiae., Mammalian cells : Despite the practical, difficulties to work with and high cost factor,, mammalian cells (such as mouse cells) are also, employed as hosts. The advantage is that certain, complex proteins which cannot be synthesized, by bacteria can be produced by mammalian, cells e.g. tissue plasminogen activator. This is, mainly because the mammalian cells possess the, machinery to modify the protein to the active, form (post-translational modifications)., , VECTORS — THE CLONING, VEHICLES, Vectors are the DNA molecules, which can, carry a foreign DNA fragment to be cloned., They are self-replicating in an appropriate host, cell. The most important vectors are plasmids,, bacteriophages,, cosmids, and, artificial, chromosome vectors., , Plasmid, Plasmids are extrachromosomal, doublestranded, circular, self-replicating DNA molecules. Almost all the bacteria have plasmids, containing a low copy number (1-4 per cell) or, a high copy number (10-100 per cell). The size, of the plasmids varies from 1 to 500 kb. Usually,, plasmids contribute to about 0.5 to 5.0% of the, total DNA of bacteria (Note : A few bacteria, contain linear plasmids e.g. Streptomyces sp,, Borella burgdorferi)., Nomenclature of plasmids : It is a common, practice to designate plasmid by a lower case p,, followed by the first letter(s) of researcher(s), names and the numerical number given by the, workers. Thus, pBR322 is a plasmid discovered, by Bolivar and Rodriguez who designated it as, 322. Some plasmids are given names of the places, where they are discovered e.g. pUC is plasmid, from University of California., pBR322 – the most common plasmid vector :, pBR322 of E.coli is the most popular and widely, used plasmid vector, and is appropriately regarded, as the parent or grand parent of several other, vectors., pBR322 has a DNA sequence of 4,361 bp. It, carries genes resistance for ampicillin (Ampr) and, tetracycline (Tetr) that serve as markers for the, identification of clones carrying plasmids. The, plasmid has unique recognition sites for the, action of restriction endonucleases such as, EcoRI, HindIII, BamHI, SalI and PstlI (Fig.27.3)., Other plasmid cloning vectors : The other, plasmids employed as cloning vectors include, pUC19 (2,686 bp, with ampicillin resistance, gene), and derivatives of pBR322–pBR325,, pBR328 and pBR329.
Page 593 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , EcoRI, , Artificial chromosome vectors, , Hind III, , picllin resis, ta n, Am, , Pst I, , Te, resistan, ce, cline, cy, tra, , c, , Bam HI, , e, , 583, , Sal I, , Origin of replication, , Fig. 27.3 : Genetic map of plasmid cloning, vector pBR322., , Bacteriophages, , Human artificial chromosome (HAC) :, Developed in 1997 (by H. Willard), human, artificial chromosome is a synthetically, produced vector DNA, possessing the, characteristics of human chromosome. HAC, may be considered as a self-replicating, microchromosome with a size ranging from 1/, 10th to 1/5th of a human chromosome. The, advantage with HAC is that it can carry human, genes that are too long. Further, HAC can carry, genes to be introduced into the cells in gene, therapy., Yeast artificial chromosomes (YACs) :, Introduced in 1987 (by M. Olson), yeast artificial, chromosome (YAC) is a synthetic DNA that can, accept large fragments of foreign DNA, (particularly human DNA). It is thus possible to, clone large DNA pieces by using YAC., Bacterial artificial chromosomes (BACs) : The, construction of BACs is based on one, F-plasmid which is larger than the other plasmids, used as cloning vectors. BACs can accept DNA, inserts of around 300 kb., , Bacteriophages or simply phages are the, viruses that replicate within the bacteria. In case, of certain phages, their DNA gets incorporated, into the bacterial chromosome and remains, there permanently. Phage vectors can accept, short fragments of foreign DNA into their, genomes. The advantage with phages is that they, can take up larger DNA segments than, plasmids. Hence phage vectors are preferred, for working with genomes of human cells., The most commonly used phages are, bacteriophage O (phage O) and bacteriophage, (phage M13)., , Among the several factors, the size of the, foreign DNA is very important in the choice of, vectors. The efficiency of this process is often, crucial for determining the success of cloning., The sizes of DNA insert that can be accepted by, different vectors is shown in Table 27.4., , Cosmids, , METHODS OF GENE TRANSFER, , Cosmids are the vectors possessing the, characteristics of both plasmid and bacteriophage O. Cosmids can be constructed, by adding a fragment of phage O DNA including, cos site, to plasmids. A foreign DNA (about 40, kb) can be inserted into cosmid DNA., The recombinant DNA so formed can be, packed as phages and injected into E.coli., Once inside the host cell, cosmids behave, just like plasmids and replicate. The, advantage with cosmids is that they can carry, larger fragments of foreign DNA compared to, plasmids., , Choice of vector, , Introducing a foreign DNA (i.e. the gene) into, the cells is an important task in biotechnology., The efficiency of this process is often crucial for, determining the success of cloning. The most, commonly employed gene transfer methods,, namely transformation, conjugation, electroporation and lipofection, and direct transfer of, DNA are briefly described., , Transformation, Transformation is the method of introducing, foreign DNA into bacterial cells (e.g. E.coli). The
Page 594 :
584, , BIOCHEMISTRY, , TABLE 27.4 The different cloning vectors with, the corresponding hosts and the sizes, of foreign insert DNAs, , Vector, , Host, , Foreign insert, DNA size, , Phage O, , E. coli, , 5–25 kb, , Cosmid O, , E. coli, , 35–45 kb, , Plasmid artifical, , E. coli, , 100–300 kb, , E. coli, , 100–300 kb, , S. cerevisiae, , 200–2000 kb, , chromosome (PAC), Bacterial artificial, chromosome (BAC), Yeast chromosome, , uptake of plasmid DNA by E.coli is carried out, in ice-cold CaCl2 (0-5°C), and a subsequent heat, shock (37–45°C for about 90 sec). By this, technique, the transformation frequency, which, refers to the fraction of cell population that can, be transferred, is reasonably good e.g., approximately one cell per 1000 (10–3) cells., , Conjugation, Conjugation is a natural microbial recombination process. During conjugation, two live, bacteria (a donor and a recipient) come together,, join by cytoplasmic bridges and transfer singlestranded DNA (from donor to recipient). Inside, the recipient cell, the new DNA may integrate, with the chromosome (rather rare) or may remain, free (as is the case with plasmids)., The natural phenomenon of conjugation is, exploited for gene transfer. This is achieved by, transferring plasmid-insert DNA from one cell to, another. In general, the plasmids lack, conjugative functions and therefore, they are not, as such capable of transferring DNA to the, recipient cells. However, some plasmids with, conjugative properties can be prepared and, used., , is a technique involving electric field-mediated, membrane permeabilization. Electric shocks can, also induce cellular uptake of exogenous DNA, (believed to be via the pores formed by electric, pulses), from, the, suspending, solution., Electroporation is a simple and rapid technique, for introducing genes into the cells from various, organisms (microorganisms, plants and animals)., , Liposome-mediated gene transfer, Liposomes are circular lipid molecules, which, have an aqueous interior that can carry nucleic, acids. Several techniques have been developed, to encapsulate DNA in liposomes. The liposomemediated gene transfer is referred to as, lipofection., On treatment of DNA fragment with, liposomes, the DNA pieces get encapsulated, inside liposomes. These liposomes can adhere to, cell membranes and fuse with them to transfer, DNA fragments. Thus, the DNA enters the cell, and then to the nucleus. The positively charged, liposomes very efficiently complex with DNA,, bind to cells and transfer DNA rapidly., , Direct transfer of DNA, It is possible to directly transfer the DNA into, the cell nucleus. Microinjection and particle, bombardment are the two techniques commonly, used for this purpose., , GENE CLONING STRATEGIES, A clone refers to a group of organisms, cells,, molecules or other objects, arising from a single, individual. Clone and colony are almost, synonymous., Gene cloning strategies in relation to, recombinant DNA technology broadly involve, the following aspects (Fig.27.4)., l, , l, , Electroporation, Electroporation is based on the principle that, high voltage electric pulses can induce cell, plasma membranes to fuse. Thus, electroporation, , l, , l, , Generation of desired DNA fragments., Insertion of these fragments into a cloning, vector., Introduction of the vectors into host cells., Selection or screening of the recipient cells for, the recombinant DNA molecules.
Page 595 :
585, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , GENERATION OF DNA FRAGMENTS, (Restriction endonuclease digestion,, cDNA synthesis, PCR, chemical synthesis), , useful if the gene sequence is short and the, complete sequence of amino acids is known., , INSERTION INTO A CLONING VECTOR, (Ligation of blunt ends or cohesive ends,, homopolymer tailing, linker molecules), , BASIC TECHNIQUES IN, GENETIC ENGINEERING, , INTRODUCTION INTO HOST CELLS, (Transformation, transfection, transduction), , There are several techniques used in, recombinant DNA technology or gene, manipulation. The most frequently used methods, are listed., , SELECTION OR SCREENING, (Hybridization, PCR, immunochemical, methods, protein–protein interactions,, functional complementation), Fig. 27.4 : An overview of cloning strategies in, recombinant DNA technology., , l, , Isolation and purification of nucleic acids., , l, , Nucleic acid blotting techniques., , l, , DNA sequencing., , l, , Methods of gene transfer (described already)., , l, , Polymerase chain reaction., , l, , CLONING FROM GENOMIC DNA, OR mRNA?, , l, , l, , Production, of, (Chapter 41)., , monoclonal, , antibodies, , Construction of gene library., Site-directed, engineering., , mutagenesis, , and, , protein, , DNA represents the complete genetic material, of an organism which is referred to as genome., Theoretically speaking, cloning from genomic, DNA is supposed to be ideal. But the DNA, contains non-coding sequences (introns), control, regions and repetitive sequences. This complicates, the cloning strategies, hence DNA as a source, material is not preferred, by many workers., However, if the objective of cloning is to elucidate, the control of gene expression, then genomic DNA, has to be invariably used in cloning., , Almost all the experiments dealing with gene, manipulations require pure forms of either DNA, or RNA, or sometimes even both. Hence there is, a need for the reliable isolation of nucleic acids, from the cells. The purification of nucleic acids, broadly involves three stages., , The use of mRNA in cloning is preferred for, the following reasons., , 1. Breaking or opening of the cells to expose, nucleic acids., , l, , l, , l, , l, , mRNA represents the actual, information being expressed., , genetic, , Selection and isolation mRNA are easy., , ISOLATION AND PURIFICATION, OF NUCLEIC ACIDS, , 2. Separation of nucleic acids from other, cellular components., 3. Recovery of nucleic acids in a pure form., , As introns are removed during processing,, mRNA reflects the coding sequence of the, gene., , The basic principles and procedures for, nucleic acid purification are briefly described., , The synthesis of recombinant protein is much, easier with mRNA cloning., , PURIFICATION OF CELLULAR DNA, , Besides the direct use of genomic DNA or, mRNA, it is possible to synthesize DNA in the, laboratory (by polymerase chain reaction), and, use it in cloning experiments. This approach is, , The first step for DNA purification is to open, the cells and release DNA. The method should, be gentle to preserve the native DNA. Due to, variability in cell structure, the approaches to, break the cells are also different.
Page 596 :
586, , Lysis of cells, Bacterial cells : The bacterial cells (e.g. E., coli) can be lysed by a combination of enzymatic, and chemical treatments. The enzyme lysozyme, and the chemical ethylenediamine tetraacetate, (EDTA) are used for this purpose. This is followed, by the addition of detergents such as sodium, dodecyl sulfate (SDS)., , BIOCHEMISTRY, , formation of an insoluble complex with nucleic, acids. This complex, in the form of a precipitate, is collected after centrifugation and suspended, in a high-salt solution to release nucleic acids., By treatment with RNase, RNA is degraded. Pure, DNA can be isolated by ethanol precipitation., , Methods to purify DNA, , The second technique is based on the, principle of tight binding between DNA and, silica particles in the presence of a denaturing, agent such as guanidinium thiocyanate. The, isolation of DNA can be achieved by the direct, addition of silica particles and guanidinium, thiocyanate to the cellular extract, followed by, centrifugation. Alternately, a column chromatography containing silica can be used, and, through this the extract and guanidinium, thiocyanate are passed. The DNA binds to the, silica particles in the column which can be, recovered., , There are two different approaches to purify, DNA from the cellular extracts., , PURIFICATION OF mRNA, , Animal cells : Animal cells, particularly, cultured animal cells, can be easily opened by, direct treatment of cells with detergents (SDS)., Plant cells : Plant cells with strong cell walls, require harsh treatment to break open. The cells, are frozen and then ground in a morter and, pestle. This is an effective way of breaking the, cellulose walls., , 1. Purification of DNA by removing cellular, components : This involves the degradation or, complete removal of all the cellular components, other than DNA. This approach is suitable if the, cells do not contain large quantities of lipids and, carbohydrates., The cellular extract is centrifuged at a low, speed to remove the debris (e.g. pieces of cell, wall) that forms a pellet at the bottom of the, tube. The supernatant is collected and treated, with phenol to precipitate proteins at the, interface between the organic and aqueous, layers. The aqueous layer, containing the, dissolved nucleic acids, is collected and treated, with the enzyme ribonuclease (RNase). The RNA, is degraded while the DNA remains intact. This, DNA can be precipitated by adding ethanol and, isolated after centrifugation, and suspended in, an appropriate buffer., 2. Direct purification of DNA : In this, approach, the DNA itself is selectively removed, from the cellular extract and isolated. There are, two ways for direct purification of DNA., In one method, the addition of a detergent, cetyltrimethyl ammonium (CTAB) results in the, , Among the RNAs, mRNA is frequently, required in a pure form for genetic experiments., After the cells are disrupted on lysis by, different techniques (described above), the, cellular extract is deproteinised by treatment with, phenol or phenol/chloroform mixtures. On, centrifugation, the nucleic acids get concentrated, in the upper aqueous phase which may then be, precipitated by using isopropanol or ethanol., The purification of mRNA can be achieved by, affinity chromatography using oligo (dT)cellulose (Fig.27.5). This is based on the, principle that oligo (dT)-cellulose can specifically, bind to the poly (A) tails of eukaryotic mRNA., Thus, by this approach, it is possible to isolate, mRNA from DNA, rRNA and tRNA., As the nucleic acid solution is passed through, an affinity chromotographic column, the, oligo(dT) binds to poly(A) tails of mRNA. By, washing the column with high-salt buffer, DNA,, rRNA and tRNA can be eluted, while the mRNA, is tightly bound. This mRNA can be then eluted, by washing with low-salt buffer. The mRNA is, precipitated with ethanol and collected by, centrifugation (Fig.27.5).
Page 597 :
587, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , d AT, ifie A T, gn, a, M, AT, AT, AT, AT, , Cellulose bead, with oligo (dT), , support (nitrocellulose or nylon membranes)., The blotted nucleic acids are then used as targets, in the hybridization experiments for their specific, detection. An outline of the nucleic acid blotting, technique is depicted in Fig.27.6., , Types of blotting techniques, The most comonly used blotting techniques, are listed below, , Polyadenylated, mRNA, Column with, oligo(dT)-cellulose, , l, , Southern blotting (for DNA), , l, , Northern blotting (for RNA), , l, , Dot blotting (DNA/RNA), , The Southern blotting is named after the, scientist Ed Southern (1975) who developed it., The other names Northern blotting and Western, blotting are laboratory jargons which are now, accepted. Western blotting involves the transfer, of protein blots and their identification by using, specific antibodies., A diagrammatic representation of a typical, blotting apparatus is depicted in Fig.27.7., High-salt wash, , SOUTHERN BLOTTING, Southern blotting technique is the first nucleic, acid blotting procedure developed in 1975 by, Southern. It is depicted in Fig.27.8, and briefly, described., , Ethanol, supernatant, , Low-salt wash, , mRNA, , Fig. 27.5 : Purification of mRNA by affinity, chromatography with oligo(dT)-cellulose., , Immobilization of nucleic acids, , Southern blot (DNA), Northern blot (RNA), Dot-blot (DNA/RNA), , Prehybridization, Labeled DNA, or RNA probes, , Hybridization, , NUCLEIC ACID BLOTTING, TECHNIQUES, Blotting techniques are very widely used, analytical tools for the specific identification of, desired DNA or RNA fragments from thousands, of molecules. Blotting refers to the process of, immobilization of sample nucleic acids on solid, , Stringency washes, , Detection, , Fig. 27.6 : An outline of the nucleic acid, blotting techniques.
Page 598 :
588, , BIOCHEMISTRY, , l, , Weight, Glass plate, , Highly useful for the determination of, restriction fragment length polymorphism, (RFLP) associated with pathological conditions., , Paper tissues, Filter paper, Membrane, Gel, Plastic, tray, Support block, Filter paper, wick, , Genomic DNA, Transfer, buffer, , Restriction endonuclease, , Fig. 27.7 : Diagrammatic representation of, a typical blotting apparatus., , The genomic DNA isolated from cells/tissues, is digested with one or more restriction enzymes., This mixture is loaded into a well in an agarose, or polyacrylamide gel and then subjected to, electrophoresis. DNA, being negatively charged, migrates towards the anode (positively charged, electrode); smaller DNA fragments move faster., The separated DNA molecules are denatured, by exposure to a mild alkali and transferred to, nitrocellulose or nylon paper. This results in an, exact replica of the pattern of DNA fragments on, the gel. The DNA can be annealed to the paper, on exposure to heat (80°C). The nitrocellulose or, nylon paper is then exposed to labeled, cDNA probes. These probes hybridize with, complementary DNA molecules on the paper., The paper after thorough washing is exposed, to X-ray film to develop autoradiograph. This, reveals specific bands corresponding to the DNA, fragments recognized by cDNA probe., Zoo blot : This is a specialized Southern blot, technique used to compare DNA sequences, (genomes) between humans and other organisms., e.g. hemoglobin gene sequences in humans, compared to that of chimpanzee, horse and pig., Zoo blot technique is also useful to distinguish, between coding and non-coding regions and, their evolution in different organisms., , DNA fragments, Gel electrophoresis, Long DNA fragments, , Short DNA fragments, Agarose gel, Denature by mild alkali, Blot transfer, , Nitrocellulose, (or nylon membrane), DNA probe, , Hybridized bands, , Applications of Southern blotting, l, l, l, , It is an invaluable method in gene analysis., Important for confirmation of DNA cloning., Forensically applied to detect minute, quantities of DNA (to identify parenthood,, thieves, rapists etc.)., , Autoradiograph, Fig. 27.8 : Southern blotting technique.
Page 599 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , NORTHERN BLOTTING, Northern blotting is the technique for the, specific identification of RNA molecules. The, procedure adopted is almost similar to that, described for Southern blotting and is depicted, in Fig.27.9. RNA molecules are subjected to, electrophoresis, followed by blot transfer,, hybridization and autoradiography., RNA molecules do not easily bind to, nitrocellulose paper or nylon membranes. Blottransfer of RNA molecules is carried out by using, a chemically reactive paper prepared by, diazotization of aminobenzyloxymethyl to create, diazobenzyloxymethyl (DBM) paper. The RNA, can covalently bind to DBM paper., Northern blotting is theoretically, a good, technique for determining the number of genes, (through mRNA) present on a given DNA. But, this is not really practicable since each gene may, give rise to two or more RNA transcripts. Another, drawback is the presence of exons and introns., , 589, , this approach, the nucleic acids (DNA or RNA), are directly spotted onto the filters, and not, subjected to electrophoresis. The hybridization, procedure is the same as in original blotting, techniques., Dot-blotting technique is particularly useful, in obtaining quantitative data for the evaluation, of gene expression., , Western blotting, Western blotting involves the identification of, proteins. It is very useful to understand the, nucleic acid functions, particularly during the, course of gene manipulations., The technique of Western blotting involves the, transfer of electrophoresed protein bands from, polyacrylamide gel to nylon or nitrocellulose membrane. These proteins can be, detected by specific protein-ligand interactions., Antibodies or lectins are commonly used for this, purpose., , DOT-BLOTTING, Dot-blotting is a modification of Southern and, Northern blotting techniques described above. In, , RNA extract, Agarose gel, electrophoresis, , rRNA bands, , Autoradiography, Autoradiography is the process of localization, and recording of a radiolabel within a solid, specimen, with the production of an image in a, photographic emulsion. These emulsions are, composed of silver halide crystals suspended in, gelatin., When a E-particle or a J-ray from a radiolabel, passes through the emulsions, silver ions are, converted to metallic silver atoms. This results in, the development of a visible image which can, be easily detected., , Applications of autoradiography, Blotting, Hybridization, Autoradiography, , DNA probe, hybridizes to RNA, , Fig. 27.9 : An outline of Northern blotting., , As already described, autoradiography is, closely associated with blotting techniques for, the detection of DNA, RNA and proteins., , DNA SEQUENCING, Determination of nucleotide sequence in a, DNA molecule is the basic and fundamental
Page 600 :
590, , BIOCHEMISTRY, , requirement in biotechnology. DNA sequencing, is important to understand the functions of genes,, and basis of inherited disorders. Further, DNA, cloning and gene manipulation invariably, require knowledge of accurate nucleotide, sequence., , Double-stranded DNA, , Single-stranded DNA (labeled), Distributed into 4 tubes, , MAXAM AND GILBERT TECHNIQUE, The first DNA sequencing technique, using, chemical reagents, was developed by Maxam, and Gilbert (1977). This method is briefly, described below (Fig.27.10)., A strand of source DNA is labeled at one end, with 32P. The two strands of DNA are then, separated. The labeled DNA is distributed into, four samples (in separate tubes). Each sample is, subjected to treatment with a chemical that, specifically destroys one (G, C) or two bases, (A + G, T + C) in the DNA. Thus, the DNA strands, are partially digested in four samples at sites G,, A + G, T + C and C. This results in the formation, of a series of labeled fragments of varying lengths., The actual length of the fragment depends on the, site at which the base is destroyed from the, labeled end. Thus for instance, if there are C, residues at positions 4, 7, and 10 away from the, labeled end, then the treatment of DNA that, specifically destroys C will give labeled pieces of, length 3, 6 and 9 bases. The labeled DNA, fragments obtained in the four tubes are subjected, to electrophoresis side by side and they are, detected by autoradiograph. The sequence of the, bases in the DNA can be constructed from the, bands on the electrophoresis., , G, , A+G, , T+C, , C, , (Specific bases destroyed and fragments formed), Fragments separated, by electrophoresis, G, , A+G, , T+C, , C, , Longer, , T, C, A, G, C, G, T, C, A, T, A, , Shorter, Bands on autoradiograph, , DIDEOXYNUCLEOTIDE METHOD, , ATACTGCGACT Sequenced strand, , Currently, the preferred technique for, determining nucleotide sequence in DNA is the, one developed by Sanger (1980). This is an, enzymatic procedure commonly referred to as, the dideoxynucleotide method or chain, termination method (Note : Fredrick Sanger won, Nobel prize twice, once for determining the, structure of protein, insulin; the second time for, sequencing the nucleotides in an RNA virus)., , at both the 2c and 3c carbons of the sugar, (Fig.27.11). This is in contrast to the natural, deoxyribonucleotide that possesses at 3c, hydroxyl group on the sugar., , A dideoxynucleotide is a laboratory-made, chemical molecule that lacks a hydroxyl group, , Termination role of dideoxynucleotide : In, the normal process of DNA replication, an, , TATGACGCTGA Complementary strand, , Fig. 27.10 : Maxam and Gilbert method, for DNA sequencing.
Page 601 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , (A ), P, , P, , 5c, , O, , P O H2C, , H, , H, , H, 2c, , 3c, , P, , P, , 5c, , O, , P O H2C, , H, , H, 3c, , H, , H, , H, , (B), , Base, (A, C, G, T), , OH, , H, 2c, , Base, (A, C, G, T), H, , H, , Fig. 27.11 : Structure of (A) dideoxynucleotide, triphosphate and (B) deoxynucleotide triphosphate, (Note the difference at 3c-carbon)., , incoming nucleoside triphosphate is attached by, its 5c-phosphate group to the 3c-hydroxyl group, of the last nucleotide of the growing chain (Refer, Chapter 24) when a dideoxynucleotide is, incorporated to the growing chain, no further, replication, occurs., This, is, because, dideoxynucleotide, lacking a 3c-hydroxyl group,, cannot form a phosphodiester bond and thus the, DNA synthesis terminates., Sequencing method : The process of, sequencing DNA by dideoxynucleotide method, is briefly described. A single-stranded DNA to be, sequenced is chosen as a template. It is attached, to a primer (a short length of DNA, oligonucleotide) complementary to a small, section of the template. The 3c-hydroxyl group of, the primer initiates the new DNA synthesis., DNA synthesis is carried out in four reaction, tubes. Each tube contains the primed, DNA, Klenow subunit (the larger fragment of, DNA polymerase of E. coli), four dideoxyribonucleotides (ddATP, ddCTP, ddGTP or, ddTTP). It is necessary to radiolabel (with 32P), the primer or one of the deoxyribonucleotides., As the new DNA synthesis is completed, each, one of the tubes contains fragments of DNA of, varying length bound to primer. Let us consider, the first reaction tube with dideoxyadenosine, (ddATP). In this tube, DNA synthesis terminates, whenever the growing chain incorporates ddA, (complementary to dT on the template strand)., Therefore, this tube will contain a series of, different length DNA fragments, each ending, , 591, , with ddA. In a similar fashion, for the other 3, reaction tubes, DNA synthesis stops as the, respective dideoxynucleotides are incorporated., The synthesis of new DNA fragments in the, four tubes is depicted in Fig.27.12., The DNA pieces are denatured to yield free, strands with radiolabel. The samples from each, tube are separated by polyacrylamide gel, electrophoresis. This separation technique, resolves DNA pieces, different in size even by a, single nucleotide. The shortest DNA will be the, fastest moving on the electrophoresis., The sequence of bases in a DNA fragment is, determined by identifying the electrophoretic, (radiolabeled) bands by autoradiography. In the, Fig.27.13, the sequence of the newly synthesized, DNA fragment that is complementary to the, original DNA piece is shown. It is conventional, to read the bands from bottom to top in 5c to 3c, direction. By noting the order of the bands first, C, second G, third T and so on, the sequence of, the DNA can be determined accurately. As many, as 350 base sequences of a DNA fragment can, be clearly identified by using autoradiographs., Modifications of dideoxynucleotide method :, Replacement of 32P-radiolabel by 33P or 35S, improves the sharpness of autoradiographic, images. DNA polymerase of the thermophilic, bacterium, Thermus aquaticus (in place of, Klenow fragment of E. coli DNA polymerase I) or, a modified form of phage T7 DNA polymerase, (sequenase) improves the technique., , AUTOMATED DNA SEQUENCING, DNA sequencing in the recent years is carried, out by an automated DNA sequencer. In this, technique, flourescent tags are attached to chainterminating nucleotides (dideoxynucleotides)., This tag gets incorporated into the DNA, molecules, while terminating new strand, synthesis. Four different fluorescent dyes are, used to identify chain-terminating reactions in a, sequencing gel. The DNA bands are separated, by electrophoresis and detected by their, fluorescence. Recently, four dyes that exhibit, strong absorption in laser are in use for, automated sequencing.
Page 602 :
592, , BIOCHEMISTRY, , 3c, , 3c, , 5c, Primer, , Reaction tube, with dideoxynucleotide, , Template, G C AT C G A AT 5c, , OH Newly synthesized DNA, , Primer with nucleotide, extended, , ddATP, , ddCTP, , ddGTP, , ddTTP, , *, Primer with sequence of, nucleotides extended, , Primer + 4, , Primer–CGTddA, , Primer + 9, , Primer–CGTAGCTTddA, , Primer + 1, , Primer–ddC, , Primer + 6, , Primer–CGTAGddC, , Primer + 2, , Primer–CddG, , Primer + 5, , Primer–CGTAddG, , Primer + 3, , Primer–CGddT, , Primer + 7, , Primer–CGTAGCddT, , Primer + 8, , Primer–CGTAGCTddT, , Fig. 27.12 : Synthesis of new DNA fragments in the presence of dideoxynucleotides (*the size of the, new DNA is variable, depending on the chain termination)., , ddATP, Largest, , ddCTP, , ddGTP, , ddTTP, , Sequence, 3c, A, T, T, C, G, A, T, G, C, 5c, , Smallest, Fig. 27.13 : Sequence of the newly synthesized DNA fragment (complementary to original strand).
Page 603 :
593, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , Advantages of automated sequencing : It is a, rapid and accurate technique. Automated DNA, sequencer can accurately sequence up to, 100,000 nucleotides per day. The cost works out, to be not more than $0.2 per nucleotide., Automated DNA sequencing has been, successfully used in the human genome project., , DNA CHIPS (MICROARRAYS), , Test, Fluorescently molecule, labeled DNA, , DNA chips or DNA microarrays are recent, developments for DNA sequencing as result of, advances made in automation and miniarization., A large number of DNA probes, each one with, different sequence, are immobilized at defined, positions on the solid surface, made up of either, nylon or glass. The probes can be short DNA, molecules such as cDNAs or synthetic oligonucleotides., , Hybridization, , Confocal, microscopy, , For the preparation of high density arrays,, oligonucleotides are synthesized in situ on the, surface of glass or silicon. This results in an, oligonucleotide chip rather than a DNA chip., , Hybridizing, signals, , Technique of DNA sequencing, A DNA chip carrying an array of different, oligonucleotides can be used for DNA, sequencing. For this purpose, a fluorescently, labeled DNA test molecule, whose sequence is, to be determined, is applied to the chip., Hybridization occurs between the complementary sequences of the test DNA molecule, and oligonucleotides of the chip. The positions, of these hybridizing oligonucleotides can be, determined by confocal microscopy. Each, hybridizing oligonucleotide represents an 8nucleotide sequence that is present in the DNA, probe. The sequence of the test DNA molecule, can be deduced from the overlaps between the, sequences of the hybridizing oligonucleotides, (Fig.27.14)., , Applications of DNA chips, There have been many successes with this, relatively new technology of DNA chips. Some, of them are listed., l, , Identification of genes responsible for the, development of nervous systems., , AGTCCCTT, GTCCCTTG Hybridizing, TCCCTTGG oligonucleotides (8), CCCTTGGC, AGTCCCTTGGC, DNA sequence, Fig. 27.14 : Microarray (or chip) technology, in DNA sequencing., , l, , l, , l, , l, , l, , Detection, of, genes, inflammatory diseases., , responsible, , for, , Construction of microarrays for every gene in, the genome of E. coli, and almost all the genes, of the yeast Saccharomyces cerevisiae., Expression of several genes in prokaryotes has, been identified., Detection and screening of single nucleotide, polymorphisms (SNPs)., Rapid detection of microorganisms, environmental monitoring., , for
Page 604 :
594, , The future of DNA chips, The major limitation of DNA chips at present, is the unavailability of complete genome arrays, for higher eukaryotes, including humans. It is, expected that within the next few years such, DNA chips will be available. This will help the, biotechnologists to capture the functional, snapshots of the genome in action for higher, organisms., , POLYMERASE CHAIN REACTION, (DNA AMPLIFICATION), The polymerase chain reaction (PCR) is a, laboratory (in vitro) technique for generating, large quantities of a specified DNA. Obviously,, PCR is a cell-free amplification technique for, synthesizing multiple identical copies (billions), of any DNA of interest. Developed in 1984 by, Karry Mullis (Nobel Prize, 1993), PCR is now, considered as a basic tool for the molecular, biologist. As is a photocopier a basic, requirement in an office, so is the PCR machine, in a molecular biology laboratory!, , Principle of PCR, The double-stranded DNA of interest is, denatured to separate into two individual, strands. Each strand is then allowed to hybridize, with a primer (renaturation). The primer-template, duplex is used for DNA synthesis (the enzymeDNA polymerase). These three steps—, denaturation, renaturation and synthesis are, repeated again and again to generate multiple, forms of target DNA., , Technique of PCR, The essential requirements for PCR are listed, below, 1. A target DNA (100–35,000 bp in length)., 2. Two primers (synthetic oligonucleotides of, 17–30, nucleotides, length), that, are, complementary to regions flanking the target, DNA., 3. Four deoxyribonucleotides (dATP, dCTP,, dGTP, dTTP)., , BIOCHEMISTRY, , 4. A DNA polymerase that can withstand at a, temperature up to 95°C (i.e., thermostable)., The actual technique of PCR involves, repeated cycles for amplification of target DNA., Each cycle has three stages., 1. Denaturation : On raising the temperature, to about 95°C for about one minute, the DNA, gets denatured and the two strands separate., 2. Renaturation or annealing : As the, temperature of the mixutre is slowly cooled to, about 55°C, the primers base pair with the, complementary regions flanking target DNA, strands. This process is called renaturation or, annealing. High concentration of primer ensures, annealing between each DNA strand and the, primer rather than the two strands of DNA., 3. Synthesis : The initiation of DNA synthesis, occurs at 3c-hydroxyl end of each primer. The, primers are extended by joining the bases, complementary to DNA strands. The synthetic, process in PCR is quite comparable to the, DNA replication of the leading strand (Refer, Chapter 24). However, the temperature has to, be kept optimal as required by the enzyme DNA, polymerase. For Taq DNA polymerase, the, optimum temperature is around 75°C (for E. coli, DNA polymerase, it is around 37°C). The, reaction can be stopped by raising the, temperature (to about 95°C)., The 3 stages of PCR in relation to temperature, and time are depicted in Fig.27.15. Each cycle, of PCR takes about 3-5 minutes. In the normal, practice, the PCR is carried out in an automated, machine., As is evident from the Fig.27.16 (cycle I), the, new DNA strand joined to each primer is beyond, the sequence that is complementary to the, second primer. These new strands are referred to, as long templates, and they will be used in the, second cycle., For the second cycle of PCR, the DNA strands, (original + newly synthesized long template) are, denatured, annealed with primers and subjected, to DNA synthesis. At the end of second round,, long templates, and short templates (DNA
Page 605 :
595, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , 100, , Denaturation, (1 min), , Variations of PCR, The basic technique of PCR has been, described. Being a versatile technique, PCR is, modified as per the specific demands of the, situation. Some of the variants of PCR are listed, , Temperature (qC), , 90, 80, DNA synthesis, (2 min), , 70, , Denaturation, Primer annealing, , 60, Renaturation, (1 min), , 50, 1, , 2, , 3, 5, 4, Time (minutes), , 6, , 7, , C, Y, C, L, E, 1, , Original strand, Long template, , Fig. 27.15 : The three stages in each cycle of PCR in, relation to temperature and time, (Each cycle takes approximately 3-5 minutes)., , strands with primer sequence at one end, and, sequence complementary to the other end, primer) are formed., In the third cycle of PCR, the original DNA, strands along with long and short templates are, the starting materials. The technique of, denaturation, renaturation and synthesis are, repeated. This procedure is repeated again and, again for each cycle. It is estimated that at the, end of 32nd cycle of PCR, about a million-fold, target DNA is synthesized. The short templates, possessing precisely the target DNA as doublestranded molecules accumulate., , Sources of DNA polymerase, In the original technique of PCR, Klenow, fragment of E. coli DNA polymerase was used., This enzyme, gets denatured at higher, temperature, therefore, fresh enzyme had to be, added for each cycle. A breakthrough occurred, (Lawyer 1989) with the introduction of Taq DNA, polymerase from thermophilic bacterium,, Thermus aquaticus. The Taq DNA polymerase, is heat resistant, hence it is not necessary, to freshly add this enzyme for each cycle of, PCR., , DNA synthesis, , Original strand, Denaturation, Annealing, Synthesis, Long template, C, Y, C, L, E, 2, , Short templates, Long template, Denaturation, Annealing, Synthesis, , C, Y, C, L, E, 3, , Fig. 27.16 : The polymerase chain reaction (PCR), representing the initial three cycles, (, indicate primers).
Page 606 :
596, , BIOCHEMISTRY, , 18 in follicular lymphoma) involving known, genes are identified by PCR., , l, , Nested PCR, , l, , Inverse PCR, , l, , Anchored PCR, , l, , Reverse transcription PCR (RT-PCR), , l, , Asymmetric PCR, , l, , Real-time quantitative PCR, , l, , Random amplified polymorphic DNA (RAPD), , l, , l, , Amplified, (AFLP), , fragment, , length, , polymorphism, , Rapid amplification of cDNA ends (RACE)., , APPLICATIONS OF PCR, The advent of PCR had, and continues to have, tremendous impact on molecular biology. The, applications of PCR are too many to be listed, here. Some of them are selectively and very, briefly described. Other applications of PCR are, discussed at appropriate places., , PCR in clinical diagnosis, The specificity and sensitivity of PCR is highly, useful for the diagnosis of various diseases in, humans. These include diagnosis of inherited, disorders (genetic diseases), viral diseases,, bacterial diseases etc., Prenatal diagnosis of inherited diseases :, PCR is employed in the prenatal diagnosis of, inherited diseases by using chorionic villus, samples or cells from amniocentesis. Thus,, diseases like sickle-cell anemia, E-thalassemia, and phenylketonuria can be detected by PCR in, these samples., , PCR in sex determination of embryos : Sex of, human and live stock embryos fertilized in vitro,, can be determined by PCR, by using primers and, DNA probes specific for sex chromosomes., Further, this technique is also useful to detect, sex — linked disorders in fertilized embryos., , PCR in DNA sequencing, As the PCR technique is much simpler and, quicker to amplify the DNA, it is conveniently, used for sequencing. For this purpose, singlestrands of DNA are required., , PCR in comparative studies, of genomes, The differences in the genomes of two, organisms can be measured by PCR with random, primers. The products are separated by, electrophoresis for comparative identification., Two genomes from closely related organisms are, expected to yield more similar bands., PCR is very important in the study of, evolutionary biology, more specifically referred, to as phylogenetics. As a technique which can, amplify even minute quantities of DNA from any, source (hair, mummified tissues, bone, or any, fossilized material), PCR has revolutionized the, studies in palaentology and archaelogy. The, movie ‘Jurassic Park’, has created public, awareness of the potential applications of PCR!, , PCR in forensic medicine, , Diagnosis of retroviral infections : PCR from, cDNA is a valuable tool for diagnosis and, monitoring of retroviral infections, e.g., HIV, infection., , A single molecule of DNA from any source, (blood strains, hair, semen etc.) of an individual, is adequate for amplification by PCR. Thus, PCR, is very important for identification of criminals., , Diagnosis of bacterial infections : PCR is used, for the detection of bacterial infections e.g.,, tuberculosis by Mycobacterium tuberculosis., , The reader may refer DNA finger printing, technique described later in this chapter., , Diagnosis of cancers : Several virally-induced, cancers (e.g., cervical cancer caused by human, papilloma virus) can be detected by PCR., Further, some cancers which occur due to, chromosomal translocation (chromosome 14 and, , GENE LIBRARIES, The collection of DNA fragments (specifically, genes) from a particular species represents gene, libraries. The creation or construction of, gene libraries (broadly genomic libraries) is
Page 607 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , accomplished by isolating the complete genome, (entire DNA from a cell) which is cut into, fragments, and cloned in suitable vectors. Then, the specific clone carrying the desired (target), DNA can be identified, isolated and, characterized. In this manner, a library of genes, or clones (appropriately considered as gene, bank) for the entire genome of a species can be, constructed., , Establishing a gene library, for humans, The human cellular DNA (the entire genome), may be subjected to digestion by restriction, endonucleases (e.g., EcoRI). The fragments, formed on an average are of about 4 kb size., (i.e., 4000 nitrogenes bases). Each human, chromosome, containing approximately 100,000, kb can be cut into about 25,000 DNA fragments., As the humans have 23 different chromosomes, (24 in man), there are a total of 575,000, fragments of 4 kb length formed. Among these, 575,000 DNA fragments is the DNA or gene of, interest (say insulin gene)., Now is the selection of a vector and cloning, process. E.coli, a harmless bacterium to humans, is most commonly used. The plasmids from E., coli are isolated. They are digested by the same, restriction enzyme as was used for cutting, human genome to form open plasmids. The, human chromosomal DNA fragments and open, plasmids are joined to produce recombined, plasmids. These plasmids contain different DNA, fragments of humans. The recombined plasmids, are inserted into E. coli and the cells multiply, (Fig.27.17). The E. coli cells possess all the, human DNA in fragments. It must, however be, remembered that each E. coli cell contains, different DNA fragments. All the E. coli cells put, together collectively represent genomic library, (containing about 575,000 DNA fragments)., , Screening strategies, Once a DNA library is created, the clones, (i.e., the cell lines) must be screened for, identification of specific clones. The screening, techniques are mostly based on the sequence of, the clone or the structure/function of its product., , 597, , Screening by DNA hybridization : The target, sequence in a DNA can be determined with a, DNA probe (Fig.27.18). To start with, the, double-stranded DNA of interest is converted, into single strands by heat or alkali, (denaturation). The two DNA strands are kept, apart by binding to solid matrix such as, nitrocellulose or nylon membrane. Now, the, single strands of DNA probe (100–1,000 bp), labeled, with, radioisotope, are, added., Hybridization (i.e., base pairing) occurs between, the complementary nucleotide sequences of the, target DNA and the probe. For a stable base, pairing, at least 80% of the bases in the two, strands (target DNA and the probe) should be, matching. The hybridized DNA can be detected, by autoradiography., (Note : DNA probe or gene probe represents, a segment of DNA that is tagged with a label, (i.e. isotope) so as to detect a complementary, base sequence with sample DNA after, hybridization), , SITE-DIRECTED MUTAGENESIS, AND PROTEIN ENGINEERING, Modifications in the DNA sequence of a gene, are ideal to create a protein with desired, properties. Site-directed mutagenesis is the, technique for generating amino acid coding, changes in the DNA (gene). By this approach, specific (site-directed) change (mutagenesis), can be made in the base (or bases) of the, gene to produce a desired enzyme. The net, result, in, site-directed, mutagenesis, is, incorporation of a desired amino acid (of one’s, choice) in place of a specific amino acid in a, protein or a polypeptide. By employing this, technique, enzymes that are more efficient and, more suitable than the naturally occurring, counterparts can be created for industrial, applications. But it must be remembered that, site-directed mutagenesis is a trial and error, method that may or may not result in a better, protein., A couple of proteins developed by sitedirected mutagenesis and protein engineering are, given next.
Page 608 :
598, , BIOCHEMISTRY, , Human cell, , E. coli cells, , DNA, , Plasmids, , Restriction, endonuclease, 1, , Restriction, endonuclease, , 6, , 3, , 5, 7, 4, DNA fragments, 2, , Open, plasmids, , 3, , 7, 1, , 4, , 2, 5, 6, Recombined plasmids, , 4, 7, , 6, , 3, 7, , 1, , 2, 6, 2, , 3, , 7, 4, , 7, , 5, , 6, , 7, 7, , 4, 5, , 5, , 2, , 5, , 1, , 1, , 6, 4, , 3, 3, 5, , 3, 1, 7, , 6, , Gene library, , Fig. 27.17 : Creation of a genomic library for humans. (Note : Double-stranded DNA is represented by, single lines or circles for clarity; human DNA fragments are coloured).
Page 609 :
599, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , Source DNA (double-stranded), Denaturation, membrane binding, , Single-stranded DNAs, , Hybridization, Single-stranded, labeled DNA probes, , the specific organism. Thus, the presence of a, disease-causing pathogen can be detected by, identifing a gene or a set of genes of the, organism. Likewise an inherited genetic defect, can be diagnosed by identifying the alterations, in the gene. In the modern laboratory, diagnostics, DNA analysis is a very useful and a, sensitive tool., The basic principles underlying the DNA, diagnostic systems, and their use in the diagnosis, of certain pathogenic and genetic diseases are, described. Besides these, the various approaches, for DNA fingerprinting (or DNA profiling) are, also discussed., , METHODS OF DNA ASSAY, Hybrid DNAs, Fig. 27.18 : Screening by DNA hybridization, (, indicates radioisotope label in the DNA probe), , The specific identification of the DNA, sequence is absolutely essential in the, laboratory diagnostics. This can be achieved by, employing the following principles/tools., , Tissue plasminogen activator (tPA), , Nucleic acid hybridization, , Tissue plasminogen activator is therapeutically, used to lyse the blood clots that cause, myocardial infarction. Due to its shorter half-life, (around 5 minutes), tPA has to be repeatedly, administered. By replacing asparagine residue (at, position 120) with glutamine, the half-life of tPA, can be substantially increased. This is due to the, fact that glutamine is less glycosylated than, asparagine and this makes a difference in the, half-life of tPA., , Hybridization of nucleic acids (particularly, DNA) is the basis for reliable DNA analysis., Hybridization is based on the principle that a, single-stranded DNA molecule recognizes and, specifically binds to a complementary DNA, strand amid a mixture of other DNA strands. This, is comparable to a specific key and lock, relationship. The general procedure adopted, for nucleic acid hybridization has been, described (See p. 597 and Fig.27.18). Some more, information is given below (Fig.27.19)., , Hirudin, , The single-stranded target DNA is bound to a, membrane support. Now the DNA probe (single-, , Hirudin is a protein secreted by leech salivary, gland, and is a strong thrombin inhibitor (i.e.,, acts as an anticoagulant). By replacing, asparagine (at 47 position) with lysine, the, potency of hirudin can be increased severalfold., , A C G T TA G C A, Target DNA, T G C A AT C G T, DNA probe, Label, , DNA IN DISEASE DIAGNOSIS AND, MEDICAL FORENSICS, DNA, being the genetic material of the living, organisms, contains the information that, contributes to various characteristic features of, , A C G T TA G C A, T G C A AT C G T, Complementary pairing, Fig. 27.19 : Hybridization of target DNA with DNA probe, (with radioactive isotope label).
Page 610 :
600, stranded and labeled with a detector substance), is added. Under appropriate conditions, (temperature, ionic strength), the DNA probe, pairs with the complementary target DNA. The, unbound DNA probe is removed. Sequence of, nucleotides in the target DNA can be identified, from the known sequence of DNA probe., There are two types of DNA hybridizationradioactive and non-radioactive respectively, using DNA probes labeled with isotopes and, non-isotopes as detectors., , THE DNA CHIP-MICROARRAY, OF GENE PROBES, The DNA chip or Genechip contains, thousands of DNA probes (4000,000 or even, more) arranged on a small glass slide of the size, of a postage stamp. By this recent and advanced, approach, thousands of target DNA molecules, can be scanned simultaneously., , Technique for use of DNA chip, The unknown DNA molecules are cut into, fragments, by, restriction, endonucleases., Fluorescent markers are attached to these DNA, fragments. They are allowed to react with the, probes of the DNA chip. Target DNA fragments, with complementary sequences bind to DNA, probes. The remaining DNA fragments are, washed away. The target DNA pieces can be, identified by their fluorescence emission by, passing a laser beam. A computer is used to, record the pattern of fluoresence emission and, DNA identification., The technique of employing DNA chips is, very rapid, besides being sensitive and specific, for the identification of several DNA fragments, simultaneously. Scientists are trying to develop, Genechips for the entire genome of an organism., , Applications of DNA chip, The presence of mutations in a DNA, sequence can be conveniently identified. In fact,, Genechip probe array has been successfully used, for the detection of mutations in the p53 and, BRCA I genes. Both these genes are involved in, cancer (See p. 593 also)., , BIOCHEMISTRY, , DNA IN THE DIAGNOSIS, OF INFECTIOUS DISEASES, The use of DNA analysis (by employing DNA, probes) is a novel and revolutionary approach, for specifically identifying the disease-causing, pathogenic organisms. This is in contrast to the, traditional methods of disease diagnosis by, detection of enzymes, antibodies etc., besides, the microscopic examination of pathogens., Although at present not in widespread use, DNA, analysis may soon take over the traditional, diagnostic tests in the years to come. Diagnosis, of selected diseases by genetically engineered, techniques or DNA probes or direct DNA, analysis is briefly described., , Tuberculosis, Tuberculosis is caused by the bacterium, Mycobacterium tuberculosis. The commonly, used diagnostic tests for this disease are very, slow, and sometimes may take several weeks., This is because M. tuberculosis multiplies very, slowly (takes about 24 hrs. to double; E. coli, takes just 20 minutes to double)., A novel diagnostic test for tuberculosis was, developed by genetic engineering, and is, illustrated in Fig.27.20. A gene from firefly,, encoding the enzyme luciferase is introduced, into the bacteriophage specific for M., tuberculosis. The bacteriophage is a bacterial, virus, frequently referred to as luciferase reporter, phage or mycophage. The genetically engineered, phage is added to the culture of M. tuberculosis., The phage attaches to the bacterial cell wall,, penetrates inside, and inserts its gene (along with, luciferase gene) into the M. tuberculosis, chromosome. The enzyme luciferase is produced, by the bacterium. When luciferin and ATP are, added to the culture medium, luciferase cleaves, luciferin. This reaction is accompanied by a flash, of light which can be detected by a luminometer., This diagnostic test is quite sensitive for the, confirmation of tuberculosis., The flash of light is specific for the, identification of M. tuberculosis in the culture., For other bacteria, the genetically engineered, phage cannot attach and enter in, hence no flash, of light would be detected.
Page 611 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , Phage, genome, , Chromosome, Phage, Mycobacterium, tuberculosis, , Viral, genome, Luciferase, gene, , Luciferase, Luciferin, +ATP, , Flash of light, Fig. 27.20 : Diagnosis of tuberculosis by using a, genetically engineered bacteriophage (phage)., , Malaria, Malaria, mainly caused by Plasmodium, falciparum, and P. vivax, affects about one-third of, the world’s population. The commonly used, laboratory tests for the diagnosis of malaria include, microscopic examination of blood smears, and, detection of antibodies in the circulation. While, the former is time consuming and frequently gives, false-negative tests, the latter cannot distinguish, between the past and present infections., A specific DNA diagnostic test for, identification of the current infection of P., falciparum has been developed. This is carried, out by using a DNA probe that can bind and, hybridize with a DNA fragment of P. falciparum, genome, and not with other species of, Plasmodium. It is reported that this DNA probe, can detect as little as 1 ng of P. falciparum in, blood or 10 pg of its purified DNA., , Acquired immunodeficiency, syndrome (AIDS), DNA probes, with radioisotope label, for HIV, DNA are now available. By using PCR and DNA, probes, AIDS can be specifically diagnosed in, the laboratory., , 601, , DNA IN THE DIAGNOSIS OF, GENETIC DISEASES, Traditional laboratory tests for the diagnosis, of genetic diseases are mostly based on, the estimation of metabolites and/or enzymes., This is usually done after the onset of, symptoms., The laboratory tests based on DNA analysis, can specifically diagnose the inherited diseases, at the genetic level. DNA-based tests are useful, to discover, well in advance, whether the, individuals or their offsprings are at risk for any, genetic disease. Further, such tests can also be, employed for the prenatal diagnosis of hereditary, disorders, besides identifying the carriers of, genetic diseases., Although not in routine use in the laboratory, service, methods have been developed or being, developed for the analysis of DNA in the, diagnosis of several genetic diseases. These, include sickle-cell anemia, cystic fibrosis,, Duchenne’s muscular dystrophy, Huntington’s, disease, fragile X syndrome, Alzheimer’s disease,, certain cancers (e.g. breast cancer, colon, cancer), type II diabetes, obesity, Parkinson’s, disease and baldness., , Sickle-cell anemia, Sickle-cell anemia is a genetic disease, characterized by the irregular sickle (crescent, like) shape of the erythrocytes. Biochemically,, this disease results in severe anemia and, progressive damage to major organs in the body, (heart, brain, lungs, joints)., Sickle-cell anemia occurs due to a single, amino acid change in the E-chain of, hemoglobin. Specifically, the amino acid, glutamate at the 6th position of E-chain is, replaced by valine. At the molecular level,, sickle-cell anemia is due to a single-nucleotide, change (A o T) in the E-globin gene of coding, (or antisense) strand. In the normal E-globin, gene the DNA sequence is CCTGAGGAG,, while in sickle-cell anemia, the sequence is, CCTGTGGAG. This single-base mutation can be, detected by using restriction enzyme MstII to cut
Page 612 :
602, , BIOCHEMISTRY, , E-Globin gene, , MstII, , a person’s cells (usually white blood cells) is, stored. As and when a DNA probe for the, detection of a specific disease is available, the, stored DNA can be used for the diagnosis or risk, assessment of the said genetic disease., , MstII, , MstII, , Pro Glu Glu, CCT GAG GAG, 5, , 6, , 7, , CCT GTG GAG, Pro Val Glu, , Base sequence, in normal gene, Amino acid number, Base sequence in, sickle-cell gene, (E–globin gene), , Fig. 27.21 : Single-base change resulting in sickle-cell, anemia. (Note : A small and relevant DNA fragment of, E-globin gene magnified and shown with, encoded amino acids)., , DNA fragments in and around E-globin gene,, followed by the electrophoretic pattern of the, DNA fragments formed. The change in the base, from A to T in the E-globin gene destroys, the recognition site (CCTGAGG) for MstII, (Fig.27.21). Consequently, the DNA fragments, formed from a sickle-cell anemia patient for, E-globin gene differ from that of a normal, person. Thus, sickle-cell anemia can be detected, by digesting mutant and normal E-globin, genes by restriction enzyme and performing, a hybridization with a cloned E-globin DNA, probe., , GENE BANKS — A NOVEL CONCEPT, As the search continues by scientists for the, identification of more and more genes, responsible for various diseases, the enlightened, public (particularly in the developed countries),, is very keen to enjoy the fruits of this research, outcome. As of now, DNA probes are available, for the detection a limited number of diseases., Researchers continue to develop DNA probes for, a large number of genetically predisposed, disorders., , Gene banks are the centres for the storage of, individual’s DNAs for future use to diagnose, diseases. For this purpose, the DNA isolated from, , In fact, some institutions have established, gene banks. They store the DNA samples of, the interested customers at a fee (one firm, was charging $200) for a specified period (say, around 20–25 years). For the risk assessment of, any disease, it is advisable to have the, DNAs from close relatives of at least 2-3, generations., , DNA FINGERPRINTING OR, DNA PROFILING, DNA fingerprinting is the present day genetic, detective in the practice of modern medical, forensics. The underlying principles of DNA, fingerprinting are briefly described., The structure of each person’s genome is, unique. The only exception being monozygotic, identical twins (twins developed from a, single fertilized ovum). The unique nature, of genome structure provides a good opportunity for the specific identification of an, individual., It may be remembered here that in the, traditional fingerprint technique, the individual, is identified by preparing an ink impression of, the skin folds at the tip of the person’s finger., This is based on the fact that the nature of these, skin folds is genetically determined, and thus the, fingerprint is unique for an individual. In, contrast, the DNA fingerprint is an analysis of, the nitrogenous base sequence in the DNA of, an individual., , History and terminology, The original DNA fingerprinting technique, was developed by Alec Jaffreys in 1985., Although the DNA fingerprinting is commonly, used, a more general term DNA profiling is, preferred. This is due to the fact that a wide, range of tests can be carried out by DNA, sequencing with improved technology.
Page 613 :
603, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , Applications of DNA fingerprinting, The amount of DNA required for DNA, fingerprint is remarkably small. The minute, quantities of DNA from blood strains, body, fluids, hair fiber or skin fragments are enough., Polymerase chain reaction is used to amplify, this DNA for use in fingerprinting. DNA profiling, has wide range of applications—most of them, related to medical forensics. Some important, ones are listed below., l, , Identification of criminals, rapists, thieves etc., , l, , Settlement of paternity disputes., , l, , Use in immigration test cases and disputes., , In general, the fingerprinting technique is, carried out by collecting the DNA from a suspect, (or a person in a paternity or immigration, dispute) and matching it with that of a reference, sample (from the victim of a crime, or a close, relative in a civil case)., , DNA MARKERS IN DISEASE, DIAGNOSIS AND FINGERPRINTING, The DNA markers are highly useful for, genetic mapping of genomes. There are four, types of DNA sequences which can be used as, markers., 1. Restriction fragment length polymorphisms, (RFLPs, pronounced as rif-lips)., 2. Minisatellites or variable number tandem, repeats (VNTRs, pronounced as vinters)., 3. Microsatellites or simple tandem repeats, (STRs)., 4. Single nucleotide polymorphisms (SNPs,, pronounced as snips)., The general aspects of the above DNA, markers are described along with their utility in, disease diagnosis and DNA fingerprinting., , R1, , R2, , R3, , DNA 1, Restriction, endonuclease, , 4 fragments, R1, , R3, DNA 2, Restriction, endonuclease, 3 fragments, , Fig. 27.22 : An outline of the restriction fragment length, polymorphism (RFLP) (R1, R2, R3 represent, the sites for the action of restriction endonucleases)., , person’s chromosomes and have no apparent, function., A DNA molecule can be cut into different, fragments by a group of enzymes called, restriction endonucleases (See Table 27.1). These, fragments are called polymorphisms (literally, means many forms)., An outline of RFLP is depicted in Fig.27.22., The DNA molecule 1 has three restriction sites, (R1, R2, R3), and when cleaved by restriction, endonucleases forms 4 fragments. Let us now, consider DNA 2 with an inherited mutation (or, a genetic change) that has altered some base, pairs. As a result, the site (R2) for the recognition, by restriction endonuclease is lost. This DNA, molecule 2 when cut by restriction endonuclease, forms only 3 fragments (instead of 4 in DNA 1)., As is evident from the above description, a, stretch of DNA exists in fragments of various, lengths (polymorphisms), derived by the action, of restriction enzymes, hence the name, restriction fragment length polymorphisms., , RESTRICTION FRAGMENT LENGTH, POLYMORPHISMS (RFLPs), , RFLPs in the diagnosis of diseases, , A RFLP represents a stretch of DNA that, serves as a marker for mapping a specified gene., RFLPs are located randomly throughout a, , If the RFLP lies within or even close to the, locus of a gene that causes a particular disease,, it is possible to trace the defective gene by the
Page 614 :
604, , BIOCHEMISTRY, , R1, , R2, , R3, , (A), DNA probe, , DNA with, restriction (R) sites, One band, , Hybridization, Two bands, Nylon membrane, , (B ), , Suspected site, R1, , R2, , One band, R3, , PCR followed, by treatment with, restriction endonucleases,, and electrophoresis, , Hybridization, , Two bands, , PCR primers, DNA with restriction map, , Fig. 27.23 : Two common methods used for scoring restriction fragment length polymorphism (RFLP), (A) RFLP by Southern hybridization (B) RFLP by polymerase chain reaction (PCR)., , analysis of RFLP in DNA. The person’s cellular, DNA is isolated and treated with restriction, enzymes. The DNA fragments so obtained are, separated by electrophoresis. The RFLP patterns, of the disease suspected individuals can be, compared with that of normal people (preferably, with the relatives in the same family). By this, approach, it is possible to determine whether the, individual has the marker RFLP and the disease, gene. With 95% certainity, RFLPs can detect, single gene-based diseases., Methods of RFLP scoring : Two methods are, in common use for the detection of RFLPs, (Fig.27.23)., 1. Southern hybridization : The DNA is, digested with appropriate restriction enzyme,, and separated by agarose gel electrophoresis., The so obtained DNA fragments are transferred, to a nylon membrane. A DNA probe that spans, the suspected restriction site is now added, and, the hybridized bands are detected by, autoradiograph. If the restriction site is absent,, then only a single restriction fragment is, detected. If the site is present, then two fragments, are detected (Fig.27.23A)., , 2. Polymerase chain reaction : RFLPs can, also be scored by PCR. For this purpose, PCR, primers that can anneal on either side of the, suspected restriction site are used. After, amplification by PCR, the DNA molecules are, treated with restriction enzyme and then, analysed by agarose gel electrophoresis. If the, restriction site is absent only one band is seen,, while two bands are found if the site is found, (Fig.27.23B)., Applications of RFLPs : The approach by, RFLP is very powerful and has helped many, genes to be mapped on the chromosomes. e.g., sickle-cell anemia (chromosome 11), cystic, fibrosis (chromosome 7), Huntington’s desease, (chromosome 4), retinoblastoma (chromosome, 13), Alzheimer’s disease (chromosome 21)., VARIABLE NUMBER, TANDEM REPEATS (VNTRs), , VNTRs, also known as minisatellites, like, RFLPs, are DNA fragments of different length., The main difference is that RFLPs develop from, random mutations at the site of restriction, enzyme activity while VNTRs are formed due to
Page 615 :
605, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , R1, , R2, , Use of RFLPs and VNTRs, in genetic fingerprinting, , DNA 1, , 4 bands, (VNTRs), Restriction enzyme, cuts here, 10 bands (VNTRs), DNA 2, R1, , R2, , Fig. 27.24 : A diagrammatic representation of variable, number tandem repeats (VNTRs). Each band (or copy), represents a repeating sequence in the DNA (e.g. 100, base pairs each). R1 and R2 indicate the sites cut by a, restriction enzyme., , RFLPs caused by variations in the number of, VNTRs between two restriction sites can be, detected (Fig.27.25). The DNAs from three, individuals with different VNTRs are cut by the, specific restriction endonuclease. The DNA, fragments are separated by electrophoresis, and, identified after hybridization with a probe, complementary to a specific sequence on the, fragments., , (A), , different number of base sequences between two, points of a DNA molecule. In general, VNTRs, are made up of tandem repeats of short base, sequences (10–100 base pairs). The number of, elements in a given region may vary, hence they, are known as variable number tandem repeats., , 2, , In the Fig.27.24, two different DNA molecules, with different number of copies (bands) of, VNTRs are shown. When these molecules are, subjected to restriction endonuclease action (at, two sites R1 and R2), the VNTR sequences are, released, and they can be detected due to, variability in repeat sequence copies. These can, be used in mapping of genomes, besides their, utility in DNA fingerprinting., , 3, , Limitations of VNTRs : The major drawback, of VNTRs is that they are not evenly distributed, throughout the genome. VNTRs tend to be, localized in the telomeric regions, at the ends of, the chromosomes., , R2, , 1, , An individual’s genome has many different, VNTRs and RFLPs which are unique to the, individual. The pattern of VNTRs and RFLPs, forms the basis of DNA fingerprinting or DNA, profiling., , VNTRs are useful for the detection of certain, genetic diseases associated with alterations in, the degree of repetition of microsatellites, e.g. Huntington’s chorea is a disorder which, is found when the VNTRs exceed 40 repeat, units., , R1, , ( B), , 1, , 2, , 3, , Fig. 27.25 : Use of restriction fragment length, polymorphisms (RFLPs) caused by variable number, tandem repeats (VNTRs) in genetic fingerprinting, (A) An illustration of DNA structure from three, individuals (B) Hybridized pattern of DNA fragment, with a probe complementary to the sequence shown in, green circles (1, 2 and 3 represent the individuals;, R1 and R2 indicate restriction sites; coloured, squares are the number of VNTRs)
Page 616 :
606, , BIOCHEMISTRY, , DNA 1, , GGCGAGAGAGAGATCT, (5 repeating units of GA), DNA 2, , GGCGAGAGAGAGAGAGAGAGAGATCT, (10 repeating units of GA), Fig. 27.26 : Two alleles of DNA molecules representing, 5 and 10 dimer repeating units., , Microsatellites are short repeat units (10–30, copies) usually composed of dinucleotide or, tetranucleotide units. These simple tandem, repeats (STRs) are more popular than, minisatellites (VNTRs) as DNA markers for two, reasons., evenly, , DNA chip technology is most commonly, used to screen SNPs hybridization with oligonucleotide (See p. 593)., , CURRENT TECHNOLOGY, OF DNA FINGERPRINTING, , MICROSATELLITES, (SIMPLE TANDEM REPEATS), , 1. Microsatellites are, throughout the genome., , An oligonucleotide is a short single-stranded, DNA molecule, synthesized in the laboratory, with a length not usually exceeding 50, nucleotides. Under appropriate conditions, this, nucleotide sequence will hybridize with a target, DNA strand if both have completely base paired, structure. Even a single mismatch in base pair, will not allow the hybridization to occur., , distributed, , In the forensic analysis of DNA, the original, techniques based on RFLPs and VNTRs are now, largely replaced by microsatellites (short tandem, repeats). The basic principle involves the, amplification of microsatellites by polymerase, chain reaction followed by their detection., It is now possible to generate a DNA, profile by automated DNA detection system, (comparable to the DNA sequencing equipment)., , 2. PCR can be effectively and conveniently, used to identify the length of polymorphism., Two variants (alleles) of DNA molecules with, 5 and 10 repeating units of a dimer nucleotides, (GA) are depicted in Fig.27.26., By use of PCR, the region surrounding the, microsatellites is amplified, separated by agarose, gel electrophoresis and identified., , SINGLE NUCLEOTIDE, POLYMORPHISMS (SNPs), , (A), CAGCTGTCGAT, CAGCTCTCGAT, (B), SNP, CAGCTGTCGAT, , Target DNA, , GTCGACAGCTA, , Oligonucleotide, , Matched base, , SNPs represent the positions in the genome, where some individuals have one nucleotide, (e.g. G) while others have a different nucleotide, (e.g. C). There are large numbers of SNPs in, genomes. It is estimated that the human genome, contains at least 3 million SNPs. Some of these, SNPs may give rise to RFLPs., , Complete and stable, hybridization of base pairs, , SNPs are highly useful as DNA markers since, there is no need for gel electrophoresis and this, saves a lot of time and labour. The detection of, SNPs is based on the oligonucleotide, hybridization analysis (Fig.27.27)., , Hybridization not formed, due to mismatch base pair, , SNP, CAGCTGTCGAT, , Target DNA, , GTCGAGAGCTA, , Oligonucleotide, , Mismatched base, , Fig. 27.27 : (A) An illustration of single nucleotide, polymorphism (SNP) (B) Oligonucleotide, hybridization to detect SNP.
Page 617 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , PHARMACEUTICAL PRODUCTS, OF DNA TECHNOLOGY, The advent of recombinant DNA technology, heralded a new chapter for the production of a, wide range of therapeutic agents in sufficient, quantities for human use. The commercial, exploitation of recombinant DNA (rDNA), technology began in late 1970s by a few, biotechnological companies to produce proteins., There are at least 400 different proteins being, produced (by DNA technology) which may serve, as therapeutic agents for humans. A selected list, of some important human proteins produced by, recombinant DNA technology potential for the, treatment of human disorders is given in, Table 27.5. As of now, only a selected few of, them (around 30) have been approved for human, use, and the most important among these are, given in Table 27.6., , INSULIN AND DIABETES, Diabetes mellitus is characterized by, increased blood glucose concentration (hyperglycemia) which occurs due to insufficient or, inefficient insulin. In the early years, insulin, isolated and purified from the pancreases of pigs, and cows was used for the treatment of severe, diabetics. This often resulted in allergies., Recombinant DNA technology has become a, boon to diabetic patients., , Production of recombinant insulin, Attemps to produce insulin by recombinant, DNA technology started in late 1970s. The basic, technique consisted of inserting human insulin, gene and the promoter gene of lac operon on to, the plasmids of E. coli. By this method human, insulin was produced. It was in July 1980,, seventeen human volunteers were, for the first, time, administered recombinant insulin for, treatment of diabetes at Guy’s Hospital, London., And in fact, insulin was the first ever, pharmaceutical product of recombinant DNA, technology, administered, to, humans., Recombinant insulin worked well, and this gave, hope to scientists that DNA technology could be, successfully employed to produce substances of, , 607, , TABLE 27.5 A selected list of human proteins, produced by recombinant DNA technology for, treatment of human disorders, , Disorder, Anemia, Asthma, Atherosclerosis, Delivery, Blood clots, Burns, Cancer, , Diabetes, Emphysema, Female infertility, Free radical damage, (minimizing), Growth defects, , Recombinant protein(s), Hemoglobin, erythropoietin, Interleukin-I receptor, Platelet-derived growth, factor, Relaxin, Tissue plasminogen, activator, urokinase, Epidermal growth factor, Interferons, tumor necrosis, factor, colony stimulating, factors, interleukins,, lymphotoxin, macrophageactivating factor, Insulin, insulin-like growth, factor, D1-Antitrypsin, Chorionic gonadotropin, Superoxide dismutase, , Growth hormone, growth, hormone-releasing factor,, somatomedin-C, Heart attacks, Prourokinase, Hemophilia A, Factor VIII, Hemophilia B, Factor IX, Hepatitis B, Hepatitis B vaccine, Hypoalbuminemia, Serum albumin, Immune disorders, Interleukins, E-cell growth, factors, Kidney disorders, Erythropoietin, Lou Gehrig’s disease, Brain-derived neurotropic, (amytrophic lateral sclerosis) factor, Multiple sclerosis, Interferons (D, E, J), Nerve damage, Nerve growth factor, Osteomalacia, Calcitonin, Pain, Endorphins and, enkephalins, Rheumatic disease, Adrenocorticotropic, hormone, Ulcers, Urogastrone, Viral infections, Interferons (D, E, J)
Page 618 :
608, , BIOCHEMISTRY, , TABLE 27.6 A selected list of rDNA-derived therapeutic agents, (approved by FDA) with trade names and their applications in humans, , rDNA product, Insulin, Growth hormone, D-Interferon, Hepatitis B vaccine, Tissue plasminogen activator, Factor VIII, DNase, Erythropoietin, , Trade name(s), , Applications/uses, , Humulin, Protropin/Humatrope, Intron A, Recombinax HB/Engerix B, Activase, Kogenate/Recombinate, Pulmozyme, Epogen/Procrit, , medical and commercial importance. An, approval, by the concerned authorities, for using, recombinant insulin for the treatment of, diabetes mellitus was given in 1982. And in, 1986, Eli Lilly company received approval to, market human insulin under the trade name, Humulin., , Diabetes, Pituitary dwarfism, Hairy cell leukemia, Hepatitis B, Myocardial infarction, Hemophilia, Cystic fibrosis, Severe anemia with kidney damage, , O, , Z, , O, Gene for, insulin, A chain, , P, I, , RECOMBINANT VACCINES, Recombinant DNA technology in recent, years, has become a boon to produce new, generation vaccines. By this approach, some of, , I, , Plasmid, , Gene for, insulin, B chain, , Plasmid, Transform into, E. coli, , Technique for production of recombinant, insulin : The orginal technique (described briefly, above) of insulin synthesis in E. coli has, undergone several changes, for improving the, yield. e.g. addition of signal peptide, synthesis of, A and B chains separately etc., The procedure employed for the synthesis of, two insulin chains A and B is illustrated in, Fig.27.28. The genes for insulin A chain and B, chain are separately inserted to the plasmids of, two different E. coli cultures. The lac operon, system (consisting of inducer gene, promoter, gene, operator gene and structural gene Z for, E-galactosidase) is used to express both the, genes. The presence of lactose in the culture, medium induces the synthesis of insulin A and B, chains in separate cultures. The so formed, insulin chains can be isolated, purified and, joined together to give a full-fledged human, insulin., , Z, , P, , E. coli, , E. coli, , Culture cells in, a nutrient medium, containing lactose., Isolate and purify, A and B chains, , B chain, , A chain, , Joining of chains, and, reconstitution, , Human insulin, , Fig. 27.28 : The production of recombinant insulin in, E. coli (I-Inducer gene, P-Promoter gene,, O-Operator gene, Z-E-Galactosidase gene; all these, genes are of lac operon system).
Page 619 :
609, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , the limitations (low yield, high cost, side effects), of traditional vaccine production could be, overcome., The list of diseases for which recombinant, vaccines are developed or being developed is, given in Table 22.7. It may be stated here that, due to very stringent regulatory requirements to, use in humans, the new generation vaccines are, first tried in animals, and it may take some more, years before most of them are approved for use, in humans., , Hepatitis B vaccine, —the first synthetic vaccine, In 1987, the recombinant vaccine for hepatitis, B (i.e. HBsAg) became the first synthetic vaccine, for public use. It was marketed by trade names, Recombivax and Engerix-B. Hepatitis B vaccine, is safe to use, very effective and produces no, allergic reactions. For these reasons, this, recombinant vaccine has been in use since, 1987. The individuals must be administered, three doses over a period of six months., Immunization against hepatitis B is strongly, recommended to anyone coming in contact with, blood or body secretions. All, the health, professionals—physicians, surgeons, medical, laboratory technicians, nurses, dentists, besides, police officers, firefighters etc., must get, vaccinated against hepatitis B., , Hepatitis B vaccine in India, India is the fourth country (after USA, France, and Belgium) in the world to develop an, indigenous hepatitis B vaccine. It was launched, in 1997, and is now being used., , DNA VACCINES, (GENETIC IMMUNIZATION), Genetic immunization by using DNA, vaccines is a novel approach that came into, being in 1990. The immune response of the, body is stimulated by a DNA molecule sequence, of pathogen’s genome. This DNA is basically a, , TABLE 27.7 A selected list of diseases along, with the pathogenic organisms for which, recombinant vaccines are developed, or being developed, , Disease, , Pathogenic organism, , Viral diseases, Acute infantile, gastroenteritis, , Rotavirus, , Acute respiratory, diseases, , Influenza A and B viruses, , AIDS, , Human immunodeficiency virus, , Chicken pox, , Varicella–zoster virus, , Encephalitis, , Japanese encephalitis virus, , Genital ulcers, , Herpes simplex virus type–2, , Hemorrhagic fever, , Dengue virus, , Liver damage, , Hepatitis A virus, , Liver damage, , Hepatitis B virus, , Upper and lower, Yellow fever virus, respiratory tract lesions, Bacterial diseases, Cholera, , Vibrio cholerae, , Diarrhea, , E. coli, , Dysentery, , Shigella strain, , Gonorrhea, , Niesseria gonorroheae, , Leprosy, , Mycobacterium leprae, , Meningitis, , Neisseria meningitidis, , Pneumonia, , Streptococcus pneumoniae, , Rheumatic fever, , Streptococcus group A, , Tetanus, , Clostridium tetani, , Tuberculosis, , Mycobacterium tuberculosis, , Typhoid, , Salmonella typhi, , Urogenital tract, infection, , Streptococcus group B, , Parasitic diseases, Filariasis, , Wuchereria bancrofti, , Malaria, , Plasmodium sp, , River blindness, , Onchocerca volvulus, , Schistosomiasis, , Schistosoma mansoni, , Sleeping sickness, , Trypanosoma sp
Page 620 :
610, , BIOCHEMISTRY, , bacterial plasmid engineered to include the, sequence of an antigenic protein from the, pathogen. After its entry into different cell types,, this DNA can be expressed there using cellular, transcription and translation machinary. Thus,, DNA vaccines behave like viruses. They cannot,, however become infectious due to limited amout, of genetic information they contain., DNA vaccine—plasmids can be administered, to the animals by one of the following delivery, methods., , l, , Nasal spray, , l, , Intramuscular injection, , l, , Intravenous injection, , l, , Intradermal injection, , l, , Gene gun or biolistic delivery (involves, pressure delivery of DNA-coated gold beads)., , DNA VACCINE AND IMMUNITY, An illustration of a DNA vaccine and, the mechanism of its action in developing, , + Biotechnology is a newly discovered discipline for age-old practices (e.g. preparation of, curd, wine, beer), with special emphasis on genetic manipulations., , + Human artificial chromosome (HAC) is a synthetic vector, possessing the characteristics, of human chromosome. HAC is capable of carrying large-sized human genes that may, be useful in gene therapy., , + Southern blotting technique (that specifically detects DNA) is employed for the, identification of thieves, rapists, and settlement of parenthood., , + Polymerase chain reaction is useful for the diagnosis of inherited diseases, in DNA, sequencing, and in forensic medicine., , + By employing site-directed mutagenesis, it is possible to produce more efficient and, more suitable enzymes for therapeutic and industrial purposes., , + The analysis of genetic material DNA (gene/genes) is employed for the diagnosis of, certain diseases, and in medical forensics e.g. AIDS, sickle-cell anemia, certain cancers,, DNA fingerprinting., , + The pharmaceutical products of rDNA technology have revolutionized the treatment of, certain diseases e.g. diabetes, asthma, atherosclerosis, heart attacks, hemophilia., , + Recombinant vaccine for hepatitis B is the first synthetic vaccine. It is effective, safe, and produces no allergic reactions., , + Genetic immunization by using DNA vaccines is a novel concept. It has been shown that, the immune response (humoral and cellular) of the body can be stimulated by a DNA, molecule., , + Transgenic mice that serve as animal models for human diseases have been developed., These include human mouse (model for immune system), Alzheimer’s mouse,, oncomouse (model for cancer), prostate mouse, knockout mice (for allergy,, transplantation etc.)., , + Transgenic animals serve as bioreactors for the production of therapeutically important, proteins e.g. interferon, lactoferrin, urokinase., , + Certain pet animals (cats, dogs) are being cloned by some companies.
Page 621 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , immunity is given in Fig.27.29. The plasmid, vaccine carrying the DNA (gene) for antigenic, protein enters the nucleus of the inoculated, target cell of the host. This DNA produces, RNA, and in turn the specific antigenic, protein. The antigen can act directly for, developing humoral immunity or as fragments in, association with major histocompatability class, (MHC) molecules for developing cellular, immunity. DNA vaccines may hold some, promise for vaccination against cancer, HIV,, malaria, tuberculosis etc., , Humoral immunity, As the antigens bind to B-lymphocytes, they, trigger the production of antibodies which, can destroy the pathogens. Some of the, B-lymphocytes become memory cells that can, protect the host against future infections., , Cellular immunity, The protein fragments of the antigen bound to, MHC molecules can activate the cytotoxic, T-lymphocytes. They are capable of destroying, the infected pathogenic cells. Some of, the activated T-lymphocytes become memory, cells which can kill the future infecting, pathogens., , TRANSGENIC ANIMALS, With the advent of modern biotechnology, it, is now possible to carry out manipulations at the, genetic level to get the desired characteristics in, animals. Transgenesis refers to the phenomenon, of introduction of exogeneous DNA into the, genome to create and maintain a stable, heritable character. The foreign DNA that is, introduced is called transgene. And the animal, whose genome is altered by adding one or more, transgenes is said to be transgenic. The, transgenes behave like other genes present in the, animals’ genome, and are passed on to the, offsprings. Thus, transgenic animals are, genetically engineered or genetically modified, organisms (GMOs) with a new heritable, character., , 611, , Importance of transgenic, animals—general, Transgenesis has now become a powerful tool, for studying the gene expression and, developmental processes in higher organisms,, besides the improvement in their genetic, characteristics. Transgenic animals serve as good, models for understanding the human diseases., Further, several proteins produced by transgenic, animals are important for medical and, pharmaceutical, applications., Thus,, the, transgenic farm animals are a part of the, lucrative world-wide biotechnology industry,, with great benefits to mankind., , TRANSGENIC MICE AND, THEIR APPLICATIONS, Mouse, although not close to humans in its, biology, has been and continues to be the most, exploited animal model in transgenesis, experiments. The common feature between man, and mouse is that both are mammals. Transgenic, mice are extensively used as animal models for, understanding human diseases, and for the, production of therapeutic agents. Adequate care,, however, must be exercised before extrapolating, data of transgenic mice to humans., Mouse models for several human diseases, (cancers,, muscular, dystrophy,, arthritis,, Alzheimer’s disease, hypertension, allergy,, coronary heart disease, endocrine diseases,, neurodegenerative, disorders, etc.), have, been developed. A selected few of them are, listed., l, , l, , l, , l, , l, , The human mouse, the transgenic mouse that, displays human immune system., The Alzheimer’s mouse to understand the, pathological basis of Alzheimer’s disease., The oncomouse, the animal model for cancer., The prostate mouse, the transgenic mouse to, understand prostate cancer., The knockout mice, (developed by eliminating, specific genes) for certain diseases e.g., SCID mouse, knockout mouse for transplantation.
Page 622 :
B-lymphocytes, with antigens, , T-lymphocyte, bound to antigen, , Fragments, of antigen, , Antigen, , Activated cytotoxic, T-lymphocytes, , CELLULAR IMMUNITY, , Memory cytotoxic T-lymphocyte, (protects against future infection), , Kill pathogenic, cells, , HUMORAL IMMUNITY, , Antibodies, , Memory B-lymphocyte, (protects against future infection), , Fig. 27.29 : DNA vaccine and mechanism of its action in developing immunity (MHC–Major histocompatability complex molecule), , Cellular, Nucleus, DNA, Inoculated, cell, , MHC, , Antigenic protein, , mRNA, , Plasmid vaccine, , DNA for, antigenic protein, , 612, BIOCHEMISTRY
Page 623 :
613, , Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , ANIMAL BIOREACTORS, Transgenesis is wonderfully utilized for, production of proteins for pharmaceutical and, medical use. In fact, any protein synthesized in, the human body can be made in the transgenic, animals, provided that the genes are correctly, programmed. The advantage with transgenic, animals is to produce scarce human proteins in, huge quantities. Thus, the animals serving as, factories for production of biologically, important products are referred to as animal, bioreactors or sometimes pharm animals. Some, transgenic animals that serve as bioreactors are, listed, l, , l, , l, , l, , Transgenic cow for the, lactoferrin and interferons., , production, , Mammary gland cell, , Ovum with nucleus, , Five days, nutrient, deprivation, , of, , Transgenic, goat, to, synthesize, tissue, plasminogen activator, and antithrombin III., Transgenic mouse for the production of, immunoglobulins, and urokinase., , Dormant totipotent cell, , Enucleated ovum, Fuse, Activate, , Transgenic pig to produce hemoglobin., , DOLLY – THE TRANSGENIC CLONE, Dolly, the first ever mammal clone was, developed by Wilmut and Campbell in 1997. It, is a sheep (female lamb) with a mother and no, father., The technique primarily involves nuclear, transfer and the phenomenon of totipotency., The character of a cell to develop into different, cells, tissues, organs, and finally an organism is, referred to as totipotency or pluripotency., Totipotency is the basic character of embryonic, cells. As the embryo develops, the cells, specialize to finally give the whole organism. As, such, the cells of an adult lack totipotency., Totipotency was induced into the adult cells for, developing Dolly., The cloning of sheep for producing Dolly,, illustrated in Fig.27.30, is briefly described here., The mammary gland cells from a donor ewe, were isolated. They were subjected to total, nutrient deprivation (starvation) for five days. By, this process, the mammary cells abandon their, , Fused cell, (mammary cell nucleus with ovum envelope), In vitro, embryo culture, , Embryo, Implant, , Foster mother, , Dolly, , Fig. 27.30 : The cloning of sheep for, developing Dolly.
Page 624 :
614, , BIOCHEMISTRY, , normal growth cycle, enter a dormant stage and, regain totipotency character. An ovum (egg cell), was taken from another ewe, and its nucleus was, removed to form an enucleated ovum. The, dormant mammary gland cell and the enucleated, ovum were fused by pulse electricity. The, mammary cell outer membrane was broken,, allowing the ovum to envelope the nucleus. The, fused cell, as it had gained totipotency, can, multiply and develop into an embryo. This, embryo was then implanted into another ewe, which served as a surrogate/foster mother. Five, months later, Dolly was born., As reported by Wilmut and Campbell, they, fused 277 ovum cells, achieved 13 pregnancies,, and of these only one pregnancy resulted in live, birth of the offspring-Dolly., , CLONING OF PET ANIMALS, Some of the companies involved in transgenic, experiments have started cloning pet animals like, cats and dogs. Little Nicky was the first pet cat, that was cloned at a cost of $50,00 by an, American company (in Dec. 2004). More cloned, cats and dogs will be made available to, interested parties (who can afford) in due course., Some people who own pet animals are, interested to continue the same pets which is, possible through cloning. There is some, opposition to this approach as the cloned, animals are less healthy, and have shorter life, span, besides the high cost factor., , BIOTECHNOLOGY AND SOCIETY, Advances in biotechnology, and their, applications are most frequently associated with, controversies. Based on their perception to, biotechnology, the people may be grouped into, three broad categories., 1. Strong opponents who oppose the new, technology, as it will give rise to problems, issues, and concerns humans have never faced before., They consider biotechnology as an unnatural, manipulative technology., , 2. Strong proponents who consider that the, biotechnology will provide untold benefits to, society. They argue that for centuries the society, has safely used the products and processes of, biotechnology., 3. A neutral group of people who have a, balanced approach to biotechnology. This group, believes that research on biotechnology (with, regulatory systems), and extending its fruits to, the society should be pursued with a cautious, approach., , BENEFITS OF BIOTECHNOLOGY, The fruits of biotechnology are beneficial to, the fields of healthcare, agriculture, food, production, manufacture of industrial enzymes, and appropriate environmental management., It is a fact that modern technology in various, forms is woven tightly into the fabric of our lives., Our day-to-day life is inseparable from, technology. Imagine life about 1-2 centuries ago, where there was no electricity, no running water,, sewage in the streets, unpredictable food supply, and an expected life span of less than 40 years., Undoubtedly, technology has largely contributed, to the present day world we live in. Many, pepople consider biotechnology as a technology, that will improve the quality of life in every, country, besides maintaining living standards at, a reasonably higher level., , ELSI OF BIOTECHNOLOGY, Why so much uproar and negativity to, biotechnology? This is mainly because the major, part of the modern biotechnology deals with, genetic manipulations. These unnatural genetic, manipulations, as many people fear, may lead to, unknown consequences., ELSI is the short form to represent the ethical,, legal and social implications of biotechnology., ELSI broadly covers the relationship between, biotechnology and society with particular, reference to ethical and legal aspects., , Risks and ethics of biotechnology, The modern biotechnology deals with genetic, manipulations of viruses, bacteria, plants,
Page 625 :
Chapter 27 : RECOMBINANT DNA AND BIOTECHNOLOGY, , animals, fish and birds. Introduction of foreign, genes into various organisms raises concerns, about the safety, ethics and unforeseen, consequences. Some of the popular phrases used, in the media while referring to experiments on, recombinant DNA technology are listed., l, , Manipulation of life, , l, , Playing God, , l, , Man-made evolution, , The major apprehension of genetic, engineering is that through recombinant DNA, experiments, unique microorganisms or viruses, (either inadvertently, or sometimes deliberately, for the purpose of war) may be developed that, would cause epidemics and environmental, catastrophes. Due to these fears, the regulatory, , 615, , guidelines for research dealing with DNA, manipulation were very stringent in the earlier, years., So far, risk assessment studies have failed to, demonstrate any hazardous properties acquired, by host cells/organisms due to transfer of, DNA. Thus, the fears of genetic manipulations, may be unfounded to a large extent., Consequently, there has been some relaxation in, the regulatory guidelines for recombinant DNA, research., It is now widely accepted that biotechnology, is certainly beneficial to humans. But it should, not cause problems of safety to people and, environment, and create unacceptable social,, moral and ethical issues., , 1. Recombinant DNA (rDNA) technology is primarily concerned with the manipulation of, genetic material (DNA) to achieve the desired goal in a pre-determined way., 2. The procedure for rDNA technology involves molecular tools (enzymes e.g. restriction, endonucleases), host cells (E. coli, S. cerevisiae), vectors (plasmids, bacteriophages), gene, transfer (transformation, electroporation) and the strategies of gene cloning., 3. Blotting techniques are employed for the identification of desired DNA (Southern blot),, RNA (Northern blot), and protein (Western blot)., 4. Polymerase chain reaction is an in vitro technique for generating large quantities of a, specified DNA i.e. cell-free amplification., 5. Gene libraries or genomic libraries represents the collection of DNA fragments (i.e., genes) from a genome of a particular species., 6. Site-directed mutagenesis is the technique for generating amino acid coding changes in, the DNA (gene) to produce a desired protein/enzyme., 7. Analysis of DNA (i.e. detection of gene/genes) can be used as a diagnostic system for, the detection of many pathogenic and genetic diseases e.g. tuberculosis, malaria, AIDS,, sickle-cell anemia, certain cancers., 8. DNA fingerprinting or DNA profiling is the present day genetic detective in the practice, of modern medical forensics. Four types of DNA markers are used in DNA, fingerprinting–RFLFs, VNTRs, STRs, and SNPs., 9. Many pharmaceutical compounds of health importance (for disease treatment) are being, produced by rDNA technology e.g. insulin, growth hormone, interferons, erythropoietin,, hepatitis B vaccine., 10. Transgenic animals can be developed by introducing a foreign DNA (transgene). These, animals are genetically modified or engineered with new heritable characters e.g., oncomouse, knockout mouse, prostate mouse.
Page 626 :
616, , BIOCHEMISTRY, , I. Essay questions, 1., 2., 3., 4., 5., , Describe the basic principles underlying the recombinant DNA technology., Give an account of the nucleic acid blotting techniques. Add a note on their importance., Describe the polymerase chain reaction along with its applications., Write briefly on the utility of DNA in disease diagnosis and medical forensics., Give an account of the pharmaceutical products of DNA technology., , III. Short notes, (a) Restriction endonucleases, (b) Plasmids, (c) Methods of gene transfer, (d) Purification of nucleic, acids, (e) Western blotting, (f) DNA sequencing, (g) DNA chips (h) Gene libraries, (i) Restriction, fragment length polymorphisms, (j) Recombinant vaccines., , III. Fill in the blanks, 1. The most commonly used prokaryotic host in rDNA technology is ______________., 2. Northern blotting technique is used for the detection of ______________., 3. Name the blotting technique in which nucleic acids (DNA or RNA) are directly blotted onto the, filters without electrophoresis______________., 4. The bacterial source of the enzyme Taq DNA polymerase, that is widely used in polymerase, chain reaction ______________., 5. The collection of DNA fragments from the genome of a particular species represents, ______________., 6. The technique for generating amino acid coding changes in the DNA (gene) is regarded as, ______________., 7. The trade name for insulin produced by rDNA technology ______________., 8. The first synthetic vaccine developed by rDNA technology ______________., 9. The most commonly used animal model in transgenesis to represent humans ______________., 10. Name the first ever mammmal that has been cloned ______________., , IV. Multiple choice questions, 11. One of the following enzyme produces single-stranded nicks in DNA, (a) DNA ligase (b) DNA polymerase (c) DNase I (d) SI nuclease., 12. Western blotting is the technique for the identification of, (a) DNA (b) RNA (c) Carbohydrates (d) Proteins., 13. The DNA markers used in the diagnosis of diseases and DNA fingerprinting, (a) Restriction fragment length polymorphisms, (b) Minisatellites and microsatellites, (c) Single, nucleotide polymorphisms, (d) Any one of the above., 14. The first pharmaceutical product of recombinant DNA technology approved for human use, (a) Insulin (b) Growth hormone (c) Interferon (d) Hypatitis B vaccine., 15. Genetic immunization involves the administration of, (a) Antigens (b) Antibodies (c) DNA (d) RNA.
Page 627 :
CURRENT TOPICS, 28, ■, 29, ■, 30, ■, 31, ■, 32, ■, 33, ■, 34, ■, 35, ■, 36, ■, 37, ■, 38, ■, , Human Genome Project, , 619, , Gene Therapy, , 625, , Bioinformatics, , 634, , Metabolism of Xenobiotics, (Detoxification), , 638, , Prostaglandins and Related, Compounds, , 644, , Biological Membranes and, Transport, , 650, , Free Radicals and, Antioxidants, , 655, , Environmental Biochemistry, , 662, , Insulin, Glucose Homeostasis,, and Diabetes Mellitus, 669, Cancer, , 685, , Acquired Immunodeficiency, Syndrome (AIDS), , 695, , Section, , VI, VI
Page 628 :
“This page intentionally left blank"
Page 629 :
Section 6, , Current Topics, , Chapter, , Human Genome Project, , 28, , The Human Genome speaks :, , “I am the outcome of international collaborative research;, Composed of 3.2 billion nucleotide base pairs;, With 30,000 to 40,000 protein coding genes;, That represents only 1.1 to 1.5% of the genome”, , T, , he most important features of a DNA, molecule are the nucleotide sequences, and, the identification of genes and their activities., Since 1920, scientists have been working to, determine the sequences of pieces of DNA., , THE BIRTH AND ACTIVITY OF, HUMAN GENOME PROJECT, The human genome project (HGP) was, conceived in 1984, and officially begun in, earnest in October 1990. The primary objective, of HGP was to determine the nucleotide, sequence of the entire human nuclear genome., In addition, HGP was also entrusted to elucidate, the genomes of several other model organisms, e.g. Escherichia coli, Saccharomyces cerevisiae, (yeast), Caenorhabditis elegans (roundworm),, Mus musculus (mouse). James Watson (who, elucidated DNA structure) was the first Director, of HGP., In 1997, United States established the, National Human Genome Research Institute, (NHGRI). The HGP was an international venture, , involving research groups from six countries —, USA, UK, France, Germany, Japan and China,, and several individual laboratories and a large, number of scientists and technicians from, various disciplines. This collaborative venture, was named as International Human Genome, Sequencing Consortium (IHGSC) and was, headed by Francis Collins. A total expenditure of, $3 billion, and a time period of 10–15 years for, the completion of HGP was expected. A second, human genome project was set up by a private, company — Celera Genomics, of Maryland USA, in 1998. This team was led by Craig Venter., , Announcement of the draft, sequence of human genome, The date 26th June 2000 will be remembered, as one of the most important dates in the history, of science or even mankind. It was on this day,, Francis Collins and Craig Venter, the leaders of, the two human genome projects, in the presence, of the President of U.S., jointly announced the, working drafts of human genome sequence. The, detailed results of the teams were later published, , 619
Page 630 :
620, , BIOCHEMISTRY, , Cytogenetic map, , Gene linkage map, Gene, , Gene, , Restriction fragments, , Restriction fragment, map, , Physical map, Base sequence, , Fig. 28.1 : Different types of genome maps., , in February 2001 in scientific journals Nature, (IHGSC) and Science (Celera Genomics)., The human genome project results attracted, worldwide attention. This achievement was, hailed with many descriptions in the media., l, , The mystery of life unravelled., , l, , The library of life., , l, , The periodic table of life., , l, , The Holy grail of human genetics., , MAPPING OF THE HUMAN GENOME, The most important objective of human, genome project was to construct a series of maps, for each chromosome. In Fig.28.1, an outline of, the different types of maps is given., 1. Cytogenetic map : This is a map of the, chromosome in which the active genes respond, to a chemical dye and display themselves as, bands on the chromosome., 2. Gene linkage map : A chromosome map, in which the active genes are identified by, locating closely associated marker genes. The, most commonly used DNA markers are, restriction fragment length polymorphism, (RFLP), variable number tandem repeats, (VNTRs) and short tandem repeats (STRs)., VNTRs are also called as minisatellites while, STRs are microsatellites., 3. Restriction fragment map : This consists of, the random DNA fragments that have been, sequenced., , 4. Physical map : This is the ultimate map of, the chromosome with highest resolution base, sequence. Physical map depicts the location of, the active genes and the number of bases, between the active genes., , APPROACHES FOR, GENOME SEQUENCING, A list of different methods used for mapping, of human genomes is given in Table 28.1. These, techniques are also useful for the detection of, normal and disease genes in humans., For elucidating human genome, different, approaches were used by the two HGP groups., IHGSC predominantly employed map first and, sequence later approach. The principal method, was heirarchical shotgun sequencing. This, technique involves fragmentation of the genome, into small fragments (100–200 kb), inserting, them into vectors (mostly bacterial artificial, chromosomes, BACs) and cloning. The cloned, fragments could be sequenced., , Celera Genomics used whole genome shotgun, approach. This bypasses the mapping step and, saves time. Further, Celera group was lucky to, have high-throughput sequenators and powerful, computer programmes that helped for the early, completion of human genome sequence., , Whose genome was sequenced?, One of the intriguing questions of human, genome project is whose genome is being, sequenced and how will it relate to the 6 billion, or so population with variations in world? There, is no simple answer to this question. However,, looking from the positive side, it does not matter, whose genome is sequenced, since the, phenotypic differences between individuals are, due to variations in just 0.1% of the total genome, sequences. Therefore many individual genomes, can be used as source material for sequencing., Much of the human genome work was, performed on the material supplied by the Centre, for Human Polymorphism in Paris, France. This, institute had collected cell lines from sixty, different French families, each spanning three, generations. Thus, the material supplied from, Paris was used for human genome sequencing.
Page 631 :
621, , Chapter 28 : HUMAN GENOME PROJECT, , TABLE 28.1 A list of principal methods used for, mapping of genomes (and also normal and, disease genes in humans), , Method, , Comments, , DNA sequencing, , Physical map of DNA can be, identified with highest, resolution., , Use of probes, , To identify RFLPs, STS and, SNPs., , HUMAN GENOME SEQUENCE—, RESULTS SUMMARISED, The information on the human genome, projects is too vast, and only some highlights, can be given (Table 28.2). Some of them are, briefly described., TABLE 28.2 Major highlights of human genome, l, , Radiation hybrid mapping Fragment genome into large, pieces and locate markers, and genes. Requires somatic, cell hybrids., , l, , Fluorescence in situ, hybridization (FISH), , To localize a gene on, chromosome., , l, , Sequence tagged site, (STS) mapping, , Applicable to any part of DNA, sequence if some sequence, information is available., , Expressed sequence, tag (EST) mapping, , A variant of STS mapping;, expressed genes are actually, mapped and located., , Pulsed-field gel, electrophoresis (PFGE), , For the separation and, isolation of large DNA, fragments., , Cloning in vectors, (plasmids, phages,, cosmids, YACs, BACs), , To isolate DNA fragments of, variable lengths., , Polymerase chain, reaction (PCR), , To amplify gene fragments, , Chromosome walking, , Useful for cloning of, overlapping DNA fragments, (restricted to about 200 kb)., , Chromosome jumping, , DNA can be cut into large, fragments and circularized for, use in chromosome walking., , Detection of cytogenetic, abnormalities, , Certain genetic diseases can, be identified by cloning the, affected genes e.g. Duchenne, muscular dystrophy., , Databases, , Existing databases facilitate, gene identification by, comparison of DNA and, protein sequences., , (RFLP–Restriction fragment length polymorphism; STS–Sequence, tagged site; SNP–Single nucleotide polymorphism; YAC–Yeast, artificial chromosome; BAC–Bacterial artificial chromosome), , l, , l, , l, , l, , l, , l, , l, , l, , l, , l, , l, , The draft represents about 90% of the entire human, genome. It is believed that most of the important parts, have been identified., The remaining 10% of the genome sequences are at the, very ends of chromosomes (i.e. telomeres) and around, the centromeres., Human genome is composed of 3200 Mb (or 3.2 Gb) i.e., 3.2 billion base pairs (3,200,000,000)., Approximately 1.1 to 1.5% of the genome codes for, proteins., Approximately 24% of the total genome is composed of, introns that split the coding regions (exons), and appear, as repeating sequences with no specific functions., The number of protein coding genes is in the range of, 30,000–40,000., An average gene consists of 3000 bases, the sizes, however vary greatly. Dystrophin gene is the largest, known human gene with 2.4 million bases., Chromosome 1 (the largest human chromosome) contains, the highest number of genes (2968), while the Y, chromosome has the lowest. Chromosomes also differ in, their GC content and number of transposable elements., Genes and DNA sequences associated with many, diseases such as breast cancer, muscle diseases,, deafness and blindness have been identified., About 100 coding regions appear to have been copied, and moved by RNA–based transposition (retrotransposons)., Repeated sequences constitute about 50% of the human, genome., A vast majority of the genome (~ 97%) has no known, functions., Between the humans, the DNA differs only by 0.2% or, one in 500 bases., More than 3 million single nucleotide polymorphisms, (SNPs) have been identified., , l, , Human DNA is about 98% identical to that of chimpanzees., , l, , About 200 genes are close to that found in bacteria.
Page 632 :
622, , BIOCHEMISTRY, , Human genome, 3200 Mb, , Genes and related, gene sequences, 1200 Mb, , Genes, 48 Mb, , Intergenic DNA, 2000 Mb, , Related gene, sequences, 1152 Mb, , LINEs, 640 Mb, , SINEs, 420 Mb, , LTR elements, 250 Mb, , Others, 510 Mb, , DNA transposons, 90 Mb, Microsatellites, 90 Mb, , Fig. 28.2 : An overview of the organization of human genome (LINEs-Long interspersed nuclear elements;, SINEs–Short interspersed nuclear elements; LTR-Long terminal repeats)., , Most of the genome sequence, is identified, About 90% of the human genome has been, sequenced. It is composed of 3.2 billion base, pairs (3200 Mb or 3.2 Gb). If written in the, format of a telephone book, the base sequence, of human genome would fill about 200, telephone books of 1000 pages each. Some other, interesting analogs/sidelights of genome are, given in Table 28.3., Individual differences in genomes : It has to, be remembered that every individual, except, identical twins, have their own versions of, genome sequences. The differences between, individuals are largely due to single nucleotide, polymorphisms (SNPs). SNPs represent positions, in the genome where some individuals have one, nucleotide (i.e. an A), and others have a different, nucleotide (i.e. a G). The frequency of, occurrence of SNPs is estimated to be one per, 1000 base pairs. About 3 million SNPs are, believed to be present and at least half of them, have been identified., , Organization of human genome, An outline of the organization of the human, genome is given in Fig.28.2. Of the 3200 Mb,, , TABLE 28.3 Some interesting analogs/sidelights, about human genome, l, , l, , l, , l, , l, , l, , The base sequence in human genome would fill about, 200 telephone books of 1000 pages each., If the genome is recited at the rate of one base per, second for 24 hours a day, it would take a century to, recite the book of life., If a typist types at the rate of 60 words per minute, (i.e. 360 letters) for 8 hours a day, he/she would take, around 50 years to type human genome., If the DNA sequence is typed in lines 10 cm containing, 60 nucleotide bases and printed, the human genome, sequence (from a single cell) would stretch a distance, of 5000 km., If the DNA in the entire human body is put end to end,, it would reach to the sun and back over 600 times, (Note : The human body contains 100 trillion cells; the, length of DNA in a cell is 6 feet; the distance between, the sun and earth is 93 million miles)., The total expenditure for human genome project was, $3 billion. The magnitude of this huge amount has to be, appreciated. If one starts counting at a non–stop rate of, a dollar per second, it would take about 90 years to, complete.
Page 633 :
623, , Chapter 28 : HUMAN GENOME PROJECT, , Upstream, , Exon, , Intron, , Exon, , Intron, , Exon, , Start of, biological information, , Downstream, , End of biological, information, , Fig. 28.3 : A diagrammatic representation of a typical structure of an average human gene., , A broad categorization of human gene catalog, in the form of a pie chart is depicted in Fig.28.4., About 17.5% of the genes participate in the, general biochemical functions of the cells, 23%, in the maintenance of genome, 21% in signal, transduction while the remaining 38% are, involved in the production of structural proteins,, transport proteins, immunoglobins etc., , BENEFITS/APPLICATIONS OF, HUMAN GENOME SEQUENCING, It is expected that the sequencing of human, genome, and the genomes of other organisms, will dramatically change our understanding and, perceptions of biology and medicine. Some of, the benefits of human genome project are given., Identification of human genes and their, functions., mical, che, lls, bio f the ce, l, a o, s, n, , G, fun en, cti er, o, , l, , 17.5%, , Sig, na, l tr, an, , 21.1%, , 38.2%, , Mainten, replica ance, tion of, e g, x, pre eno, ss, i, , A diagrammatic representation of a typical, structure of an average human gene is given in, Fig.28.3. It has exons and introns., , The major categories of the proteins encoded, by human genes are listed in Table 28.4. The, functions of at least 40% of these proteins are, not known., , tion, uc, sd, , Before the results of the HGP were, announced, the best guess of human genes was, in the range of 80,000–100,000. This estimate, was based on the fact that the number of proteins, in human cells is 80,000–100,000, and thus so, many genes expected. The fact that the number, of genes is much lower than the proteins suggests, that the RNA editing (RNA processing) is, widespread, so that a single mRNA may code for, more than one protein., , As already described, a huge portion of the, genome is composed of introns, and intergenic, sequences (junk DNA)., , ns, ctio, bulins, fun unoglo, .), m, c, t, im ins e, e, t, o, , The two genome projects differ in their, estimates of the total number of genes in, humans. Their figures are in the range of 30,000–, 40,000 genes. The main reason for this variation, is that it is rather difficult to specifically, recognize the DNA sequences which are genes, and which are not., , It is now clear that only 1.1-1.5% of the, human genome codes for proteins. Thus, this, figure 1.1-1.5% represents exons of genome., , 23.2%, , ,, me n, o, , Genes present in human genome, , Human genes encoding proteins, , (structu Vari, r, a, l ou, tran prot s ot, e h, s, p, or ins, er, tp, r, , only a small fraction (48 Mb) represents the, actual genes, while the rest is due to gene-related, sequences (introns, pseudogenes) and intergenic, DNA (long interspersed nuclear elements, short, interspread nuclear elements, microsatellites,, DNA transposons etc.). Intergenic DNA, represents the parts of the genome that lie, between the genes which have no known, function. This is appropriately regarded as junk, DNA., , Fig. 28.4 : A pie chart showing a broad categorization, of the human gene catalog (About 13000 genes whose, functions are not known are not included).
Page 634 :
624, , l, , l, , BIOCHEMISTRY, , Understanding of polygenic disorders e.g., cancer, hypertension, diabetes., Improvements in gene therapy, , TABLE 28.4 Different categories of proteins, encoded by human genes (based on the, Human Genome Project report, 2001), , Category of, proteins, Unknown functions, , Percentage Actual number, of genes, 41.0%, , 12,809, , Nucleic acid enzymes, , 7.5%, , 2,308, , Transcription factors, , 6.0%, , 1,850, , Receptors, , 5.0%, , 1,543, , Hydrolases, , 4.0%, , 1,227, , Regulatory proteins, (G-proteins, cell cycle, regulators etc.), , 3.2%, , 988, , Protooncogenes, , 2.9%, , 902, , Structural proteins of, cytoskeleton, , 2.8%, , 876, , Kinases, , 2.8%, , 868, , (Note : This table is based on the rough draft of human genome, reported by Celera Genomics. The percentages are derived from, a total of 26,383 genes), , l, , Improved diagnosis of diseases, , l, , Development of pharmacogenomics, , l, , Genetic basis of psychiatric disorders, , l, , Understanding of complex social trait, , l, , Improved knowledge on mutations, , l, , Better understanding of developmental biology, , l, , Comparative genomics, , l, , Development of biotechnology, , ETHICS AND HUMAN GENOME, The research on human genomes will make, very sensitive data available that will affect the, personal and private lives of individuals. For, instance, once it is known that a person carries, genes for an incurable disease, what would be, the strategy of an insurance company? How will, the society treat him/her? There is a possibility, that individuals with substandard genome, sequences may be discriminated. Human, genome results may also promote racial, discrimination categorizing the people with good, and bad genome sequences. Considering the, gravity of ethics related to a human genome,, about 3% of the HGP budget was earmarked for, ethical research., , 1. Human Genome Project is an international venture involving several laboratories, and, a large number of scientists and technicians from various disciplines., 2. About 90% of the human genome has been sequenced. It is composed of 3.2 billion base, pairs., 3. The total number of genes in the humans is in the range of 30,000–40,000., 4. About 1.1–1.5% of the human genome codes for proteins while the remaining portion, is regarded as junk DNA (composed of introns and intergenic sequences)., 5. Human genome sequencing has wide range of applications–better understanding of, genetic diseases, improvements in gene therapy, development of pharmacogenomics,, and advancement of biotechnology.
Page 635 :
Section 6, , Current Topics, , Chapter, , Gene Therapy, , 29, , The gene therapy speaks :, , “I represent the insertion of genes into cells;, The preferred being somatic cells to treat diseases;, Although unsuccessful and unable to satisfy now;, I am highly optimistic about my future!”, , A, , dvances in biochemistry and molecular, biology have helped to understand the, genetic basis of inherited diseases. It was a, dream of the researchers to replace the defective, genes with good ones, and cure the genetic, disorders., , Gene therapy is the process of inserting, genes into cells to treat diseases. The newly, introduced genes will encode proteins and, correct the deficiencies that occur in genetic, diseases. Thus, gene therapy primarily involves, genetic manipulations in animals or humans to, correct a disease, and keep the organism in good, health. The initial experiments on gene therapy, are carried out in animals, and then in humans., Obviously, the goal of the researchers is to, benefit the mankind and improve their health., An overview of gene therapy strategies is, depicted in Fig.29.1. In gene augmentation, therapy, a DNA is inserted into the genome to, replace the missing gene product. In case of gene, inhibition therapy, the antisense gene inhibits, the expression of the dominant gene., , APPROACHES FOR GENE THERAPY, There are two approaches to achieve gene, therapy., 1. Somatic cell gene therapy : The nonreproductive (non-sex) cells of an organism are, referred to as somatic cells. These are the cells of, an organism other than sperm or egg cells, e.g.,, bone marrow cells, blood cells, skin cells,, intestinal cells. At present, all the research on, gene therapy is directed to correct the genetic, defects in somatic cells. In essence, somatic cell, gene therapy involves the insertion of a fully, functional and expressible gene into a target, somatic cell to correct a genetic disease, permanently., 2. Germ cell gene therapy : The reproductive (sex) cells of an organism constitute, germ cell line. Gene therapy involving the, introduction of DNA into germ cells is passed on, to the successive generations. For safety, ethical, and technical reasons, germ cell gene therapy is, not being attempted at present., , 625
Page 636 :
626, , BIOCHEMISTRY, , (A), , The genetic alterations in somatic cells are, not carried to the next generations. Therefore,, somatic cell gene therapy is preferred and, extensively studied with an ultimate objective of, correcting human diseases., , Functional, gene, , Defective gene, (B), , A large number of genetic disorders and other, diseases are currently at various stages of gene, therapy trials. A selected list of some important, ones is given in Table 29.1., , Antisense, gene, , There are two types of gene therapies., , Dominant, functional gene, , Inhibitory action, , Fig. 29.1 : Overview of two major gene therapy, strategies (A) Gene augmentation therapy (B) Gene, inhibition therapy., , I. Ex vivo gene therapy : This involves the, transfer of genes in cultured cells (e.g., bone, marrow cells) which are then reintroduced into, the patient., , TABLE 29.1 Human gene therapy trials, , Disease, Severe combined immunodeficiency (SCID), , Adenosine deaminase (ADA)., , Cystic fibrosis, , Cystic fibrosis transmembrane regulator (CFTR)., , Familial hypercholesterolemia, , Low density lipoprotein (LDL) receptor., D1-Antitrypsin, , Emphysema, Hemophilia B, , Factor IX, D- or E-Globin, , Thalassemia, Sickle-cell anemia, , E-Globin, , Lesch-Nyhan syndrome, , Hypoxanthine-guanine phosphoribosyltransferase (HGPRT)., , Gaucher’s disease, , Glucocerebrosidase, , Peripheral artery disease, Fanconi anemia, , Vascular endothelial growth factor (VEGF), Fanconi anemia C, , Melanoma, , Tumor necrosis factor (TNF), , Melanoma, renal cancer, , Interleukin-2 (IL-2), , Glioblastoma (brain tumor), AIDS, ovarian cancer, , Thymidine kinase (herpes simplex virus), , Head and neck cancer, , p53, , Breast cancer, , Multidrug resistance I, , AIDS, , rev and env, , Colorectal cancer, melanoma, renal cancer, Duchenne muscular dystrophy, , Histocompatability locus antigen-B 7 (HLA-B7), Dystrophin, , Short stature, , Growth hormone, , Diabetes, , Glucose transporter-2, (GLUT-2), glucokinase, , *, , *, , Phenylketonuria, , *, , Citrullinemia, , *, , *, , Gene therapy, , Mostly confined to animal experiments, , Phenylalanine hydroxylase, Arginosuccinate synthetase
Page 637 :
627, , Chapter 29 : GENE THERAPY, , II. In vivo gene therapy : The direct, delivery of genes into the cells of a, particular tissue is referred to as in vivo, gene therapy., , Isolated, cells, , in vitro, culture, , EX VIVO GENE THERAPY, The ex vivo gene therapy can be, applied to only selected tissues (e.g.,, bone marrow) whose cells can be, cultured in the laboratory., , Tr, an, ta spl, tio an, n A man with a, genetic defect, , The technique of ex vivo gene, therapy involves the following steps, (Fig.29.2)., 1. Isolate cells with genetic defect, from a patient., 2. Grow the cells in culture., 3. Introduce the therapeutic gene to correct, gene defect., 4. Select the genetically corrected cells, (stable transformants) and grow., 5. Transplant the modified cells to the, patient., The procedure basically involves the use of, the patient’s own cells for culture and genetic, correction, and then their return back to the, patient. This technique is therefore, not, associated, with, adverse, immunological, responses after transplanting the cells. Ex vivo, gene therapy is efficient only if the therapeutic, gene (remedial gene) is stably incorporated and, continuously expressed. This can be achieved by, use of vectors., , VECTORS IN GENE THERAPY, The carrier particles or molecules used to, deliver genes to somatic cells are referred to as, vectors. The important vectors employed in ex, vivo gene therapy are listed below and briefly, described next., l, , Viruses, , l, , Human artificial chromosome, , l, , Bone marrow cells., , Therapeutic, gene constructs, , Genetically, transformed, cells selected, , Fig. 29.2 : The procedure for ex vivo gene therapy., , VIRUSES, The vectors frequently used in gene therapy, are viruses, particularly retroviruses. RNA is the, genetic material in retroviruses. As the retrovirus, enters the host cell, it synthesizes DNA from, RNA (by reverse transcription). The so formed, viral DNA (referred to as provirus) gets, incorporated into the DNA of the host cell. The, proviruses are normally harmless. However,, there is a tremendous risk, since some of the, retroviruses can convert normal cells into, cancerous ones. Therefore, it is absolutely, essential to ensure that such a thing does not, happen., , HUMAN ARTIFICIAL CHROMOSOME, The human artificial chromosome (HAC), is a synthetic chromosome that can replicate, with other chromosomes, besides encoding, a human protein. As already discussed, above, use of retroviruses as vectors in, gene therapy is associated with a heavy risk., This problem can be overcome if HAC is used., Some success has been achieved in this, direction., , BONE MARROW CELLS, Bone marrow contains totipotent embryonic, stem (ES) cells. These cells are capable of, dividing and differentiating into various cell, types (e.g., red blood cells, platelets, macro-
Page 638 :
628, , BIOCHEMISTRY, , phages, osteoclasts, B- and T-lymphocytes)., For this reason, bone marrow transplantation is, the most widely used technique for several, genetic diseases. And there is every reason to, believe that the genetic disorders that respond to, bone marrow transplantation are likely to, respond to ex vivo gene therapy also e.g. sicklecell anemia, SCID, thalassemia., , has been depicted in Fig.29.2. The same, procedure with suitable modifications can also, be applied for other gene therapies., , SELECTED EXAMPLES OF, EX VIVO GENE THERAPY, , A diagrammatic representation of the, treatment of ADA deficient patient is depicted in, Fig.29.3., , THERAPY FOR ADENOSINE, DEAMINASE DEFICIENCY, The first and the most publicised human, gene therapy was carried out to correct the, deficiency of the enzyme adenosine deaminase, (ADA). This was done on September 14,, 1990 by a team of workers led by Blaese, and Anderson at the National Institute of Health,, USA (The girl’s name is Ashanti, 4 years old then)., , Severe combined, immunodeficiency (SCID), SCID is rare inherited immune disorder, associated with T-lymphocytes, and (to a, lesser extent) B-lymphocytes dysfunction. About, 50% of SCID patients have a defect in the gene, (located on chromosome 20, and has 32,000, base pairs and 12 exons) that encodes for, adenosine deaminase. In the deficiency of ADA,, deoxyadenosine and its metabolites (primarily, deoxyadenosine 5c-triphosphate) accumulate and, destroy T-lymphocytes. T-Lymphocytes are, essential for body’s immunity. Besides, participating directly in body’s defense, they, promote the function of B-lymphocytes to, produce antibodies. Thus, the patients of SCID, (lacking ADA) suffer from infectious diseases and, die at an young age. Previously, the children, suffering from SCID were treated with conjugated, bovine ADA, or by bone marrow transplantation., , Technique of therapy, for ADA deficiency, The general scheme of gene therapy adopted, for introducing a defective gene in the patient, , A plasmid vector bearing a proviral DNA is, selected. A part of the proviral DNA is replaced, by the ADA gene and a gene (G 418) coding for, antibiotic resistance, and then cloned. The, antibiotic resistance gene will help to select the, desired clones with ADA gene., , Circulating lymphocytes are removed from a, patient suffering from ADA deficiency. These, cells are transfected with ADA gene by exposing, to billions of retroviruses carrying the said gene., The genetically-modified lymphocytes are grown, in cultures to confirm the expression of ADA, gene and returned to the patient. These, lymphocytes persist in the circulation and, synthesize ADA. Consequently, the ability of the, patient to produce antibodies is increased., However, there is a limitation. The lymphocytes, have a short life span (just live for a few months),, hence the transfusions have to be carried out, frequently., , Transfer of ADA gene, into stem cells, In 1995, ADA gene was transferred into, the stem cells, obtained from the umbilical cord, blood, at the time of baby’s delivery. Four days, after birth, the infant received the modified cells, back. By this way, a permanent population of, ADA gene producing cells was established., , IN VIVO GENE THERAPY, The direct delivery of the therapeutic gene, (DNA) into the target cells of a particular tissue, of a patient constitutes in vivo gene therapy, (Fig.29.4). Many tissues are the potential, candidates for this approach. These include liver,, muscle, skin, spleen, lung, brain and blood cells., Gene delivery can be carried out by viral or nonviral vector systems. The success of in vivo gene
Page 639 :
629, , Chapter 29 : GENE THERAPY, , Vector DNA, Human, ADA+ gene, Retrovirus, containing ADA+, gene, Vectors, , Isolation, , Transfection, , Lymphocytes, , Lymphocytes with, viral DNA and ADA+ gene, Child with SCID, (ADA– gene), , Synthesis of, ADA, , Correction of, SCID, , Growth of cells, Infuse lymphocytes, with ADA+ gene, expression into, patient, , Cell cultures to, verify expression, of ADA+ transgene, , Fig. 29.3 : Treatment of adenosine deaminase (ADA) deficient patient by somatic, ex vivo gene therapy (SCID-Severe combined immunodeficiency)., , therapy mostly depends on the following, parameters, l, , l, , l, , The efficiency of the uptake of the remedial, (therapeutic) gene by the target cells., Intracellular degradation of the gene and its, uptake by nucleus., The expression capability of the gene., , GENE DELIVERY BY VIRUSES, Many viral vector systems have been, developed for gene delivery. These include, , retroviruses, adenoviruses, adeno-associated, viruses and herpes simplex virus., , GENE DELIVERY BY, NON-VIRAL SYSTEMS, There are certain limitations in using viral, vectors in gene therapy. In addition to the, prohibitive cost of maintaining the viruses, the, viral proteins often induce inflammatory, responses in the host. Therefore, there is a, continuous search by researchers to find, alternatives to viral vector systems.
Page 640 :
630, , BIOCHEMISTRY, , Target, tissue, , p, , Patient, , Fig. 29.4 : Diagrammatic representation of in vivo gene, therapy. (p-Promoter gene specific for therapeutic gene), , The non-viral gene delivery systems are listed, , l, , l, , Tumor necrosis factor gene therapy, Tumor necrosis factor (TNF) is a protein, produced by human macrophages. TNF provides, defense against cancer cells. This is brought out, by enhancing the cancer-fighting ability of, tumor-infiltrating lymphocytes (TILs), a special, type of immune cells., , Therapeutic, gene, , l, , treatment strategies (surgery, chemotherapy,, radiation therapy). Gene therapy is the latest and, a new approach for cancer treatment. Some, of the developments are briefly described, hereunder., , Pure DNA constructs that can be directly, introduced into target tissues., , The tumor-infiltrating lymphocytes were, transformed with a TNF gene (along with a, neomycin resistant gene) and used for the, treatment of malignant melanoma (a cancer of, melanin producing cells, usually occurs in skin)., TNF as such is highly toxic, and fortunately no, toxic side effects were detected in the melanoma, patients injected with genetically altered TILs, with TNF gene. Some improvement in the cancer, patients was observed., , Lipoplexes, lipid-DNA complexes that have, DNA surrounded by lipid layers., , Suicide gene therapy, , Human artificial chromosome which can, carry large DNA (one or more genes)., , The gene encoding the enzyme thymidine, kinase is often referred to as suicide gene, and is, used for the treatment of certain cancers., , GENE THERAPY, STRATEGIES FOR CANCER, Cancer is the leading cause of death, throughout the world, despite the intensive, , Thymidine kinase (TK) phosphorylates, nucleosides to form nucleotides which are used, for the synthesis of DNA during cell division., The drug ganciclovir (GCV) bears a close, structural resemblance to certain nucleosides, , + Theoretically, gene therapy is the permanent solution for genetic diseases., + A large number of genetic disorders and other diseases are at various stages of gene, therapy trials e.g. sickle-cell anemia, cystic fibrosis, AIDS, cancer., , + Ganciclovir (a drug with structural resemblance to thymidine) has been used (suicide, gene therapy) for the treatment of brain tumors, although with limited success., , + Despite extensive research and trials, as of now, no disease has been permanently cured, by gene therapy. However, a breakthrough may come at anytime.
Page 641 :
631, , Chapter 29 : GENE THERAPY, , DNA synthesis, Nucleoside, , Nucleotide, Thymidine kinase, , Inhibits DNA, polymerase, , Phosphates, Ganciclovir, , DNA synthesis, blocked, , False nucleotide, , Cancer, cells die, , Fig. 29.5 : The action of ganciclovir mediated by thymidine kinase to inhibit the growth of cancer cells., , (thymidine). By mistake, TK phosphorylates, ganciclovir to form triphosphate-GCV, a false, and unsuitable nucleotide for DNA synthesis., Triphosphate-GCV inhibits DNA polymerase, (Fig.29.5). The result is that the elongation of the, DNA molecule abruptly stops at a point, containing the false nucleotide (of ganciclovir)., Further, the triphospate-GCV can enter and kill, the neighbouring cancer cells, a phenomenon, referred to as bystander effect. The ultimate, result is that the cancer cells cannot multiply,, and therefore die. Thus, the drug ganciclovir can, be used to kill the cancer cells., Ganciclovir is frequently referred to as a, prodrug and this type of approach is called, prodrug activation gene therapy. Ganciclovir, has been used for treatment of brain tumors (e.g.,, glioblastoma, a cancer of glial cells in brain),, although with a limited success., , Gene replacement therapy, A gene named p53 codes for a protein with a, molecular weight of 53 kilodaltons (hence p53)., p53 is considered to be a tumor-suppressor gene,, since the protein it encodes binds with DNA and, inhibits replication. The tumor cells of several, tissues (breast, brain, lung, skin, bladder, colon,, bone) were found to have altered genes of p53, (mutated p53), synthesizing different proteins, from the original. These altered proteins cannot, inhibit DNA replication. It is believed that the, damaged p53 gene may be a causative factor in, tumor development., , Some workers have tried to replace the, damaged p53 gene by a normal gene by, employing adenovirus vector systems. There are, some encouraging results in the patients with, liver cancer., The antisense therapy for cancer is discussed, as a part of antigene and antisense therapy., , ANTIGENE AND, ANTISENSE THERAPY, In general, gene therapy is carried out by, introducing a therapeutic gene to produce the, defective or the lacking protein. But there are, certain disorders (cancer, viral and parasitic, infections, inflammatory diseases) which result, in an overproduction of certain normal proteins., It is possible to treat these diseases by blocking, transcription using a single-stranded nucleotide, sequence (antigene oligonucleotide) that, hybridizes with the specific gene, and this is, called antigene therapy. Antisense therapy refers, to the inhibition of translation by using a singlestranded nucleotide (antisense oligonucleotide)., Further, it is also possible to inhibit both, transcription and translation by blocking (with, oligonucleotides), the, transcription, factor, responsbile for the specific gene expression., , Nucleic acid therapy refers to the use of DNA, or RNA molecules for therapeutic purposes, as, stated above. The naturally occurring sequences, of DNA and RNA (with suitable modifications)
Page 642 :
632, , (A), , BIOCHEMISTRY, , I, , E1, , AS cDNA, , E2, DNA, Transcription, , Transcription, , I, 2, 1, Primary transcript, Processing, , Antisense RNA, 1, , 2, mRNA, , mRNA–antisense, RNA complex, 1, , 2, , No translation, , (B), , I, , E1, , Antisense RNA, , E2, DNA, , 1, , 2, mRNA, , mRNA–antisense, RNA complex, 1, , 2, , No translation, , Fig. 29.6 : Inhibition of translation by antisense RNA, (A) The cloned AS cDNA introduced into cells to, produce antisense RNA (B) Antisense RNA, directly introduced into cells. (AS cDNA = Antisense, complementary DNA; E1, E2-Exons in a gene; I-Intron), , or the synthetic ones can be employed in nucleic, acid therapy. Theoretically, there is a vast, potential for use of nucleic acids as therapeutic, agents. But most of the work that is being carried, out relates to the use of RNA in antisense, therapy. Some of these are described below, (Note : Some authors use antisense therapy in a, , broad sense to reflect antigene therapy as well as, antisense therapy, discussed in the previous, paragraph)., , ANTISENSE THERAPY FOR CANCER, Oncogenes are the genes responsible for the, causation of cancer. The dominantly acting, oncogenes can be targeted in antisense, technology by using antisense transgenes or, oligonucleotides. Antisense oligonucleotides are, used for the treatment of myeloid leukemia in as, early as 1991., Antisense RNA molecules are more frequently, used in cancer therapy. This approach is, effective only if the antisense oligonucleotide, (antisense mRNA) specifically binds to the, target mRNA, and blocks protein biosynthesis, (translation). This can be achieved in two ways,, as illustrated in Fig.29.6., The antisense cDNA can be cloned and, transfected into cells. Antisense mRNA is, synthesized by transcription. This can readily, bind with the specific mRNA and block, translation (Fig.29.6A). The mRNA is actually, formed by a gene containing exons and introns, through transcription, followed by processing., The other way to block translation is to, directly introduce antisense RNA into the cells., This hybridizes with target mRNA and blocks, translation (Fig.29.6B)., The antisense mRNA therapy was tried for the, treatment of a brain tumor namely malignant, glioma and the cancer of prostate gland. In case, malignant glioma, the protein insulin-like growth, factor I (IGF-I) is overproduced, while in prostate, cancer, insulin-like growth factor I receptor (IGFIR) protein is more synthesized. For both these, cancers, the respective antisense cDNAs can be, used to synthesize antisense mRNA molecules., These in turn, are used to block translation, as, briefly described above, and illustrated in, Fig.29.6., Peptide nucleic acid (PNA) therapy : PNAs, are artificial analogs of nucleic acid with a, polypeptide backbone. They possess standard
Page 643 :
633, , Chapter 29 : GENE THERAPY, , nucleic acid bases attached to a polypeptide in, place of sugar-phosphate backbone. Antisense, PNAs have been developed to inhibit translation, of HIV viral transcript, gag-pol, and to block, translation of cancer genes e.g. Has-ras, and bcl-2., , THE FUTURE OF GENE THERAPY, Theoretically, gene therapy is the permanent, solution for genetic diseases. But it is not as, simple as it appears since gene therapy has, several inbuilt complexicities. Gene therapy, broadly involves isolation of a specific gene,, making its copies, inserting them into target, tissue cells to make the desired protein. The story, does not end here. It is absolutely essential to, ensure that the gene is harmless to the patient, and it is appropriately expressed (too much or, too little will be no good). Another concern in, gene therapy is the body’s immune system which, reacts to the foreign proteins produced by the, new genes., , The public, in general, have exaggerated, expectations on gene therapy. The researchers,, at least for the present, are unable to satisfy, them. As per the records, by 1999 about 1000, Americans had undergone clinical trails, involving various gene therapies. Unfortunately,, the gene therapists are unable to categorically, claim that gene therapy has permanently cured, any one of these patients! Some people in the, media (leading news papers and magazines), have openly questioned whether it is worth to, continue research on gene therapy!!, It may be true that as of now, gene therapy, due to several limitations, has not progressed the, way it should, despite intensive research. But a, breakthrough may come anytime, and of course,, this is only possible with persistent research. And, a day may come (it might take some years) when, almost every disease will have a gene therapy,, as one of the treatment modalities. And gene, therapy will revolutionize the practice of, medicine!, , 1. Gene therapy is the process of inserting genes into cells to treat diseases. Somatic cell, gene therapy, involving the insertion of an expressible gene into somatic cells, is the, preferred approach., 2. Ex vivo gene therapy involves the transfer of genes in cultured cells which are then, reintroduced into the patient. The direct delivery of genes into the cells of a particular, tissue is regarded as in vivo gene therapy., 3. Gene therapy was successfully carried out in a patient of severe combined, immunodeficiency (caused by the deficiency of the enzyme adenosine deaminase)., 4. Antigene therapy involves blocking of transcription (by antigene oligonucleotide) while, in antisense therapy, translation is inhibited (by antisense oligonucleotide). These, approaches are in the experimental stages for the therapy of cancer and AIDS., 5. Although as of now, gene therapy has not offered any permanent cure to any human, patients, a breakthrough may come anytime. And gene therapy may revolutionize the, practice of medicine.
Page 644 :
Section 6, , Current Topics, , Chapter, , Bioinformatics, , 30, , The bioinformatics speaks :, , “I am the product of biology and informational technology;, International computer network (internet) is my brain;, Biological databases represent my body;, I have revolutionized the advances in biology.”, , B, , ioinformatics is the combination (or, marriage!) of biology and information, technology. Basically, bioinformatics is a, recently developed science using information to, understand biological phenomenon. It broadly, involves the computational tools and methods, used to manage, analyse and manipulate, volumes and volumes of biological data., , Bioinformatics may also be regarded as a part, of the computational biology. The latter is, concerned with the application of quantitative, analytical techniques in modeling and solving, problems, in, the, biological, systems., Bioinformatics is an interdisciplinary approach, requiring advanced knowledge of computer, science, mathematics and statistical methods for, the understanding of biological phenomena at, the molecular level., , History and relevance, of bioinformatics, in, , The term bioinformatics was first introduced, 1990s. Originally, it dealt with the, , management and analysis of the data pertaining, to DNA, RNA and protein sequences. As the, biological data is being produced at an, unprecedented rate, their management and, interpretation invariably requires bioinformatics., Thus, bioinformatics now includes many other, types of biological data. Some of the most, important ones are listed below, l, , Gene expression profiles, , l, , Protein structure, , l, , Protein interactions, , l, , Microarrays (DNA chips), , l, , Functional analysis of biomolecules, , l, , Drug designing., , Bioinformatics is largely (not exclusively) a, computer-based discipline. Computers are in fact, very essential to handle large volumes of, biological data, their storage and retrieval., We have to accept the fact that there is no, computer on earth (however advanced) which, , 634
Page 645 :
635, , Chapter 30 : BIOINFORMATICS, , can store information, and perform the functions, like a living cell. Thus a highly complex, information technology lies right within the cells, of an organism. This primarily includes the, organism’s genes and their dictates for the, organisms biological processes and behaviour., , 1. Creation of databases : This involves the, organizing, storage and management of the, biological data sets. The databases are accessible, to researchers to know the existing information, and submit new entries. e.g. protein sequence, data bank for molecular structure. Databases will, be of no use until analysed., , BROAD COVERAGE, OF BIOINFORMATICS, , 2. Development of algorithms and statistics :, This involves the development of tools and, resources to determine the relationship among, the members of large data sets e.g. comparison, of protein sequence data with the already, existing protein sequences., , Bioinformatics covers many specialized and, advanced areas of biology., Functional genomics : Identification of genes, and their respective functions., Structural genomics : Predictions related to, functions of proteins., Comparative genomics : For understanding, the genomes of different species of organisms., DNA microarrays : These are designed to, measure the levels of gene expression in different, tissues, various stages of development and in, different diseases., , 3. Analysis of data and interpretation : The, appropriate use of components 1 and 2 (given, above) to analyse the data and interpret the, results in a biologically meaningful manner. This, includes DNA, RNA and protein sequences,, protein structure, gene expression profiles, and, biochemical pathways., , BIOINFORMATICS, AND THE INTERNET, , Medical informatics : This involves the, management of biomedical data with special, reference to biomolecules, in vitro assays and, clinical trials., , The internet is an international computer, network. A computer network involves a group, of computers that can communicate (usually, over a telephone system) and exchange data, between users., , COMPONENTS OF BIOINFORMATICS, , It is the internet protocol (IP) that determines, how the packets of information are addressed, , Bioinformatics comprises three components, , + Bioinformatics has largely benefited biological and medical sciences, particularly related, to molecular biology and biotechnology. Some applications are listed :, l, , Sequencing of macromolecules (proteins, DNA, RNA), , l, , Human genome sequencing, , l, , Molecular modelling of biomolecules, , l, , Handling of vast biological data, , l, , Designing of drugs for the treatment of diseases, , l, , Development of models for the functioning of cells, tissues and organs, , + As such, there is no field of biological science that is not benefited by bioinformatics.
Page 646 :
636, , BIOCHEMISTRY, , and routed over the network. To access the, internet, a computer must have the correct, hardware (modem/network card), appropriate, software and permission for access to network., For this purpose, one has to subscribe to an, internet service provider (ISP)., World wide web (www) : www involves the, exchange of information over the internet using, a programme called browser. The most widely, used browsers are Internet explorer and, Netscape navigator., www works on the basis of Uniform resource, locator (URL) which is a document with a, unique address. URLs takes the format http.//, , (hypertext transfer protocol) that can identify the, protocol for communication over www., , BIOLOGICAL DATABASES, The collection of the biological data on a, computer which can be manipulated to appear, in varying arrangements and subsets is regarded, as a database. The biological information can be, stored in different databases. Each database has, its own website with unique navigation tools., The biological databases are, in general,, publicly accessible. Selected examples of, biological databases are briefly described, (Table 30.1)., , TABLE 30.1 Selected examples of biological databases in bioinformatics, , Database(s), , Salient features, , Primary nucleotide sequence databases, GenBank, (www.ncbi.nih.gov/GeneBank/), , Provides nucleotide sequence databases maintained by the, National Center for Biotechnology Information (NCBI), USA., , Other nucleotide sequence databases, UniGene, (www.ncbi.nih.gov/UniGene/), , The nucleotide sequences of GenBank in the form of clusters,, representing genes are available., , Genome Biology, (www.ncbi.nlm.nih.gov/Genomes/), , The information about the completed genomes is available., , Protein sequence database, SWISS-PROT, (www.expasy.ch/sport), , Provides the description of the structure of a protein, its domains, structure, post-translational modifications, variants etc. It has high, level of integration with other databases and minimal level of, redundancy., , Protein sequence motif databases, PROSITE, (www.expasy.ch/prosite/), , Provides information on protein families and domains. It also has, patterns and profiles for sequences and biological functions., , Macromolecular databases, PDB, (www.rcsb.org/pdb), , This is the primary database for 3-dimensional (3-D) structures, of biological macromolecules (determined by X-ray and NMR, studies)., , Other databases, KEGG, (www.genome.ad.jp/kegg/), , The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a, database with latest computerised information on biomolecules, and cell biology. KEGG provides details on information pathways,, interacting molecules and the connecting links with genes.
Page 647 :
637, , Chapter 30 : BIOINFORMATICS, , Nucleotide sequence databases, , APPLICATIONS OF BIOINFORMATICS, , The nucleotide sequence data submitted by, the scientists and genome sequencing groups is, at the databases namely GenBank, EMBL, (European Molecular Biology Laboratory) and, DDBJ (DNA Data Bank of Japan). There is a good, coordination between these three databases as, they are synchronized on daily basis., Besides the primary nucleotide databases, (referred above), there are some other databases, also to provide information on genes, genomes, and ongoing research projects., , The, advent, of, bioinformatics, has, revolutionized the advancements in biological, science. And biotechnology is largely benefited, by bioinformatics. The best example is the, sequencing of human genome in a record time, which would not have been possible without, bioinformatics. A selected list of applications of, bioinformatics is given below., l, , l, , Protein sequence databases, Protein sequence databases are usually, prepared from the existing literature and/or in, consultation with the experts. In fact, these, databases represent the translated DNA, databases., , Molecular structure of databases, The three dimensional (3-D) structures of, macromolecules are determined by X-ray, crystallography and nuclear magnetic resonance, (NMR). PDB and SCOP are the primary, databases of 3-D structures of biological, molecules., , Other databases, KEGG database is an important one that, provides information on the current knowledge, of molecular biology and cell biology with, special reference to information on metabolic, pathways, interacting molecules and genes., , l, , Sequence mapping of biomolecules (DNA,, RNA, proteins)., Identification of nucleotide sequences of, functional genes., Finding of sites that can be cut by restriction, enzymes., , l, , Prediction of functional gene products., , l, , To trace the evolutionary trees of genes., , l, , For the prediction of 3-dimensional structure, of proteins., , l, , Molecular modelling of biomolecules., , l, , Designing of drugs for medical treatment., , l, , l, , Handling of vast biological data which, otherwise is not possible., Development of models for the functioning, various cells, tissues and organs., , The above list of applications however, may, be treated as incomplete, since at present there, is no field in biological sciences that does not, involve bioinformatics., , APPLICATIONS OF BIOINFORMATICS, , 1. Bioinformatics (a computer-based discipline) represents an alliance between biology and, information technology., 2. The storage, management and interpretation of vast biological data invariably requires, bioinformatics., 3. Bioinformatics comprises three components-creation of data base, development of, algorithms and statistics, and analysis of data and interpretation., 4. Biological databases, containing the biological information, are publicly accessible e.g., GenBank (www.ncbi.nih.gov/GeneBank)., 5. Bioinformatics has revolutionized the advancements of biological and medical sciences, e.g. sequencing of human genome.
Page 648 :
Section 6, , Current Topics, , Chapter, , Metabolism of Xenobiotics, (Detoxification), , 31, , XENOBIOTICS, , PHASE I, , PHASE II, , Oxidation, Reduction, Hydrolysis, Conjugation Conjugation, Excreted, , Excreted, , The detoxification speaks :, , “I deal with the metabolism of foreign compounds;, Through oxidation, reduction, hydrolysis and conjugation,, To convert xenobiotics into soluble forms;, For their effective elimination from the body.”, , M, , an is continuously exposed to several, foreign compounds such as drugs,, pollutants, food additives, cosmetics, pesticides, etc. Certain unwanted compounds are produced, in the large intestine by the bacteria which enter, the circulation. These include indole from, tryptophan, cadaverine from lysine, tyramine, from tyrosine, phenol from phenylalanine etc. In, the normal metabolism of the body, certain, waste compounds (e.g. bilirubin) are formed. A, vast majority of the foreign compounds or the, unwanted substances, produced in the body, are, toxic and, therefore, they should be quickly, eliminated from the body., The term detoxication or detoxification refers, to the series of biochemical reactions occurring, in the body to convert the foreign (often toxic), compounds to non-toxic or less toxic, and more, easily excretable forms., , Detoxification—a misnomer?, Detoxification is rather misleading, since, sometimes a detoxified product is more toxic, , than the original substance (e.g. procarcinogens, to carcinogens). It appears that the body tries to, get rid of a foreign substance by converting, it into a more soluble (often polar), and, easily excretable compound, and this may, be sometimes associated with increased, toxicity (e.g. conversion of methanol to formaldehyde)., In recent years, the term detoxification, is replaced by biotransformation or metabolism, of xenobiotics (Greek : xenos—strange,, foreign) or simply metabolism of foreign, compounds., , Site of detoxification, The detoxification reactions are carried out, mainly in the liver which is equipped with the, enzyme machinery. Kidney and other organs, may sometimes be involved. The products, formed by detoxification are mostly excreted by, the kidneys, less frequently excreted via feces or, expired air., , 638
Page 649 :
639, , Chapter 31 : METABOLISM OF XENOBIOTICS (DETOXIFICATION), , XENOBIOTICS, , C2H5OH, , o CH3COOH, , Ethanol, , Acetic acid, , C6H5CH2OH o C6H5COOH, Benzyl alcohol, , PHASE I, , Oxidation, Reduction, Hydrolysis, , Benzoic acid, , Aldehydes : Aldehydes are oxidized to, produce the corresponding acids., C6H5CHO, , o C6H5COOH, , Benzaldehyde, , PHASE II, , Conjugation Conjugation, , C.CI3CHO, , Benzoic acid, , o CCI3COOH, , Chloral, , Excreted, , Excreted, , Fig. 31.1 : Phase I and phase II reactions, in the metabolism of xenobiotics., , Amines and their derivatives : Alipahtic, amines are converted to the corresponding acids,, liberating urea while aromatic amino acids are, oxidized to phenols., RCH2NH2 o R–COOH + NH2–CO–NH2, Aliphatic amine, , MECHANISM OF DETOXIFICATION, , C6H5NH2, , Phase I : The reactions of phase I are, oxidation, reduction and hydrolysis., , Aliphatic acid, , p-Amino phenol, , Aromatic hydrocarbons : Benzene may be, oxidized to mono, di- and trihydroxy phenols as, shown below, OH, , Phase II : These are the conjugation reactions,, involving compounds such as glucuronic acid,, amino acids (glycine), glutathione, sulfate,, acetate and methyl group., Generally, detoxification of a compound, involves phase I as well as phase II reactions. For, instance, oxidation followed by conjugation is, the most frequent process in the metabolism of, xenobiotics., , OH, +, , +, OH, , Benzene, , Phenol, , Quinol, , OH, , OH, OH, +, , OH, , OH, , Oxidation, A large number of foreign substances are, detoxified by oxidation. These include alcohols,, aldehydes, amines, aromatic hydrocarbons and, sulfur compounds. In general, aliphatic, compounds are more easily oxidized than, aromatic ones., Alcohols : Aliphatic and aromatic alcohols, undergo oxidation to form the corresponding, acids., CH3OH o HCOOH, Methanol, , Urea, , o HO–C6H4–NH2, , Aniline, , The metabolism of xenobiotics may be, divided into two phases which may occur, together or separately (Fig.31.1)., , Trichloroacetic acid, , Formic acid, , Catechol, , Hydroxyquinol, , Sulfur compounds : Organic sulfur is oxidized, to sulfuric acid., Drugs : Meprobamate is a tranquilizer. It is, oxidized to hydroxymeprobamate and excreted, in urine., , Role of cytochrome P450, Most of the oxidation reactions of, detoxification are catalysed by monooxygenase, or cytochrome P450. This enzyme, also called
Page 652 :
642, , BIOCHEMISTRY, , Sulfate : The active form of sulfate—, 3’-phosphoadenosine 5-phosphosulfate (PAPS)—, participates in conjugation reactions and the, enzyme sulfotransferase is involved in this, process. Several aliphatic and aromatic, compounds undergo sulfation., OH, , O SO3H, , + PAPS, Phenol, , Sulfotransferase, , +, Phenyl, sulfate, , PAP, Phosphoadenosylphosphate, , Acetic acid : Acetyl CoA is the active form of, acetic acid that takes part in conjugating, reactions. Drugs such as sulfanilamide are, converted to acetyl derivatives., Sulfanilamide + Acetyl CoA, Acetyl sulfanilamide + CoASH, , Thiosulfate : The highly toxic cyanides are, conjugated with thiosulfate to form less toxic, thiocyanate., Cyanide +, Sodium thiosulfate, , Thiocyanate +, Sodium sulfate, , Detoxification by drugs : It may be surprising, to know that some drugs are administered to, detoxify foreign substances. The toxic effects of, certain metals such as arsenic, mercury and, cadminum could be overcome by administering, BAL (British antilewisite). This compound was, developed during the World War II and was, used as a detoxifying agent for certain war, poisons. The mechanism of action of BAL is not, clearly known. It is believed that BAL readily, combines with metals and gets easily excreted, into urine., , + Knowledge of the metabolism of xenobiotics is essential for the understanding of, toxicology, pharmacology and drug addiction., + The body possesses the capability to get rid of the foreign substances by converting, them into more easily excretable forms., + Detoxification is not necessarily associated with the conversion of toxic into non-toxic, compounds. For instance, methanol is metabolized to a more toxic formaldehyde., + Detoxification primarily occurs in the liver through one or more of the reactions,, namely oxidation, reduction, hydrolysis and conjugation., + British antilewisite (BAL), a compound developed during Second World War, was used, to detoxify certain war poisons.
Page 653 :
Chapter 31 : METABOLISM OF XENOBIOTICS (DETOXIFICATION), , 1. Detoxification deals with the series of biochemical reactions occurring in the body to, convert the foreign (often toxic) compounds to non-toxic or less toxic and more easily, excretable forms. Liver is the major site of detoxification. In recent years, the term, detoxification is replaced by biotransformation or metabolism of xenobiotics., 2. Detoxification may be divided into phase I (oxidation, reduction, hydrolysis) and phase, II reactions (conjugation). Oxidation is a major process of detoxification, involving the, microsomal enzyme cytochrome P450 which is an inducible, NADPH dependent, hemoprotein., 3. Conjugation is a process in which a foreign compound combines with a substance, produced in the body. The process of conjugation may occur either directly or after, phase I reactions. At least 8 different conjugating agents have been identified in the, body—glucuronic acid, glycine, cysteine, glutamine, methyl group, sulfate, acetic acid, and thiosulfate., , 643
Page 654 :
Section 6, , Current Topics, , Chapter, , Prostaglandins and, Related Compounds, , 32, , The prostaglandins speak :, , O, R1, , R2, OH, , “Twenty carbon compounds are we!, Synthesized from arachidonic acid;, Act as local hormones in function;, Widely used as therapeutic agents.”, , P, , rostaglandins and their related compounds—, prostacyclins (PGI), thromboxanes (TXA),, leukotrienes (LT) and lipoxins are collectively, known as eicosaniods, since they all, contain 20 carbons (Greek : eikosi-twenty)., Eicosanoids are considered as locally acting, hormones with a wide range of biochemical, functions., History : Prostaglandins (PGs) were first, discovered in human semen by Ulf von Euler (of, Sweden) in 1930. These compounds were found, to stimulate uterine contraction and reduce, blood pressure. von Euler presumed that they, were synthesized by prostate gland and hence, named them as prostaglandins. It was later, realized that PGs and other eicosanoids are, synthesized in almost all the tissues (exception–, erythrocytes). By then, however, the name, prostaglandins was accepted worldwide, and, hence continued., The prostaglandins E and F were first isolated, from the biological fluids. They were so named, due to their solubility in ether (PGE) and, , phosphate buffer (PGF, F for fosfat, in Swedish)., All other prostaglandins discovered later were, denoted by a letter—PGA, PGH etc., , Structure of prostaglandins, Prostaglandins, are, derivatives, of, a, hypothetical 20-carbon fatty acid namely, prostanoic acid, hence known as prostanoids., This has a cyclopentane ring (formed by carbon, atoms 8 to 12) and two side chains, with, carboxyl group on one side. Prostaglandins differ, in their structure due to substituent group and, double bond on cyclopentane ring. The different, prostaglandins are given in Fig.32.1., The structures of the most important, prostaglandins (PGF2 and PGF2D), prostacyclins, (PGI2), thromboxanes (TXA2) and leukotrienes, (LTA4) along with arachidonic acid are depicted, in Fig.32.2. A subscript numeral indicates the, number of double bonds in the two side chains., A subscript D-denotes that the hydroxyl group at, C9 of the ring and the carboxyl group are on the, same side of the ring., , 644
Page 656 :
646, , BIOCHEMISTRY, , COOH, , Many non-steroidal anti-inflammatory drugs, inhibit the synthesis of prostaglandins,, prostacyclins and thromboxanes. They do so by, blocking the enzyme cyclooxygenase., , Arachidonic acid, , O, COOH, , OH, , OH, PGE2, , OH, COOH, , OH, , OH, PGF2 D, , COOH, , O, , OH, , OH, PGI2, , COOH, , O, , cortisol) prevent the formation of arachidonic, acid by inhibiting the enzyme phospholipase A2., , Aspirin inhibits PG synthesis : Aspirin (acetyl, salicylic acid) has been used since nineteenth, century as an antipyretic (fever-reducing) and, analgesic (pain relieving). The mechanism of, action of aspirin however, was not known for a, long period. It was only in 1971, John Vane, discovered that aspirin inhibits the synthesis of, PG from arachidonic acid. Aspirin acetylates, serine at the active site of cyclooxygenase and, irreversibly inhibits. Other antiinflammatory, drugs, such as indomethacin and phenylbutazone act as reversible inhibitors of the, enzyme cyclooxygenase. Paracetamol is also a, reversible inhibitor., Degradation of prostaglandins : Almost all, the eicosanoids are metabolized rapidly. The, lung and liver are the major sites of PG, degradation. Two enzymes, namely 15-Dhydroxy PG dehydrogenase and 13-PG, reductase, convert hydroxyl group at C15 to keto, group and then to C13 and C14 dihydroderivative., , O, , Biochemical actions, of prostaglandins, , OH, TXA2, , Fig. 32.2 : The structures of arachidonic acid, common, prostaglandins (PGE2 and PGF2D), prostacyclins (PGI2),, thromboxanes (TXA2) and leukotrienes (LTA4)., , Prostaglandins act as local hormones in their, function. They, however, differ from the true, hormones in many ways. Prostaglandins are, produced in almost all the tissues in contrast to, hormonal synthesis which occurs in specialized, glands. PGs are not stored and they are degraded, to inactive products at the site of their, production. Further, PGs are produced in very, small amounts and have low half-lives., , the enzyme cyclooxygenase. This enzyme is, capable of undergoing self-catalysed destruction, to switch off PG synthesis., , Prostaglandins are involved in a variety of, biological functions. The actions of PGs differ in, different tissues. Sometimes, PGs bring about, opposing actions in the same tissue., , Inhibition of PG synthesis : A number of, structurally unrelated compounds can inhibit, prostaglandin synthesis. Corticosteroids (e.g., , Overproduction of PGs results in many, symptoms which include pain, fever, nausea,, vomiting and inflammation., , O, , COOH, , LTA 4
Page 657 :
647, , Chapter 32 : PROSTAGLANDINS AND RELATED COMPOUNDS, , Prostaglandins mediate the, regulation of blood pressure,, inflammatory response, blood, clotting, reproductive functions,, response to pain, fever etc., 1. Regulation, of, blood, pressure : The prostaglandins, (PGE, PGA and PGI2) are, vasodilator in function. This, results in increased blood flow, and, decreased, peripheral, resistance to lower the blood, pressure. PGs serve as agents in, the treatment of hypertension., , Phospholipids, (membrane bound), Phospholipase A2, , Corticosteroids, , Lysophospholipid, 5-Lipoxygenase, , Arachidonic acid, , Cyclooxygenase, , 5-HPETE, , Aspirin, Phenylbutazone, Indomethacin, Ibuprofen, , Leukotrienes (LT), , Lipoxins (LX), , PGG2, 2GSH, , 2. Inflammation, :, The, Peroxidase, prostaglandins, PGE1, and, G-S-S-G, PGE2 induce the symptoms, of, inflammation, (redness,, PGH 2, swelling, edema etc.) due to, PGI2 synthase, TXA synthase, arteriolar vasodilation. This led, Reductase Isomerase, to the belief that PGs are, natural, mediators, of, TXA 2, PGI2, PGF 2D, PGE2, inflammatory, reactions, of, Fig. 32.3 : Overview of biosynthesis of prostaglandins and related, rheumatoid arthritis (involving, compounds (5-HPETE–5-Hydroxyperoxyeicosatetraenoic acid;, joints),, psoriasis, (skin),, PG–Prostaglandins; PGI2–Prostacyclin I2; TXA2–Thromboxane A2)., conjunctivitis, (eyes), etc., Corticosteroids are frequently, used to treat these inflammatory reactions, since secretion. PGs are used for the treatment of, gastric ulcers. However, PGs stimulate, they inhibit prostaglandin synthesis., pancreatic secretion and increase the motility of, 3. Reproduction : Prostaglandins have wideintestine which often causes diarrhea., spread applications in the field of reproduction., on, immune, system, :, 6. Influence, PGE2 and PGF2 are used for the medical, termination of pregnancy and induction of Macrophages secrete PGE which decreases the, labor. Prostaglandins are administered to cattle immunological functions of B-and T-lymphocytes., to induce estrus and achieve better rate of, 7. Effects on respiratory function : PGE is a, fertilization., bronchodilator whereas PGF acts as a constrictor, 4. Pain and fever : It is believed that of bronchial smooth muscles. Thus, PGE and, pyrogens (fever producing agents) promote PGF oppose the actions of each other in the, prostaglandin biosynthesis leading to the lungs. PGE1 and PGE2 are used in the treatment, formation of PGE in the hypothalamus, the site of asthma., 2, , of regulation of body temperature. PGE2 along, with histamine and bradykinin cause pain., Migraine is also due to PGE2. Aspirin and other, non-steroidal drugs inhibit PG synthesis and thus, control fever and relieve pain., 5. Regulation of gastric secretion : In, general, prostaglandins (PGE) inhibit gastric, , 8. Influence on renal functions : PGE, increases glomerular filtration rate (GFR) and, promotes urine output. Excretion of Na+ and K+, is also increased by PGE., 9. Effects on metabolism : Prostaglandins, influence certain metabolic reactions, probably, through the mediation of cAMP. PGE decreases
Page 658 :
648, , BIOCHEMISTRY, , lipolysis, increases glycogen formation and, promotes calcium mobilization from the bone., 10. Platelet aggregation and thrombosis : The, prostaglandins, namely prostacyclins (PGI2),, inhibit platelet aggregation. On the other hand,, thromboxanes (TXA2) and prostaglandin E2, promote platelet aggregation and blood clotting, that might lead to thrombosis. PGI2, produced, by endothelial cells lining the blood vessels,, prevents the adherence of platelets to the blood, vessels. TXA2 is released by the platelets and is, responsible for their spontaneous aggregation., Thus, prostacyclins and thromboxanes are, antagonists in their action. In the overall effect, PGI2 acts as a vasodilator, while TXA2 is a, vasoconstrictor. The balance between PGI2 and, TXA2 is important in the regulation of hemostasis, and thrombosis., The mechanism of action of prostaglandins is, not known for certain. It is believed that PGs, may act through the mediation of cyclic, nucleotides. PGE increases cAMP levels whereas, PGF elevates cGMP., , aggregation and thrombus formation. This helps, to prevent heart attacks to some extent., , Biomedical applications of PGs, Prostaglandins perform diversified functions., And for this reason, PGs (or other derivatives), are the most exploited in therapeutic, applications. They are used in the treatment of, gastric ulcers, hypertension, thrombosis, asthma, etc. Prostaglandins are also employed in the, medical termination of pregnancy, prevention of, conception, induction of labor etc., Inhibitors of prostaglandin synthesis (e.g., aspirin, ibuprofen) are utilized in controlling, fever, pain, migraine, inflammation etc., , LEUKOTRIENES, , Low doses of aspirin reduce, heart attacks, , Leukotrienes are synthesized by leucocytes,, mast cells, lung, heart, spleen etc., by, lipoxygenase pathway of arachidonic acid. The, synthesis of different leukotrienes (A4, B4, C4,, D4 and E4) through the intermediate, 5–hydroperoxyeicosatetraenoic acid (5–HPETE) is, depicted in Fig.32.4., , At low doses (80–325 mg/day), aspirin inhibits, platelet cyclooxygenase, thereby reduces, thromboxane (TXA2) formation, and thus platelet, , Anaphylaxis is a violent and fatal allergic, reaction. It is now known that leukotrienes (C4,, D4 and E4) are the components of slow-reacting, , + Prostaglandins, synthesized in almost all the tissues (exception–erythrocytes) of the, body, act as local hormones., , + PGs perform diversified biochemical functions. These include lowering of blood, pressure, inhibition of gastric HCI secretion, decrease in immunological response and, induction of labor., , + Overproduction of PGs causes symptoms such as pain, fever, vomiting, nausea,, inflammation etc. Aspirin/ibuprofen/corticosteroid administration inhibits PG synthesis, and relieves these symptoms., , + Platelet aggregation that may lead to thrombosis is promoted by thromboxanes and, prostaglandins E1 and inhibited by prostacyclins., + Leukotrienes are implicated in hypersensitivity (allergy) and asthma., , + Consumption of fish foods containing the unsaturated fatty acid namely, eicosapentaenoic acid is advocated to prevent heart attacks.
Page 660 :
Section 6, , Current Topics, , Chapter, , Biological Membranes, and Transport, , 33, , The plasma membrane speaks :, , “I earmark the cell territory;, For protection from hostile environment;, Regulate solute import and export, By passive or active transport.”, , T, , he plasma membrane is an envelope, surrounding the cell (Refer Fig.1.1). It, separates and protects the cell from the external, hostile environment. Besides being a protective, barrier, plasma membrane provides a connecting, system between the cell and its environment., The subcellular organelles such as nucleus,, mitochondria, lysosomes are also surrounded by, membranes., , Chemical composition, The membranes are composed of lipids,, proteins and carbohydrates. The actual composition differs from tissue to tissue. Among the, lipids, amphipathic lipids (containing hydrophobic and hydrophilic groups) namely phospholipids, glycolipids and cholesterol, are found, in the animal membranes., Many animal cell membranes have thick, coating of complex polysaccharides referred to, as glycocalyx. The oligosaccharides of, glycocalyx interact with collagen of intercellular, matrix in the tissues., , Structure of membranes, A lipid bilayer model originally proposed for, membrane structure in 1935 by Davson and, Danielle has been modified., , Fluid mosaic model, proposed by Singer and, Nicolson, is a more recent and acceptable model, for membrane structure. The biological, membranes usually have a thickness of 5-8 nm. A, membrane is essentially composed of a lipid, bilayer. The hydrophobic (nonpolar) regions of the, lipids face each other at the core of the bilayer, while the hydrophilic (polar) regions face outward., Globular proteins are irregularly embedded in the, lipid bilayer (Fig.33.1). Membrane proteins are, categorized into two groups., 1. Extrinsic (peripheral) membrane proteins, are loosely held to the surface of the membrane, and they can be easily separated e.g. cytochrome, c of mitochondria., 2. Intrinsic (integral) membrane proteins are, tightly bound to the lipid bilayer and they can, be separated only by the use of detergents or, , 650
Page 661 :
Chapter 33 : BIOLOGICAL MEMBRANES AND TRANSPORT, , 651, , organic solvents e.g. hormone, receptors, cytochrome P450., , Lipid, , The membrane is asymmetric, due to the irregular distribution of, proteins. The lipid and protein, subunits of the membrane give an, appearance of mosaic or a ceramic, tile. Unlike a fixed ceramic tile, the, membrane freely changes, hence, the structure of the membrane is, considered as fluid mosaic., , Peripheral, protein, Integral, protein, , Fig. 33.1 : The fluid mosaic model of membrane structure., , Transport across membranes, The biological membranes are relatively, impermeable. The membrane, therefore, forms a, barrier for the free passage of compounds across, it. At least three distinct mechanisms have been, identified for the transport of solutes, (metabolites) through the membrane (Fig.33.2)., 1. Passive diffusion, 2. Facilitated diffusion, 3. Active transport., 1. Passive diffusion : This is a simple process, which depends on the concentration gradient of, a particular substance across the membrane., Passage of water and gases through membrane, occurs by passive diffusion. This process does, not require energy., , 2. Facilitated diffusion : This is somewhat, comparable with diffusion since the solute, moves along the concentration gradient (from, higher to lower concentration) and no energy is, needed. But the most important distinguishing, feature is that facilitated diffusion occurs through, the mediation of carrier or transport proteins., Specific carrier proteins for the transport of, glucose, galactose, leucine, phenylalanine etc., have been isolated and characterized., Mechanism of facilitated diffusion : A pingpong model is put forth to explain the occurrence, of facilitated diffusion (Fig.33.3). According to, this mechanism, a transport (carrier) protein exists, in two conformations. In the pong conformation,, it is exposed to the side with high solute, , Concentration, gradient, , Membrane, , Passive, diffusion, , Facilitated, diffusion, , Energy, Active, transport, , Passive transport, , Fig. 33.2 : Mechanism of transport across biological membrane, (Note : Transport molecule are represented in blue; the carrier proteins in red).
Page 662 :
652, , BIOCHEMISTRY, , Pong, , Ping, , Fig. 33.3 : A diagrammatic representation of ‘ping-pong’ model for facilitated diffusion., , concentration. This allows the binding of solute, to specific sites on the carrier protein. The protein, then undergoes a conformational change (ping, state) to expose to the side with low solute, concentration where the solute molecule is, released. Hormones regulate facilitated diffusion., For instance, insulin increases glucose transport, in muscle and adipose tissue; amino acid, transport in liver and other tissues., 3. Active transport : Active transport occurs, against a concentration gradient and this is, dependent on the supply of metabolic energy, (ATP). Active transport is also a carrier mediated, process like facilitated diffusion. The most, important primary active transport systems are, ion-pumps (through the involvement of pump, ATPases or ion transporting ATPases)., Na+-K+ pump : The cells have a high intracellular K+ concentration and a low Na+ concentration. This is essentially needed for the survival, of the cells. High cellular K+ is required for the, optimal glycolysis (pyruvate kinase is dependent, on K+) and for protein biosynthesis. Further, Na+, and K+ gradients across plasma membranes are, needed for the transmission of nerve impulse., The Na+-K+ pump is responsible for the, maintenance of high K+ and low Na+, concentrations in the cells. This is brought about, by an integral plasma membrane protein, namely, the enzyme Na+-K+ ATPase (mol. wt. 250,000)., It consists of two D and two E subunits which, may be represented as (DE)2. Na+-K+ ATPase, pumps 3Na+ ions from inside the cell to outside, and brings 2K+ ions from the outside to the, inside with a concomitant hydrolysis of, intracellular ATP. The Na+-K+ pump, depicted in, Fig.33.4, is summarized., , 3 Na+ (in) + 2K+ (out) + ATP o 3Na+ (out), + 2K+ (in) + ADP + Pi, A major portion of the cellular ATP (up to, 70% in nerve cells) is in fact utilized by Na+-K+, pump to maintain the requisite cytosolic Na+, and K+ levels. Ouabain (pronounced as Wahbáin) inhibits Na+-K+ ATPase. Ouabain is a, steroid derivative extracted from the seeds of an, African shrub. It is a poison used to tip the, hunting arrows by the tribals in Africa. Digoxin, a steroid glycoside and an inhibitor of Na+-K+, ATPase, is used in the treatment of congestive, cardiac failure (digoxin improves cardiac, contractility)., Na+-cotransport system : The amino acids, and sugars are transported into the cells by a, Na+-cotransport system. This process essentially, consists of the passage of glucose (or amino acid), into the cell with a simultaneous movement of, Na+. ATP is required to pump out the, intracellular Na+ through the mediation of, Na+-K+ ATPase. More details on the cotransport, system are given under digestion and absorption, (Chapter 8)., 3Na+, , 2K+, , Outside, , Membrane, , ATP, 3Na+, , ADP + Pi, 2K+, , Inside, , Fig. 33.4 : Diagrammatic representation of, Na+—K+ pump (Note : Red colour block, represents the enzyme Na+—K+ ATPase).
Page 663 :
653, , Chapter 33 : BIOLOGICAL MEMBRANES AND TRANSPORT, , Membrane, , Uniport, , Symport, , Antiport, , Cotransport, , Fig. 33.5 : Diagrammatic representation of transport systems., , Transport systems, The transport systems may be divided into 3, categories (Fig.33.5)., 1. Uniport system : This involves the, movement of a single molecule through the, membrane e.g. transport of glucose to the, erythrocytes., 2. Symport system : The simultaneous, transport of two different molecules in the same, direction e.g. transport of Na+ and glucose to the, intestinal mucosal cells from the gut., 3. Antiport system : The simultaneous transport of two different molecules in the opposite, direction e.g exchange of Cl– and HCO3– in the, erythrocytes. Uniport, symport and antiport, systems are considered as secondary active, transport systems., , Cotransport system : In cotransport, the, transport of a substance through the membrane, is coupled to the spontaneous movement of, another substance. The symport and antiport, systems referred to above are good examples of, cotransport system., Proton pump in the stomach : This is, an antiport transport system of gastric parietal, cells. It is brought out by the enzyme, H+–K+ ATPase to maintain highly acidic, (pH|1) conditions in the lumen of the stomach., Proton pump antiports two cytoplasmic protons, (2H+) and two extracellular potassium (2K+) ions, for a molecule of ATP hydrolysed. The chloride, ions secreted by Cl– channels combine with, protons to form gastric HCl. Omeprazole is a, drug used in the treatment of peptic ulcer. It, inhibits H+-K+ ATPase and results in reduced, secretion of HCl., , + Biological membranes are relatively impermeable protective barriers that provide a, connecting link between the cell (or its organelle) and its environment., , + The cells must contain high K+ and low Na+ concentrations for their survival. Na+-K+, pump, which consumes a major portion of the cellular metabolic energy (ATP), is, responsible for this., , + Ouabain inhibits Na+-K+ ATPase (Na+-K+ pump). It is extracted from the seeds of an, African shrub and used as poison to tip the hunting arrows by the tribals., , + Disturbances is osmosis are associated with diarrhea, edema, inflammation. Changes in, membrane fluidity have been suggested to be involved in LCAT deficiency, hypertension, and Alzheimer’s disease.
Page 664 :
654, , BIOCHEMISTRY, , Passive transport, of water-osmosis, , Endocytosis, , Osmosis is the phenomenon of, movement of water from low osmotic, pressure (dilute solution) to high osmotic, pressure (concentrated solution) across, biological membranes. The movement of, water in the body occurs through osmosis,, and this process does not require energy, (ATP). Certain medical and health, complications are due to disturbances in, osmosis. e.g. edema, diarrhea, cholera,, inflammation of tissues. The reader may, refer Chapter 40 for more information on, osmosis, water and electrolyte imbalance in, cholera/diarrhea., , Diseases due to loss of membrane, transport systems, Alterations in transport systems result in a, number of pathological conditions, selected, examples are listed., , Exocytosis, , Inside, , Fig. 33.6 : Diagrammatic representation of, endocytosis and exocytosis, (Note : The red coloured particles indicate the, transport material)., , Transport of macromolecules, The transport of macromolecules such as, proteins, polysaccharides and polynucleotides, across the membranes is equally important., This is brought about by two independent, mechanisms namely endocytosis—intake of, macromolecules by the cells (e.g. uptake of, LDL by cells) and exocytosis—release of, macromolecules from the cells to the outside, (e.g. secretion of hormones-insulin, PTH)., , Outside, , l, , l, , l, , Hartnup’s disease due to a decrease in the, transport of neutral amino acids in the, intestinal cells and renal tubules., Cystinuria, characterized, by, increased, excretion of cystine, lysine, arginine and, ornithine. This results in the formation of renal, cystine stones., Decreased glucose uptake in some individuals, due to lack of the specific sodium-glucose, transporter., Renal reabsorption of phosphate is decreased, in vitamin D resistant rickets., , 1. The biological membranes are the barriers that protect the cell and the subcellular, organelles from the hostile environment. The membranes are primarily composed of a, lipid bilayer onto which the globular proteins are irregularly embedded to form a fluid, mosaic model., 2. Transport of molecules through membranes occurs either by passive diffusion, facilitated, diffusion or active transport. Active transport occurs against a concentration gradient, which is dependent on the supply of metabolic energy (ATP). Na+-K+ pump is, responsible for the maintenance of high K+ and low Na+ concentrations inside the cells,, an essential requisite for the survival of cells., 3. The transport systems are divided into 3 categories—uniport, symport and antiport., 4. The transport of macromolecules takes place by endocytosis (ingestion by the cells) and, exocytosis (release from the cells).
Page 665 :
Section 6, , Current Topics, , Chapter, , Free Radicals, and Antioxidants, , 34, –, , O2 (Superoxide), e–, 2H+, H2O2 (Hydrogen peroxide), e–, H+, H2O, OH– (Hydroxyl radical), , The free radicals speaks :, , “We exist as independent molecular species;, Generated by cellular metabolism and environmental effects;, Implicated in the causation of several diseases;, Destroyed by antioxidants to protect cells/tissues/body.”, , T, , O2 (Molecular oxygen), , he supply of oxygen is absolutely essential, for the existence of higher organisms. As, the saying goes too much of even the best is, bad. Very high concentrations of O2 are found, to be toxic, and can damage tissues. The present, day concept of oxygen toxicity is due to the, involvement of oxygen free radicals or reactive, oxygen species (ROS). In fact, the generation of, reactive metabolites of O2 is an integral part of, our daily life., A free radical is defined as a molecule or, a molecular species that contains one or, more unpaired electrons, and is capable of, independent existence., , Types of free radicals, Oxygen is required in many metabolic, reactions, particularly for the release of energy., During these processes, molecular O2 is, completely reduced, and converted to water., However, if the reduction of O2 is incomplete, a, series of reactive radicals are formed, as shown, in the next column., , e–, –, , O2 (Superoxide), e–, 2H+, H2O2 (Hydrogen peroxide), e–, H+, H2O, OH– (Hydroxyl radical), e–, H+, H2O (Water), , Besides the above (O–2, H2O2, OH–), the other, free radicals and reactive oxygen species of, biological importance include singlet oxygen, (1O2), hydroperoxy radical (HOO–), lipid, peroxide radical (ROO–), nitric oxide (NO–) and, peroxynitrite (ONOO–)., The common characteristic features of free, radicals are listed, l, , Highly reactive, , l, , Very short half-life, , 655
Page 666 :
656, , l, , l, , BIOCHEMISTRY, , Can generate new radicals by chain reaction, Cause damage to biomolecules, cells and, tissues, , Free radicals and reactive oxygen species, (ROS)—not synonymous : By definition, a free, radical contains one or more unpaired electrons., e.g. O–2 , OH–, ROO–. There are certain nonradical derivatives of O2 which do not contain, unpaired electrons e.g. H2O2, 1O2. The term, reactive oxygen species is used in a broad sense, to collectively represent free radicals, and nonfree radicals (which are extremely reactive) of, the biological systems. However, most authors, do not make a clear distinction between, free radicals and ROS, and use them interchangebly., , SOURCES AND GENERATION, OF FREE RADICALS, The major sources responsible for the, generation of free radicals may be considered, under two categories, I. Due to normal biological processes (or, cellular metabolism)., II. Due to environmental effects., It is estimated that about 1-4% of the O2, taken up by the body is converted to free, radicals. A summary of the sources for, generation of free radicals is given in the, Table 34.1, and a couple of the processes are, briefly described., , Lipid peroxidation, Free, radical-induced, peroxidation, of, membrane lipids occurs in three stages-initiation,, propagation and termination, Initiation phase : This step involves the, removal of hydrogen atom (H) from, polyunsaturated fatty acids (LH), caused by, hydroxyl radical, LH + OH– o L– + H2O, Propagation phase : Under aerobic, conditions, the fatty acid radical (L–) takes up, oxygen to form peroxy radical (LOO–). The, , TABLE 34.1 Sources along with some examples, for generation of free radicals, I Cellular metabolism, l, , l, , l, , Leakage of electrons from the respiratory chain, (ETC)., Production of H2O2 or O2– by oxidase enzymes, (e.g. xanthine oxidase, NADPH oxidase)., Due to chain reactions of membrane lipid, peroxidation., , l, , Peroxisomal generation of O2 and H2O2., , l, , During the synthesis of prostaglandins., , l, , Production of nitric oxide from arginine., , l, , l, l, , During the course of phagocytosis (as a part of, bactericidal action)., In the oxidation of heme to bile pigments., As a result of auto-oxidation e.g. metal ions, [Fe2+, Cu2+]; ascorbic acid, glutathione, flavin, coenzymes., , II Environmental effects, l, , l, , As a result of drug metabolism e.g. paracetamol,, halothane, cytochrome P450 related reactions., Due to damages caused by ionizing radiations, (e.g. X-rays) on tissues., , l, , Photolysis of O2 by light., , l, , Photoexcitation of organic molecules, , l, , l, , Cigarette smoke contains free radicals, and trace, metals that generate OH–., Alcohol, promoting lipid peroxidation., , latter, in turn, can remove H-atom from another, PUFA (LH) to form lipid hydroperoxide (LOOH)., L– + O2 o LOO–, LOO– + LH o LOOH + L–, The hydroperoxides are capable of further, stimulating lipid peroxidation as they can form, alkoxy (LO–) and peroxyl (LOO–) radicals., 2LOOH, , Fe, Cu, , LO– + LO–2 + H2O, , LOOH o LO– + LOO– + aldehydes, Termination phase : Lipid peroxidation, proceeds as a chain reaction until the available, PUFA gets oxidized.
Page 667 :
657, , Chapter 34 : FREE RADICALS AND ANTIOXIDANTS, , Malondialdehyde, (MDA) as a marker, for lipid peroxidation, , NADPH, , O2, , Respiratory, burst, , NADPH, , oxidase, Most of the products, of lipid peroxidation are, Superoxide, Myeloperoxidase, NADP+, HClO, H2O2, O2–, unstable, e.g., carbonyls,, dismutase, Hypochlorous acid, Cl, esters, alkanes, alkenes, 2alkenal, 2,4-alkadienal, MDA., Of these, malondialdehyde, Bacteria, destroyed, ( CHO CH2 CHO ) is the, most extensively studied, and, Fig. 34.1 : Generation of free radicals by macrophages and respiratory burst., is used as a biochemical, marker for the assessment of, lipid peroxidation. MDA and, other aldehydes react with thiobarbituric acid, 3. Nuclear accidents explosions result in, and produce red-coloured products namely ionizing radiations. This causes oxidative, thiobarbituric acid reactive substances (TBARS) damage to DNA and mutations which may lead, which can be measured colorimetrically. The to cancers., estimation of serum MDA is often used to assess, oxidative stress, and free radical damage to the HARMFUL EFFECTS, body., OF FREE RADICALS, , Generation of ROS by macrophages, During the course of phagocytosis, macrophages produce superoxide (O–2), by a reaction, catalysed by NADPH oxidase (Fig.34.1). This O–2, radical gets converted to H2O2, and then to, hypochlorous acid (HClO). The superoxide, radical along with hypochlorous ions brings, about bactericidal action. This truly represents, the beneficial affects of the free radicals, generated by the body. A large amount of O2 is, consumed by macrophages during their, bactericidal function, a phenomenon referred to, as respiratory burst. It is estimated that about, 10% of the O2 taken up by macrophages is, utilized for the generation of free radicals., , Medical applications of ROS, 1. Radiation therapy uring cobalt-60 or J-rays, to destroy tumor tissue involves ROS (hydroxyl, radicals, organic radicals). Thus, the biochemical, basis of radiation therapy is localized oxidative, stress, causing damage to all biomolecules. Most, important is the damage to DNA that prevents, tumor cell replication and tumor growth., 2. Sterilization of foods by irradiation, destroying viral or bacterial contaminants also, involves ROS., , Free radicals and biomolecules, Free radicals are highly reactive, and are, capable of damaging almost all types of, biomolecules (proteins, lipids, carbohydrates,, nucleic acids). Free radicals beget free radicals, i.e. generate free radicals from normal, compounds which continues as a chain reaction., Proteins : Free radicals cause oxidation of, sulfhydryl groups, and modification of certain, amino acids (e.g. methionine, cysteine, histidine,, tryptophan, tyrosine). ROS may damage proteins, by fragmentation, cross-linking and aggregation., The net result is that free radicals may often, result in the loss of biological activity of proteins., Lipids : Polyunsaturated fatty acids (PUFA), are highly susceptible to damage by free, radicals. (see lipid peroxidation), Carbohydrates : At physiological pH,, oxidation of monosaccharides (e.g. glucose) can, produce H2O2 and oxoaldehydes. It appears that, the linkage of carbohydrates to proteins, (glycation) increases the susceptibility of proteins, to the attack by free radicals. This character, assumes significance in diabetes mellitus where
Page 668 :
658, protein glycation is associated with many health, complications e.g. diabetic microangiopathy,, diabetic nephropathy., Nucleic acids : Free radicals may cause DNA, strand breaks, fragmentation of bases and, deoxyribose. Such damages may be associated, with cytotoxicity and mutations., , Free radicals and diseases, , BIOCHEMISTRY, , the causes for the pathogenesis of insulindependent diabetes mellitus., Cataract : Increased exposure to oxidative, stress contributes to cataract formation, which is, mostly related to aging., Male infertility : Free radicals are known to, reduce sperm motility and viability, and thus, may contribute to male infertility., , As discussed above, free radicals are harmful, to biomolecules, and in turn cells and tissues., Free radicals have been implicated in the, causation and progress of several diseases., Cardiovascular diseases (CHD) : Oxidized, low density lipoproteins (LDL), formed by the, action of free radicals, promote atherosclerosis, and CHD., , Aging process : Free radicals are closely, associated with the various biochemical and, morphological changes that occur during normal, aging., , Cancer : Free radicals can damage DNA, and, cause mutagenicity and cytotoxicity, and thus, play a key role in carcinogenesis. It is believed, that ROS can induce mutations, and inhibit DNA, repair process, that results in the inactivation of, certain tumor suppressor genes leading to, cancer., Further,, free, radicals, promote, biochemical and molecular changes for rapid, growth of tumor cells., , ANTIOXIDANTS IN, BIOLOGICAL SYSTEM, , Inflammatory diseases : Rheumatoid arthritis, is a chronic inflammatory disease. The free, radicals produced by neutrophils are the, predominant causative agents. The occurrence, of other inflammatory disorders—chronic, glomerulonephritis and ulcerative colitis is also, due to the damages caused by ROS on the, extracellular, components, (e.g., collagen,, hyaluronic acid)., Respiratory diseases : Direct exposure of, lungs to 100% oxygen for a long period (more, than 24 hrs) is known to destroy endothelium, and cause lung edema. This is mediated by free, radicals. ROS are also responsible for adult, respiratory distress syndrome (ARDS), a disorder, characterized by pulmonary edema., Cigarette smoke, as such, contains free, radicals, and further it promotes the generation, of more free radicals. The damages caused to, lungs in the smokers are due to ROS., Diabetes : Destruction of islets of pancreas, due to the accumulation of free radicals is one of, , Other diseases : Free radicals play a key role, in Parkinson’s disease, Alzheimer’s disease,, multiple sclerosis, liver cirrhosis, muscular, dystrophy, toxemia of pregnancy etc., , To mitigate the harmful/damaging effects of, free radicals, the aerobic cells have developed, antioxidant defense mechanisms. A biological, antioxidant may be defined as a substance, (present in low concentrations compared to an, oxidizable substrate) that significantly delays or, inhibits oxidation of a substrate. Antioxidants, may be considered as the scavengers of free, radicals., The production of free radicals and their, neutralization by antioxidants is a normal bodily, process. There are different ways of classifying, antioxidants., , I. Antioxidants in relation to, lipid peroxidation, 1. Preventive antioxidants that will block the, initial production of free radicals e.g. catalase,, glutathione peroxidase., 2. Chain breaking antioxidants that inhibit, the propagative phase of lipid peroxidation e.g., superoxide dismutase, vitamin E, uric acid., , II. Antioxidants according to, their location, 1. Plasma antioxidants e.g. E-carotene,, ascorbic acid, bilirubin, uric acid, ceruloplasmin,, transferrin.
Page 670 :
660, , BIOCHEMISTRY, , D-Lipoic acid : It is vitamin-like compound,, produced in the body, besides the supply from, plant and animal sources. D-Lipoic acid plays a, key role in recycling other important antioxidants, such as ascorbic acid, D-tocopherol and, glutathione., Caffeine : Coffee contains flavonoids which, are antioxidant in nature. Recent studies indicate, that caffeine can directly act as an antioxidant., Besides the above, there are many other, important nutrient antioxidants, some of them, are listed below, , TABLE 34.2 Nutrient antioxidants and, their dietary sources, Antioxidant, , Dietary Source, , Vitamin E, (tocopherols), , Unprocessed vegetable oils, (cotton seed oil, peanut oil,, sunflower oil) whole grains,, leafy vegetables, legumes, , Vitamin C, (ascorbic acid), , Citrus fruits (oranges, grapes), gooseberry (amla), guava, green, vegetables (cabbage, spinach),, cauliflower, melons, , l, , Coenzyme Q10 of ubiquinone family, , E-Carotene, (provitamin A), , Carrots, green fruits and vegetables, spinach, turnip, apricots., , l, , Proanthocyanidins of grape seeds, , Lycopene, , l, , Catechins of green tea, , l, , Curcuminoids of turmeric, , Tomatoes, and their products, (tomato sauce), papaya, pink, guava, watermelon., , l, , Quercetin of onions, , Leutein and zeaxanthin Egg yolk, fruits, green leafy, vegetables, corn, green peas., , In the Table 34.2, some important nutrient, antioxidants and their dietary sources are, given. Consumption of a variety of nutrient, antioxidants is important, since each antioxidant targets certain types of damaging free, radicals., , Selenium, , Sea foods, meats, organ meats,, whole grains, , D-Lipoic acid, , Red meat, liver, yeast, , Coenzyme Q10, , Organ meats (best heart), beef,, chicken., , N-Acetylcysteine, , Available as supplement or drug., , Metabolic antioxidants, , Proanthocyanidins, , Grape seeds, , Glutathione : Reduced glutathione (GSH), plays a key role in the biological antioxidant, enzyme system (See Fig.34.2C). GSH and H2O2, are the twin substrates for glutathione, peroxidase. The reduced glutathione (GSH) gets, regenerated from the oxidized glutathione, , Catechins, , Green tea, , Curcuminoids, , Turmeric, , Quercetin, , Onions, red wine, green tea, , Ellagic acid, , Berries, walnuts, pomegranates, , Hesperidin, , Citrus fruits (oranges), lemon., , + Free radicals have been implicated in the causation and progress of several diseases e.g., atherosclerosis and CHD, cancer, respiratory diseases, aging., , + The estimation of serum malondialdehyde is often used to assess oxidative stress and, free radical damage to the body., , + The respiratory burst of macrophages, accompanied by the generation of ROS (H2O2, and HClO), brings about bactericidal action, and is beneficial to the body., , + Dietary consumption of a variety of nutrient antioxidants (vitamins C and E, E-, , carotene, lycopenes, Se, E-lipoic acid) is desirable since each antioxidant targets certain, types of damaging free radicals.
Page 671 :
Chapter 34 : FREE RADICALS AND ANTIOXIDANTS, , (GS SG) through the participation of glutathione, reductase and NADPH. It is sugested that the, ability to synthesize GSH decreases as age, advances, and this has been implicated in certain, diseases e.g. cataract., There are many more metabolic antioxidants, of biological importance. A selected few of them, are listed below, l, , l, , l, , l, , l, , l, , Uric acid, a powerful scavenger of singlet, oxygen (1O2) and OH– radicals., Ceruloplasmin inhibits iron and copper, dependent lipid peroxidation., Transferrin binds to iron and prevents ironcatalysed free radical formation., Albumin can scavange the free radicals, formed on its surface., Bilirubin protects the albumin bound free fatty, acids from peroxidation., Haptoglobin binds to free hemoglobin and, prevents the acceleration of lipid peroxidation., , 661, , DIETARY SUPPLEMENTATION, OF ANTIOXIDANTS, Free radicals damage biomolecules (proteins,, nucleic acids, lipids), and are implicated in the, causation and progress of several diseases (CHD,, cancer, autoimmune diseases). To counter the, action of free radicals, many protective, antioxidant nutrients (vitamins E and C,, E-carotene, selenium) are in use as dietary, supplements., Some recent studies show that antioxidant, supplements may be beneficial to the people, who are on deficient states, but not to all. On, the other hand, some clinical trials indicate that, supplementation of E-carotene and vitamin E is, associated with increased mortality. However,, this is controversial, and needs to be proved, beyond doubt., In any case, caution should be exercised in, the supplementation of antioxidants, and their, overuse should be avoided., , 1. Free radicals are the molecules or molecular species containing one or more unpaired, electrons with independent existence. e.g. O2–, H2O2, OH–, 1O2., 2. ROS are constantly formed during the normal cellular metabolism, (e.g. lipid, peroxidation) and due to various environmental influences (e.g. ionizing radiations)., 3. Free radicals are highly reactive and are capable of damaging almost all types of, biomolecules (proteins, lipids, carbohydrates, nucleic acids), and have been implicated, in the causation of many diseases e.g. cardiovascular diseases, cancer, inflammatory, diseases., 4. To mitigate the harmful effects of free radicals, the aerobic cells have developed, antioxidant defense mechanisms-enzymatic antioxidants (superoxide dismutase,, catalase) and non-enzymatic antioxidants (glutathione, Se, D-tocopherol, E-carotene).
Page 672 :
Section 6, , Current Topics, , Chapter, , Environmental Biochemistry, , 35, , The environment speaks :, , “Composed of living and non-living entities;, Co-existing and friendly with you all;, Continuously insulted by your activities of pollution;, That threatens only your healthy existence!”, , E, , nvironment constitutes the non-living (air,, water, land, energy etc.) as well as the living, (biological and social) systems surrounding man., Environmental biochemistry primarily deals with, the metabolic (biochemical) responses and, adaptations in man (or other organisms) due to, the environmental factors., A healthy environment is required for a, healthy life which is however, not really possible, or practicable. This is mainly because of the, atmospheric, (climatic), changes, and, environmental pollution., Environmental biochemistry is a very vast, subject. The basic concepts regarding the, atmospheric, changes, and, environmental, pollution on humans are dealt with here., , normal temperature (despite cold and heat, surroundings) for optimal physiological and, biochemical functions., , EXPOSURE TO COLD, Short-term exposure to cold causes shivering, (mainly due to skeletal muscle) to produce extra, heat. Heat is generated by the hydrolysis of ATP., , Non-shivering phase, Chronic exposure to cold results in nonshivering phase which is characterized by, several metabolic adaptations., l, , ATMOSPHERIC, (CLIMATIC) CHANGES, The climatic changes include cold, heat etc., The body makes every effort to maintain its, , l, , 662, , Energy metabolism : Heat generation by a, process called chemical thermogenesis occurs, in non-shivering phase. The foodstuffs undergo, oxidation to generate heat at the expense of, growth and other anabolic processes., Elevation in BMR, and increased intake of, foods are observed., Lipid metabolism : Stored fat (triacylglycerol), in the adipose tissue is mobilized to supply
Page 673 :
663, , Chapter 35 : ENVIRONMENTAL BIOCHEMISTRY, , free fatty acids for oxidation and production of, energy. Brown edipose tissue, particularly in, neonatal life, significantly contributes to, thermogenesis., l, , Hormonal changes : Thyroxine, a hormone, closely associated with energy metabolism, is, elevated. Further, corticosteroids are increased, on exposure to cold., , EXPOSURE TO HEAT, There is a continuous generation of heat by, the body due to the ongoing biochemical, processes, referred to as metabolic heat. This, heat has to be exchanged with the environment, to maintain a constant body temperature. On, exposure to heat in surroundings, as happens in, summer, the body is subjected to an, uncomfortable situation (since temperature of the, surroundings is much higher than that of the, body). However, heat is still lost from the body, through sweating and evaporation. Normally, the, body (thermoregulation) gets acclimatized to, higher temperature within 3-5 days., Heat stroke : It is characterized by the failure, of the heat regulatory system (thermoregulation), of the body. The manifestations of heat stroke, include high body temperature, convulsions,, partial (some times total) loss of consciousness., In extreme cases, heat stroke may cause, irreversible damage to brain. The treatment for, the heat stroke involves rapid cooling of the, body., The milder form of heat stroke is referred to, as heat syncope. Although the body temperature, is not raised much in this condition, the blood, pressure falls and the person may collapse, suddenly. Heat syncope is easily reversible., , ENVIRONMENTAL POLLUTION, Environmental pollution may be regarded as, the addition of extraneous (foreign) materials to, air, water or land which adversely affects the, quality of life. Pollution may be caused by, physical, chemical or biological processes., , The term pollutant refers to a substance, which increases in quantity due to human, activity and adversely affects the environment, (e.g. carbon monoxide, sulfur dioxide, lead). A, substance which is not present in nature but, released during human activity is the, contaminant (e.g. methyl isocyanate, DDT,, malathion). A contaminant however, is regarded, as a pollutant when it exerts detrimental effects., Environmental pollution may be considered in, different ways—industrial pollution; agricultural, pollution; pollution due to gaseous wastes, liquid, wastes and solid wastes. Environmental pollution, with reference to air, water and foodstuffs is, briefly discussed., , AIR POLLUTION, The major components of air include nitrogen, (78.1%), oxygen (20.93%) and carbon dioxide, (0.03%), along with water vapour and suspended, particles. The rapid growth of industries coupled, with changing lifestyles of man (urbanization,, smoking, use of motor vehicles etc.) largely, contribute to air pollution. The major chemical, constituents of air pollution are sulfur dioxide,, oxides of carbon (CO2 and CO), oxides of, nitrogen, hydrocarbons and particulates. The, biochemical affects of air pollution are, described., , Sulfur dioxide, Sulfur dioxide (SO2) is the most dangerous, pollutant gas to man. Industrial activities such as, burning of coal and oil emit large quantities of, SO2., Sulfur dioxide pollution primarily affects, respiratory system in man. Irritation of the, respiratory tract and increasing airway resistance, (breathing difficulty) are observed. Lung tissue, may get damaged due to acidic pH. Further,, dipalmityl lecithin, the phospholipid acting as, the lung surfactant, gets affected. Continuous, exposure to SO2 (> 1 ppm) for several days, causes bronchitis and in some individuals lung, cancer. Atmospheric SO2 when dissolved in rain, water becomes very acidic (acid rain) damaging, soil, plants and vegetables. Exposure of plants to, SO2 destroys leaves.
Page 674 :
664, , Carbon monoxide, Carbon monoxide (CO) is mostly produced, by incomplete combustion of fuel or carboncontaining compounds. Automobiles, aircrafts,, rail engines and burning of coal in factories, contribute to CO pollution., Carbon monoxide combines with hemoglobin, to form carboxyhemoglobin (Refer Chapter 10)., This causes a drastic reduction in the supply of, O2 to tissues. At a CO concentration around 1, ppm, impairment in mental performance and, visual perception take place. With a further, increase in CO level, headache, dizziness and, loss of consciousness occur. Death may be, inevitable in persons exposed to above 750 ppm, of CO., , Carbon dioxide, Carbon dioxide (CO2), constituting only a, fraction (0.03%) of the atmospheric gases, plays, a significant role in controlling the climate. This, is done by trapping the heat radiation from the, earth’s surface. Without the presence of CO2,, the earth would be as cold as moon!, Carbon dioxide is often referred to as, greenhouse gas. The term greenhouse, effect refers to an elevation in CO2 near earth’s, surface that traps sunlight and increases, atmospheric, temperature., Deforestation,, burning of coal, oils etc., elevate atmospheric, CO2 resulting in greenhouse effect. Hence the, global propaganda for increased plantation of, trees!, Fortunately, marginal variations in atmospheric, CO2 are tolerated by the cells. The body gets, adapted to prolonged exposure to higher, concentrations of CO2 (even upto 1%) with minor, alterations in electrolyte balance., , Nitrogen dioxide, Nitrogen dioxide (NO2) like carbon monoxide, (CO), combines with hemoglobin and reduces, the supply of O2 to the tissues. NO2 is more, harmful to human health than CO. It is fortunate, that the atmospheric concentration of NO2 is, relatively lower., , BIOCHEMISTRY, , Nitrogen dioxide (in the form of HNO3) along, with SO2 (as H2SO4) contributes to acid rain., , Hydrocarbons, Many hydrocarbons polluting the environment, affect human life. The aromatic hydrocarbons, cause irritation to injuries., , Particulates, The solid dust particles suspended in the, atmosphere constitute particulates. The sources, of particulates are grinding, spraying, erosion,, smoking etc., The particulates have ill-affects on humans., These include interference in respiratory function, (coughing, sneezing) and toxicity caused by the, absorption particulate chemicals. Further, the, dust particles carry microorganisms and other, infective agents to spread diseases., , Ozone layer, Ozone is formed from atmospheric oxygen, during high energy radiations of electrical, discharges. This ozone forms a layer above the, earth’s surface (15-35 km). It absorbs harmful, ultraviolet radiations of sun which would, otherwise cause skin diseases and mutations,, besides increasing the temperature of earth., In recent years, a decrease in the ozone layer, is observed due to chemical pollution in the air., Nitrogen oxides (released from engines of aeroplanes) and chlorofluoro carbons (used in refrigerators and air conditioners) deplete the ozone, layer., , WATER POLLUTION, Water is the most predominant constituent of, living matter. The very existence of life is, unimaginable without water., As such, pure water does not exist in nature., The available water contains dissolved gases,, minerals and some suspended particles., Pollution of water occurs due to waste disposal, from industries, agriculture and municipalities., The pollutants may be organic, inorganic,, sediments, radioactive, thermal etc., in nature.
Page 675 :
665, , Chapter 35 : ENVIRONMENTAL BIOCHEMISTRY, , ORGANIC POLLUTANTS, The organic pollutants include agents carrying, water borne diseases, oxygen demanding wastes, and organic chemicals., , rodenticides. Based on their structure, pesticides, are classified as follows., (a) Chlorinated hydrocarbons : e.g. aldrin,, dieldrin, endrin, dichlorodiphenyl trichloroethane (DDT)., , Water-borne disease agents, Several pathogenic organisms find their entry, into water and cause diseases. The water borne, disease include typhoid, paratyphoid, cholera,, amoebiasis, giardiasis and infectious hepatitis., These diseases can be prevented by disinfection, techniques employed for the treatment of water., , Oxygen demanding wastes, Sewage, and wastes from industries and, agriculture provide good nutrients for algae. As, the algae grow utilizing the wastes, oxygen, depletion occurs. This phenomenon of water, deoxygenation is technically referred to as, eutrophication., As, a, consequence, of, eutrophication, fish and other acquatic animals, die (due to lack of O2), causing foul smell., , Organic chemicals, , (b) Organophosphates, diazinon., , :, , e.g., , malathion,, , (c) Carbamates e.g. baygon, carbaryl (sevin), (d) Chlorophenoxy e.g. 2,4-dichlorophenoxy, acetic acid., The use of pesticides has helped in controlling, certain diseases (malaria, typhus), besides, boosting food production. However, pesticides, pollute water and cause several health, complications to humans, besides damaging, acquatic life., Dichloro-diphenyl trichloroethane (DDT) is a, widely used pesticide to control cotton and, peanut pests, besides malaria. However,, continuous use of DDT leads to its accumulation, in foods causing ill effects (hence banned in, some countries like USA)., , The organic chemical pollutants of water, include pesticides and several synthetic, compounds, (detergents,, paints,, plastics,, pharmaceuticals, food additives etc.), , DDT, being fat soluble, accumulates in the, adipose tissue and is not excreted. Thus, its, concentration in the body goes on increasing., DDT causes nervous irritability, muscle twitching, and convulsions., , Pesticides, , Aldrin and dialdrin are also fat soluble and, their effects on humans are comparable with that, of DDT., , Pesticides, insecticides,, , is a broad term used for, herbicides,, fungicides, and, , + The body makes every effort to maintain its normal temperature, despite cold and heat, surroundings, for optimal physiological and biochemical functions., , + Failure of heat regulatory system (thermoregulation) leads to heat stroke characterized, by high body temperature, convulsions etc., , + Sulfur dioxide (SO2) is the most dangerous industrial pollutant gas to man. It primarily, affects the respiratory system, and may result in bronchitis, and even lung cancer., , + Carbon monoxide (CO) combines with hemoglobin to form carboxyHb. This reduces O2, supply to tissues., , + Pollution of water with pathogenic organisms causes many diseases e.g. typhoid,, cholera, amoebiasis., , + Lead toxicity affects central nervous system—learning disabilities, mental retardation etc., + Radioactive pollution may lead to gene mutations, cancer etc.
Page 676 :
666, Organophosphates and carbamates are, powerful neurotoxic agents. They prevent the, transmission of nerve impulse by inhibiting the, enzyme cholinesterase., , INORGANIC POLLUTANTS, Heavy metals (lead, mercury, cadmium,, aluminium, arsenic etc.) are the most dangerous, among the inorganic pollutants., , Lead, Lead is the most common inorganic pollutant, found in water, air, foods and soils. The sources, of lead pollution include petrol, gasoline, paints,, cigarettes, news papers, lead pipes and xerox, copies. The plasma concentration of > 25 Pg/dl, in adults and > 10 Pg/dl in children results in, toxic manifestations., The principal target of lead toxicity is central, nervous system. In the growing children, Pb, causes learning disabilities, behavioural changes, (hyperexcitability) and mental retardation. In, adults, confusion, irritability, abdominal colic, and severe anemia are associated with lead, toxicity., Lead inhibits several enzymes, particularly,, G-aminolevulinate, (ALA), synthase,, ALA, dehydratase and ferrochelatase of heme synthesis, (Refer Chapter 10 also). This results in severe, anemia. There has been an increasing awareness, worldover on the toxic manifestations of lead., This has lead to the supply of unleaded petrol in, most countries., , Mercury, Mercury is a common industrial (plastic,, paints, electrical apparatus, fungicides) pollutant., Acute mercuric poisoning causes gastritis,, vomiting and pulmonary edema. Chronic, manifestations of Hg include emotional changes,, loss of memory and other neuropsychiatric, disturbances. In addition, deposition of mercuric, salts may cause renal failure., Organic mercuric poisoning is commonly, referred to as minamata disease (as it first, occurred in Minamata, Japan in 1953-60 by, , BIOCHEMISTRY, , consuming fish containing methyl mercury, as, industrial pollutant)., , Cadmium, The outbreak of cadmium toxicity was, reported in Japan in the form of itai itai or ouch, disease. Cadmium poisoning causes fragile, bones, anemia, bone marrow disorders and, kidney damage. Biochemically, cadmium, replaces zinc and adversely influences several, metabolic reactions., , Aluminium, The sources of aluminium include cooking, vessels, building materials, food additives and, cosmetics. Aluminium toxicity is associated with, Alzheimer’s disease, anemia and osteomalacia., , Arsenic, Arsenic,, commonly, found, in, many, insecticides and fungicides, is toxic to the body., Arsenic binds with–SH groups of several, enzymes and inhibits biochemical reactions e.g., pyruvate dehydrogenase. Further, arsenic causes, coagulation of proteins and blockage of ATP, generation (functions as an uncoupler)., , NOISE POLLUTION, The unwanted sound is noise, which is a, major urban environmental pollutant. Man can, tolerate noise upto 100 decibels (speaking – 60, decibels; telephone bell 70 decibels; motor cycle, 110 decibels; rockets 170 decibels). A noise, above 150 decibels is uncomfortable., The affects of noise pollution include, headache, increased blood pressure, irritability,, neuromuscular tension, confusion, disturbed, vision and digestion, depression and loss of, hearing., , RADIOACTIVE POLLUTION, The pollution due to radioactive substances is, the most dangerous to human life. The health, hazards of radioactive pollution include, gene mutations, cancer, destruction of living, cells etc.
Page 677 :
667, , Chapter 35 : ENVIRONMENTAL BIOCHEMISTRY, , TOXIC COMPOUNDS, IN FOODSTUFFS, The foodstuffs consumed by humans contain, several toxic compounds which may be either, normally present or enter foodstuffs during the, course of cultivation, processing or storage., , Natural toxins in foodstuffs, Neurotoxins : Kesari dal (Lathyrus sativus) is, a pulse grown in some parts of Madhya Pradesh,, Bihar and Uttar Prodesh. Excessive consumption, of kesari dal causes paralysis of lower limbs, referred to as lathyrism. This is due to a, neurotoxin, namely, E-oxalylaminoalanine, (BOAA). BOAA damages upper motor neurons,, and inhibits the enzyme lysyl oxidase (reduces, collagen cross-linking). Cooking of kesari dal, 2-3 times and removal of the supernatant water, will eliminate the toxin., Protease inhibitors : Certain legumes (soya, bean, peanut) contain inhibitors of protease, enzymes particularly trypsin. Normally, protease, inhibitors are destroyed by cooking. However,, partial cooking does not totally destroy them. In, such a case, protease inhibitors can inhibit, digestion and proteins., Goitrogens : These compounds prevent, uptake and utilization of iodine by thyroid gland., Goitrogens are found in cabbage and turnips, (thioglycosides), mustard and rape seed oils, (thiocyanates), ground nuts and almonds, (polyphenolic glycosides)., Biogenic amines : Bananas and cheese, contain biogenic amines namely histamine,, tryptamine, tyramine serotonin and epinephrine., In normal metabolism, they are degraded by, monoamine oxidase (MAO). However, in, persons taking MAO—inhibitors, the foodstuffs, with amines may cause hypertension., , Anti-vitamins : Avidin of raw egg is a good, example of anti-vitamin of biotin., , Toxic pollutants of foodstuffs, The foodstuffs may get polluted with several, toxic chemicals which might occur during, cultivation, processing or storage., Cultivation : Pesticides and other unnatural, chemicals used during cultivation do find an, entry into the foodstuffs. It is fortunate that most, of these chemicals can be removed by peeling, the outer layers of vegetables and fruits, besides, repeated washings., Processing : Defects in freezing, and packing, provide a suitable environment for the growth of, several organisms which release toxic products, e.g. milk contamination by Salmonella., Several food additives are in use for, preservation and enchancing flavour. Not all of, them are safe e.g. aniline dyes used as colouring, agents are carcinogenic; sweetening agent, cyclamate may cause bladder cancer., Storage : Contamination of stored foods, occurs mostly due to fungal infections., Aflatoxins are produced by Aspergillus favus, when ground nuts or coconuts are stored in, moist conditions. Aflatoxins are hepatotoxic and, carcinogenic., , CARCINOGENS, The group of chemicals that cause cancer in, man and animals are collectively referred to as, carcinogens (Refer Chapter 37). Environmental, pollution is undoubtedly associated with, increased risk of cancer. The topic ‘cancer’ may, be considered as a part of environmental, biochemistry for learning purpose.
Page 678 :
668, , BIOCHEMISTRY, , 1. Environmental biochemistry deals with the biochemical responses and adaptations in, man (and other organisms) due to environmental factors., 2. The atmospheric (climatic) changes like cold and heat influence the body. Several, metabolic adaptations occur to overcome the adverse affects., 3. The major chemical constituents of air pollution include SO2, CO, CO2 and oxides of, nitrogen. Among these, sulfur dioxide is the most dangerous., 4. Water pollution occurs mainly due to waste disposal from industries, agriculture and, municipalities. The pollutants may be organic (pathogenic organisms, pesticides), or, inorganic (lead, mercury)., 5. The foodstuffs consumed by humans may contain several toxic compounds. These may, be normally present (e.g. BOAA causing lathyrism) or enter the foodstuffs during the, course of cultivation (e.g. pesticides), or storage (e.g. aflatoxins).
Page 679 :
Section 6, , Current Topics, , Chapter, , Insulin, Glucose Homeostasis,, and Diabetes Mellitus, , 36, , The Diabetes speaks :, Diabetes mellitus, Insulin-dependent, diabetes mellitus (IDDM), Non-insulin dependent, diabetes mellitus (NIDDM), , “Dubbed as a disease of fuel scarcity in plenty;, Starving the cells bathed in ample glucose quantity;, Attributed to insufficient or inefficient insulin;, Affecting several tissues by metabolic complications.”, , D, , iabetes mellitus is the third leading cause, of death (after heart disease and cancer) in, many developed countries. It affects about 6 to, 8% of the general population. The complications, of diabetes affect the eye, kidney and nervous, system. Diabetes is a major cause of blindness,, renal failure, amputation, heart attacks and, stroke. (The term diabetes, whenever used, refers, to diabetes mellitus. It should, however, be, noted that diabetes insipidus is another, disorder characterized by large volumes of, urine excretion due to antidiuretic hormone, deficiency)., , Diabetes mellitus is a clinical condition, characterized by increased blood glucose level, (hyperglycemia) due to insufficient or inefficient, (incompetent) insulin. In other words, insulin is, either not produced in sufficient quantity or, inefficient in its action on the target tissues. As a, consequence, the blood glucose level is elevated, which spills over into urine in diabetes mellitus, (Greek : diabetes—a siphon or running through;, mellitus—sweet)., , An important feature of diabetes is that the, body cells are starved of glucose despite, its very high concentration around i.e. scarcity in, plenty. For a comprehensive understanding of, diabetes, the relevant hormones, namely insulin, and glucagon, homeostasis of blood glucose,, besides the biochemical aspects of diabetes, are, discussed in this chapter., , INSULIN, Insulin is a polypeptide hormone produced, by the E-cells of islets of Langerhans of, pancreas. It has profound influence on the, metabolism of carbohydrate, fat and protein., Insulin is considered as anabolic hormone, as it, promotes, the, synthesis, of, glycogen,, triacylglycerols and proteins. This hormone has, been implicated in the development of diabetes, mellitus., Insulin occupies a special place in the history, of biochemistry as well as medicine. Insulin was, the first hormone to be isolated, purified and, , 669
Page 680 :
670, , BIOCHEMISTRY, , +, , synthesized; first hormone to be sequenced; first, hormone to be produced by recombinant DNA, technology., , H 3N, , Structure of insulin, Human insulin (mol. wt. 5,734) contains 51, amino acids, arranged in two polypeptide, chains. The chain A has 21 amino acids while B, has 30 amino acids. Both are held together by, two interchain disulfide bridges, connecting A7, to B7 and A20 to B19. In addition, there is an, intrachain disulfide link in chain A between the, amino acids 6 and 11., , –, , COO, , Preproinsulin, Endoplasmic, reticulum, , Biosynthesis of insulin, Insulin is produced by the E-cells of the islets, of Langerhans of pancreas. The gene for this, protein synthesis is located on chromosome 11., The synthesis of insulin involves two precursors,, namely preproinsulin with 108 amino acids, (mol. wt. 11,500) and proinsulin with 86 amino, acids (mol. wt. 9,000). They are sequentially, degraded (Fig.36.1) to form the active hormone, insulin and a connecting peptide (C-peptide)., Insulin and C-peptide are produced in equimolar, concentration. C-peptide has no biological, activity, however its estimation in the plasma, serves as a useful index for the endogenous, production of insulin., , Signal, sequence, , S S, , S, S S, , Proinsulin, Golgi, apparatus, , In the E-cells, insulin (and also proinsulin), combines with zinc to form complexes. In this, form, insulin is stored in the granules of the, cytosol which is released in response to various, stimuli (discussed below) by exocytosis., , 7, 19, , Glucose is the most important stimulus for, insulin release. The effect is more predominant when glucose is administered orally, (either direct or through a carbohydrate-rich, , 7, , S S, , S, S, , 20, 21, , 30, , A-chain, , B-chain, Insulin, , About 40-50 units of insulin is secreted daily, by human pancreas. The normal insulin concentration in plasma is 20-30 PU/ml. The important, factors that influence the release of insulin from, the E-cells of pancreas are discussed hereunder., , l, , 6, , S S, , 11, , Regulation of insulin secretion, , 1. Factors stimulating insulin secretion :, These include glucose, amino acids and, gastrointestinal hormones., , S, , C-peptide, , Fig. 36.1 : Formation of insulin from preproinsulin., , meal). A rise in blood glucose level is a signal, for insulin secretion., l, , Amino acids induce the secretion of insulin., This is particularly observed after the ingestion, of protein-rich meal that causes transient rise, in plasma amino acid concentration. Among, the amino acids, arginine and leucine are, potent stimulators of insulin release.
Page 681 :
Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , 671, , TABLE 36.1 Metabolic effects of insulin—a summary, , Metabolism, , Net effect, , Effect on important enzyme(s), , Carbohydrate metabolism, 1. Glycolysis, , Increased, , Glucokinase n, Phosphofructokinase n, Pyruvate kinase n, , 2. Gluconeogenesis, , Decreased, , 3. Glycogenesis, 4. Glycogenolysis, 5. HMP shunt, , Increased, Decreased, Increased, , Pyruvate carboxylase p, Phosphoenol pyruvate carboxykinase p, Glucose 6-phosphatase p, Glycogen synthetase n, Glycogen phosphorylase p, Glucose 6- phosphate dehydrogenase n, , Increased, Decreased, Decreased, , Acetyl CoA carboxylase n, Hormone sensitive lipase p, HMG CoA synthetase p, , Increased, Decreased, , RNA polymerase n, Transaminases p, Deaminases p, , Lipid metabolism, 6. Lipogenesis, 7. Lipolysis, 8. Ketogenesis, Protein metabolism, 9. Protein synthesis, 10. Protein degradation, , l, , Gastrointestinal hormones (secretin, gastrin,, pancreozymin) enhance the secretion of, insulin. The GIT hormones are released after, the ingestion of food., , 2. Factors inhibiting insulin secretion : Epinephrine is the most potent inhibitor of insulin, release. In emergency situations like stress,, extreme exercise and trauma, the nervous system, stimulates, adrenal, medulla, to, release, epinephrine. Epinephrine suppresses insulin, release and promotes energy metabolism by, mobilizing energy-yielding compounds—glucose, from liver and fatty acids from adipose tissue., , Degradation of insulin, In the plasma, insulin has a normal half-life of, 4-5 minutes. This short half-life permits rapid, metabolic changes in accordance to the, alterations in the circulating levels of insulin., This is advantageous for the therapeutic, purposes. A protease enzyme, namely insulinase, (mainly found in liver and kidney), degrades, insulin., , Metabolic effects of insulin, Insulin plays a key role in the regulation of, carbohydrate, lipid and protein metabolisms, (Table 36.1). Insulin exerts anabolic and, anticatabolic influences on the body metabolism., 1. Effects on carbohydrate metabolism : In a, normal individual, about half of the ingested, glucose is utilized to meet the energy demands, of the body (mainly through glycolysis). The, other half is converted to fat (~ 40%) and, glycogen (~ 10%). This relation is severely, impaired in insulin deficiency. Insulin influences, glucose metabolism in many ways. The net effect, is that insulin lowers blood glucose level, (hypoglycemic effect) by promoting its, utilization and storage and by inhibiting its, production., l, , Effect on glucose uptake by tissues : Insulin is, required for the uptake of glucose by muscle, (skeletal, cardiac and smooth), adipose tissue,, leukocytes and mammary glands. Surprisingly,, about 80% of glucose uptake in the body is
Page 682 :
672, not dependent on insulin. Tissues into which, glucose can freely enter include brain, kidney,, erythrocytes, retina, nerve, blood vessels and, intestinal mucosa. As regards liver, glucose, entry into hepatocytes does not require, insulin. However, insulin stimulates glucose, utilization in liver and, thus, indirectly, promotes its uptake., l, , l, , Effect on glucose utilization : Insulin increases, glycolysis in muscle and liver. The activation, as well as the quantities of certain key, enzymes of glycolysis, namely glucokinase, (not hexokinase) phosphofructokinase and, pyruvate kinase are increased by insulin., Glycogen production is enhanced by insulin, by increasing the activity of glycogen, synthetase., Effect on glucose production : Insulin, decreases gluconeogenesis by suppressing the, enzymes pyruvate carboxylase, phosphoenol, pyruvate carboxykinase and glucose 6phosphatase. Insulin also inhibits glycogenolysis by inactivating the enzyme glycogen, phosphorylase., , 2. Effects on lipid metabolism : The net effect, of insulin on lipid metabolism is to reduce the, release of fatty acids from the stored fat and, decrease the production of ketone bodies., Among the tissues, adipose tissue is the most, sensitive to the action of insulin., l, , l, , l, , Effect on lipogenesis : Insulin favours the, synthesis of triacylglycerols from glucose by, providing more glycerol 3-phosphate (from, glycolysis) and NADPH (from HMP shunt)., Insulin increases the activity of acetyl CoA, carboxylase, a key enzyme in fatty acid, synthesis., Effect on lipolysis : Insulin decreases the, activity of hormone-sensitive lipase and thus, reduces the release of fatty acids from stored, fat in adipose tissue. The mobilization of fatty, acids from liver is also decreased by insulin., In this way, insulin keeps the circulating free, fatty acids under a constant check., Effect on ketogenesis : Insulin reduces, ketogenesis by decreasing the activity of HMG, CoA synthetase. Further, insulin promotes the, , BIOCHEMISTRY, , utilization of acetyl CoA for oxidation (Krebs, cycle) and lipogenesis. Therefore, the, availability of acetyl CoA for ketogenesis, in, the normal circumstances, is very low., 3. Effects on protein metabolism : Insulin is, an anabolic hormone. It stimulates the entry of, amino acids into the cells, enhances protein, synthesis and reduces protein degradation., Besides the metabolic effects described, above, insulin promotes cell growth and, replication. This is mediated through certain, factors such as epidermal growth factor (EGF),, platelet derived growth factor (PDGF) and, prostaglandins., , Mechanism of action of insulin, It is now recognized that insulin binds to, specific plasma membrane receptors present on, the target tissues, such as muscle and adipose., This results in a series of reactions ultimately, leading to the biological action. Three distinct, mechanisms of insulin action are known. One, concerned with the induction of transmembrane, signals (signal transduction), second with the, glucose transport across the membrane and the, third with induction of enzyme synthesis., 1. Insulin receptor mediated signal transduction, Insulin receptor : It is a tetramer consisting of, 4 subunits of two types and is designated as, D2E2. The subunits are in the glycosylated form., They are held together by disulfide linkages. The, D-subunit (mol. wt. 135,000) is extracellular and, it contains insulin binding site. The E-subunit, (mol. wt. 95,000) is a transmembrane protein, which is activated by insulin. The cytoplasmic, domain of E-subunit has tyrosine kinase activity., The insulin receptor is synthesized as a single, polypeptide and cleaved to D and E subunits, which are then assembled. The insulin receptor, has a half-life of 6-12 hours. There are about, 20,000 receptors per cell in mammals., Signal transduction : As the hormone insulin, binds to the receptor, a conformational change, is induced in the D-subunits of insulin receptor., This results in the generation of signals which
Page 683 :
673, , Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , D, Membrane, , E E, Tyr, , Tyr, , Cytoplasm, , Insulin, (, ), D, , Membrane, , Cytoplasm, , activity of intracellular E-subunit of insulin, receptor. This causes the autophosphorylation of, tyrosine residues on E-subunit. It is believed that, receptor tyrosine kinase also phosphorylates, insulin receptor substrate (IRS). The phosphorylated IRS, in turn, promotes activation of other, protein kinases and phosphatases, finally leading, to biological action (Fig.36.2)., , D, , 2. Insulin-mediated glucose transport : The, binding of insulin to insulin receptors signals the, translocation of vesicles containing glucose, transporters from intracellular pool to the, plasma membrane. The vesicles fuse with the, membrane recruiting the glucose transporters., The glucose transporters are responsible for the, insulin–mediated uptake of glucose by the cells., As the insulin level falls, the glucose transporters, move away from the membrane to the, intracellular pool for storage and recycle, (Fig.36.3)., , D, , E E, Tyr, , Tyr, , P, , P, , IRS—Tyr, IRS—Tyr, P, , Protein kinases, Phosphatases, (activated), , Biological, effects, , 3. Insulin mediated enzyme synthesis :, Insulin promotes the synthesis of enzymes such, as glucokinase, phosphofructokinase and, pyruvate kinase. This is brought about by, increased transcription (mRNA synthesis),, followed by translation (protein synthesis)., , Fig. 36.2 : Insulin receptor mediated signal, transduction (IRS—Insulin receptor substrate)., , are transduced to E-subunits. The net effect is, that insulin binding activates tyrosine kinase, , Glucose, (), , (), , (), , Glucose, , (), , (), , (), , (), Signal, Insulinreceptor, complex, , Translocation, , (), , Fission, , (), , (), , (), , Plasma, membrane, , (), , Insulin, , () (), , Intracellular pool of vesicles, , Fig. 36.3 : Insulin mediated glucose transport., , (), , (), , (), , (), , Glucose transporters
Page 684 :
674, , BIOCHEMISTRY, , GLUCAGON, Glucagon, secreted by D-cells of the pancreas,, opposes the actions of insulin. It is a polypeptide, hormone composed of 29 amino acids (mol. wt., 3,500) in a single chain. Glucagon is actually, synthesized as proglucagon (mol. wt. 9,000), which on sequential degradation releases active, glucagon. Unlike insulin, the amino acid, sequence of glucagon is the same in all, mammalian species (so far studied). Glucagon has, a short half-life in plasma i.e. about 5 minutes., , Regulation of glucagon secretion, The secretion of glucagon is stimulated by, low blood glucose concentration, amino acids, derived from dietary protein and low levels of, epinephrine. Increased blood glucose level, markedly inhibits glucagon secretion., , Metabolic effects of glucagon, Glucagon influences carbohydrate, lipid and, protein metabolisms. In general, the effects of, this hormone oppose that of insulin., 1. Effects on carbohydrate metabolism :, Glucagon is the most potent hormone that, enhances the blood glucose level (hyperglycemic)., Primarily, glucagon acts on liver to cause, increased synthesis of glucose (gluconeogenesis), and enhanced degradation of glycogen, (glycogenolysis). The actions of glucagon are, mediated through cyclic AMP (Chapter 13)., 2. Effects on lipid metabolism : Glucagon, promotes fatty acid oxidation resulting in, energy production and ketone body synthesis, (ketogenesis)., 3. Effects on protein metabolism : Glucagon, increases the amino acid uptake by liver which,, in turn, promotes gluconeogenesis. Thus,, glucagon lowers plasma amino acids., , Mechanism of action of glucagon, Glucagon binds to the specific receptors on, the plasma membrane and acts through the, mediation of cyclic AMP, the second messenger., The details are given elsewhere (Chapter 19)., , REGULATION OF BLOOD GLUCOSE, LEVEL (HOMEOSTASIS OF, BLOOD GLUCOSE), Glucose is carbohydrate currency of the, body. An adult human body contains about 18 g, free glucose. This amount is just sufficient to, meet the basal energy requirements of the body, for one hour! The liver has about 100 g, stored glycogen. Besides this, it is capable of, producing about 125-150 mg glucose/minute or, 180-220 g/24 hrs., Expression of glucose concentration : In most, developed countries, plasma glucose (instead of, blood glucose) is estimated and expressed as SI, units (mmol/l). This is not however so, in, developing countries for practical reasons. It, may be noted that the plasma concentration, of glucose is slightly higher (about 15%), than blood glucose. Further, a glucose, concentration of 180 mg/dl (plasma or blood), corresponds to 10 mmol/l. In this book,, expression of blood glucose as mg/dl is more, frequently used., A healthy individual is capable of maintaining, the blood glucose concentration within a narrow, range. The fasting blood glucose level in a postabsorptive state is 70-100 mg/dl (plasma glucose, 80-120 mg/dl). Following the ingestion of a, carbohydrate meal, blood glucose may rise to, 120-140 mg/dl. The fasting blood glucose value, is comparatively lower in ruminant animals, (sheep 30-40 mg/dl; cattle 50-60 mg/dl), while it, is higher in birds (250-300 mg/dl)., The term hyperglycemia refers to an increase, in the blood glucose above the normal level., Hypoglycemia represents a decreased blood, glucose concentration. Excretion of glucose in, urine is known as glycosuria. The concentration, of blood glucose is dependent on the quantity of, glucose that enters the circulation from various, sources (dietary carbohydrates, glycogenolysis,, gluconeogenesis etc.) and the amount that is, utilized for different metabolic purposes, (glycolysis, glycogenesis, fat synthesis etc.) as, illustrated in Fig.36.4.
Page 685 :
675, , Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , Dietary carbohydrates, (starch, sucrose, glucose), Digestion and absorption, Glycogenolysis, in muscle, , Glycolysis and, TCA cycle, Glucose o CO2, H2O, , Hormonal, regulation, , Glucose in liver, Glycogenesis in, liver and kidney, , Lactate, BLOOD GLUCOSE, Gluconeogenesis, , Synthesis of other, monosaccharides and, aminosugars, , Fasting 70-100 mg/dl, Post-prandial 120-140 mg/dl, , Amino acids,, glycerol, propionate, , HMP shunt for pentoses, and NADPH, , Glycogenolysis, in liver, , Excreted into, urine (>180 mg/dl, blood glucose), , Synthesis of fat, Utilization of, blood glucose, , Sources of blood, glucose, , Fig. 36.4 : Overview of blood glucose homeostasis., , 1. Dietary sources : The dietary, carbohydrates are digested and absorbed as, monosaccharides, (glucose,, fructose,, galactose etc.). The liver is capable of, converting fructose and galactose into, glucose, which can readily enter blood., 2. Gluconeogenesis : The degradation of, glycogen in muscle results in the formation, of lactate. Breakdown of fat in adipose tissue, will produce free glycerol and propionate., Lactate, glycerol, propionate and some amino, acids are good precursors for glucose, synthesis (gluconeogenesis) that actively, occurs in liver and kidney. Gluconeogenesis, continuously adds glucose to the blood. Cori, cycle is responsible for the conversion of, muscle lactate to glucose in liver., 3. Glycogenolysis : Degradation of glycogen, in liver produces free glucose. This is in contrast, to muscle glycogenolysis where glucose is not, formed in sufficient amount due to lack of the, enzyme glucose 6-phosphatase. However, the, contribution of liver glycogenolysis to blood, glucose is rather limited and can meet only the, short intervals of emergency. This is due to the, limited presence of glycogen in liver. An adult, , Sources of blood glucose (%), during normal day, , Sources of blood glucose, , Dietary, Gluconeogenesis, Glycogenolysis, , 100, , 50, , 0, Midnight, , Breakfast, , Lunch, , Dinner, , Midnight, , Fig. 36.5 : Sources of blood glucose during, a normal day (24 hours)., , liver (weighing about 1.5 kg) can provide only, 40-50 g of blood glucose from glycogen, that, can last only for a few hours to meet the body, requirements., In the Fig.36.5, the sources of blood glucose, during a normal day (24 hours) are given., Glucose is primarily derived from glycogenolysis, (of hepatic glycogen) between the meals., Gluconeogenesis becomes a predominant source, of glucose in late night (after depletion of hepatic
Page 686 :
676, , BIOCHEMISTRY, , Blood glucose (mg/dl), , 40, , 50, , 60, , 70, , 80, , 90, , Fasting, (<100 mg/dl), , 100, , 110, , 120, , 130, , 140, , 150 160, , Post-prandial, (<130 mg/dl), , 170, , 180, , 190, , 200, , Renal threshold, , To urine, , Hypoglycemic effect, , Hyperglycemic effect, , Insulin, , Glucagon, , Glucose uptake n, Glycolysis n, Glycogenesis n, , Gluconeogenesis n, Glycogenolysis n, Epinephrine, Glycogenolysis n, , HMP shunt n, Lipid synthesis n, Gluconeogenesis p, Glycogenolysis p, , Thyroxine, Gluconeogenesis n, Glucocorticoids, Gluconeogenesis n, Glucose utilization n, (extrahepatic), Growth hormone and ACTH, Glucose uptake p, Glucose utilization p, , Fig. 36.6 : Hormonal regulation of blood glucose., , glycogen). During day time, gluconeogenesis, may be more or less active, depending on the, frequency of consumption of snacks, coffee, tea,, fruit juices etc., , Utilization of blood glucose, Certain tissues like brain, erythrocytes, renal, medulla and bone marrow are exclusively, dependent on glucose for their energy needs., When the body is at total rest, about two-thirds, of the blood glucose is utilized by the brain. The, remaining one-third by RBC and skeletal muscle., A regular supply of glucose, by whatever means, it may be, is absolutely required to keep the, brain functionally intact., The different metabolic pathways (glycolysis,, glycogenesis, HMP shunt etc.) responsible for the, utilization of blood glucose are already discussed, (Chapter 13). The synthesis of fat from acetyl, CoA and glycerol is described in lipid, metabolism (Chapter 14)., , Kidney plays a special role in the homeostasis, of blood glucose. Glucose is continuously, filtered by the glomeruli, reabsorbed and, returned to the blood. If the level of glucose in, blood is above 160-180 mg/dl, glucose, is excreted in urine (glycosuria). This value, (160-180 mg/dl) is referred to as renal, threshold for glucose. The maximum ability of, the renal tubules to reabsorb glucose per, minute is known as tubular maximum for, glucose (TmG). The value for TmG is 350, mg/minute., , Role of hormones in, blood glucose homeostasis, Hormones play a significant role in the, regulation of blood glucose concentration, (Figs.36.6 and 36.7). Primarily, insulin lowers, blood glucose level (hypoglycemic) while the, rest of the hormones oppose the actions of, insulin (hyperglycemia).
Page 687 :
40, , Hypoglycemic, , Insulin, , 70, 100, 160, 190, , Blood glucose concentration (mg/dl), , 130, , Hyperglycemic, , Epinephrine, , 220, , 250, , Thyroxine, , 280, , Growth hormone, , Fig. 36.7 : A cartoon of tug of war illustrating hormonal action on blood glucose regulation., , Normal, , Glucagon, , Glucocorticoids, , ACTH, , Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , 677
Page 688 :
678, Insulin : Insulin is produced by E-cells of the, islets, of, Langerhans, in, response, to, hyperglycemia (elevated blood glucose level)., Some amino acids, free fatty acids, ketone, bodies, drugs such as tolbutamide also cause the, secretion of insulin., Insulin is basically a hypoglycemic hormone, that lowers in blood glucose level through, various means. It is an anti-diabetogenic, hormone. For details of insulin action on glucose, homeostasis refer metabolic effects of insulin, (carbohydrate metabolism) in this chapter., Glucagon : Glucagon is synthesized by D-cells, of the islets of Langerhans of the pancreas., Hypoglycemia (low blood glucose level), stimulates its production. Glucagon is basically, involved, in, elevating, blood, glucose, concentration. It enhances gluconeogenesis and, glycogenolysis., Epinephrine : This hormone is secreted by, adrenal medulla. It acts both on muscle and liver, to bring about glycogenolysis by increasing, phosphorylase activity. The end product is, glucose in liver and lactate in muscle. The net, outcome is that epinephrine increases blood, glucose level., Thyroxine : It is a hormone of thyroid gland., It elevates blood glucose level by stimulating, hepatic glycogenolysis and gluconeogenesis., Glucocorticoids : These hormones are, produced by adrenal cortex. Glucocorticoids, stimulate protein metabolism and increase, gluconeogenesis (increase the activities of, enzymes—glucose, 6-phosphatase, and, fructose 1,6-bisphosphatase). The glucose, utilization by extrahepatic tissues is inhibited, by glucocorticoids. The overall effect of, glucocorticoids is to elevate blood glucose, concentration., Growth hormone and adrenocorticotropic, hormone (ACTH) : The anterior pituitary gland, secretes growth hormone and ACTH. The uptake, of glucose by certain tissues (muscle, adipose, tissue etc.) is decreased by growth hormone., ACTH decreases glucose utilization. The net, effect of both these hormones is hyperglycemic., , BIOCHEMISTRY, , [In Fig.36.7, regulation of blood glucose level, by hormones is depicted as a game of tug of war, with elephant (representing insulin) on one side, and the other animals (as rest of the hormones), on the opposite side. This is just an illustration (a, cartoon) for a quick understanding of glucose, homeostasis]., , HYPOGLYCEMIA, When the blood glucose concentration falls to, less than 45 mg/dl, the symptoms of, hypoglycemia appear. The manifestations include, headache, anxiety, confusion, sweating, slurred, speech, seizures and coma, and, if not corrected,, death. All these symptoms are directly and, indirectly related to the deprivation of glucose, supply to the central nervous system (particularly, the brain) due to a fall in blood glucose level., The mammalian body has developed a well, regulated system for an efficient maintenance of, blood glucose concentration (details already, described). Hypoglycemia, therefore, is not, commonly observed. The following three types, of hypoglycemia are encountered by physicians., 1. Post-prandial hypoglycemia : This is also, called reactive hypoglycemia and is observed in, subjects with an elevated insulin secretion, following a meal. This causes transient, hypoglycemia and is associated with mild, symptoms. The patient is advised to eat, frequently rather than the 3 usual meals., 2. Fasting hypoglycemia : Low blood glucose, concentration in fasting is not very common., However, fasting hypoglycemia is observed in, patients with pancreatic E-cell tumor and, hepatocellular damage., 3. Hypoglycemia due to alcohol intake : In, some individuals who are starved or engaged in, prolonged exercise, alcohol consumption may, cause hypoglycemia. This is due to the, accumulation of NADH (during the course of, alcohol metabolism by alcohol dehydrogenase), which diverts the pyruvate and oxaloacetate, (substrates of gluconeogenesis) to form,
Page 689 :
Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , respectively, lactate and malate. The net effect is, that gluconeogenesis is reduced due to alcohol, consumption., 4. Hypoglycemia due to insulin overdose :, The most common complication of insulin, therapy in diabetic patients is hypoglycemia., This is particularly observed in patients who are, on intensive treatment regime., 5. Hypoglycemia in premature infants :, Premature and underweight infants have smaller, stores of liver glycogen, and are susceptible to, hypoglycemia., , CLASSIFICATION OF, DIABETES MELLITUS, Diabetes mellitus is a metabolic disease, more, appropriately a disorder of fuel metabolism. It is, mainly characterized by hyperglycemia that, leads to several long term complications., Diabetes mellitus is broadly divided into 2, groups, namely insulin-dependent diabetes, mellitus (IDDM) and non-insulin dependent, diabetes mellitus (NIDDM). This classification is, mainly based on the requirement of insulin for, treatment., , Insulin-dependent, diabetes mellitus (IDDM), IDDM, also known as type I diabetes or (less, frequently) juvenile onset diabetes, mainly, occurs in childhood (particularly between 12-15, yrs age). IDDM accounts for about 10 to 20% of, the known diabetics. This disease is, characterized by almost total deficiency of, insulin due to destruction of E-cells of pancreas., The E-cell destruction may be caused by drugs,, viruses or autoimmunity. Due to certain genetic, variation, the E-cells are recognized as non-self, and they are destroyed by immune mediated, injury. Usually, the symptoms of diabetes appear, when 80-90% of the E-cells have been, destroyed. The pancreas ultimately fails to, secrete insulin in response to glucose ingestion., The patients of IDDM require insulin therapy., , 679, , Non-insulin dependent, diabetes mellitus (NIDDM), NIDDM, also called type II diabetes or (less, frequently) adult-onset diabetes, is the most, common, accounting for 80 to 90% of the, diabetic population. NIDDM occurs in adults, (usually above 35 years) and is less severe than, IDDM. The causative factors of NIDDM include, genetic and environmental. NIDDM more, commonly occurs in obese individuals. Overeating coupled with underactivity leading to, obesity is associated with the development of, NIDDM. Obesity acts as a diabetogenic factor, and leads to a decrease in insulin receptors on, the insulin responsive (target) cells. The patients, of NIDDM may have either normal or even, increased insulin levels. Many a times weight, reduction by diet control alone is often sufficient, to correct NIDDM., Recent research findings on NIDDM suggest, that increased levels of tumor necrosis factor-D, (TNF-D) and resistin, and reduced seretion of, adiponectin by adipocytes of obese people cause, insulin resistance (by impairing insulin receptor, function)., The comparison between IDDM and NIDDM, is given in Table 36.2. (For metabolic syndrome, refer p-326), , GLUCOSE TOLERANCE TEST (GTT), The diagnosis of diabetes can be made on the, basis of individual’s response to oral glucose, load, the oral glucose tolerance test (OGTT)., , Preparation of the subject for GTT, The person should have been taking, carbohydrate-rich diet for at least 3 days prior to, the test. All drugs known to influence, carbohydrate, metabolism, should, be, discontinued (for at least 2 days). The subject, should avoid strenuous exercise on the previous, day of the test. He/she should be in an overnight, (at least 10 hr) fasting state. During the course, of GTT, the person should be comfortably, seated and should refrain from smoking, and exercise.
Page 690 :
680, , BIOCHEMISTRY, , TABLE 36.2 Comparison of two types of diabetes mellitus, , Character, , Insulin-dependent, diabetes mellitus (IDDM), , Non-insulin dependent, diabetes mellitus (NIDDM), , 10-20% of diabetic population, Usually childhood (<20 yrs), Normal or low, Mild or moderate, , 80-90% of diabetic population, Predominantly in adults (>30yrs), Obese, Very strong, , Defect, , Insulin deficiency due to, destruction of E-cells, , Plasma insulin, Auto antibodies, Ketosis, Acute complications, , Decreased or absent, Frequently found, Very common, Ketoacidosis, , Impairment in the production of, insulin by E-cells and/or, resistance of target cells to insulin, Normal or increased, Rare, Rare, Hyperosmolar coma, , Weeks, , Months to years, , Rare, Not useful for treatment, Always required, , Found in 10-20% cases, Suitable for treatment, Usually not necessary, , General, Prevalence, Age at onset, Body weight, Genetic predisposition, Biochemical, , Clinical, Duration of symptoms, Diabetic complications at, diagnosis, Oral hypoglycemic drugs, Administration of insulin, , Procedure for GTT, , Interpretation of GTT, The graphic representation of the GTT results, is depicted in Fig.36.8. The fasting plasma, glucose level is 75–110 mg/dl in normal persons., On oral glucose load, the concentration, increases and the peak value (140 mg/dl) is, reached in less than an hour which returns to, normal by 2 hours. Glucose is not detected in, any of the urine samples., , Plasma glucose mg/dl (mmol/l), , Glucose tolerance test should be conducted, preferably in the morning (ideal 9 to 11 AM). A, fasting blood sample is drawn and urine, collected. The subject is given 75 g glucose, orally, dissolved in about 300 ml of water, to be, drunk in about 5 minutes. Blood and urine, samples are collected at 30 minute intervals for, at least 2 hours. All blood samples are subjected, to glucose estimation while urine samples are, qualitatively tested for glucose., , 250, (13.8), , 200, (11.1), , Diabetes, , Impaired, glucose, tolerance, , 150, (8.3), , 100, (5.5), Normal, 50, (2.7), , 0, , 1, 2, , 1, Hours, , 1 12, , 2, , Fig. 36.8 : Oral glucose tolerance test.
Page 691 :
681, , Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , TABLE 36.3 Diagnostic criteria for oral glucose tolerance test (WHO 1999), , Condition, , Plasma glucose concentration as mmol/l (mg/dl), Normal, , Impaired glucose, tolerance, , Diabetes, , <6.1, , <7.0, , >7.0, , (<110), , (<126), , (>126), , 2 hours after, , <7.8, , <11.1, , >11.1, , glucose, , (<140), , (<200), , (>200), , Fasting, , In individuals with impaired glucose, tolerance, the fasting (110-126 mg/dl) as well as, 2 hour (140-200 mg/dl) plasma glucose levels, are elevated. These subjects slowly develop frank, diabetes at an estimated rate of 2% per, year. Dietary restriction and exercise are, advocated for the treatment of impaired glucose, tolerance., The WHO criteria for the diagnosis of, diabetes by OGTT is presented in Table 36.3. A, person is said to be suffering from diabetes, mellitus if his/her fasting plasma glucose exceeds, 7.0 mmol/l (126 mg/dl) and, at 2 hrs. 11.1, mmol/l (200 mg/dl)., , Other relevant aspects of GTT, 1. For conducting GTT in children, oral, glucose is given on the basis of weight (1.5 to, 1.75 g/kg)., 2. In case of pregnant women, 100 g oral, glucose is recommended. Further, the diagnostic, criteria for diabetes in pregnancy should be more, stringent than WHO recommendations., 3. In the mini GTT carried out in some, laboratories, fasting and 2 hrs. sample (instead of, 1/ hr. intervals) of blood and urine are collected., 2, 4. The GTT is rather unphysiological. To, evaluate the glucose handling of the body under, physiological conditions, fasting blood sample is, drawn, the subject is allowed to take heavy, breakfast, blood samples are collected at 1 hour, and 2 hrs (post-prandial—meaning after food)., Urine samples are also collected. This type of, test is commonly employed in established, diabetic patients for monitoring the control., , 5. For individuals with suspected malabsorption, intravenous GTT is carried out., 6. Corticosteroid stressed GTT is employed, to detect latent diabetes., , Glycosuria, The commonest cause of glucose excretion in, urine (glycosuria) is diabetes mellitus. Therefore,, glycosuria is the first line screening test for, diabetes. Normally, glucose does not appear in, urine until the plasma glucose concentration, exceeds renal threshold (180 mg/dl). As age, advances, renal threshold for glucose increases, marginally., Renal glycosuria : Renal glycosuria is a, benign condition due to a reduced renal, threshold for glucose. It is unrelated to diabetes, and, therefore, should not be mistaken as, diabetes. Further, it is not accompanied by the, classical symptoms of diabetes., Alimentary glycosuria : In certain individuals,, blood glucose level rises rapidly after meals, resulting in its spill over into urine. This, condition is referred to as alimentary (lag, storage) glycosuria. It is observed in some, normal people, and in patients of hepatic, diseases, hyperthyroidism and peptic ulcer., , Metabolic changes in diabetes, Diabetes mellitus is associated with several, metabolic alterations. Most important among, them are hyperglycemia, ketoacidosis and hypertriglyceridemia (Fig.36.9)., 1. Hyperglycemia : Elevation of blood glucose, concentration is the hallmark of uncontrolled, diabetes. Hyperglycemia is primarily due to
Page 692 :
682, , BIOCHEMISTRY, , INSULIN p, , Glucose uptake, by tissues p, , Plasma, amino acids n, , Gluconeogenesis n, , Glycogenolysis n, , Glucose production n, , Lipolysis n, Plasma free, fatty acids n, , Hepatic ketone, bodies n, HYPERGLYCEMIA, , KETOACIDOSIS, , Triacylglycerol, synthesis n, HYPERTRIGLYCERIDEMIA, , Fig. 36.9 : Major metabolic alterations in diabetes mellitus., , reduced glucose uptake by tissues and its, increased production via gluconeogenesis and, glycogenolysis. When the blood glucose level, goes beyond the renal threshold, glucose is, excreted into urine (glycosuria)., Glucose toxicity : High concentrations of, glucose can be harmful causing osmotic effects/, hypertonic effects (water drawn from cells, into extracellular fluid and excreted into urine,, resulting in dehydration), E-cell damage by, free radicals (due to enhanced oxidative, phosphorylation, oxidative stress, and increased, free radicals) and glycation of proteins, (associated, with, diabetic, complicationsneuropathy, nephropathy, retinopathy etc.)., 2. Ketoacidosis : Increased mobilization of, fatty acids results in overproduction of ketone, bodies which often leads to ketoacidosis., 3. Hypertriglyceridemia : Conversion of fatty, acids to triacylglycerols and the secretion of, VLDL and chylomicrons is comparatively higher, in diabetics. Further, the activity of the enzyme, lipoprotein lipase is low in diabetic patients., Consequently, the plasma levels of VLDL, chylomicrons and triacylglycerols are increased., Hypercholesterolemia is also frequently seen in, diabetics., , include atherosclerosis, retinopathy, nephropathy and neuropathy. The biochemical basis of, these complications is not clearly understood. It is, believed that at least some of them are related to, microvascular changes caused by glycation of, proteins., , Management of diabetes, Diet, exercise, drug and, finally, insulin, are the management options in diabetics., Approximately, 50% of the new cases of diabetes, can be adequately controlled by diet alone, 2030% need oral hypoglycemic drugs while the, remaining 20-30% require insulin., Dietary management : A diabetic patient is, advised to consume low calories (i.e. low, carbohydrate and fat), high protein and fiber, rich diet. Carbohydrates should be taken in the, form of starches and complex sugars. As far as, possible, refined sugars (sucrose, glucose) should, be avoided. Fat intake should be drastically, reduced so as to meet the nutritional, requirements of unsaturated fatty acids. Diet, control and exercise will help to a large extent, obese NIDDM patients., , Long term effects of diabetes, , Hypoglycemic drugs : The oral hypoglycemic, drugs are broadly of two categories-sulfonylureas, and biguanides. The latter are less commonly, used these days due to side effects., , Hyperglycemia is directly or indirectly, associated with several complications. These, , Sulfonylureas such as acetohexamide, tolbutamide and glibenclamide are frequently used.
Page 693 :
Chapter 36 : INSULIN, GLUCOSE HOMEOSTASIS, AND DIABETES MELLITUS, , 683, , They promote the secretion of endogenous, insulin and thus help in reducing blood glucose, level., , HbA1c is produced by the condensation of, glucose with N-terminal valine of each E-chain, of HbA., , Management with insulin :, Two types, of insulin preparations are commercially, available—short acting and long acting. The, short acting insulins are unmodified and their, action lasts for about 6 hours. The long acting, insulins are modified ones (such as adsorption to, protamine) and act for several hours, which, depends on the type of preparation., , Diagnostic importance of HbA1c : The rate of, synthesis of HbA1c is directly related to the, exposure of RBC to glucose. Thus, the, concentration of HbA1c serves as an indication, of the blood glucose concentration over a, period, approximating to the half-life of, RBC (hemoglobin) i.e. 6–8 weeks. A close, correlation between blood glucose and HbA1c, concentrations has been observed when, simultaneously monitored for several months., , The advent of genetic engineering is a boon, to diabetic patients since bulk quantities of, insulin can be produced in the laboratory., , Biochemical indices, of diabetic control, For a diabetic patient who is on treatment, (drug or insulin therapy), periodical assessment, of the efficacy of the treatment is essential., Urine glucose detection and blood glucose, estimations are traditionally followed in several, laboratories. In recent years, more reliable and, long-term biochemical indices of diabetic, control are in use., Glycated hemoglobin : Glycated or, glycosylated hemoglobin refers to the glucose, derived products of normal adult hemoglobin, (HbA). Glycation is a post-translational, nonenzymatic addition of sugar residue to amino, acids of proteins. Among the glycated, hemoglobins, the most abundant form is HbA1c., , Normally, HbA1c concentration is about, 3–5% of the total hemoglobin. In diabetic, patients, HbA1c is elevated (to as high as 15%)., Determination of HbA1c is used for monitoring, of diabetes control. HbA1c reflects the mean, blood glucose level over 2 months period prior, to its measurement., In the routine clinical practice, if the HbA1c, concentration is less than 7%, the diabetic, patient is considered to be in good control., Estimated average glucose(eAG) : eAG is a, new term (introduced by American Diabetic, Association) used in diabetic management. It is a, laboratory tool to understand the approximate, relationship between HbA1c and glucose, concentrations, and is given by the following, formula, eAG (mg/dl) = (28.7 u HbA1c) – 46.7, , + Diabetes affects about 2-3% of the population and is a major cause of blindness, renal, failure, heart attack and stroke., , + The hormone insulin has been implicated in the development of diabetes., + Diabetic ketoacidosis is frequently encountered in severe uncontrolled diabetics. The, management includes administration of insulin, fluids and potassium., , + The hypoglycemic drugs commonly used in diabetic patients include tolbutamide,, glibenclamide and acetohexamide., , + Measurement of glycated hemoglobin (HbA1c) serves as a marker for diabetic control.
Page 694 :
684, , BIOCHEMISTRY, , (e.g. for HBA1c values of 6%, 8% and 10%, the, eAG values respectively are 126 mg/dl, 183 mg/, dl and 240 mg/dl), Fructosamine : Besides HbA1c, several other, proteins in the blood are glycated. Glycated, serum proteins (fructosamine) can also be, measured in diabetics. As albumin is the most, abundant plasma protein, glycated albumin, largely contributes to plasma fructosamine, measurements. Albumin has shorter half-life, than Hb. Thus, glycated albumin represents, glucose status over 3 weeks prior to its, determination., Microalbuminuria, , :, , Microalbuminuria, , is, , defined as the excretion of 30-300 mg of, albumin in urine per day. It may be noted that, microalbuminuria represents an intermediary, stage between normal albumin excretion (2.5–30, mg/d) and macroalbuminuria (> 300 mg/d). The, small increase in albumin excretion predicts, impairment in renal function in diabetic, patients. Microalbuminuria serves as a signal of, early reversible renal damage., Serum lipids : Determination of serum lipids, (total cholesterol, HDL, triglycerides) serves as, an index for overall metabolic control in diabetic, patients. Hence, serum lipids should be, frequently measured., , 1. Diabetes mellitus is a common metabolic disorder, characterized by insufficient or, inefficient insulin., 2. Insulin is a polypeptide hormone, secreted by the E-cells of pancreas. It has a profound, influence on carbohydrate, fat and protein metabolisms. Insulin lowers blood glucose, concentration (hypoglycemic effect)., 3. Glucagon, secreted by the D-cells of pancreas, in general opposes the actions of insulin., The net effect of glucagon is to increase blood glucose concentration (hyperglycemic effect)., 4. In a healthy person, the blood glucose level (fasting 70-100 mg/dl) is maintained by a, well coordinated hormonal action regulating the sources that contribute to glucose, (gluconeogenesis, glycogenolysis), and the utilization pathways (glycolysis, glycogenesis,, lipogenesis). Insulin is hypoglycemic while other hormones (glucagon, epinephrine,, thyroxine, glucocorticoids) are hyperglycemic., 5. In hypoglycemia (blood glucose <45 mg/dl), there is deprivation of glucose supply to, brain resulting in symptoms such as headache, confusion, anxiety and seizures., 6. Diabetes mellitus is broadly classified into 2 categories—insulin dependent diabetes, mellitus (IDDM) and non-insulin dependent diabetes mellitus (NIDDM)., 7. The diagnosis of diabetes is frequently carried out by oral glucose tolerance test (GTT)., As per WHO criteria, a person is said to be suffering from diabetes if his/her fasting, blood glucose exceeds 126 mg/dl, and 2 hrs. after OGTT goes beyond 200 mg/dl., 8. Diabetes is associated with several metabolic derangements such as ketoacidosis and, hypertriglyceridemia, besides hyperglycemia. The chronic complications of diabetes, include atherosclerosis, retinopathy, nephropathy and neuropathy., 9. Diet, exercise, drug and insulin are the options for diabetic control. It is estimated that, about half of the new diabetic patients can be controlled by diet and exercise., 10.Estimation of glycated hemoglobin (HbA1C), plasma fructosamine, microalbumin in, urine, and serum lipids serve as biochemical indices to monitor diabetic control.
Page 695 :
Section 6, , Current Topics, , Chapter, , Cancer, , 37, Oncogenic, viruses, , The dreaded disease cancer speaks :, , Environmental, factors (physical, and chemical), , Mutations, , ONCOGENE ACTIVATION, , Inactive, antioncogenes CARCINOGENESIS, , Diminished regulation, by apoptosis genes, , “I am the world’s second largest killer;, Characterized by uncontrolled proliferation of cells;, Implicating environmental and genetic factors;, Perhaps due to oncogene and antioncogene imblance.”, , I, , n the normal circumstances, the proliferation, of body cells is under strict control. The cells, differentiate, divide and die in a sequential, manner in a healthy organism. Cancer is, characterized by loss of control of cellular, growth and development leading to excessive, proliferation and spread of cells. Cancer is, derived from a Latin word meaning crab. It is, presumed that the word cancer originated from, the character of cancerous cells which can, migrate and adhere and cause pain (like a crab), to any part of the body., Neoplasia literally means new growth., Uncontrolled growth of cells results in tumors (a, word originally used to represent swelling)., Oncology (Greek : oncos—tumor) deals with the, study of tumors., The tumors are of two types., 1. Benign tumors : They usually grow by, expansion and remain encapsulated in a layer of, connective tissue. Normally benign tumors are, not life-threatening e.g. moles, warts. These types, of benign tumors are not considered as cancers., , 2. Malignant tumors or cancers : They are, characterized by uncontrolled proliferation and, spread of cells to various parts of the body, a, process referred to as metastasis. Malignant, tumors are invariably life-threatening e.g. lung, cancer, leukemia., About 100 different types of human cancers, have been recognized. Cancers arising from, epithelial cells are referred to as carcinomas, while that from connective tissues are known as, sarcomas. Methods for the early detection and, treatment of cancers have been developed., However, little is known about the biochemical, basis of cancer., , Incidence, Cancer is the second largest killer disease (the, first being coronary heart disease) in the, developed countries. It is estimated that cancer, accounts for more than 20% of the deaths in, United States. Based on the current rate of, incidence, it is believed that one in every 3, persons will develop cancer at sometime during, his life., , 685
Page 696 :
686, , BIOCHEMISTRY, , Although humans of all ages develop cancer,, the incidence increases with advancement of, age. More than 70% of the new cancer cases, occur in persons over 60 years. Surprisingly,, cancer is a leading cause of death in children in, the age group 3-13 years, half of them die due, to leukemia., , ETIOLOGY, In general, cancers are multifactorial in origin., The causative agents include physical, chemical,, genetic and environmental factors. A survey in, USA has shown that about 90% of all cancer, deaths are due to avoidable factors such as, tobacco, pollution, occupation, alcohol and diet., Most of the cancers are caused by chemical, carcinogens, radiation energy and viruses. These, agents may damage DNA or interfere with its, replication or repair., , Chemical carcinogens, It is estimated that almost 80% of the human, cancers are caused by chemical carcinogens in, nature. The chemicals may be organic (e.g., dimethylbenzanthracene, benzo (a) pyrene,, dimethyl nitrosamine) or inorganic (arsenic,, cadmium) in nature. Entry of the chemicals into, the body may occur by one of the following, mechanisms., 1. Occupation e.g. asbestos, benzene., 2. Diet e.g. aflatoxin B produced by fungus, (Aspergillus flavus) contamination of foodstuffs,, particularly peanuts., 3. Drugs—certain therapeutic drugs can be, carcinogenic e.g. diethylstibesterol., 4. Life style e.g. cigarette smoking., Mechanism of action : Although a few of the, chemicals are directly carcinogenic, majority of, them require prior metabolism to become, carcinogenic. The enzymes such as cytochrome, P450 responsible for the metabolism of, xenobiotics (Chapter 31) are involved in dealing, with the chemical carcinogens. In general, a, , chemically non-reactive procarcinogen is, converted to an ultimate carcinogen by a series, of reactions., The carcinogens can covalently bind to, purines, pyrimidines and phosphodiester bonds, of DNA, often causing unrepairable damage. The, chemical, carcinogens, frequently, cause, mutations (a change in the nucleotide sequence, of DNA) which may finally lead to the, development of cancer, hence they are regarded, as mutagens., Ames assay : This is a laboratory test to check, the carcinogenecity of chemicals. Ames assay, employs the use of a special mutant strain of, bacterium, namely Salmonella typhimurium, (His–). This organism cannot synthesize histidine;, hence the same should be supplied in the, medium for its growth. Addition of chemical, carcinogens causes mutations (reverse mutation), restoring the ability of the bacteria to synthesize, histidine (His+). By detecting the strain of, Salmonella (His+) in the colonies of agar plates,, the chemical mutagens can be identified. The, Ames assay can detect about 90% of the, chemical carcinogens. This test is regarded as a, preliminary screening procedure., Animal, experiments are conducted for the final, assessment of carcinogenecity., Promoters of carcinogenesis : Some of the, chemicals on their own are not carcinogenic., Certain substances known as promoting agents, make them carcinogenic. The application of, benzo- (a)pyrene to the skin, as such, does not, cause tumor development. However, if this is, followed by the application of croton oil, tumors, will develop. In this case, benzo(a)pyrene is the, initiating agent while croton oil acts as a, promoting, agent, or, promoter., Several, compounds that act as promoting agents in, various organs of the body have been identified., These include saccharin and phenobarbital., , Radiation energy, Ultraviolet rays, X-rays and J-rays have been, proved to be mutagenic in nature causing, cancers. These rays damage DNA which is the, basic mechanism to explain the carcinogenicity
Page 697 :
687, , Chapter 37 : CANCER, , TABLE 37.1 Selected tumor viruses, , Class, , Members, , DNA viruses, , 1. Cancers are transmitted from mother to, daughter cells. In other words, cancer cells beget, cancer cells., 2. Chromosomal abnormalities are observed, in many tumor cells., , Adenovirus, , Adenovirus 12 and 18, , Herpesvirus, , Epstein-Barr virus, herpes, simplex virus, , 3. Damage to DNA caused by mutations, often results in carcinogenesis., , Papovirus, , Papilloma virus, polyoma virus, , 4. Laboratory experiments have proved that, purified oncogenes can transform normal cells, into cancer cells., , RNA viruses, Retrovirus type B, , Mammary tumor virus of mouse, , Retrovirus type C, , Leukemia, sarcoma., , MOLECULAR BASIS OF CANCER, of radiation energy. For instance, exposure to, UV rays results in the formation of pyrimidine, dimers in DNA while X-rays cause the, production of free radicals. This type of, molecular damages are responsible for the, carcinogenic effects of radiations., , Carcinogenic viruses, The involvement of viruses in the etiology of, cancer was first reported by Rous in 1911. He, demonstrated that the cell-free filtrates from, certain chicken sarcomas (tumors of connective, tissues) promote new sarcomas in chickens., Unfortunately, this epoch-making discovery of, Rous was ignored for several years. This is, evident from the fact that Rous was awarded the, Nobel Prize in 1966 at the age of 85 for his, discovery in 1911!, The presence of viral particles and the, enzyme reverse transcriptase, besides the, occurrence of base sequence in the DNA of, malignant cells, complementary to tumor viruses, indicate the involvement of viruses in cancer., The viruses involved in the development of, cancer, commonly known as oncogenic viruses,, may contain either DNA or RNA. A selected list, of tumor viruses is given in Table 37.1., , Cancer is caused by a genetic change in a, single cell resulting in its uncontrolled, multiplication. Thus, tumors are monoclonal., Two types of regulatory genes—oncogenes and, antioncogenes are involved in the development, of cancer (carcinogenesis). In recent years, a, third category of genes that control the cell death, or apoptosis are also believed to be involved in, carcinogenesis., , Oncogenes, The genes capable of causing cancer are, known as oncogenes (Greek : oncos—tumor or, mass). Oncogenes were originally discovered in, tumor causing viruses. These viral oncogenes, were found to be closely similar to certain genes, present in the normal host cells which are, referred to as protooncogenes. Now, about 40, viral and cellular protooncogenes have been, identified. Protooncogenes encode for growthregulating, proteins., The, activation, of, protooncogenes to oncogenes is an important, step in the causation of cancer., In the Table 37.2, a selected list of, oncoproteins,, protooncogenes, and, the, associated human cancers is given., , DNA—the ultimate in, carcinogenesis, , Activation of, protooncogenes to oncogenes, , DNA is the ultimate critical macromolecule, in carcinogenesis. This fact is supported by, several evidences., , There are several mechanisms for converting, the protooncogenes to oncogenes, some of the, important ones are described next.
Page 698 :
688, , BIOCHEMISTRY, , TABLE 37.2 Selected oncoproteins, protooncogenes and associated cancers, , Oncoproteins, , Protooncogene, , Associated human cancer(s), , Growth factors, Platelet derived growth factor (PDGF), , sis, , Osteosarcoma, , Epidermal growth factor (EGF), , hst-1, , Cancers of stomach, breast and bladder, , erb–B1, , Lung cancer, , erb–B2, , Stomach cancer, , erb–B3, , Breast cancer, , GTP— binding proteins, , ras, , Leukemias, cancers of lung, pancreas and colon, , Non-receptor tyrosine kinase, , abl, , Leukemia, , Growth factor receptors, , Signal—transducing proteins, , 1. Viral insertion into chromosome : When, certain retroviruses (genetic material RNA) infect, cells, a complementary DNA (cDNA) is made, from their RNA, by the enzyme reverse, transcriptase. The cDNA so produced gets, inserted into the host genome (Fig.37.1). The, integrated double-stranded cDNA is referred to, as provirus. This pro-viral DNA takes over the, control of the transcription of cellular, chromosomal DNA and transforms the cells., Activation of protooncogene myc to oncogene, by, viral, insertion, ultimately, causing, carcinogenesis is well known (e.g. avian, leukemia)., Some DNA viruses also get inserted into the, host chromosome and activate the protooncogenes., 2. Chromosomal translocation : Some of the, tumors exhibit chromosomal abnormalities. This, is due to the rearrangement of genetic material, (DNA) by chromosomal translocation i.e., splitting off a small fragment of chromosome, which is joined to another chromosome., Chromosomal translocation usually results in, overexpression of protooncogenes., , Burkitt’s lymphoma, a cancer of human, B-lymphocytes, is a good example of, chromosomal translocation. In this case, a, fragment from chromosome 8 is split off and, joined to chromosome 14 containing myc gene, , (Fig.37.2). This results in the activation of, inactive myc gene leading to the increased, synthesis of certain proteins which make the cell, malignant., 3. Gene amplification : Severalfold amplifications of certain DNA sequences are observed, in some cancers. Administration of anticancer, drugs methotrexate (an inhibitor of the enzyme, dihydrofolate reductase) is associated with gene, amplification. The drug becomes inactive due, to gene amplification resulting in a severalfold, (about 400) increase in the activity of, dihydrofolate reductase., 4. Point mutation : The ras protooncogene is, the best example of activation by point mutation, (change in a single base in the DNA). The, mutated ras oncogene produces a protein, myc, , Host DNA, Viral DNA, , Provirus, , Activated, myc, , Fig. 37.1 : Integration of viral DNA into host DNA.
Page 699 :
689, , Chapter 37 : CANCER, , Chromosome 8, , Chromosome 14, , Break, , A selected list of polypeptide growth factors,, their sources and major functions is given in, Table 37.3., The cell proliferation is stimulated by growth, factors. In general, a growth factor binds to a, protein receptor on the plasma membrane. This, binding activates cytoplasmic protein kinases, leading to the phosphorylation of intracellular, target proteins. The phosphorylated proteins, in, turn, act as intracellular messengers to stimulate, cell division, the mechanism of which is not, clearly known., Transforming growth factor (TGF-D) is a, protein synthesized and required for the growth, of epithelial cells. TGF-D is produced in high, concentration in individuals suffering from, psoriasis, a disease characterized by excessive, proliferation of epidermal cells., , Fig. 37.2 : Diagrammatic representation of reciprocal, translocation occurring in Burkitt’s lymphoma., , (GTPase) which differs in structure by a single, amino acid. This alteration diminishes the, activity of GTPase, a key enzyme involved in the, control of cell growth (details described later)., The presence of ras mutations is detected in, several human tumors—90% of pancreatic, 50%, of colon and 30% of lung. However, ras, mutations have not been detected in the breast, cancer., , Mechanism of action of oncogenes, Oncogenes encode for certain proteins,, namely oncoproteins. These proteins are the, altered versions of their normal counterparts and, are involved in the transformation and, multiplication of cells. Some of the products of, oncogenes are discussed below., Growth factors : Several growth factors, stimulating the proliferation of normal cells are, known. They regulate cell division by transmitting, the message across the plasma membrane to the, interior of the cell (transmembrane signal, transduction). It is believed that growth factors, play a key role in carcinogenesis., , Growth factor receptors : Some oncogenes, encoding growth factor receptors have been, identified. Overexpression and/or structural, alterations in growth factor receptors are, associated with carcinogenesis. For instance, the, overexpression of gene erb-B, encoding EGFreceptor is observed in lung cancer., GTP-binding proteins : These are a group of, signal, transducing, proteins., Guanosine, triphosphate (GTP)-binding proteins are found in, about 30% of human cancers. The mutation of, ras protooncogene is the single-most dominant, cause of many human tumors., The involvement of ras protein (product of ras, gene) with a molecular weight 21,000 (P21) in, cell multiplication is illustrated in Fig.37.3. The, inactive ras is in a bound state with GDP. When, the cells are stimulated by growth factors, ras, P21 gets activated by exchanging GDP for GTP., This exchange process is catalysed by guanine, nucleotide releasing factor (GRF). The active ras, P21 stimulates regulators such as cytoplasmic, kinases, ultimately causing DNA replication and, cell division. In normal cells, the activity of ras, P21 is shortlived. The GTPase activity, which is, an integral part (intrinsic) of ras P21, hydrolyses, GTP to GDP, reverting ras 21 to the original, state. There are certain proteins, namely GTPase, activating proteins (GAP), which accelerate the
Page 700 :
690, , BIOCHEMISTRY, , TABLE 37.3 Selected polypeptide growth factors, , Growth factor, , Source(s), , Major function(s), , Epidermal growth factor (EGF), , Salivary gland, fibroblasts, , Stimulates growth of epidermal, and epithelial cells, , Platelet derived growth factor (PDGF), , Platelets, , Stimulates growth of mesenchymal cells,, promotes wound healing, , Transforming growth factor-D (TGF-D), , Epithelial cell, , Similar to EGF, , Transforming growth factor-E (TGF-E), , Platelets, kidney, placenta, , Inhibitory (sometimes stimulatory) effect, on cultured tumor cells, , Erythropoietin, , Kidney, , Stimulates development erythropoietic cells, , Nerve growth factor (NGF), , Salivary gland, , Stimulates the growth of sensory and, sympathetic neurons, , Insulin like growth factors (IGF-I and IGF-II,, respectively known as somatomedins C and A), , Serum, , Stimulates incorporation of sulfates into, cartilage; exerts insulin-like action on, certain cells, , Tumor necrosis factor (TNF-D), , Monocytes, , Necrosis of tumor cells, , Interleukin-1 (IL-1), , Monocytes, leukocytes, , Stimulates synthesis of IL-2., , Interleukin-2 (IL-2), , Lymphocytes, (mainly T-helper cells)., , Stimulates growth and maturation of T-cells, , Growth, factor, , hydrolysis of GTP of ras P21. Thus, in normal, cells, the activity of ras P21 is well regulated., Plasma, membrane, , Receptor Stimulus, , GRF catalyses, GDP, Inactive, ras P21, , Pi, Block in, mutated ras, , GDP, GTP, , GAP, Activated, ras P21, Activation, (cytoplasmic kinases), DNA synthesis, and cell multiplication, , Fig. 37.3 : Model for the mechanism of action of, ras P21 protein (GRF—Guanine nucleotide releasing, factor; GAP—GTPase activating proteins)., , Point mutations in ras gene result in the, production of altered ras P21, lacking GTPase, activity. This leads to the occurrence of ras P21, in a permanently activated state, causing, uncontrolled multiplication of cells., Non-receptor tyrosine kinases : These, proteins are found on the interior of the inner, plasma membrane. They phosphorylate the, cellular target proteins (involved in cell division), in response to external growth stimuli. Mutations, in the protooncogenes (e.g. abl) encoding nonreceptor tyrosine kinases increase the kinase, activity and, in turn, phosphorylation of target, proteins causing unlimited cell multiplication., , Antioncogenes, A special category of genes, namely cancer, suppressor genes (e.g. p53 gene) or, more, commonly, antioncogenes, have been identified., The products of these genes apply breaks and, regulate cell proliferation. The loss of these
Page 701 :
691, , Chapter 37 : CANCER, , Oncogenic, viruses, , Environmental, factors (physical, and chemical), , Mutations, , ONCOGENE ACTIVATION, , Inactive, CARCINOGENESIS Diminished regulation, antioncogenes, by apoptosis genes, , Fig. 37.4 : A simplified hypothesis for, the development of cancer., , suppressor genes removes the growth control of, cells and is believed to be a key factor in the, development, of, several, tumors,, e.g., retinoblastoma, one type of breast cancer,, carcinoma of lung, Wilms’ kidney tumor., With the rapid advances in the field of genetic, engineering, introducing antioncogenes to a, normal chromosome to correct the altered, growth rate of cells may soon become a reality., , Genes that regulate apoptosis, A new category of genes that regulate, programmed cell death (apoptosis) have been, discovered. These genes are also important in, the development of tumors., The gene, namely bcl-2, causes B-cell, lymphoma by preventing programmed cell, death. It is believed that overexpression of bcl-2, allows other mutations of protooncogenes that,, ultimately, leads to cancer., , Unified hypothesis, of carcinogenesis, The multifactorial origin of cancer can be, suitably explained by oncogenes. The physical, and chemical agents, viruses and mutations all, lead to the activation of oncogenes causing, carcinogenesis. The antioncogenes and the genes, regulating apoptosis are intimately involved in, development of cancer. A simplification of a, unified hypothesis of carcinogenesis is depicted, in Fig.37.4., , TUMOR MARKERS, The biochemical indicators employed to, detect the presence of cancers are collectively, referred to as tumor markers. These are the, abnormally produced molecules of tumor cells, such as surface antigens, cytoplasmic proteins,, enzymes and hormones. Tumor markers can be, measured in serum (or plasma). In theory, the, tumor markers must ideally be useful for, screening the population to detect cancers. In, practice, however, this has not been totally true., As such, the tumor markers support the diagnosis, of cancers, besides being useful for monitoring, the response to therapy and for the early, detection of recurrence., A host of tumor markers have been described, and the list is evergrowing. However, only a few, of them have proved to be clinically useful. A, selected list of tumor markers and the associated, cancers are given in Table 37.4., A couple of the most commonly used tumor, markers are discussed hereunder., 1. Carcinoembryonic antigen (CEA) : This is, a complex glycoprotein, normally produced by, the embryonic tissue of liver, gut and pancreas., The presence of CEA in serum is detected in, several cancers (colon, pancreas, stomach, lung)., In about 67% of the patients with colorectal, cancer, CEA can be identified. Unfortunately,, serum CEA is also detected in several other, disorders such as alcoholic cirrhosis (70%),, emphysema (57%) and diabetes mellitus (38%)., Due to this, CEA lacks specificity for cancer, detection. However, in established cancer, patients (particularly of colon and breast), the, serum level of CEA is a useful indicator to detect, the burden of tumor mass, besides monitoring, the treatment., 2. Alpha-fetoprotein (AFP) : It is chemically a, glycoprotein, normally synthesized by yolk sac, in early fetal life. Elevation in serum levels of, AFP mainly indicates the cancers of liver and, germ cells of testis and, to some extent,, carcinomas of lung, pancreas and colon. As is, the case with CEA, alpha-fetoprotein is not, specific for the detection of cancers. Elevated
Page 702 :
692, , BIOCHEMISTRY, , TABLE 37.4 Selected tumor markers and associated cancers, , Tumor marker, , Associated cancer(s), , Oncofetal antigens, Carcinoembryonic antigen (CEA), Alpha fetoprotein (AFP), Cancer antigen-125 (CA-125), , Cancers of colon, stomach, lung, pancreas and breast, Cancer of liver and germ cells of testis, Ovarian cancer, , Hormones, Human chorionic gonadotropin (hCG), Calcitonin, Catecholamines and their, metabolites (mainly vanillyl mandelic acid), , Choriocarcinoma, Carcinoma of medullary thyroid, Pheochromocytoma and, neuroblastoma, , Enzymes, Prostatic acid phosphatase, Neuron specific enolase, Alkaline phosphatase, , Prostate cancer, Neuroblastoma, Bone secondaries, , Specific proteins, Prostate specific antigen (PSA), Immunoglobulins, Bence-Jones proteins, , Prostate cancer, Multiple myeloma, Multiple myeloma, , they form monolayers and cannot move away, from each other. The cancer cells form, multilayers due to loss of contact inhibition, (Fig.37.5). As a result, the cancer cells freely, move and get deposited in any part of the, body, a property referred to as metastasis., , levels of AFP are observed in cirrhosis, hepatitis, and pregnancy. However, measurement of serum, AFP provides a sensitive index for tumor therapy, and detection of recurrence., , CHARACTERISTICS OF, GROWING TUMOR CELLS, The morphological and biochemical changes, in the growing tumor cells are briefly described, here. These observations are mostly based on, the in vitro culture studies. Knowledge on the, biochemical profile of tumor cells guides in the, selection of chemotherapy of cancers., , l, , l, , Loss of anchorage dependence : The cancer, cells can grow without attachment to the, surface. This is in contrast to the normal cells, which firmly adhere to the surface., Alteration in permeability properties : The, tumor cells have altered permeability and, transport., , (A), , 1. General and morphological changes, l, , l, , l, , Shape of cells : The tumor cells are much, rounder in shape compared to normal cells., Alterations in cell structures : The cytoskeletal, structure of the tumor cells with regard to, actin filaments is different., Loss of contact inhibition : The normal cells, are characterized by contact inhibition i.e., , (B), Fig. 37.5 : Growth cells in culture (A) Normal, cells forming monolayer (exhibiting contact, inhibition); (B) Cancer cells forming, multilayers (loss of contact inhibition).
Page 703 :
693, , Chapter 37 : CANCER, , 2. Biochemical changes, l, , l, , l, , l, , l, , Increased replication and transcription : The, synthesis of DNA and RNA is increased in, cancer cells., Increased glycolysis : The fast growing tumor, cells are characterized by elevation in aerobic, and anaerobic glycolysis due to increased, energy demands of multiplying cells., Reduced requirement of growth factors : The, tumor cells require much less quantities of, growth factors. Despite this fact, there is an, increased production of growth factors by, these cells., Synthesis of fetal proteins : During fetal life,, certain genes are active, leading to the, synthesis of specific proteins. These genes are, suppressed in adult cells. However, the tumor, cells synthesize the fetal proteins e.g., carcinoembryonic antigen, alfa fetoprotein., Alterations in the structure of molecules :, Changes in the structure of glycoproteins and, glycolipids are observed., , Metastasis, Metastasis refers to the spread of cancer cells, from the primary site of origin to other tissues of, the body where they get deposited and grow as, secondary tumors. Metastasis is the major cause, of cancer related morbidity and mortality. It is, believed that the morphological changes in, tumor cells, loss of contact inhibition, loss of, , anchorage dependence and alterations in the, structure of certain macromolecules are among, the important factors responsible for metastasis., , CANCER THERAPY, Chemotherapy, employing certain anticancer, drugs, is widely used in the treatment of cancer., In the Table 37.5, a selected list of the most, commonly used drugs, and their mode of action, is given. The effectiveness of anticancer drugs is, inversely proportional to the size of the tumor, i.e. the number of cancer cells. The major, limitation of cancer chemotherapy is that the, rapidly dividing normal cells (of hematopoietic, system, gastrointestinal tract, hair follicles) are, also affected. Thus, the use of anticancer drugs is, associated with toxic manifestations., , Cisplatin is used in the treatment of testicular,, ovarian and several other cancers (bone, lung)., The side effects of cisplatin include bone marrow, depletion, loss of hearing and impairment in, kidney function. About 80% of testicular cancer, patients survive with a new combination therapy, of cisplatin, etoposide, and bleomycin., The term tumor lysis syndrome (TLS), represents all the metabolic consequences that, occur during cancer treatment. These include, increased uric acid levels in serum and urine,, acute renal failure, hyperkalemia, hyperphosphatemia etc. Recombinant urate oxidase, (converts uric acid to soluble allantion) is, successfully used in the treatment of TLS., , +, +, , About 80% of the human cancers are caused by chemical carcinogens., , +, , The physical and chemical agents, viruses and mutations result in the activation of, oncogenes causing carcinogenesis., , +, , The abnormal products of tumor cells, referred to as tumor markers (CEA, AFP, PSA), are useful for the diagnosis and prognosis of cancer., , +, , Anticancer drugs (e.g. methotrexate, cisplatin) are commonly used in the treatment, of cancer. Antioxidants (vitamins E and C, E-carotene, Se) decrease the risk of, carcinogenesis and hence their increased consumption is advocated., , The products of oncogenes (e.g. growth factors) have been implicated in the, development of cancer. Antioncogenes apply breaks and regulate the cell proliferation.
Page 704 :
694, , BIOCHEMISTRY, , TABLE 37.5 A selected list of the most commonly used anticancer drugs and their mode of action, , Anticancer drug, , Chemical nature, , Mode of action, , Methotrexate, , Folic acid analogue, , 6-Mercaptopurine, 6-Thioguanine, Mitomycin C, Actinomycin D, Vinblastine and vincristine, Cisplatin, Cyclophosphamide, Imatinib, , Purine analogue, Purine analogue, Antibiotic, Antibiotic, Alkaloids, Platinum compound, Alkylating agent, Monoclonal antibody, , Blocks the formatin of tetrahydrofolate (inhibits the, enzyme dihydrofolate reductase). THF is required for, nucleotide synthesis., Inhibits the formation of AMP from IMP., Blocks thymidylate synthase reaction., Results in cross bridges between DNA base pairs., Blocks transcription, Inhibit cell division and cytoskeleton formation, Results in the formation of intrastrand DNA adducts., Cross-links bases and inhibits DNA strand separation, Tyrosine kinase inhibitor, , PREVENTION OF CANCER, In recent years, certain precautionary, measures are advocated to prevent or reduce the, occurrence of cancer. The most important, among them, from the biochemical perspective,, are the antioxidants namely vitamin E,, E-carotene, vitamin C and selenium. They, prevent the formation or detoxify the existing free, , radicals (free radicals are known to promote, carcinogenesis). In addition, antioxidants, stimulate body’s immune system, and promote, detoxification of various carcinogens., In general, most of the vegetables and fruits, are rich in antioxidants. Their increased, consumption is advocated to prevent cancer., (For more details on free radicals and, antioxidants, Refer Chapter 34)., , 1., , Cancer is characterized by uncontrolled cellular growth and development, leading to, excessive proliferation and spread of cells. Cancer is the second largest killer disease, (next to heart disease) in the developed world., , 2., , Regulatory genes—namely oncogenes, antioncogenes and genes controlling cell death—, are involved in the development of cancer. Activation of oncogenes is a fundamental, step in carcinogenesis. This may occur by insertion of viral DNA into host chromosome,, translocation of chromosomes, gene amplification and point mutation., , 3., , The products of activated oncogenes such as growth factors, growth factor receptors,, GTP-binding proteins, non-receptor tyrosine kinases have all been implicated in the, development of cancer., , 4., , Tumor markers of cancers include carcinoembryonic antigen (CEA), alpha fetoprotein, (AFP), cancer antigen-125 and prostate specific antigen (PSA). They are mainly useful, to support diagnosis, monitor therapy and detect recurrence., , 5., , There are several morphological and biochemical changes in the tumor cells which, distinguish them from the normal cells. The cancer cells are characterized by loss of, contact inhibition, altered membrane transport, increased DNA and RNA synthesis,, increased glycolysis, alteration in the structure of certain molecules etc.
Page 705 :
Section 6, , Current Topics, , Chapter, , Acquired Immunodeficiency, Syndrome (AIDS), , 38, , AIDS speaks :, , “I am the most feared disease of the world;, Due to a retrovirus, causing immunodeficiency;, With no cure in sight, except prevention;, I challenge the scientists worldwide to conquer me!”, , cquired immunodeficiency syndrome (AIDS), was first reported in 1981 in homosexual, men. AIDS is a retroviral disease caused by, human immunodeficiency virus (HIV). The, disease is characterized by immunosuppression,, secondary, neoplasma, and, neurological, manifestations. AIDS is invariably fatal, since, there is no cure. In the USA, it is the fourth, leading cause of death in men between the ages, 15 to 55 years., , A, , No other disease has attracted as much, attention as AIDS by the governments, public and, scientists. AIDS has stimulated an unprecedented, amount of biomedical research which led to a, major understanding of this deadly disease within, a short period of time. So rapid is the research on, AIDS (particularly relating to molecular biology),, any review is destined to be out of date by the, time it is published!, The isolation of human immunodeficiency, virus (HIV) from lymphocytes of AIDS patients, was independently achieved by Gallo (USA) and, Montagnier (France) in 1984., , Epidemiology, AIDS was first described in USA and this, country has the majority of reported cases. The, prevalence of AIDS has been reported from, almost every country. The number of people, living with HIV worldwide is estimated to be, around 40 million by the end of the year 2005., (India alone has about 5 million persons). At, least 5 million deaths occurred in 2005, due to, AIDS. AIDS is truely a global disease with an, alarming increase in almost every country., Transmission of HIV : Transmission of AIDS, essentially requires the exchange of body fluids, (semen, vaginal secretions, blood, milk), containing the virus or virus-infected cells. There, are three major routes of HIV transmission—, sexual contact, parenteral inoculation, and from, infected mothers to their newborns., The distribution of risk factors for AIDS transmission are as follows., Sex between men (homosexuals), , — 60%, , Sex between men and women, , — 15%, , 695
Page 706 :
696, Intravenous drug abusers, , BIOCHEMISTRY, , — 15%, , Transfusion of blood and blood products — 6%, All others, , Lipid membrane, , — 4%, , p18, p24, p66, RNA, , The, predominant, methods, of, HIV, transmission (about 75%) are through anal or, vaginal intercourse. The risk for the transmission, is much higher with anal than with vaginal, intercourse. The practice of ‘needle sharing’ is, mainly responsible for the transmission of HIV in, drug abusers. Pediatric AIDS is mostly caused by, vertical transmission (mother to infant)., It should, however, be noted that HIV cannot, be transmitted by casual personal contact in the, household or work place. Further, the, transmission of AIDS from an infected individual, to health personnel attending on him is, extremely rare., , Virology of HIV, AIDS is caused by a retrovirus, namely human, immunodeficiency virus (HIV), belonging to, lentivirus family. Retroviruses contain RNA as, the genetic material. On entry into the host cell,, they transcribe DNA which is a complementary, copy of RNA. The DNA, in turn is used, as a, template to produce new viral RNA copies., Two different forms of HIV, namely HIV-1, and HIV-2 have been isolated from AIDS, patients. HIV-1 is more common, being found in, AIDS patients of USA, Canada, Europe and, Central Africa while HIV-2 is mainly found in, West Africa. Both the viruses are almost similar, except they differ in certain immunological, properties., HIV-1 is described in some detail., Structure of HIV : The virus is spherical with, a diameter of about 110 nm. It contains a core,, surrounded by a lipid envelop derived from the, host plasma membrane (Fig.38.1). The core of, the HIV has two strands of genomic RNA and, four core proteins, p24, p18, reverse transcriptase, (p66/p51) and endonuclease (p32). Note that the, naming of the proteins is based on the molecular, weight. For instance, a protein with a molecular, weight of 24,000 is designated as p24., , p32, gp42, gp120, , Fig. 38.1 : Diagrammatic representation of HIV, (p represents protein with molecular weight, e.g. p18 is with molecular weight 18,000; gp, represents glycoprotein)., , The lipid membrane of the virus is studded, with two glycoproteins gp120 and gp41. The, surface antigen gp120 is very important for the, viral infection and the detection of AIDS., Genome and gene products of HIV : The HIV, genome contains 3 structural genes–gag, pol and, env that, respectively, code for core proteins,, reverse transcriptase and envelop proteins. On, either side of the HIV genome are long terminal, repeat (LTR) genes which control transcription., Besides the structural genes, HIV contains, several regulatory genes including vif, vpr, tat,, rev, vpu and nef (Fig.38.2). These genes control, the synthesis and assembly of infectious viral, proteins. In fact, the regulatory genes of HIV play, a key role in the development of AIDS., , Immunological abnormalities, in AIDS, As is evident from the name AIDS,, immunodeficiency (or immunosuppression) is, the hallmark of this disease. AIDS primarily, affects the cell-mediated immune system which, protects the body from intracellular parasites, such as viruses, protozoa and mycobacteria. This, is caused by a reduction in CD4 (cluster, determinant antigen 4) cells of T-lymphocytes,, besides impairment in the functions of surviving, CD4 cells.
Page 707 :
697, , Chapter 38 : ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS), , CD4 cells may be regarded as, master cells of cell mediated, immunity. They produce cytokines,, macrophage chemotactic factors,, hemopoietic growth factors, and, others involved in the body, immunity., , LTR, , gag, , Core, proteins, , Entry of HIV and lysis of CD4 cells : The virus, enters the CD4 T-lymphocytes. HIV binds to the, specific receptors on CD4 cells by using its, surface, membrane, glycoprotein, (gp120)., Following the entry into the host cells, RNA of, HIV is transcribed into DNA by the viral enzyme, reverse transcriptase. The viral DNA gets, incorporated into the host genomic DNA. The, virus may remain locked in the host genome for, months or years and this is considered as the, latent period. The viral DNA may undergo, replication, and, translation,, respectively,, producing viral RNA and viral proteins. The, latter two, on assembly, result in new viruses., The newly synthesized viruses leave the host, cells by forming buds on plasma membrane., Extensive viral budding is associated with lysis, and death of CD4 cells (Fig.38.3). The new viral, particles infect other host cells and repeat the, whole process, ultimately resulting in a profound, loss of CD4 cells from the blood. Most of the, immunodeficiency symptoms of AIDS are, associated with the reduction in CD4 cells., , pol, , vif vpr tat vpu, , Reverse, transcriptase, , env, , tat rev nef LTR, , Envelope, glycoproteins, , Fig. 38.2 : Genome of HIV., , gp120, HIV, , CD4 cell, , Latent infection, , Other immunological abnormalities, The viral membrane protein gp120 binds, with normal T-helper cells and kills them., AIDS patients also display abnormalities in, antibody production by B-lymphocytes (humoral, immunity)., Abnormalities of central nervous system :, HIV also infects the cells of central nervous, system. It is believed that HIV infected, monocytes enter the brain and cause damage,, the mechanism of which remains obscure., Consequences of immunodeficiency : The, various clinical symptoms (fever, diarrhea,, weight loss, neurological complications, multiple, opportunistic infections, generalized lymphadenopathy, secondary neoplasma etc.) of AIDS, , Extensive viral, multiplication, , Lysis of CD4 cells, , Fig. 38.3 : Immunological abnormalities, in CD4 cells on HIV infection.
Page 708 :
698, , BIOCHEMISTRY, , Acute, phase, , Chronic, phase, , Crisis, phase, , CD4 T-Lymphocyte count in peripheral blood, (___), , 1,200, 1,100, Primary, infection, , 1,000, , Death, , 900, , 1 : 512, 1 : 256, , Clinical latency, , 1 : 128, , 700, 600, , Opportunistic, diseases, , 500, , 1 : 64, 1 : 32, , 400, , 1 : 16, , 300, , 1:8, , 200, , 1:4, , 100, , 1:2, , Plasma viremia titer, (___), , 800, , 0, , 0, 2, , 4, , 6, , 8, , 10 12, , 1, , 2, , 3, , Weeks, , 4, , 5, , 6 7, , 8, , 9 10 11 12 13 14, , Years, , Fig. 38.4 : Graphic representation of a typical course of HIV infection., , are directly or indirectly related to the, immunosuppression caused by HIV. Due to the, deficiency in the immune system, the body of, AIDS patient is freely exposed to all sorts of, infections (viral, bacterial, fungal)., , Natural course of AIDS, Three distinct phases of HIV interaction with, the immune system of infected body have been, identified. These are the early, acute phase; the, intermediate, chronic phase; the final, crisis, phase (Fig.38.4)., 1. Acute phase : This represents the initial, body response to HIV infection. It is, characterized by high rate of production of, viruses which are lodged in the lymphoid tissues, and the antiviral immune response of the body., This period may last for about 8-12 weeks., 2. Chronic phase : During this period that, may last for 5 to 10 years or even more, the, body tries to contain the virus. The immune, system is largely intact. The person obviously, appears normal, although he/she is the carrier of, HIV which can be transmitted to others., Antibodies to HIV are found in the circulation,, , hence this phase is, seropositive period., , also, , referred, , to, , as, , 3. Crisis phase : A failure in the defense, system of the body, caused by immunosuppression by HIV, represents the crisis phase., The plasma level of virus is tremendously, increased. CD4 T-lymphocyte concentration, drastically falls. A patient with lower than 200, CD4 T-lymphocytes/Pl blood is considered to, have developed AIDS. Crisis phase is, characterized by opportunistic infections and, the related clinical manifestations. In Western, countries, a cancer—Kaposis sarcoma—is, associated with AIDS., In general, AIDS patients die between 5-10, years after HIV infection. Treatment may,, however, prolong the life., , Laboratory diagnosis of AIDS, The following laboratory tests are employed, to diagnose the HIV infection., 1. The detection of antibodies in the, circulation by ELISA (enzyme-linked immunosorbant assay).
Page 709 :
699, , Chapter 38 : ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS), , O, HN, O, , O, CH3, , N, , N, , N, , O, HOH2C, H, , H, , N N, , H, , H, N, , N, , O, , HOH2C, , –, , NH, , +, , H, , 3c-Azido 2c,3c-diodeoxythymidine (AZT), , H, , H, , H, , H, , H, , H, , 2c,3c-Dideoxyinosine (DDI), , Fig. 38.5 : Structure of anti-AIDS drugs., , 2. Western blot technique, a more specific, test for the HIV antibodies, is employed for, confirmation of ELISA positive cases., 3. A more recent and sophisticated PCR can, be used to detect the presence of the HIV, genome in the peripheral blood lymphocytes., , Drugs for the treatment of AIDS, Although there is no cure for AIDS, use of, certain drugs can prolong the life of AIDS, patients. Zidovudine or AZT (3’-azido 2’,, 3’-dideoxy thymidine), a structural analog of, deoxythymidine was the first drug used and, continues to be the drug of choice for the, treatment of AIDS. Didanosine (dideoxyionosine,, DDI) is another drug employed to treat AIDS., The structures of AZT and DDI are shown in, Fig.38.5., Mechanism of action : AZT is taken up by the, lymphocytes and converted to AZT triphosphate, which inhibits the enzyme HIV reverse, transcriptase. AZT triphosphate competes with, dTTP for the synthesis of DNA from viral RNA., Further, AZT is added to the growing DNA chain, and the synthesis is halted. This drug is not toxic, , to the T-lymphocytes since cellular DNA, polymerase has low affinity for AZT. However,, AZT is found to be toxic to the bone marrow, cells, therefore, the patients develop anemia., The mechanism of action of dideoxyinosine is, almost similar to that of AZT., , Vaccine against AIDS, —a failure so far, HIV exhibits genetic heterogenecity with a, result that several species of virus may be found, in the same AIDS patient. The principal cause for, the genetic variation is the lack of proof-reading, activity by the enzyme reverse transcriptase. This, leads to very frequent alterations in the DNA, base sequence synthesized from viral RNA, which, in turn, influences the amino acid, sequence of proteins. Thus, the protein products, of HIV are highly variable in the amino acid, composition and, therefore, the antigenic, properties. For this reason, it has not been, possible to develop a vaccine against AIDS., However, there have been some encouraging, animal and in vitro experiments which raise fresh, hopes for a vaccine in the near future.
Page 710 :
700, , BIOCHEMISTRY, , +, , AIDS is a global disease with an alarming increase in the incidence of occurrence. By, the year 2005, more than 40 million people were globally affected by AIDS., , +, , Homosexuality (predominantly in men) and intravenous drug abuse are the major, factors in the risk of AIDS transmission., , +, , The patients of AIDS are destined to die (within 5–10 years after infection), since there, is no cure. However, administration of certain drugs (AZT, DDI) prolongs life., , +, , The clinical manifestations of AIDS are directly or indirectly related to, immunosuppression (mostly due to reduced CD4 cells). AIDS patients are freely, exposed to all sorts of infections (viral, bacterial, fungal)., , 1., , AIDS is a retorviral disease caused by human immunodeficiency virus (HIV). It is, characterized by immunosuppression, secondary neoplasms and neurological, manifestations. Transmission of HIV occurs by sexual contact (more in male, homosexuals), parental inoculation (intravenous drug abusers) and from infected, mothers to their newborns., , 2., , HIV enters CD4 T-lymphocytes where its genetic material RNA is transcribed into DNA, by the enzyme reverse transcriptase. The viral DNA gets incorporated into the host, genome ultimately leading to the multiplication of the virus and the destruction of CD4, cells. This is the root cause of immunosuppression leading to opportunistic infections, in AIDS., , 3., , The natural course of AIDS has 3 distinct phases—acute, chronic and crisis. A patient, with lower than 200 CD4 T-lymphocytes/Pl is considered to have developed AIDS. The, sensitive laboratory tests for AIDS detection are—ELISA, Western blot technique and,, recently PCR., , 4., , There is no cure for AIDS. The patients generally die within 5–10 years after HIV, infection. Administration of drugs (zidovudine and didanosine), however, prolongs the, life of AIDS patients. These drugs inhibit the viral enzyme reverse transcriptase and, halt the multiplication of the virus., , 5., , The attempts to produce vaccine for AIDS have been unsuccessful due to the variations, in the genome (and, therefore, the protein products) of the HIV.
Page 711 :
BASICS TO LEARN BIOCHEMISTRY, 39, ■, 40, ■, 41, ■, 42, ■, 43, ■, , Introduction to Bioorganic, Chemistry, , 703, , Overview of Biophysical, Chemistry, , 708, , Tools of Biochemistry, , 719, , Immunology, , 732, , Genetics, , 737, , Section, , VI, VIII
Page 712 :
“This page intentionally left blank"
Page 713 :
Section 7, , Basics to Learn Biochemistry, , Chapter, , Introduction to, Bioorganic Chemistry, , 39, , OH, , Naphthalene, , D-Naphthol, , Phenanthrene Cyclopentane, , Carbon, the official spokesperson, of organic chemistry, speaks :, , “I am the unique and versatile element;, Capable of forming covalent C C chains;, To produce unlimited number of compounds;, Thus, I am the mother of organic molecules.”, , A, , s life comes from the existing life, it was, believed for a long that the carbon compounds, of organisms (hence the name organic) arose from, life only. This is referred to as vital force theory., Friedrich Wohler (1825) first discovered that urea, (NH2 CO NH2), the organic compound, could, be prepared by heating ammonium cyanate, (NH4NCO), in the laboratory. Thereafter, thousands, and thousands of organic compounds have been, synthesized outside the living system., , Organic chemistry broadly deals with the, chemistry of carbon compounds, regardless of, their origin. Biochemistry, however, is, concerned with the carbon chemistry of life, only. The general principles of organic chemistry, provide strong foundations for understanding, biochemistry. However, biochemistry exclusively, deals with the reactions that occur in the living, system in aqueous medium., , Most common organic compounds, found in living system, The organic compounds, namely carbohydrates, lipids, proteins, nucleic acids and, , vitamins are the most common organic, compounds of life. Their chemistry has been, discussed in Section I (Chapters 1-7)., , Common functional groups, in biochemistry, Most of the physical and chemical properties, of organic compounds are determined by their, functional groups. Biomolecules possess certain, functional groups which are their reactive, centres. The common functional groups of, importance in biomolecules are presented in, Table 39.1., , Common ring structures, in biochemistry, There are many homocyclic and heterocyclic, rings, commonly encountered in biomolecules., A selected list of them is given in Fig.39.1., Homocyclic rings : Phenyl ring derived from, benzene is found in several biomolecules, (phenylalanine,, tyrosine,, catecholamines)., Phenanthrene and cyclopentane form the, backbone of steroids (cholesterol, aldosterone)., , 703
Page 715 :
705, , Chapter 39 : INTRODUCTION TO BIOORGANIC CHEMISTRY, , OH, , OH, , O, , (A), O, Benzene, , Phenol, , Benzoquinone, , Naphthalene, , D-Naphthol, , O, , O, Anthracene, , Naphthoquinone, , Phenanthrene, , Cyclopentane, , N, , (B), S, , N, , Thiophene, , Imidazole, , N, , O, , H, Furan, , H, , Pyrrole, , N, O, , N, , Pyran, , N, , Pyridine, , N, , N, N, , Pyrimidine, , N, , N, , H, , H, , Purine, , Indole, , Fig. 39.1 : Common ring structures found in biomolecules (A) Homocyclic rings (B) Heterocyclic rings., , Consider the molecular formula—C2H6O. There, are two important isomers of this—ethyl alcohol, (C2H5OH) and diethyl ether (CH3OCH3), depicted next., H, , H OH, , H, , isomerism) or difference in the position of, functional groups (position isomerism) or, difference in both molecular chains and, functional groups (functional isomerism)., , Isomerism is broadly divided into two categories-structural isomerism and stereoisomerism., , Structural isomerism, as such, is more, common in general organic molecules., Tautomerism, a type of structural isomerism,, occurs due to the migration of an atom or group, from one position to the other e.g. purines and, pyrimidines (Chapter 5)., , Structural isomerism, , Stereoisomerism, , The difference in the arrangement of the, atoms in the molecule (i.e. molecular framework), is responsible for structural isomerism. This, may be due to variation in carbon chains (chain, , Stereoisomerism (Greek : stereos—space occupying) is, perhaps, more relevant and important, to biomolecules. The differential space, arrangement of atoms or groups in molecules, , H C C H, , H C O C H, H, , H H, Ethyl alcohol, , H, , Diethyl ether
Page 716 :
706, , BIOCHEMISTRY, , gives rise to stereoisomerism. Thus, stereoisomers have the same structural formula but, differ in their spatial arrangement., Stereoisomerism is of two types—geometric, isomerism and optical isomerism., Geometrical isomerism : This is also called, cis-trans isomerism and is exhibited by certain, molecules possessing double bonds. Geometrical, isomerism is due to restriction of freedom of, rotation of groups around a carbon-carbon double, bond (C C). Maleic acid and fumaric acid are, classical examples of cis-trans isomerism., H C COOH, H C COOH, Maleic acid (cis), , H C COOH, HOOC C H, Fumaric acid (trans), , When similar groups lie on the same side, it, is called cis isomer (Latin : cis—on the same, side). On the other hand, when similar groups, lie on the opposite sides, it is referred to as trans, isomer (Latin : trans—across). As is observed, from the above structure, maleic acid is a cis, form while fumaric acid is a trans form., Geometric isomerism is also observed in, sterols and porphyrins. cis-trans isomers differ in, physical and chemical properties., Optical isomerism : Optical isomers or, enantiomers occur due to the presence of an, asymmetric carbon (a chiral carbon). Optical, isomers differ from each other in their optical, activity to rotate the plane of polarized light., , What is an asymmetric carbon?, An object is said to be symmetrical if it can, be divided into equal halves e.g. a ball. Objects, which cannot be divided into equal halves are, asymmetric, e.g. hand (Fig.39.2). An asymmetric, object cannot coincide with its mirror image. For, instance, left hand is the mirror image of right, hand and these two can never be superimposed., In contrast, a symmetrical object like a ball, superimposes its image., A carbon is said to be chiral (Greek : hand) or, asymmetric when it is attached to four different, groups. Their mirror images do not superimpose, with each other., , Symmetric, , Asymmetric, , Fig. 39.2 : Asymmetric and symmetric objects., , B, , B, , A C D, , D C A, , E, , E, Mirror, , The number of possible optical isomers of a, molecule depends upon the specific number of, chiral carbon (n). It is given by 2n., , What is optical activity?, The ordinary light propagates in all directions., However, on passing ordinary light through a, Nicol prism, the plane of polarized light vibrates, in one direction only (Fig.39.3)., Certain organic compounds (optical isomers), which are said to exhibit optical activity rotate, the plane of polarized light either to the left or to, the right., The term levorotatory (indicated by 1 or, (–) sign) is used for the substances which rotate, the plane of polarized light to the left. On the, other hand, the term dextrorotatory (indicated, by d or (+) sign) is used for substances rotating, the plane of polarized light to right (Fig.39.3)., The term racemic mixture represents equal, concentration of d and l forms which cannot, rotate the plane of polarized light., , Configuration of chiral molecules, While representing the configuration of chiral, molecules, the configuration of glyceraldehyde, is taken as a reference standard.
Page 717 :
707, , Chapter 39 : INTRODUCTION TO BIOORGANIC CHEMISTRY, , CHO, H C OH, , CHO, HO C H, , CH2OH, D-Glyceraldehyde, , CH2OH, L-Glyceraldehyde, , It must, however, be remembered that, D- and L- do not represent the direction, of the rotation of plane of polarized light., , Ordinary light waves Nicol prism, vibrating in all directions, , Plane of polarized light, vibrating in one direction, , Existence of chiral, biomolecules, As you know, you can never come, across anybody who is your mirror image., The same is true with biomolecules. Only, one type of molecules (D or L) are found, in the living system. Thus, the naturally, occurring amino acids are of L-type while, the carbohydrates are of D-type., , Plane rotated to, the left (levorotatory), , Plane rotated to, the right (dextrorotatory), , Fig. 39.3 : Diagrammatic illustration of optical activity.
Page 718 :
Section 7, , Basics to Learn Biochemistry, , Chapter, , Overview of, Biophysical Chemistry, , 40, , The radioactive isotopes speak :, Isotope Radiation, 3H, 14C, 22Na, , E, E, J, , Half-life, 12.2 years, 5,700 years, 2.5 years, , “We are elements of same atomic numbers;, Undergo decay to emit D or E or J rays;, Exhibit identical chemical properties;, Exploited as tracers in biomedical research.”, , T, , he general laws and principles of chemistry, and physics are applicable to biochemistry, as well. It is, therefore, worthwhile to have a, brief understanding of some of the basic, chemical and physical principles that have direct, relevance to life., It must, however, be remembered that this, chapter deals with quite unrelated topics to each, other., , WATER, Water is the most abundant fluid on earth. It, is justifiably regarded as the solvent of life. As, much as 70% of a typical cell is composed of, water. The unique physical and chemical, properties of water have profound biological, importance. The structures of biomolecules, (proteins, nucleic acids, lipids and carbohydrates) are maintained due to their interaction, with water, which forms an aqueous, environment. This is essential for sustaining life., , Structure of water, The H2O molecule exists in a bent geometry., The bond angle of H O H is 104.5q and the, O H bond has a distance of 0.958Aq. There, exists electrical polarity in H2O due to electronegativity (the power of an atom in a molecule, to attract electrons) difference between H and O., This results in the polarization of a positive, charge on H and a negative charge on O. Thus, H2O molecule, although carrying no net charge,, possesses an electrical dipole. The polar, character of water has tremendous biological, significance., Hydrogen bonds between H2O molecules :, The presence of electrical dipoles on H2O, molecules is responsible for their attraction., Hydrogen bonds are formed due to polarity, between, two, atoms, with, different, electronegativities. Thus, in H2O, the transient, negative charge on the O atom of one H2O, molecule and the transient positive charge on, the H atom of another H2O molecule attract, each other to form a hydrogen bond. The water, , 708
Page 719 :
709, , Chapter 40 : OVERVIEW OF BIOPHYSICAL CHEMISTRY, , b–, 0.958 A°, , O, , +, , b, , b, , 104.5°, , H, H, O, , +, , b–, , H-bonds, , H, H, b–, , O, , H, , H, , Fig. 40.1 : Diagrammatic representation of water, structure along with hydrogen bonds., , molecules are interlinked with each other by, profuse hydrogen bonding. The energy of, each hydrogen bond is very small compared to, that of a covalent bond. But the collective, strength of H-bonds is due to their large, numbers. Hydrogen bonds are important for, the three-dimensional structures of biomolecules., Water expands on freezing : Water is one of, the very few substances that expands on, freezing. Thus, ice has a density of 0.92 g/ml,, while water at 0°C has density of 1.0 g/ml. For, this reason, ice floats on water. And this property, is essential to maintain water equilibrium in the, environment, and to sustain life., (Imagine that water contracted on cooling and, becomes denser. In such a case, ice would sink, to the bottom of seas and lakes and would never, get exposed to sun rays. Thus, frozen water, would permanently remain as ice. If this were to, happen, earth would have a permanent ice age!), , Acid, , Base, , HCl, , H+ + Cl –, , H2CO3, , H+ + HCO 3–, , H2PO4, , H+ + HPO4–, , NH+4, , H+ + NH3, , H2 O, , H+ + OH–, , CH3COOH, , H+ + CH3COO–, , HA, , H+ + A –, , (general), , (general), , It is evident that an acid dissociates to form, proton and base. On the other hand, the base, combines with proton to form acid. The, difference between an acid and its corresponding, base (more commonly referred to as conjugate, base) is the presence or absence of a proton. In, general, a strong acid has a weak base while a, weak acid has a strong base. For instance, strong, acid HCl has weak base Cl–, weak acid HCN, has a strong base CN–., Alkalies : The metallic hydroxides such as, NaOH and KOH are commonly referred to as, alkalies. These compounds do not directly satisfy, the criteria of bases. However, they dissociate to, form metallic ion and OH– ion. The latter, being, a base, accepts H+ ions., Ampholytes : The substances which can, function both as acids and bases are referred to, as ampholytes. Water is the best example for, ampholytes., , Dissociation of water, Water is a weak electrolyte and dissociates as, follows., H+ + OH, , H2 O, , The proton reacts with another molecule of, water to form hydronium ion (H3O+)., , ACIDS AND BASES, According to Lowry and Bronsted, an acid is, defined as a substance that gives off protons, while base is a substance that accepts protons., Thus, an acid is a proton (H+) donor and a base, is a proton acceptor. A few examples of, acids and their corresponding bases are given in, the next column., , H+ + H2O, , H3O+, , For the sake of convenience, the presence of, proton as H3O+ is ignored., By applying the law of mass action for the, dissociation of water., , [H ][OH ], +, , K=, , [H2O], , –
Page 720 :
710, , BIOCHEMISTRY, , Here K is a constant; the concentrations are, expressed in molarity. Since the degree of, dissociation is very small, the concentration of, undissociated [H2O] may be taken as constant., KZ = [H+] [OH–], KZ is the dissociation constant for water. Its, value is 10–14 at 25qC., [H+] [OH–] = 10–14, In a neutral solution, [H+] = [OH–] = 10–7, , Hydrogen ion concentration (pH), The acidic or basic nature of a solution is, measured by H+ ion concentration. The strength, of H+ ions in the biological fluids is exceedingly, low. For this reason, the conventional units such, as moles/l or g/l are not commonly used to, express H+ ion concentration., Sorenson (1909) introduced the term pH to, express H+ ion concentration. pH is defined as, the negative logarithm of H+ ion concentration., pH = – log [H+], The pH (may be considered as potential of H+, ions) is a narrow scale, ranging from 0 to 14, which corresponds to 1 M solution to 10–14 M, solution of [H+] concentration., As explained under dissociation of water,, pure water has an equal concentration of H+ and, OH– ions i.e. 10–7 M each. Thus, pure water has, a pH 7 which is neutral. Solutions with pH less, than 7 are said to be acidic while those with pH, greater than 7 are alkaline. It must be, remembered that the term acidic or alkaline are, not absolute but only relative. Thus, a solution, with pH 3.0 is more acidic when compared with, a solution of pH 4.5., A rise in H+ concentration decreases pH, while a fall in H+ concentration increases pH., The reverse is true for OH– concentration. The, pH of a solution containing 1N [H+] is 0 while, that containing 1N [OH–] is 14., The pH of important biological fluids is, presented in Table 40.1., , TABLE 40.1 pH of important biological fluids, , Fluid, , pH, , Pancreatic juice, Blood plasma (or whole blood), Cerebrospinal fluid, Tears, Interstitial fluid, Human milk, Saliva, Intracellular fluid (cytosol), Gastric juice, Urine, , 7.5, 7.35, 7.2, 7.2, 7.2, 7.2, 6.4, 6.5, 1.5, 5.0, , –, –, –, –, –, –, –, –, –, –, , 8.0, 7.45, 7.4, 7.4, 7.4, 7.4, 7.0, 6.9, 3.0, 7.5, , BUFFERS, The pH of a given solution can be easily, altered by the addition of acids or bases. Buffers, are defined as the solutions which resist change, in pH by the addition of small amounts of acids, or bases. A buffer usually consists of a weak acid, and its salt (e.g. acetic acid and sodium acetate), or a weak base and its salt (e.g. ammonium, hydroxide and ammonium chloride). Several, buffers can be prepared in the laboratory. Nature, has provided many buffers in the living system., , Mechanism of buffer action, Let us consider the buffer pair of acetic acid, and sodium acetate. Acetic acid, being a weak, acid, feebly ionizes. On the other hand, sodium, acetate ionizes to a large extent., CH3COOH, , CH3COO– + H+, , CH3COONa, , CH3COO– + Na+, , When an acid (say HCl) is added, the acetate, ions of the buffer bind with H+ ions (of HCl) to, form acetic acid which is weakly ionizing., Therefore, the pH change due to acid is resisted, by the buffer., H+ + CH3COO– o CH3COOH, When a base (say NaOH) is added the H+, ions of the buffer (acetic acid) combine with, OH– ions to form water, which is weakly
Page 721 :
711, , Chapter 40 : OVERVIEW OF BIOPHYSICAL CHEMISTRY, , dissociated. Thus, the pH change due to base, addition is also prevented by the buffer., , solution can be prepared by dissolving 1 mole of, solute in 1,000 g of solvent., , OH– + H+ o H2O, , Normality : Molarity is based on molecular, weight while normality is based on equivalent, weight. One gram equivalent weight of an, element or compound represents its capacity to, combine or replace 1 mole of hydrogen. In, general, the gram equivalent weight of an, element or a compound is equal to its molecular, weight divided by the total positive valence of, the constituent ions. Thus, for NaOH and KOH,, the molecular and equivalent weights are the, same, while, for H2SO4, equivalent weight is half, of, the, molecular, weight., The, term, milliequivalent per liter (mEq/l) is used for, smaller concentrations., , Buffering capacity : The efficiency of a buffer, in maintaining a constant pH on the addition of, acid or base is referred to as buffering capacity., It mostly depends on the concentration of the, buffer components. The maximum buffering, capacity is usually achieved by keeping the same, concentration of the salt as well as the acid., For a comprehensive discussion on blood, buffers, refer Chapter 21., , SOLUTIONS, Solutions may be regarded as mixtures of, substances. In general, a solution is composed of, two parts—solute and solvent. The substance, that is dissolved is solute and the medium that, dissolves the solute is referred to as solvent. The, particle size of a solute in solution is < 1 nm., The relative concentrations of substances in a, solution can be measured by several ways., Per cent concentration : This represents parts, per 100. The most frequently used is weight per, volume (w/v) e.g. 9% saline (9 g/100 ml, solution). For expressing smaller concentration,, mg (10–3g), Pg (10–6 g), ng (10–9 g) and pg, (10–12 g) are used., Parts per million (ppm) : This refers to the, number of parts of a substance in one million, parts of the solution. Thus 10 ppm chlorine, means 10 Pg of chlorine in 1 g of water., , COLLOIDAL STATE, Thomas Graham (1861), regarded as the, ‘father of colloidal chemistry’, divided, substances into two classes—crystalloids and, colloids., Crystalloids are the substances which in, solution can freely pass (diffuse) through, parchment membrane e.g. sugar, urea, NaCl., Colloids (Greek : glue-like), on other hand, are, the substances that are retained by parchment, membrane e.g. gum, gelatin, albumin. The above, classification of Graham is no longer tenable,, since any substance can be converted into a, colloid by suitable means. For instance, sodium, chloride in benzene forms a colloid., , Molarity (M) : It is defined as the number of, moles of solute per liter solution. NaCl has a, molecular weight of 58.5. To get one molar, (1 M) or one mole solution of NaCl, one gram, molecular weight (58.5 g) of it should be, dissolved in the solvent (H2O) to make to a final, total volume of 1 liter. For smaller concentrations, millimole and micromole are used., , Colloidal state : As such, there are no group, of substances as colloids, rather, substances can, exist in the form of colloidal state or colloidal, system. Colloidal state is characterized by the, particle size of 1 to 100 nm. When the particle, size is <1 nm, it is in true solution. For the, particle sizes >100 nm, the matter exists as a, visible precipitate. Thus, the colloidal state is an, intermediate between true solution and, precipitate., , Molality : It represents the number of moles, of solute per 1,000 g of solvent. One molal, , Phases of colloids : Colloidal state is heterogeneous with two phases.
Page 722 :
712, 1. Dispersed phase (internal phase) which, constitutes the colloidal particles., 2. Dispersion medium (external phase) which, refers to the medium in which the colloidal, particles are suspended., , CLASSIFICATION OF COLLOIDS, Based on the affinity of dispersion medium, with dispersed phase, colloids are classified as, lyophobic and lyophilic colloids., 1. Lyophobic (Greek : solvent-hating) : These, colloids do not have any attraction towards, dispersion medium. When water is used as, dispersion medium, the colloids are referred to, as hydrophobic., 2. Lyophilic (Greek : solvent-loving) : These, colloids have distinct affinity towards dispersion, medium. The term hydrophilic is used for the, colloids when water is the dispersion medium., The terms gel and sol are, respectively, used, to jelly-like and solution-like colloids. Emulsions, are the colloids formed by two immiscible, liquids (e.g. oil + water). Frequently, emulsions, can be stabilized by using agents known as, emulsifiers. For instance, the protein casein acts, as an emulsifier for milk., , Micelles are the aggregates of colloidal, particles. Soap (sodium palmitate) in water is the, classical example for the micelles formation., , Properties of colloids, 1. Brownian movement : The continuous and, haphazard motion of the colloidal particles is, known as Brownian movement., 2. Optical properties : When light is passed, through a colloidal solution, it gets scattered., This phenomenon is referred to as Tyndal effect., 3. Electrical properties : The colloidal, particles carry electrical charges, either positive, or negative. The electrical charge may be due to, ionization of the colloidal particles or adsorption, of the ions from the medium, or both. The, stability and precipitation of colloids is, determined by the ionic charges they carry. The, , BIOCHEMISTRY, , separation of charged colloids can be achieved, by the analytical technique—electrophoresis, (Refer Chapter 41)., 4. Osmotic pressure : Since the colloidal, particles are larger in size, their contribution to, osmotic pressure is relatively less., 5. Non-dialysable nature : The colloidal, particles, being larger in size, cannot pass, through a membrane (cellophane or parchment)., The membrane, however, allows dispersion, medium and smaller particles to escape through, the pores. This process is referred to as dialysis, and is useful for the separation of colloids., 6. Donnan membrane equilibrium : The, presence of non-diffusible colloidal particles (e.g., protein) in the biological systems influences the, concentration of diffusible ions across the, membrane. This is an important phenomenon,, the details of which are given on page 714., , Biological importance of colloids, 1. Biological fluids as colloids : These, include blood, milk and cerebrospinal fluid., 2. Biological compounds as colloidal, particles : The complex molecules of life, the, high molecular weight proteins, complex lipids, and polysaccharides exist in colloidal state., 3. Blood coagulation : When blood clotting, occurs, the sol is converted finally into the gel., 4. Fat digestion and absorption : The, formation of emulsions, facilitated by the, emulsifying agents bile salts, promotes fat, digestion and absorption in the intestinal tract., 5. Formation of urine : The filtration of urine, is based on the principle of dialysis., , DIFFUSION, The molecules in liquids or gases are in, continuous motion. Diffusion may be regarded, as the movement of solute molecules from a, higher concentration to a lower concentration., Diffusion is more rapid in gases than in liquids.
Page 723 :
Chapter 40 : OVERVIEW OF BIOPHYSICAL CHEMISTRY, , The smaller particles diffuse faster than the larger, ones. The greater the temperature, the higher is, the rate of diffusion., Diffusion occurs in true solutions as well as in, colloidal solutions., , Applications of diffusion, 1. Exchange of O2 and CO2 in lungs and in, tissues occurs through diffusion., 2. Certain nutrients are absorbed by diffusion, in the gastrointestinal tract e.g. pentoses,, minerals, water soluble vitamins., 3. Passage of the waste products namely, ammonia, in the renal tubules occurs due to, diffusion., , OSMOSIS, Osmosis (Greek : push) refers to the, movement of solvent (most frequently water), through a semipermeable membrane., The flow of solvent occurs from a solution of, low concentration to a solution of high, concentration, when both are separated by a, semipermeable membrane. In a strict sense, the, semipermeable membrane is expected to be, permeable to the solvent and not to the solute., , Osmotic pressure, Osmotic pressure may be defined as the, excess pressure that must be applied to a, solution to prevent the passage of solvent into, the solution, when both are separated by a, semipermeable membrane., Osmosis is a colligative property i.e. a, character which depends on the number of, solute particles and not their nature. Osmotic, pressure is directly proportional to the, concentration (number) of the solute molecules, or ions. Low molecular weight substances (e.g., NaCl, glucose) will have more number of, molecules compared to high molecular weight, substances (albumin, globulin) for unit mass., Therefore, the substances with low molecular, , 713, , weight, in general, exhibit greater osmotic, pressure. Further, for ionizable compounds, the, total osmotic pressure is equivalent to the sum of, the individual pressures exerted by each ion. For, instance, one molar solution of NaCl will exert, double the osmotic pressure of one molar, solution of glucose. This is because NaCl ionizes, to Na+ and Cl– while glucose is non-ionizable., The solutions that exert the same osmotic, pressure are said to be isoosmotic. The term, isotonic is used when a cell is in direct contact, with an isoosmotic solution (0.9% NaCl) which, does not change the cell volume and, thus, the, cell tone is maintained. A solution with relatively, greater osmotic pressure is referred to as, hypertonic. On the other hand, a solution with, relatively lower pressure is hypotonic., The term oncotic pressure is commonly used, to represent the osmotic pressure of colloidal, substances (e.g. albumin, globulin)., Units of osmotic pressure : Osmole is the, unit of osmotic pressure. One osmole is the, number of molecules in gram molecular weight, of undissociated solute. One gram molecular, weight of glucose (180 g) is one osmole., However, one gram molecular weight of NaCl, (58.5 g) is equivalent to 2 osmoles, since NaCl, ionizes to give two particles (Na+, Cl–)., Osmotic pressure of biological fluids is, frequently expressed as milliosmoles. The, osmotic pressure of plasma is 280–300, milliosmoles/l., , Applications of osmosis, 1. Fluid balance and blood volume : The, fluid balance of the different compartments of, the body is maintained due to osmosis. Further,, osmosis significantly contributes to the regulation, of blood volume and urine excretion., 2. Red blood cells and fragility : When RBC, are suspended in an isotonic (0.9% NaCl), solution, the cell volume remains unchanged, and they are intact. In hypertonic solution (say, 1.5% NaCl), water flows out of RBC and the, cytoplasm shrinks, a phenomenon referred to as, crenation.
Page 724 :
714, , BIOCHEMISTRY, , On the other hand, when the RBC are kept in, hypotonic solution (say 0.4% NaCl), the cells, bulge due to entry of water which often causes, rupture of plasma membrane of RBC (hemolysis)., , Osmotic fragility test for RBC is employed in, laboratories for diagnostic purposes. For a, normal human blood, RBC begin to hemolyse in, 0.45% NaCl and the hemolysis is almost, complete in 0.33% NaCl. Increased fragility of, RBC is observed in hemolytic jaundice while it, is decreased in certain anemias., 3. Transfusion : Isotonic solutions of NaCl, (0.9%) or glucose (5%) or a suitable combination, of these two are commonly used in transfusion, in hospitals for the treatment of dehydration,, burns etc., 4. Action of purgatives : The mechanism of, action of purgatives is mainly due to osmotic, phenomenon. For instance, epson (MgSO4, 7H2O) or Glauber’s (Na2SO4 10H2O) salts, withdraw water from the body, besides, preventing the intestinal water absorption., 5. Osmotic diuresis : The high blood glucose, concentration causes osmotic diuresis resulting, in the loss of water, electrolytes and glucose in, the urine. This is the basis of polyuria observed, in diabetes mellitus. Diuresis can be produced, by administering compounds (e.g. mannitol), which are filtered but not reabsorbed by renal, tubules., 6. Edema due to hypoalbuminemia :, Disorders such as kwashiorkor and glomerulonephritis are associated with lowered plasma, albumin concentration and edema. Edema is, caused by reduced oncotic pressure of plasma,, leading to the accumulation of excess fluid in, tissue spaces., 7. Cerebral edema : Hypertonic solutions of, salts (NaCl, MgSO4) are in use to reduce the, volume of the brain or the pressure of, cerebrospinal fluid., 8. Irrigation of wounds : Isotonic solutions, are used for washing wounds. The pain, experienced by the direct addition of salt or, sugar to wounds is due to osmotic removal of, water., , Na+, , Na+, , Pr–, , Cl–, II, , I, , Initial, , Na+, Pr–, Cl–, I, , Na+, Cl–, II, , At equilibrium, , Fig. 40.2 : Diagrammatic representation of, Donnan membrane equilibrium., , DONNAN MEMBRANE EQUILIBRIUM, When membrane is freely permeable to ions, (say Na+, Cl–) and if the concentration of ions on, both the sides is different, the ions freely diffuse, to attain equal concentration. Gibbs-Donnan, observed that the presence of a non-diffusible, ion on one side of the membrane alters the, diffusion of diffusible ions., In the molecule sodium proteinate (Na+Pr –),, the protein (Pr–) ion is non-diffusable through the, membrane. Let us consider two sides of a, compartment separated by a membrane. Initially,, sodium proteinate is on side I while sodium, chloride is on side II (Fig.40.2). Diffusible ions, (Na+, Cl–) can freely pass through the membrane., On side I, Na+ ions will balance the incoming, Cl– ions besides Pr– ions, while on side II Na+, ions have to balance only Cl– ions. Therefore,, the concentration of Na+ on side I is greater than, on side II. However, from the thermodynamical, point of view, at equilibrium, the concentration, of Na+ Cl– on both the sides should be the same., Thus, , Na+ Cl– (I) = Na+ Cl– (II), , Since, , Na+ (I), , > Na+ (II), , Cl–, , < Cl– (II), , (I), , Consequently, the concentration of Cl– ions, should be greater on side II. Further, the total, concentration of ions on side I is higher than on, side II., The salient features of Donnan membrane, equilibrium are listed next.
Page 725 :
715, , Chapter 40 : OVERVIEW OF BIOPHYSICAL CHEMISTRY, , 1. The presence of a non-diffusible ion, influences the concentration of diffusible ions, across the membrane., 2. The concentration of oppositely charged, ions (Na+), is greater on the side of, membrane containing non-diffusible ions (Pr–)., 3. The concentration of similarly charged, ions (Cl–) is higher on the side of the membrane, not containing non-diffusible ions (Pr–)., 4. The net concentration of total ions, will be greater on the side of the membrane, containing non-diffusible ions. This leads to a, difference in the osmotic pressure on either side, of the membrane., , Applications of Donnan, membrane equilibrium, 1. Difference in the ionic concentrations of, biological fluids : The lymph and interstitial, fluids have lower concentration of inorganic, cations (Na+, K+) and higher concentration of, anions (Cl–) compared to plasma. This is due to, the higher protein (Pr–) content in plasma., 2. Membrane hydrolysis : The relative, strength of H+ and OH– ions and, therefore,, the acidic or alkaline nature on either side, of a membrane, is influenced by the presence of, non-diffusible ions. This phenomenon is referred, to as membrane hydrolysis. Donnan membrane, equilibrium explains the greater concentration, of H+ ions in the gastric juice., 3. Lower pH in RBC : Hemoglobin of RBC, is negatively charged and, this causes, accumulation of positively charged ions, including H+. Therefore, the pH of RBC is, slightly lower (7.25) than that of plasma (7.4)., 4. Osmotic imbalance : Donnan membrane, equilibrium—which results in the differential, distribution of ions in different compartments of, the body—partly explains the osmotic pressure, differences., 5. Dialysis in renal failure : Donnan, membrane equilibrium is the basic principle, involved in the artificial means of purifying, blood by dialysis in the patients of renal failure., , VISCOSITY, Liquid or fluid has a tendency to flow which, is referred to as fluidity. The term viscosity may, be defined as the internal resistance offered by, a liquid or a gas to flow. The property of, viscosity is due to frictional forces between the, layers while their movement occurs. Viscosity, may be appropriately regarded as the internal, friction of a liquid., Liquids vary widely as regards their viscosity., For instance, ether has very low viscosity while, honey and blood are highly viscous. Among the, several factors that contribute to viscosity,, density of the liquid, concentration of dissolved, substances and their molecular weight and the, molecular interactions are important. Increase in, temperature decreases viscosity while increase, in pressure increases viscosity to some extent., Viscosity of colloidal solutions, particularly, lyophilic colloids, is generally higher than true, solutions., Units of viscosity : The unit of viscosity is, poise, after the scientist Poiseuille, who first, systematically studied the flow of liquids. A poise, represents dynes/cm2., , Applications of viscosity, 1. Viscosity of blood : Blood is about 4 times, more viscous than water. The viscosity of blood, is mainly attributed to suspended blood cells and, colloidal plasma proteins. As the blood flows, through capillaries the viscosity decreases to, facilitate free flow of blood. Blood viscosity is, increased in polycythemia (elevation of RBC),, while it is reduced in anemia and nephritis. A, more viscous blood increases cardiac work load., When dehydration occurs, the viscosity of the, blood increases., 2. Viscosity change in muscle : Excitation of, the muscle is associated with increase in the, viscosity of the muscle fibres. This delays the, change in the tension of the contracting muscle., 3. Vitreous body : This is an amorphous, viscous body located in the posterior chamber of, the eye. It is rich in albumin and hyaluronic acid.
Page 726 :
716, , BIOCHEMISTRY, , salts in urine of jaundice patients. Sulfur powder,, when sprinkled on the surface of urine, possessing bile salts, sinks. This is in contrast to, a normal urine where sulfur powder floats. Hay’s, test is based on the principle that bile salts in, urine lower surface tension which is responsible, for sulfur to sink., Fig. 40.3 : Surface tension of a liquid., , 4. Synovial fluid : It contains hyaluronic acid, which imparts viscosity and helps in the, lubricating function of joints., , SURFACE TENSION, A molecule in the interior of a liquid is, attracted by other molecules in all directions. In, contrast, a molecule on the surface is attracted, only downwards and sideways and not upwards, (Fig.40.3). Due to this, the surface layer behaves, like a stretched film. Surface tension is the force, with which the molecules on the surface are, held together. It is expressed as dynes/cm., Surface tension decreases with increase in, temperature., Due to the phenomenon of surface tension,, any liquid occupies the minimum possible, volume., According to the principle of Gibbs-Thomson,, the compounds which lower the surface tension, get concentrated at the surface (or interface), layer while those compounds which increase, surface tension get distributed in the interior, portion of the liquid. In general, organic, substances (proteins, lipids) decrease whereas, inorganic substances (NaCl, KCl) increase, surface tension., , Applications of surface tension, 1. Digestion and absorption of fat : Bile salts, reduce the surface tension. They act as, detergents and cause emulsification of fat,, thereby allowing the formation of minute, particles for effective digestion and absorption., 2. Hay’s sulfur test : This is a common, laboratory test employed for the detection of bile, , 3. Surfactants and lung function : The low, surface tension of the alveoli keeps them apart, and allows an efficient exchange of gases in, lungs. In fact, certain surfactants, predominantly, dipalmitoyl phosphatidyl choline (dipalmitoyl, lecithin) are responsible for maintaining low, surface tension in the alveoli. Surfactant, deficiency causes respiratory distress syndrome, in the infants., 4. Surface tension and adsorption : Adsorption, being a surface phenomenon, is closely, related to surface tension. Due to the coupled, action of these two processes, the formation of, complexes of proteins and lipids occurs in the, biological systems., 5. Lipoprotein complex membranes : The, structure of plasma membrane is composed of, surface tension reducing substances, namely, lipids and proteins. This facilitates absorption of, these compounds., , ADSORPTION, Adsorption is a surface phenomenon. It is the, process of accumulation of a substance, (adsorbate) on the surface of another substance, (adsorbent). Adsorption differs from absorption,, as the latter involves the diffusion into the, interior of the material., The capacity of an adsorbent depends on the, surface area. Therefore, porous substances serve, as better adsorbents e.g. charcoal, alumina, silica, gel. Adsorption is a dynamic and reversible, process which decreases with rise in, temperature., , Applications of adsorption, 1. Formation of enzyme-substrate complex :, For the catalysis to occur in biological system,, formation of enzyme-substrate complex is a
Page 727 :
Chapter 40 : OVERVIEW OF BIOPHYSICAL CHEMISTRY, , 717, , prerequisite. This happens by adsorption of, substrate on the enzyme., , 1. D-Rays—an D particle possessing 2 protons, i.e. helium nuclei., , 2. Action of drugs and poisons : On adsorption at the cell surface, drugs and poisons exert, their action., , 2. E-Rays—due to the emission of electrons., 3. J-Rays—due to emission of high energy, photons., , 3. Adsorption in analytical biochemistry :, The principle of adsorption is widely employed, in the chromatography technique for the, separation and purification of compounds, (enzymes, immunoglobulins)., , The radiations emmitted by radioactive nuclei, are characteristic of the isotope. For instance,, 3H, 14C, and 32P all emit E-particles in the, respective energies of 0.018, 0.155 and 1.71, MeV., , ISOTOPES, Isotopes have revolutionized biochemistry, when they became available to investigators, soon after Second World War. Isotopes are, defined as the elements with same atomic, number but different atomic weights. They, possess the same number of protons but differ in, the neutrons in their nuclei. Therefore, isotopes, (Greek : iso—equal; tope—place) occupy the, same place in the periodic table. The chemical, properties of different isotopes of a particular, element are identical., Isotopes are of two types—stable and, unstable. The latter are more commonly referred, to as radioactive isotopes and they are of, particular interest to biochemists. Conventionally, while representing isotopes, the atomic weight is, written on upper left side of the element symbol., , The E and J emitting radioisotopes are, employed in biochemical research. These, isotopes are produced in nuclear reactors. The, simple chemicals so produced are then, converted to radiolabelled biochemicals by, chemical or enzymatic synthesis., Units of radioactivity : Curie (Ci) is the basic, unit of radioactive decay. It is defined as the, amount of radioactivity equivalent to 1 g of, radium i.e. 2.22 u 1012 disintegrations per, minute (dpm). Millicurie (mCi) and microcurie, (PCi), respectively, corresponding to 2.2 u 109, and 2.2 u 106 dpm, are more commonly used., Half-lives of isotopes : The unstable, radioisotopes undergo decay. The radioactivity, gets reduced to half of the original within a fixed, time. This represents the half-life which is, characteristic for a given isotope., Some of the commonly used radioactive, isotopes in biochemical research with their, characteristics are given in Table 40.2., , Stable isotopes, They are naturally occurring and do not emit, radiations (non-radioactive) e.g. deuterium, (heavy hydrogen) 2H; 13C; 15N; 18O. Stable, isotopes can be identified and quantitated by, mass spectrometry or nuclear magnetic, resonance (NMR). They are less frequently used, in biochemical investigations., , Measurement of, radioactivity of isotopes, , Radioactive isotopes, , Several techniques are in use for the detection, of radioactivity of the isotopes. The most, commonly employed in biochemical research, are—Geiger counters, liquid scintillation, counter and autoradiography. Geiger counters, are almost outdated. Liquid scintillation counters, are now widely used., , The atomic nucleus of radioactive isotopes is, unstable and, therefore, undergoes decay. The, radioactive decay gives rise to one of the, following 3 ionizing radiations., , In the liquid scintillation counter, the sample, is dissolved or suspended in a solution, containing one or two fluorescent organic, compounds (fluors). The fluors emit a pulse of
Page 728 :
718, , BIOCHEMISTRY, , TABLE 40.2 Commonly used, radioisotopes in biochemistry, Isotope, , Radiation, , Half-life, , 3H, , E, , 12.2 years, , 14C, , E, , 5,700 years, , 22Na, , J, , 2.5 years, , 32P, , E, , 14.5 days, , 35S, , E, , 87 days, , 45Ca, , E, , 164 days, , 59Fe, , E, J, , 45 days, , 60Co, , J, , 5.25 years, , 125I, , J, , 60 days, , 131I, , E, J, , 8.1 days, , light when struck by radiation. The light,, proportional to the radiation energy, can be, detected. The advantage with liquid scintillation, counter is that it can discriminate the particles of, different energies. Thus, two or more isotopes, can be simultaneously detected., In autoradiography, the radiations are, detected by its blackening of photographic film., This technique is commonly used for the, detection of radioactive substances separated in, polyacrylamide gel electrophoresis (PAGE)., , Applications of, radioisotopes in biochemistry, Radioactive, isotopes, have, become, indispensable tools of biochemistry. They can be, conveniently used as tracers in biochemical, research since the chemical properties of different, isotopes of a particular element are identical., Therefore, the living cells cannot distinguish the, radioactive isotope from a normal atom., , Radioisotopes are widely used in establishing, the precursor-product relationships in metabolisms and understanding of the complex, metabolic pathways., A few important application of radioisotopes, are, 1. By the use of isotope tracers, the metabolic, origin of complex molecules such as heme,, cholesterol, purines and phospholipids can be, determined. As early as 1945, it was established, that nitrogen atom of heme was derived from, glycine. This was done by feeding rats with (15N), glycine and detecting (15N) heme., 2. The precursor-product relationship in, several, metabolic, pathways, has, been, investigated by radioisotopes. e.g. Krebs cycle,, E-oxidation of fatty acids, urea cycle, fatty acid, synthesis., 3. Radioisotopes are conveniently used in, the study of metabolic pools (e.g. amino acid, pool) and metabolic turnovers (e.g. protein, turnover)., 4. Certain endocrine and immunological, studies also depend on the use of radioisotopes, e.g. radioimmunoassay., 5. Radioisotopes are employed in elucidating, drug metabolism., , Radioisotopes in, diagnosis and treatment, Certain radioisotopes are used in the scanning, of organs—thyroid gland (131I), bone (90Sr) and, kidney (131I hippuran)., Radioactivity has been employed in the, treatment of cancers. This is based on the, principle that radiations produce ionizations, which damage nucleic acids. Thus, the, uncontrolled proliferation of cells is restricted.
Page 729 :
Section 7, , Basics to Learn Biochemistry, , Chapter, , Tools of Biochemistry, , 41, , The chromatography speaks :, , “A technique I am, separating mixture of compounds;, To isolate, identify, characterize molecules, as desired;, Working on the principles of adsorption, partition, ion-exchange;, I am a key biochemical tool in laboratory experimentation.”, , B, , iochemistry is an experimental rather than a, theoretical science. The understanding and, development of concepts in biochemistry are a, result of continuous experimentation and, evidence obtained therein. It is no exaggeration, to state that the foundations for the present (and, the future, of course!) knowledge of biochemistry, are based on the laboratory tools employed, for biochemical experimentation. Thus, the, development of sensitive and sophisticated, analytical, techniques, has, tremendously, contributed to our understanding of biochemistry., A detailed discussion on the tools of, biochemistry is beyond the scope of this book., The basic principles of some of the commonly, employed tools are described in this chapter. The, reader must, however, refer Chapter 27, for the, following techniques related to molecular, biology,and recombinant DNA technology, l, , Isolation and purification of nucleic acids, , l, , Nucleic acid blotting techniques, , l, , DNA sequencing, , l, , Polymerase chain reaction, , l, , Methods of DNA assay, , l, , DNA fingerprinting or DNA profiling., , CHROMATOGRAPHY, Chromatography is one of the most useful and, popular tools of biochemistry. It is an analytical, technique dealing with the separation of closely, related compounds from a mixture. These, include proteins, peptides, amino acids, lipids,, carbohydrates, vitamins and drugs., , Historical perspective, The, credit, for, the, discovery, of, chromatography goes to the Russian botanist, Mikhail Tswett. It was in 1906, Tswett described, the separation of plant leaf pigments in solution, by passing through a column of solid adsorbents., He coined the term chromatography (Greek :, chroma—colour; graphein—to write), since the, technique dealt with the separation of colour, compounds (pigments). Coincidently, the term, Tswett means colour in Russian! Truly speaking,, , 719
Page 730 :
720, , BIOCHEMISTRY, , chromatography is a misnomer,, since it is no longer limited to, the separation of coloured, compounds., , Principles and, classification, , Chromatography, , Partition, , Adsorption, , Column, , TLC, , Ion-exchange, , Gel filtration, , Affinity, , HPLC, , Chromatography, usually, consists of a mobile phase and, Paper chromatography, a stationary phase. The mobile, phase refers to the mixture of, Single dimensional Two dimensional, substances (to be separated),, dissoved in a liquid or a gas., Ascending, The stationary phase is a, Descending, porous solid matrix through, Thin layer chromatography, which the sample contained in, the mobile phase percolates., Gas-liquid chromatography, The interaction between the, Fig. 41.1 : Important types of chromatography, mobile and stationary phases, (HPLC—High performance liquid chromatography;, results in the separation of the, TLC—Thin layer chromatography)., compounds from the mixture., These interactions include the, physicochemical principles such as adsorption,, (spotted) at one end, usually ~2 cm, partition, ion-exchange, molecular sieving and, above, a strip of filter paper (Whatman, affinity., No. 1 or 3). The paper is dried and, dipped into a solvent mixture, The interaction between stationary phase and, consisting of butanol, acetic acid and, mobile phase is often employed in the, water in 4 : 1 : 5 ratio (for the sepaclassification chromatography e.g. partition,, ration of amino acids). The aqueous, adsorption, ion-exchange. Further, the classicomponent of the solvent system binds, fication of chromatography is also based either, to the paper and forms a stationary, on the nature of the stationary phase (paper, thin, phase. The organic component that, layer, column), or on the nature of both mobile, migrates on the paper is the mobile, and stationary phases (gas-liquid chromatophase. When the migration of, graphy). A summary of the different methods, the solvent is upwards, it is referred, (classes) of chromatography is given in, to as ascending chromatography. In, Fig.41.1., descending, chromatography,, the, solvent moves downwards (Fig.41.2)., 1. Partition chromatography : The molecules, As the solvent flows, it takes along with, of a mixture get partitioned between the stationary, it the unknown substances. The rate of, phase and mobile phase depending on their, migration of the molecules depends on, relative affinity to each one of the phases., the relative solubilities in the stationary, (a) Paper, chromatography, :, This, phase (aqueous) and mobile phase, technique is commonly used for the, (organic)., separation of amino acids, sugars,, sugar derivatives and peptides. In paper, After a sufficient migration of the, chromatography, a few drops of, solvent front, the paper (chromatogram), solution containing a mixture of the, is removed, dried and developed for, compounds to be separated is applied, the identification of the specific spots.
Page 731 :
721, , Chapter 41 : TOOLS OF BIOCHEMISTRY, , Mobile phase, , Paper strip, , Mobile phase, Ascending, , Descending, , Fig. 41.2 : Paper chromatography—ascending and descending types., , Ninhydrin, which forms purple, complex with D-amino acids, is, frequently used as a colouring reagent., The chemical nature of the individual, spots can be identified by running, known standards with the unknown, mixture., The migration of a substance is, frequently expressed as Rf value (ratio, of fronts), Distance travelled by the substance, , Rf =————————————————, Distance travelled by solvent front, , The Rf value of each substance,, characteristic of a given solvent system, and paper, often helps for the, identification of unknown., Sometimes, it is rather difficult to, separate a complex mixture of, substances by a single run with one, solvent system. In such a case, a, second run is carried out by a different, solvent system, in a direction, perpendicular to the first run. This is, referred to as two dimensional, chromatography which enhances the, separation of a mixture into the, individual components., (b) Thin layer chromatography (TLC) : The, principle of TLC is the same as, described for paper chromatography, (partition). In place of a paper, an inert, , substance, such as cellulose, is, employed as supporting material., Cellulose is spread as a thin layer on, glass or plastic plates. The chromatographic separation is comparatively, rapid in TLC., In case of adsorption thin layer, chromatography, adsorbents such as, activated silica gel, alumina, kieselguhr, are used., (c) Gas-liquid chromatography (GLC) :, This is the method of choice for the, separation of volatile substances or the, volatile derivatives of certain nonvolatile substances. In GLC, the, stationary phase is an inert solid, material (diatomaceous earth or, powdered firebrick), impregnated with, a non-volatile liquid (silicon or, polyethylene glycol). This is packed in, a narrow column and maintained at, high temperature (around 200°C). A, mixture of volatile material is injected, into the column along with the mobile, phase, which is an inert gas (argon,, helium or nitrogen). The separation of, the volatile mixture is based on the, partition of the components between, the mobile phase (gas) and stationary, phase (liquid), hence the name gasliquid chromatography. The separated, compounds can be identified and
Page 732 :
722, , BIOCHEMISTRY, , Inert gas, , Sample, , Detector, , Column, , Oven, , Recorder, , Amplifier, , Fig. 41.3 : Diagrammatic representation of gas-liquid chromatography (GLC)., , quantitated by a detector (Fig.41.3)., The detector works on the principles of, ionization or thermal conductivity., Gas-liquid chromatography is sensitive,, rapid and reliable. It is frequently used, for the quantitative estimation of, biological materials such as lipids,, drugs and vitamins., 2. Adsorption column chromatography : The, adsorbents such as silica gel, alumina, charcoal, , powder and calcium hydroxyapatite are packed, into a column in a glass tube. This serves as the, stationary phase. The sample mixture in a solvent, is loaded on this column. The individual, components get differentially adsorbed on to the, adsorbent. The elution is carried out by a buffer, system (mobile phase). The individual, compounds come out of the column at different, rates which may be separately collected and, identified (Fig.41.4). For instance, amino acids, can be identified by ninhydrin calorimetric, , Buffer, Mobile, phase (buffer), , Mixture, , Stationary phase, (column), , Elution, Fig. 41.4 : Diagrammatic representation of adsorption column chromatography.
Page 733 :
723, , Chapter 41 : TOOLS OF BIOCHEMISTRY, , Pump, , Sample, , Ion exchange, column, Recorder, , Amino acid, separation, Buffer, Colour, development, , Colorimeter, , Ninhydrin, , Fig. 41.5 : Diagrammatic representation of amino acid analyser., , method. An automated column chromatography, apparatus—fraction collector—is frequently, used nowadays., 3. Ion-exchange chromatography : Ionexchange, chromatography, involves, the, separation of molecules on the basis of their, electrical charges. Ion-exchange resins—cation, exchangers and anion exchangers—are used for, this purpose. An anion exchanger (R+A–), exchanges its anion (A–) with another anion, (B–) in solution., R+A– + B–, , R+B– + A–, , Similarly, a cation exchanger (H+R–), exchanges its cation (H+) with another cation, (C+) in solution., H+R– + C+, , C+R– + H+, , Thus, in ion-exchange chromatography, ions, in solution are reversibly replaced by ionexchange resins. The binding abilities of ions, bearing positive or negative charges are highly, pH dependent, since the net charge varies with, , pH. This principle is exploited in the separation, of molecules in ion-exchange chromatography., A mixture of amino acids (protein hydrolysate), or proteins can be conveniently separated by, ion-exchange chromatography. The amino acid, mixture (at pH around 3.0) is passed through a, cation exchange and the individual amino acids, can be eluted by using buffers of different pH., The various fractions eluted, containing, individual amino acids, are allowed to react with, ninhydrin reagent to form coloured complex., This is continuously monitored for qualitative, and quantitative identification of amino acids., The amino acid analyser, first developed by, Moore and Stein, is based on this principle, (Fig.41.5)., Several types of ion exchangers are, commercially available. These include polystyrene resins (anion exchange resin, Dowex 1;, cation exchange resin, Dowex 50), DEAE (diethyl, aminoethyl) cellulose, CM (carboxy methyl), cellulose, DEAE-sephadex and CM-sephadex.
Page 734 :
724, , BIOCHEMISTRY, , Porous, beads, , Small, molecule, , Large, molecule, , Fig. 41.6 : The principle of gel-filtration chromatography., , 4. Gel filtration chromatography : In gel, filtration chromatography, the separation of, molecules is based on their size, shape and, molecular weight. This technique is also referred, to as molecular sieve or molecular exclusion, chromatography. The apparatus consists of a, column packed with spongelike gel beads, (usually cross-linked polysaccharides) containing, pores. The gels serve as molecular sieves for the, separation of smaller and bigger molecules, (Fig.41.6)., The solution mixture containing molecules of, different sizes (say proteins) is applied to column, and eluted with a buffer. The larger molecules, cannot pass through the pores of gel and,, therefore, move faster. On the other hand, the, smaller molecules enter the gel beads and are, left behind which come out slowly. By selecting, the gel beads of different porosity, the molecules, can be separated. The commercially available, gels include Sephadex (G-10, G-25, G-100), Biogel (P-10, P-30, P-100) and sepharose (6B, 4B,, 2B)., , molecular fishhooks to selectively pick up the, desired protein while the remaining proteins pass, through the column. The desired protein,, captured by the ligand, can be eluted by using, free ligand molecules. Alternately, some reagents, that can break protein-ligand interaction can also, be employed for the separation., Affinity chromatography is useful for the, purification of enzymes, vitamins, nucleic acids,, drugs, hormone receptors, antibodies etc., 6. High performance liquid chromatography, (HPLC) : In general, the chromatographic, techniques are slow and time consuming. The, separation can be greatly improved by applying, high pressure in the range of 5,000-10,000 psi, (pounds per square inch), hence this technique, is also referred (less frequently) to as high, pressure liquid chromatography. HPLC requires, the use of non-compressible resin materials and, strong metal columns. The eluants of the column, are detected by methods such as UV absorption, and fluorescence., , ELECTROPHORESIS, The movement of charged particles (ions) in, an electric field resulting in their migration, towards the oppositely charged electrode is, , Paper, , The gel-filtration chromatography can be used, for an approximate determination of molecular, weights. This is done by using a calibrated, column with substances of known molecular, weight., 5. Affinity chromatography : The principle of, affinity chromatography is based on the property, of specific and non-covalent binding of proteins, to other molecules, referred to as ligands. For, instance, enzymes bind specifically to ligands, such as substrates or cofactors., The technique involves the use of ligands, covalently attached to an inert and porous matrix, in a column. The immobilized ligands act as, , Buffer, , Negative, ions, , Point of, application, , Positive, ions, , Fig. 41.7 : Diagrammatic representation, of paper electrophoresis.
Page 735 :
Chapter 41 : TOOLS OF BIOCHEMISTRY, , known as electrophoresis. Molecules with a net, positive charge (cations) move towards the, negative cathode while those with net negative, charge (anions) migrate towards positive anode., Electrophoresis is a widely used analytical, technique for the separation of biological, molecules such as plasma proteins, lipoproteins, and immunoglobulins., The rate of migration of ions in an electric, field depends on several factors that include, shape, size, net charge and solvation of the ions,, viscosity of the solution and magnitude of the, current employed., , Different types of electrophoresis, Among the electrophoretic techniques, zone, electrophoresis (paper, gel), isoelectric focussing, and immunoelectrophoresis are important and, commonly employed in the laboratory. The, original moving boundary electrophoresis,, developed by Tiselius (1933), is less frequently, used these days. In this technique, the U-tube is, filled with protein solution overlaid by a buffer, solution. As the proteins move in solution during, electrophoresis, they form boundaries which can, be identified by refractive index., 1. Zone electrophoresis : A simple and modified method of moving boundary electrophoresis, is the zone electrophoresis. An inert supporting, material such as paper or gel are used., (a) Paper electrophoresis : In this, technique, the sample is applied on a, strip of filter paper wetted with desired, buffer solution. The ends of the strip, are dipped into the buffer reservoirs in, which the electrodes are placed. The, electric current is applied allowing the, molecules to migrate for sufficient time., The paper is removed, dried and, stained with a dye that specifically, colours the substances to be detected., The coloured spots can be identified, by comparing with a set of standards, run simultaneously., For the separation of serum proteins,, Whatman No. 1 filter paper, veronal or, tris buffer at pH 8.6 and the stains, , 725, amido black or bromophenol blue, are employed. The serum proteins, are separated into five distinct, bands—albumin, D1-, D2-, E- and, J-globulins (Refer Fig.9.1). For the, electrophoretic pattern of serum, lipoproteins, refer Fig.14.34., (b) Gel electrophoresis : This technique, involves the separation of molecules, based on their size, in addition to the, electrical charge. The movement of, large molecules is slow in gel, electrophoresis (this is in contrast to gel, filtration). The resolution is much, higher in this technique. Thus, serum, proteins can be separated to about 15, bands, instead of 5 bands on paper, electrophoresis., The gels commonly used in gel electrophoresis are agarose and polyacrylamide, sodium dodecyl sulfate, (SDS). Polyacrylamide is employed for, the determination of molecular weights, of proteins in a popularly known, electrophoresis technique known as, SDS-PAGE., 2. Isoelectric focussing : This technique is, primarily based on the immobilization of the, molecules, at, isoelectric, pH, during, electrophoresis. Stable pH gradients are set up, (usually in a gel) covering the pH range to, include the isoelectric points of the components, in a mixture. As the electrophoresis occurs, the, molecules (say proteins) migrate to positions, corresponding to their isoelectric points, get, immobilized and form sharp stationary bonds., The gel blocks can be stained and identified. By, isoelectric focussing, serum proteins can be, separated to as many as 40 bands. Isoelectric, focussing can be conveniently used for the, purification of proteins., 3. Immunoelectrophoresis : This technique, involves combination of the principles of, electrophoresis and immunological reactions., Immunoelectrophoresis is useful for the, analysis of complex mixtures of antigens and, antibodies.
Page 736 :
726, , BIOCHEMISTRY, , Trough with, antibodies, , Electrophoretically, separated proteins, , Precipitin, arc, , Fig. 41.8 : Diagrammatic representation of immunoelectrophoresis., , The complex proteins of biological samples, (say human serum) are subjected to, electrophoresis. The antibody (antihuman, immune serum from rabbit or horse) is then, applied in a trough parallel to the electrophoretic, separation. The antibodies diffuse and, when, they come in contact with antigens, precipitation, occurs, resulting in the formation of precipitin, bands which can be identified (Fig.41.8)., , depends on the concentration of the absorbing, molecules). And according to Lambert’s law, the, transmitted light decreases exponentially with, increase in the thickness of the absorbing, molecules (i.e. the amount of light absorbed is, dependent on the thickness of the medium)., By combining the two laws (Beer-Lambert, law), the following mathematical derivation can, be obtained, I = I0Hct, , PHOTOMETRY—COLORIMETER, AND SPECTROPHOTOMETER, Photometry broadly deals with the study of, the phenomenon of light absorption by, molecules in solution. The specificity of a, compound to absorb light at a particular, wavelength (monochromatic light) is exploited, in the laboratory for quantitative measurements., From the biochemist’s perspective, photometry, forms an important laboratory tool for accurate, estimation of a wide variety of compounds in, biological samples. Colorimeter and spectrophotometer are the laboratory instruments used, for this purpose. They work on the principles, discussed below., When a light at a particular wavelength is, passed through a solution (incident light), some, amount of it is absorbed and, therefore, the light, that comes out (transmitted light) is diminished., The nature of light absorption in a solution is, governed by Beer-Lambert law., Beer’s law states that the amount of, transmitted light decreases exponentially with an, increase in the concentration of absorbing, material (i.e. the amount of light absorbed, , where I = Intensity of the transmitted light, I0 = Intensity of the incident light, H = Molar, extinction, coefficient, (characteristic of the substance being, investigated), c = Concentration of the absorbing, substance (moles/l or g/dl), t = Thickness of medium, which light passes., , through, , When the thickness of the absorbing medium, is kept constant (i.e. Lambert’s law), the intensity, of the transmitted light depends only on, concentration of the absorbing material. In other, words, the Beer’s law is operative., The ratio of transmitted light (I) to that of, incident light (I0) is referred to as transmittance, (T)., I, , T = —, I0, , Absorbance (A) or optical density (OD) is, very commonly used in laboratories. The relation, between absorbance and transmittance is, expressed by the following equation., A = 2 – log10 T = 2 – log% T
Page 737 :
727, , Chapter 41 : TOOLS OF BIOCHEMISTRY, , Light, source, , Filter, , Cuvette, , Sample, holder, , Detector, , Display, , Fig. 41.9 : Diagrammatic representation of the components in a colorimeter., , Colorimeter, Colorimeter (or photoelectric colorimeter) is, the instrument used for the measurement, of coloured substances. This instrument is, operative in the visible range (400-800 nm), of the electromagnetic spectrum of light., The working of colorimeter is based on, the principle of Beer-Lambert law (discussed, above)., The colorimeter, in general consists of, light source, filter sample holder and detector, with display (meter or digital). A filament, lamp usually serves as a light source.The, filters allow the passage of a small range, of wave length as incident light. The, sample holder is a special glass cuvette, with a fixed thickness. The photoelectric, selenium cells are the most common detectors, used in colorimeter. The diagrammatic, representation of a colorimeter is depicted in, Fig.41.9., , Spectrophotometer, The spectrophotometer primarily differs from, colorimeter by covering the ultraviolet region, (200-400 nm) of the electromagnetic spectrum., Further, the spectrophotometer is more, sophisticated with several additional devices, that ultimately increase the sensitivity of its, operation severalfold when compared to, a colorimeter. A precisely selected wavelength, (say 234 nm or 610 nm) in both ultraviolet and visible range can be used for, measurements. In place of glass cuvettes (in, colorimeter), quartz cells are used in a, spectrophotometer., The spectrophotometer has similar basic, components as described for a colorimeter, (Fig.41.9), and its operation is also based on the, Beer-Lambert law (already discussed)., , FLUORIMETRY, When certain compounds are subjected to, light of a particular wavelength, some of the, molecules get excited. These molecules, while, they return to ground state, emit light in the form, of fluorescence which is proportional to the, concentration of the compound. This is the, principle in the operation of the instrument, fluorometer., , FLAME PHOTOMETRY, Flame photometry primarily deals with the, quantitative measurement of electrolytes such as, sodium, potassium and lithium. The instrument,, namely flame photometer, works on the, following principle. As a solution in air is finally, sprayed over a burner, it dissociates to give, neutral atoms. Some of these atoms get excited, and move to a higher energy state. When the, excited atoms fall back to the ground state, they, emit light of a characteristic wavelength which, can be measured. The intensity of emission light, is proportional to the concentration of the, electrolyte being estimated., , ULTRACENTRIFUGATION, The ultracentrifuge was developed by a, Swedish biochemist Svedberg (1923). The, principle is based on the generation of, centrifugal force to as high as 600,000 g (earth’s, gravity g = 9.81 m/s2) that allows the, sedimentation of particles or macromolecules., Ultracentrifugation is an indispensable tool for, the isolation of subcellular organelles, proteins, and nucleic acids. In addition, this technique is, also employed in the determination of molecular, weights of macromolecules.
Page 738 :
728, , BIOCHEMISTRY, , Homogenate, , 700 g�u 10 min, , Supernatant I, Nuclear, fraction, 15,000 g�u 15 min, , Supernatant II, , Mitochondrial, fraction, 1,00,000 g�u 60 min, , Supernatant III, , Microsomal, fraction, , Fig. 41.10 : Separation of subcellular, fractions by differential centrifugation., , The rate at which the sedimentation occurs in, ultracentrifugation primarily depends on the size, and shape of the particles or macromolecules, (i.e. on the molecular weight). It is expressed in, terms of sedimentation coefficient(s) and is given, by the formula., v, s = ——, Z2r, , where v = Migration (sedimentation) of the, molecule, Z = Rotation of the centrifuge rotor in, radians/sec, r = Distance in cm from the centre of, rotor, The sedimentation coefficient has the units of, seconds. It was usually expressed in units of 10–, 13s (since several biological macromolecules, occur in this range), which is designated as one, Svedberg unit. For instance, the sedimentation, coefficient of hemoglobin is 4 u 10–13 s or 4S;, ribonuclease is 2 u 10–13 s or 2S. Conventionally,, the subcellular organelles are often referred to by, their S value e.g. 70S ribosome., , Isolation of subcellular, organelles by centrifugation, The cells are subjected to disruption by, sonication or osmotic shock or by use of, homogenizer. This is usually carried out in an, isotonic (0.25 M) sucrose. The advantage with, sucrose medium is that it does not cause the, organelles to swell. The subcellular particles can, be separated by differential centrifugation. The, most commonly employed laboratory method, separates subcellular organelles into 3, major fractions—nuclear, mitochondrial and, microsomal (Fig.41.10)., When the homogenate is centrifuged at 700 g, for about 10 min, the nuclear fraction (includes, plasma membrane) gets sedimented. On, centrifuging the supernatant (I) at 15,000 g for, about 5 min mitochondrial fraction (that includes, lysosomes, peroxisomes) is pelleted. Further, centrifugation of the supernatant (II) at 100,000, g for about 60 min separates microsomal fraction, (that includes ribosomes and endoplasmic, reticulum). The supernatant (III) then obtained, corresponds to the cytosol., The purity (or contamination) of the, subcellular fractionation can be checked by the, use of marker enzymes. DNA polymerase is the, marker enzyme for nucleus, while glutamate, dehydrogenase and glucose 6-phosphatase are, the markers for mitochondria and ribosomes,, respectively. Hexokinase is the marker enzyme, for cytosol.
Page 739 :
729, , Chapter 41 : TOOLS OF BIOCHEMISTRY, , RADIOIMMUNOASSAY, Radioimmunoassay (RIA) was developed in, 1959 by Solomon, Benson and Rosalyn Yalow, for the estimation of insulin in human serum., This technique has revolutionized the estimation, of several compounds in biological fluids that, are found in exceedingly low concentrations, (nanogram or picogram). RIA is a highly sensitive, and specific analytical tool., , Principle, Radioimmunoassay combines the principles, of radioactivity of isotopes and immunological, reactions of antigen and antibody, hence the, name., The principle of RIA is primarily based on the, competition between the labelled and unlabelled, antigens to bind with antibody to form antigenantibody complexes (either labelled or, unlabelled). The unlabelled antigen is the, substance (say insulin) to be determined. The, antibody to it is produced by injecting the, antigen to a goat or a rabbit. The specific, antibody (Ab) is then subjected to react with, unlabelled antigen in the presence of excess, amounts of isotopically labelled (131I) antigen, (Ag+) with known radioactivity. There occurs a, competition between the antigens (Ag+ and Ag), to bind the antibody. Certainly, the labelled Ag+, will have an upper hand due to its excess, presence., Ag+ + Ab, , Ag + Ab, Ag, Ag, , +, , antigen and the same quantities of antibody and, labelled antigen., The labelled antigen-antibody (Ag+-Ab), complex is separated by precipitation. The, radioactivity of 131I present is Ag+-Ab is, determined., , Applications, RIA is no more limited to estimating of, hormones and proteins that exhibit antigenic, properties. By the use of haptens (small, molecules such as dinitrophenol, which, by, themselves, are not antigenic), several substances, can be made antigenic to elicit specific antibody, responses. In this way, a wide variety of, compounds have been brought under the net of, RIA estimation. These include peptides, steroid, hormones, vitamins, drugs, antibiotics, nucleic, acids, structural proteins and hormone receptor, proteins., Radioimmunoassay, has, tremendous, application in the diagnosis of hormonal, disorders, cancers and therapeutic monitoring of, drugs, besides being useful in biomedical, research., , ENZYME-LINKED, IMMUNOSORBANT ASSAY, Enzyme-linked immunosorbant assay (ELISA), is a non-isotopic immunoassay. An enzyme is, used as a label in ELISA in place of radioactive, isotope employed in RIA. ELISA is as sensitive, as or even more sensitive than RIA. In addition,, there is no risk of radiation hazards (as is the, case with RIA) in ELISA., , Ag-Ab, , Principle, As the concentration of unlabelled antigen, (Ag) increases the amount of labelled antigenantibody complex (Ag+-Ab) decreases. Thus, the, concentration of Ag+-Ab is inversely related to, the concentration of unlabelled Ag i.e. the, substance to be determined. This relation is, almost linear. A standard curve can be drawn by, using different concentrations of unlabelled, , ELISA is based on the immunochemical, principles of antigen-antibody reaction. The, stages of ELISA, depicted in Fig.41.11, are, summarized., 1. The antibody against the protein to be, determined is fixed on an inert solid such as, polystyrene.
Page 740 :
730, , BIOCHEMISTRY, , 2. The biological sample containing the, protein to be estimated is applied on the, antibody coated surface., , First, antibody, Protein, , 3. The protein antibody complex is, then reacted with a second protein specific, antibody to which an enzyme is covalently, linked. These enzymes must be easily, assayable and produce preferably coloured, products. Peroxidase, amylase and alkaline, phosphatase are commonly used., , Enzyme, , 4. After washing the unbound antibody, linked enzyme, the enzyme bound to the, second antibody complex is assayed., , Second, antibody, , 5. The enzyme activity is determined, by its action on a substrate to form a, product (usually coloured). This is related, to the concentration of the protein being, estimated., The principle for the use of the enzyme, peroxidase in ELISA is illustrated next., , Substrate, , Product, , Peroxidase, H2O2, (substrate), , H2O + O, (nascent oxygen), , Diaminobenzidine, (colourless), , Oxidized, diaminobenzidine, (brown), , Applications, ELISA is widely used for the determination of, small quantities of proteins (hormones, antigens,, antibodies) and other biological substances. The, most commonly used pregnancy test for the, detection of human chorionic gonadotropin, (hCG) in urine is based on ELISA. By this test,, pregnancy can be detected within few days after, conception. ELISA is also been used for the, diagnosis of AIDS., , Fig. 41.11 : Diagrammatic representation of enzyme-linked, immunosorbant assay (ELISA)., , have the desired properties but are found with, many other antibodies which undoubtedly are, not required. A simple, convenient and desirable, method for the large scale production of specific, antibodies remained a dream for immunologists, for a long period. In 1975, George Kohler and, Cesar Milstein (Nobel Prize 1984) made this, dream a reality. They created hybrid cells that, will make unlimited quantities of antibodies with, defined specificities, which are termed as, monoclonal antibodies (McAb). This discovery,, often referred to as hybridoma technology, has, revolutionized methods for antibody production., , Principle, HYBRIDOMA TECHNOLOGY, Conventional methods adopted in the, laboratory for the production of antisera against, antigens lead to the formation of heterogeneous, antibodies. Among these antibodies a few may, , This is based on the fusion between myeloma, cells (malignant plasma cells) and spleen cells, from a suitably immunized animal. Spleen cells, die in a short period under ordinary tissue culture, conditions while myeloma cells are adopted, to grow permanently in culture. Mutants of
Page 741 :
731, , Chapter 41 : TOOLS OF BIOCHEMISTRY, , Spinner culture, , Immunized animal, , Spleen, cells, , Myeloma line, Fusion, Selection of, hybrids in, HAT medium, Assay antibody, , Freeze, , Positive ‘Pots’, , ., . ..... ., .................., ., .. . .., , ., . ..... ., .................., ., .. . .., , ., . ..... ., .................., ., .. . .., , Cloning, , myeloma cells lack the enzyme hypoxanthine, guanine phosphoribosyltransferase (azaquinine, resistant) or thymidine kinase (bromodeoxyuridine resistant). These mutants cannot grow in, a medium containing aminopterin, supplemented, with hypoxanthine and thymidine (HAT, medium). Hybrids between the mutant myeloma, cells and spleen cells can be selected and, cultured in HAT medium., From the growing hybrids, individual, clones can be chosen that secrete the desired, antibodies (monoclonal origin). The selected, clones like ordinary myeloma cells can, be maintained indefinitely. In short, the, hybridoma technology for the production of, monoclonal antibodies involves the following, steps., 1. Immunization of appropriate animals with, antigen (need not be pure) under study., , Assay antibody, Freeze, , Positive clones, , Recloning, Characterize, clones Select, variants, Freeze, , Propagation of, selected clones, , Tumours of, cells producing, antibody, , 2. Fusion of suitable drug resistant myeloma, cells with plasma cells, obtained from the spleen, of the immunized animal., 3. Selection and cloning of the hybrid cells, that grow in culture and produce antibody, molecules of desired class and specificity against, the antigen of interest., Hybridoma technology can make available, highly specific antibodies in abundant amounts., The clones once developed are far cheaper, than the traditionally employed animals, (horses, rabbits) for producing antibodies., The clones developed from the hybrids will, also ensure constancy of the quality of the, product and will also avoid the batch to, batch variation inherent in the conventional, methods., , Applications of monoclonal, antibodies, , ~10 Pg/ml, Specific, antibody, Serum/Ascites, 5-20 mg/ml, specific antibody, , Fig. 41.12 : Basic protocol for the derivation of, monoclonal antibodies from hybrid myelomas., , The antibodies produced by hybridoma, technology have been widely used for a variety of, purposes. These include the early detection of, pregnancy, detection and treatment of cancer,, diagnosis of leprosy and treatment of, autoimmune diseases.
Page 742 :
Section 7, , Basics to Learn Biochemistry, , Chapter, , Immunology, , 42, , The immunology speaks :, , “I represent the defense system of the body;, Mainly composed of E-lymphocytes and T-lymphocytes;, Designed to eliminate invading microbes and moles;, My memory can distinguish self, and non-self.”, , I, , mmunology deals with the study of immunity, and immune systems of vertebrates. Immunity, (immunis literally means exempt/free from, burden) broadly involves the resistance, shown, and protection offered by the host, organism against the infectious diseases. The, immune system consists of a complex network of, cells and molecules, and their interactions., It is specifically designed to eliminate, infectious organisms from the body. This is, possible since the organism is capable of, distinguishing the self from non-self, and, eliminate non-self., Immunity is broadly divided into two types —, innate (non-specific) immunity and adaptive or, acquired (specific) immunity., , INNATE IMMUNITY, Innate immunity is non-specific, and, represents the inherent capability of the organism, to offer resistance against diseases. It consists of, defensive barriers., , First line of defense, The skin is the largest organ in the human, body, constituting about 15% of the adult body, weight. The skin provides mechanical barrier to, prevent the entry of microorganisms and viruses., The acidic (pH 3-5) environment on the skin, surface inhibits the growth of certain, microorganisms. Further, the sweat contains an, enzyme lysozyme that can destroy bacterial cell, wall., , Second line of defense, Despite the physical barriers, the microorganisms do enter the body. The body defends, itself and eliminates the invading organisms by, non-specific mechanisms such as sneezing and, secretions of the mucus. In addition, the body, also tries to kill the pathogens by phagocytosis, (involving macrophages and complement, system). The inflammatory response and fever, response of the body also form a part of innate, immunity., , 732
Page 743 :
733, , Chapter 42 : IMMUNOLOGY, , THE IMMUNE SYSTEM, , Adenoids, Tonsils, Lymph nodes, , The immune system represents the third and, most potent defense mechanism of the body., Acquired (adaptive or specific) immunity is, capable of specifically recognizing and, eliminating the invading microorganisms and, foreign molecules (antigens). In contrast to innate, immunity, the acquired immunity displays four, distinct characteristics, l, , Antigen specificity, , l, , Recognition diversity, , l, , Immunological memory, , l, , Discrimination between self and non-self., , The body possess tremendous capability to, specifically identify various antigens (antigen is, a foreign substance, usually a protein or a, carbohydrate that elicits immune response)., Exposure to an antigen leads to the development, of immunological memory. As a result, a second, encounter of the body to the same antigen results, in a heightened state of immune response. The, immune system recognizes and responds to, foreign antigens as it is capable of distinguishing, self and non-self. Autoimmune diseases are, caused due to a failure to discriminate self and, non-self antigens., , ORGANIZATION OF, IMMUNE SYSTEM, The immune system consists of several organs, distributed throughout the body (Fig. 42.1)., These lymphoid organs are categorized as, primary and secondary., , Primary lymphoid organs, These organs provide appropriate microenvironment for the development and maturation, of antigen-sensitive lymphocytes (a type of white, blood cells). The thymus (situated above the, heart) and bone marrow are the central, or primary lymphoid organs. T-lymphocyte, maturation occurs in the thymus while, B-lymphocyte maturation takes place in the bone, marrow., , Thymus, Lymph nodes, Spleen, Peyers patches, Appendix, Lymph nodes, Lymph vessels, , Bone marrow, , Fig. 42.1 : A diagrammatic representation of, human lymphatic system., , Secondary lymphoid organs, These are the sites for the initiation of immune, response. e.g. spleen, tonsils, lymph nodes,, appendix, Peyers patches in the gut. Secondary, lymphoid organs provide the microenvironment for, interaction between antigens and mature, lymphocytes., , CELLS OF THE IMMUNE SYSTEM, Two types of lymphocytes namely B-cells and, T-cells are critical for the immune system. In, addition, several accessory cells and effector, cells also participate., , B-lymphocytes, The site of development and maturation of, B-cells occurs in bursa fabricius in birds, and, bone marrow in mammals. During the course of, immune response. B-cells mature into plasma, cells and secrete antibodies (immunoglobulins)., The B-cells possess the capability to, specifically recognize each antigen and produce, antibodies (i.e. immunoglobulins) against it., B-lymphocytes are intimately associated with, humoral immunity. Immunoglobulins are, described in Chapter 9.
Page 744 :
734, , BIOCHEMISTRY, , T-lymphocytes, The maturation of T-cells occurs in the, thymus, hence the name. The T-cells can identify, viruses and microorganisms from the antigens, displayed on their surfaces. There are at least, four different types of T-cells., l, , l, , l, , l, , Inducer T-cells that mediate the development, of T-cells in the thymus., Cytotoxic T-cells (TC), capable of recognizing, and killing the infected or abnormal cells., Helper T-cells (TH) that initiate immune, responses., Suppressor T-cells mediate the suppression of, immune response., , T-lymphocytes are responsible for the cellmediated immunity., , MAJOR HISTOCOMPATIBILITY, COMPLEX, The major histocompatibility complex (MHC), represents a special group of proteins, present on, the cell surfaces of T-lymphocytes. MHC is, involved in the recognition of antigens on T-cells., It may be noted here that the B-cell receptors, (antibodies) can recognize antigens on their own,, while T-cells can do so through the mediation, of MHC., In humans, the MHC proteins are encoded by, a cluster of genes located on chromosome 6 (it, is on chromosome 17 for mice). The major histocompatibility complex in humans is referred to, as human leukocyte antigen (HLA). Three classes, of MHC molecules (chemically glycoproteins), are known in human. Class I molecules are, found on almost all the nucleated cells of the, body. Class II molecules are associated only with, leukocytes involved in cell-mediated immune, response. Class III molecules are the, secreted proteins possessing immune functions, e.g. complement components (C2, C4), tumor, necrosis factor., , function of antibodies in defending the body, from the invading antigens. The complementary, factors are heat labile and get inactivated if, heated at 56°C for about 30 minutes. The, complement system helps the body immunity in, 4 ways, 1. Complement fixation : The complement, system binds to the foreign invading cells and, causes lysis of the cell membranes., 2. Opsonization : The process of promoting, the phagocytosis of foreign cells is referred to as, opsonization., 3. Inflammatory reaction : The complement, system stimulates local inflammatory reaction, and attracts phagocytic cells., 4. Clearance of antigen-antibody complexes :, The complement system promotes the clearance, of antigen-antibody complexes from the body., Nomenclature of complement system : The, complement proteins are designated by the letter, ‘C’, followed by a component number—C1, C2,, C3 etc., Types of reaction : The complement system, brings about two sets of reactions :, 1. Antibody dependent classical pathway., 2. Antibody independent alternative pathway., Each one of the pathways consists of a series, of reactions converting inactive precursors to, active products by serine proteases which, resembles blood coagulation., , THE IMMUNE RESPONSE, , THE COMPLEMENT SYSTEM, , The immune response refers to the series of, reactions carried out by the immune system in, the body against the foreign invader. When an, infection takes place or when an antigen enters, the body, it is trapped by the macrophages in, lymphoid organs. The phagocytic cells which are, guarding the body by constant patrolling engulf, and digest the foreign substance. However, the, partially digested antigen (i.e. processed antigen), with antigenic epitopes attaches to lymphocytes., , The complement system is composed of about, 20 plasma proteins that ‘complement’ the, , T-helper cells (TH) play a key role the immune, response (Fig. 42.2). This is brought out through
Page 745 :
735, , Chapter 42 : IMMUNOLOGY, , APC, , Class I MHC, Interleukin–1, , Antigen fragment, T-cell receptor, TH cell, , Interleukin-2, Cytokines, Activation, , TC cell, , B-cell, , Cell-mediated, immunity, , Humoral, immunity, , CYTOKINES, Cytokines are a group of proteins that bring, about communication between different cell, types involved in immunity. They are low, molecular weight glycoproteins and are, produced by lymphoid and non-lymphoid, cells during the course of immune response., Cytokines may be regarded as soluble messenger, molecules of immune system. They can act, as short messengers between the cells or, long range messengers by circulating in, the blood and affecting cells at far off sites. The, latter function is comparable to that of, hormones., The term interleukin (IL) is frequently used to, represent cytokines. There are more than a dozen, interleukins (IL-I……IL12), produced by different, cells with wide range of functions. The main, function (directly or indirectly) of cytokines is to, amplify immune responses and inflammatory, responses., , Therapeutic uses of cytokines, , Fig. 42.2 : A diagrammatic representation of the central, role of helper T cells (TH cells) in, immune response (APC—Antigen presenting, cell; TC cell—Cytotoxic T–cell)., , the participation of antigen presenting cell (APC),, usually a macrophage. Receptors of TH cell bind, to class II MHC-antigen complex displayed on, the surface of APC. APC secretes interleukin-I,, which activates the TH cell. This activated TH, cell actively grows and divides to produce clones, of TH cells. All the TH cells possess receptors that, are specific for the MHC-antigen complex. This, facilitates triggering of immune response in an, exponential manner. The TH cells secrete, interleukin-2 which promotes the prolifiration of, cytotoxic T cells (TC cells) to attack the, infected cells through cell-mediated immunity., Further, interleukin-2 also activates B-cells to, produce immunoglobulins which perform, humoral immunity., , It is now possible to produce cytokines in, vitro. Some of the cytokines have potential, applications in the practice of medicine. For, instance, IL-2 is used in cancer immunotherapy,, and in the treatment of immunodeficiency, diseases. IL-2 induces the proliferation and, differentiation of T-and B-cells, besides, increasing the cytotoxic capacity of natural killer, cells., A group of cytokines namely interferons can, combat viral infection by inhibiting their, replication., , IMMUNITY IN HEALTH, AND DISEASE, The prime function of immune system is to, protect the host against the invading pathogens., The body tries its best to overcome various, strategies of infectious agents (bacteria, viruses),, and provides immunity.
Page 746 :
736, , BIOCHEMISTRY, , Some of the important immunological aspects, in human health and disease are briefly, described., , AUTOIMMUNE DISEASES, In general, the immune system is self-tolerant, i.e. not responsive to cells or proteins of self., Sometimes, for various reasons, the immune, system fails to discriminate between self and, non-self. As a consequence, the cells or tissues, of the body are attacked. This phenomenon is, referred to as autoimmunity and the diseases are, regarded as autoimmune diseases. The, antibodies produced to self molecules are, regarded as autoantibodies. Some examples of, autoimmune diseases are listed., l, , l, , l, , l, , Insulin-dependent diabetes (pancreatic E-cell, autoreactive T-cells and antibodies)., Rheumatoid arthritis (antibodies, proteins present in joints)., , against, , Myasthenia gravis (acetylcholine receptor, autoantibodies)., Autoimmune hemolytic anemia (erythrocyte, autoantibodies)., , Mechanism of autoimmunity : It is widely, accepted that autoimmunity generally occurs as, a consequence of body’s response against, bacterial, viral or any foreign antigen. Some of, the epitopes of foreign antigens are similar, (homologous) to epitopes present on certain host, proteins. This results in cross reaction of antigens, and antibodies which may lead to autoimmune, diseases., , ORGAN TRANSPLANTATION, The phenomenon of transfer of cells, tissues, or organs from one site to another (in the same, organism, autograft or from another organism, allograft) is regarded as organ transplantation., , In case of humans, majority of organ, transplantations are allografts (between two, individuals). The term xenograft is used if, tissues/organs are transferred from one species, to another e.g. from pig to man., Organ transplantation is associated with, immunological complications, and tissue, rejection. This is because the host body responds, to the transplanted tissue in a similar way as if it, were an invading foreign organism. Major, histocompatibility complex (MHC) is primarily, involved in allograft rejection. This is due to the, fact that MHC proteins are unique to each, individual, and the immune system responds, promptly to foreign MHCs., Organ transplantation between closely related, family members is preferred, since their MHCs, are also likely to be closely related. And major, immunological complications can be averted., , CANCERS, Growth of tumors is often associated with the, formation of novel antigens. These tumor, antigens (also referred to as oncofetal antigens, e.g. D-fetoprotein) are recognized as non-self by, the immune systems. However, tumors have, developed several mechanisms to evade immune, responses., , AIDS, Acquired, immunodeficiency, syndrome, (AIDS), caused by human immunodeficiency, virus, is characterized by immunosuppression,, secondary, neoplasma, and, neurological, manifestations. AIDS primarily affects the cellmediated immune system which protects the, body from intracellular parasites such as viruses,, and bacteria. Most of the immunodeficiency, symptoms of AIDS are associated with a, reduction in CD4 (cluster determinant antigen 4), cells.
Page 747 :
Section 7, , Basics to Learn Biochemistry, , Chapter, , Genetics, , 43, , , a, , a, , A, , Aa, , Aa, , a, , aa, , aa, , The genetics speaks :, , “I am the science for the study of heredity;, With DNA as the chemical of inheritance;, Transmitting characters from parents to offsprings;, Mutations and chromosomal abnormalities result in diseases.”, , G, , enetics is the study of heredity. It is, appropriately regarded as the science that, explains the similarities and differences among, the related organisms., , The blood theory of, inheritance in humans, For many centuries, it was customary to, explain inheritance in humans through blood, theory. People used to believe that the children, received blood from their parents, and it was the, union of blood that led to the blending of, characteristics. That is how the terms ‘blood, relations’, ‘blood will tell’, and ‘blood is thicker, than water’ came into existence. They are still, used, despite the fact that blood is no more, involved in inheritance. With the advances in, genetics, the more appropriate terms should be, as follows, l, , Gene relations in place of blood relations., , l, , Genes will tell instead of blood will tell., , BRIEF HISTORY AND, DEVELOPMENT OF GENETICS, Genetics is relatively young, not even 150, years. The blood theory of inheritance was, questioned in 1850s, based on the fact that the, semen contained no blood. Thus, blood was not, being transferred to the offspring. Then the big, question was what was the hereditary substance., Mendel’s experiments : It was in 1866, an, Austrian monk named Gregor Johann Mendel,, for the first time reported the fundamental laws, of, inheritance., He, conducted, several, experiments on the breeding patterns of pea, plants. Mendel put forth the theory of, transmissible factors which states that, inheritance is controlled by certain factors, passed from parents to offsprings. His results, were published in 1866 in an obscure journal, Proceedings of the Society of Natural Sciences., For about 35 years, the observations made by, Mendel went unnoticed, and were almost, , 737
Page 748 :
738, forgotten. Two European botanists (Correns and, Hugo de Vries) in 1900, independently and, simultaneously rediscovered the theories of, Mendel. The year 1900 is important as it marks, the beginning the modern era of genetics., The origin of the word gene : In the early, years of twentieth century, it was believed that, the Mendel’s inheritance factors are very closely, related to chromosomes (literally coloured, bodies) of the cells. It was in 1920s, the term, gene (derived from a Greek word gennan, meaning to produce) was introduced by Willard, Johannsen. Thus, gene replaced the earlier terms, inheritance factor or inheritance unit., Chemical basis of heredity : There was a, controversy for quite sometime on the chemical, basis of inheritance. There were two groups—, the protein supporters and DNA supporters. It, was in 1944, Avery and his associates presented, convincing evidence that the chemical basis of, heredity lies in DNA, and not in protein. Thus,, DNA was finally identified as the genetic, material. Its structure was elucidated in 1952 by, Watson and Crick., , Importance of genes in, inheritance—studies on twins, Monozygotic or identical twins contain the, same genetic material — DNA or genes. Studies, conducted on identical twins make startling, revelations with regard to inheritance. One such, study is described here., Oskar Stohr and Jack Yufe were identical, twins separated at birth. Oskar was taken to, Germany where he was brought up by his, grandmother as a Christian. Jack was raised by, his father in Israel as a Jew. The two brothers, were reunited at the age of 47. Despite the, different, environmental, influences,, their, behavioural patterns and personalities were, remarkably similar, l, , l, , l, , Both men had moustaches, wore two pocket, shirts, and wire-rimmed glasses., Both loved spicy foods and tended to fall, asleep in front of television., Both flushed the toilet before using., , BIOCHEMISTRY, , l, , Both read maganizes from back to front., , l, , Both stored rubber bands on their wrists., , l, , Both liked to sneeze in a room of strangers., , Besides Oskar and Jack, many other studies, conducted on identical twins point out the, importance of genes on the inherited characters, related to personality and mannerisms., , BASIC PRINCIPLES OF, HEREDITY IN HUMANS, The, understanding, of, how, genetic, characteristics are passed on from one, generation to the next is based on the principles, developed by Mendel., As we know now, the human genome is, organized into a diploid (2n) set of 46, chromosomes. They exist as 22 pairs of, autosomes and one pair of sex chromosomes, (XX/XY). During the course of meiosis, the, chromosome number becomes haploid (n). Thus,, haploid male and female gametes — sperm and, oocyte respectively, are formed. On fertilization, of the oocyte by the sperm, the diploid status is, restored. This becomes possible as the zygote, receives one member of each chromosome pair, from the father, and the other from the mother., As regards the sex chromosomes, the males have, X and Y, while the females have XX. The sex of, the child is determined by the father., , Monogenic and polygenic traits, The genetic traits or characters are controlled, by single genes or multiple genes. The changes, in genes are associated with genetic diseases., Monogenic disorders : These are the single, gene disease traits due to alterations in the, corresponding gene e.g. sickle-cell anemia,, phenylketonuria. Inheritance of monogenic, disorders usually follows the Mendelian pattern, of inheritance., Polygenic disorders : The genetic traits, conferred by more than on gene (i.e multiple, genes), and the disorders associated with them, are very important e.g. height, weight, skin, colours, academic performance, blood pressure,, aggressiveness, length of life.
Page 749 :
739, , Chapter 43 : GENETICS, , (A) Autosomal dominant, , , , PARENTS, , Male ( ), Genotype-Aa, Phenotype-Affected male, , CHILDREN, , a, , a, , A, , Aa, , Aa, , a, , aa, , aa, , Genotype ratio-1 : 1 Aa to aa, Phenotype-50% affected, -50% normal, , Female (), Genotype-aa, Phenotype-Normal female, (B) Autosomal recessive, PARENTS, , CHILDREN, , , , Male ( ), Genotype-Bb, Phenotype-Carrier male, , B, , b, , B, , BB, , Bb, , b, , Bb, , bb, , Female (), Genotype-Bb, Phenotype-Carrier female, , Genotype ratio-1 : 2 : 1 BB/Bb/Bb/bb, Phenotype-25% affected, -25% normal, -50% carriers, , (C) X-chromosome (sex chromosome)–linked inheritance, PARENTS, , CHILDREN, , , , Male ( ), Genotype - XY, Phenotype-Normal male, , C, , C, , X, , X, , X, , XX, , XX, , Y, , XY, , XY, , Female (), , C, , Genotype ratio-1 : 1 : 1 : 1 XX/XY/X X/X Y, Phenotype-50% of males affected, , C, , C, , Genotype-X X, Phenotype-Carrier female, , C, , Fig. 43.1 : Patterns of inheritance-autosomal dominant, autosomal recessive and, X-linked (Note : Genotype refers to the description of genetic composition, while phenotype, represents the observable character displayed by an organism)., , PATTERNS OF INHERITANCE, The heredity is transmitted from parent to, offspring as individual characters controlled by, genes. The genes are linearly distributed on, chromosomes at fixed positions called loci., A gene may have different forms referred to, as alleles. Usually one allele is transferred from, the father, and the other from the mother. The, allele is regarded as dominant if the trait is, exhibited due to its presence. On the other hand,, the allele is said to be recessive if its effect is, masked by a dominant allele. The individuals, are said to be homozygous if both the alleles are, the same. When the alleles are different they are, said to be heterozygous., , The pattern of inheritance of monogenic traits, may occur in the following ways (Fig. 43.1)., 1. Autosomal dominant, 2. Autosomal recessive, 3. Sex-linked., 1. Autosomal dominant inheritance : A, normal allele may be designated as a while an, autosomal dominant disease allele as A, (Fig. 43.1A). The male with Aa genotype is an, affected one while the female with aa is normal., Half of the genes from the affected male will, carry the disease allele. On mating, the male, and female gametes are mixed in different, combinations. The result is that half of the
Page 750 :
740, , BIOCHEMISTRY, , TABLE 43.1 Selected examples of genetic disorders (monogenic traits) in humans, , Inherited pattern/disease, , Estimated incidence, , Salient features, , Autosomal dominant, Familial hypercholesterolemia, Huntington’s disease, Familial retinoblastoma, Breast cancer genes, (BRAC 1 and 2), , 1, 1, 1, 1, , :, :, :, :, , 500, 5000, 12000, 800, , High risk for heart diseases, Nervous disorders, dementia, Tumors of retina, High risk for breast and ovarian cancers, , E-Thalassemia, , 1 : 2500 (in people of, Mediterranean descent), , A blood disorder; the blood appears to be blue, instead of red, , Autosomal recessive, Sickle-cell anemia, Cystic fibrosis, Phenylketonuria, D1-Antitrypsin deficiency, Tay-Sachs disease, Severe combined immunodeficiency, disease (SCID), , 1 : 100 (in Africans), , Severe life threatening anemia; confers, resistance to malaria, 1 : 2500 (in Caucasians), Defective ion transport; severe lung infections, and early death (before they reach 30 years), 1 : 2000, Mental retardation due to brain damage, 1 : 5000, Damage to lungs and liver, 1 : 3000 (in Ashkenazi Jews) Nervous disorder; blindness and paralysis, Rare (only 100 cases, Highly defective immune system; early death, reported worldwide), , Sex-linked, Colour blindness, Hemophilia (A/B), Duchenne muscular dystrophy, , 1 : 50 males, 1 : 10,000 males, 1 : 7000 males, , Unable to distinguish colours, Defective blood clotting, Muscle wastage, , Not known, , Damage to optic nerves, may lead to blindness, , Mitochondrial, Leber hereditary optic neuropathy, , children will be heterozygous (Aa) and have the, disease. Example of autosomal dominant, inherited, diseases, are, familial, hypercholesterolemia, E-thalassemia, breast cancer, genes., 2. Autosomal recessive inheritance : In this, case, the normal allele is designated as B while, the disease-causing one is a (Fig. 43.1B). The, gametes of carrier male and carrier female (both, with genotype Bb) get mixed. For these, heterozygous carrier parents, there is one fourth, chance of having an affected child. Cystic, fibrosis, sickle-cell anemia and phenylketonuria, are some good examples of autosomal recessive, disorders., , 3. Sex (X)-linked inheritance : In the, Fig. 43.1C, sex-linked pattern of inheritance is, depicted. A normal male (XY) and a carrier, female (XcY) will produce children wherein, half, of the male children are affected while no female, child is affected. This is due to the fact that the, male children possess only one X chromosome,, and there is no dominant allele to mark its effects, (as is the case with females). Colour blindness, and hemophilia are good examples of X-linked, diseases., A selected list of genetic disorders, (monogenic traits) due to autosomal and sexlinked inheritance in humans is given in, Table 43.1.
Page 751 :
741, , Chapter 43 : GENETICS, , GENETIC DISEASES IN HUMANS, The pattern of inheritance and monogenic, traits along with some of the associated disorders, are described above (Table 43.1). Besides, gene mutations, chromosomal abnormalities, (aberrations) also result in genetic diseases., Aneuploidy : The presence of abnormal, number of chromosomes within the cells is, referred to as aneuploidy. The most common, aneuploid condition is trisomy in which three, copies of a particular chromosome are present in, a cell instead of the normal two e.g. trisomy-21, causing Down’s syndrome; trisomy-18 that, results in Edward’s syndrome. These are the, examples of autosomal aneuploidy., , Eugenics is a highly controversial subject due, to social, ethical, and political reasons. The, proponents of eugenics argue that people with, desirable and good traits (good blood) should, reproduce while those with undersirable, characters (bad blood) should not. The advocates, of eugenics, however, do not force any policy,, but they try to convince the people to perform, their duty voluntarily. The object of eugenics is, to limit the production of people who are unfit, to live in the society., , Eugenics in Nazi Germany, , EUGENICS, , Germany developed its own eugenic, programme during 1930s. A law on eugenic, sterilization was passed in 1933. In a span of, three years, compulsory sterilization was, done on about 250,000 people, who allegedly, suffered from hereditary disabilities, feeble, mindedness, epilepsy, schizophrenia, blindness,, physical deformaties, and drug or alcohol, addiction., , Eugenics is a science of improving human, race based on genetics. Improving the traits of, plants, and, animals, through, breeding, programmes has been in practice for centuries., , The German Government committed many, atrocities in the name of racial purity. Other, countries, however do not support this kind of, eugenics., , In case of sex-linked aneuploidy, the sex, chromosomes occur as three copies. e.g. phenotypically male causing Klinefelter’s syndrome has, XXY; trisomy-X is phenotypically a female with, XXX.
Page 752 :
“This page intentionally left blank"
Page 753 :
APPENDICES, ı, n, nI, II, n, III, n, IV, n, V, n, VI, n, , Answers to Self-assessment Exercises, , 745, , Abbreviations used in this Book, , 751, , Origins of Important Biochemical Words, , 756, , Common Confusables in Biochemistry, , 759, , Practical Biochemistry—Principles, , 763, , Clinical Biochemistry Laboratory, , 769, , Case studies with Biochemical Correlations, , 772, , Section, , VIIIII, VI
Page 754 :
“This page intentionally left blank"
Page 755 :
Answers to Self-assessment Exercises, , 8. Phosphatidylinositol,, , CHAPTER 2, , 9. Gangliosides,, , Answers to III and IV, 1. Sucrose,, , 10. Cyclopentanoperhydrophenanthrene,, , 2. Glyceraldehyde,, , 11. a,, , 3. Epimers,, , 12. d,, , 4. Anomers,, , 13. d,, , 5. Aglycone,, , 14. c,, , 6. Streptomycin,, , 15. b., , 7. α-1,6-Glycosidic bond,, , CHAPTER 4, , 8. Inulin,, 9. Hyaluronic acid,, 10. N-Acetylneuraminic acid,, , Answers to III and IV, 1. 16%,, , 11. b,, , 2. L-α-Amino acids,, , 12. d,, 13. a,, , 3. Methionine,, , 14. d,, , 4. Zwitterion,, , 15. a., , 5. β-Alanine,, 6. Peptide bonds,, , CHAPTER 3, , 7. Tryptophan,, , Answers to III and IV, , 8. 9,, , 1. Triacylglycerolds,, , 9. 1-Fluro 2,4-dinitrobenzene (FDNB),, , 2. Geometric isomerism (cis-trans isomerism),, , 10. Denaturation,, , 3. Chaulmoogric acid,, , 11. b,, , 4. Triacylglycerols,, , 12. d,, , 5. Stereospecific number,, , 13. b,, , 6. Saponification number,, , 14. d,, , 7. Dipalmitoyl lecithin,, , 15. a., 745
Page 756 :
746, , BIOCHEMISTRY, , CHAPTER 5, Answers to III and IV, , CHAPTER 7, Answers to III and IV, , 1. Gene,, , 1. Acetylation,, , 2. RNA,, , 2. Riboflavin,, , 3. Nucleotides,, , 3. Vitamin E (tocopherol),, , 4. Thymine,, , 4. Pyridoxine (B6),, , 5. 2,, , 5. Avidin,, , 6. Base + sugar + phosphate,, , 6. Pantothenic acid,, , 7. Erwin Chargaff,, , 7. Cobalamin (B12),, , 8. 3 Hydrogen bonds (in place of 2 in A-T),, , 8. Dermatitis, diarrhea and dementia,, , 9. β-Form,, , 9. Vitamin K,, , 10. CCA(5′ to 3′),, , 10. Folic acid,, , 11. d,, , 11. b,, , 12. b,, , 12. d,, , 13. c,, , 13. a,, , 14. d,, , 14. d,, , 15. d., , 15. a., , CHAPTER 6, Answers to III and IV, , CHAPTER 8, Answers to III and IV, , 1. In yeast,, , 1. β-Glycosidic bonds,, , 2. Ligases,, , 2. Raffinose,, , 3. Coenzyme,, , 3. Lactase (β-galactosidase),, , 4. Denaturation,, , 4. Fiber,, , 5. Alcohol dehydrogenase, carbonic anhydrase,, , 5. Parietal (oxyntic) cells,, , 6. Active site,, , 6. Glutathione,, , 7. NADP+,, , 7. Hartnup’s disease,, , 8. E.C. 1.1.1.1,, , 8. Arginine, lysine,, , 9. AMP/ADP,, , 9. Colipase, , 10. Creatine phosphokinase (CPK),, , 10. Mixed micelles,, , 11. c,, , 11. a,, , 12. d,, , 12. d,, , 13. b,, , 13. c,, , 14. d,, , 14. b,, , 15. b., , 15. a.
Page 759 :
749, , ANSWERS TO SELF-ASSESSMENT EXERCISES, , 8. Dihydrotestosterone (DHT),, , 12. a,, , 9. Cholesterol,, , 13. c,, , 10. Cholecystokinin (CCK),, , 14. b,, , 11. a,, , 15. d., , 12. d,, , CHAPTER 22, , 13. b,, , Answers to III and IV, , 14. c,, , 1. Collagen,, , 15. a., , 2. Glycine,, , CHAPTER 20, Answers to III and IV, , 3. β-oxalyl aminoalamine,, 4. Fibrillin,, , 1. Heme,, , 5. Glycosaminoglycans,, , 2. van den Bergh reaction,, , 6. Sarcomere,, , 3. Alanine transaminase,, , 7. Actin,, , 4. Alkaline phosphatase,, , 8. Calcium caseinate,, , 5. Bromosulphthalein (BSP),, , 9. Vitamin C,, , 6. 180 mg/dl,, , 10. Lecithin/Sphingomyelin,, , 7. Inulin,, , 11. c,, , 8. 2ml/min,, , 12. d,, , 9. Ryle’s tube,, , 13. a,, , 10. Pentagastrin,, , 14. c,, , 11. a,, , 15. b., , 12. d,, , CHAPTER 23, , 13. c,, , Answers to III and IV, , 14. b,, , 1. 4.128,, , 15. b., , 2. Thyroid gland,, , CHAPTER 21, Answers to III and IV, 1. Antidiuretic hormone (ADH),, 2., , Na+,, , 3. Fiber,, 4. Carbohydrates,, 5. Chemical score,, 6. 1g/kg body weight/day,, , 3. 285–295 milliosmoles /kg,, , 7. Biological value (BV) of protein,, , 4. Aldosterone,, , 8. Sulfur containing amino acids,, , 5. Carbonic acid (H2CO3),, , 9. Iron,, , 6. Bicarbonate buffer,, , 10. Plasma albumin,, , 7. 20 : 1,, , 11. a,, , 8. Ammonium ion (NH4+),, , 12. d,, , 9. Bicarbonate (HCO3–),, , 13. c,, , 10. Carbonic acid (H2CO3) or CO2,, , 14. d,, , 11. d,, , 15. a.
Page 760 :
750, , BIOCHEMISTRY, , CHAPTER 24, Answers to III and IV, 1. DNA helicase,, , 13. a,, 14. b,, 15. a., , 2. Okazaki pieces,, , CHAPTER 26, , 3. DNA polymerase III,, 4. DNA topoisomerases,, , Answers to III and IV, , 5. Cyclins,, , 1. 30,000–40,000,, , 6. Telomere,, , 2. One cistron-one subunit concept,, , 7. Transposons or transposable elements,, , 3. Protein-DNA complex,, , 8. Mutation,, , 4. a,, , 9. Missense,, 10. Hereditary nonpolyposis colon cancer,, 11. c,, , 5. b,, 6. d., , 12. a,, , CHAPTER 27, , 13. b,, , Answers to III and IV, , 14. a,, , 1. Escherichia coli,, , 15. b., , 2. RNA,, , CHAPTER 25, Answers to III and IV, , 3. Dot-blotting,, 4. Thermus aquaticus,, , 1. Genome,, , 5. Genomic library/DNA library,, , 2. hnRNA,, , 6. Site-directed mutagenesis,, , 3. Introns,, , 7. Humulin,, , 4. Reverse transcriptase,, , 8. Hepatitis B vaccine,, , 5. Wobble hypothesis,, 6. Ribosomes,, 7. rRNA,, 8. Chaperones,, , 9. Mouse,, 10. Sheep (Dolly),, 11. c,, , 9. Prion diseases,, , 12. d,, , 10. Protein targeting,, , 13. d,, , 11. d,, , 14. a,, , 12. c,, , 15. c.
Page 769 :
Appendix III : Common Confusables in Biochemistry, , Acetone; acetate – Acetone is a ketone; acetate is, a carboxylic acid., , heme; bile salts are the sodium and potassium, salts of bile acids (glycocholate, taurocholate), produced by cholesterol., , Acetyl CoA; acyl CoA – Acetyl CoA is a specific, compound containing acetate bound to, coenzyme A; acyl CoA is a general term used, to refer to any fatty acid (acyl group) bound to, coenzyme A., , Biliverdin; bilirubin – Both are bile pigments., Biliverdin is produced from heme in the, reticuloendothelial cells; bilirubin is formed, by reduction of biliverdin., , Albumin; albinism – Albumin is a serum protein;, albinism is a genetic disease in tysosine, metabolism., , Biotin; biocytin – Biotin is a B-complex vitamin;, biocytin refers to the covalently bound biotin, to enzymes (through H-amino group of lysine)., , Amino; imino – Amino group ( NH2) is found in, majority of amino acids; imino group ( NH), is present in a few amino acids like proline, and hydroxyproline., , B-Lymphocytes; T-lymphocytes – B-lymphocytes, produce immunoglobulins (antibodies) and are, involved in humoral immunity; T-lymphocytes, are responsible for cellular immunity., , Anabolism; catabolism – Anabolism refers to the, biosynthetic reactions involving the formation, of complex molecules from simpler ones;, catabolism is concerned with the degradation, of complex molecules to simpler ones with a, concomitant release of energy., , Bisphosphate; diphosphate – Bisphosphate has two, phosphates held separately e.g. 2,3-BPG;, diphosphate has two phosphates linked, together e.g. ADP., , Anomers; epimers – Anomers refer to two, stereoisomers of a sugar that differ in, configuration around a single carbonyl atom;, epimers are two stereoisomers that differ in, configuration around one asymmetric carbon, of a sugar possessing two or more asymmetric, carbon atoms., , Calcitriol; calcitonin – Calcitriol (1,25-DHCC) is, the physiologically active form of vitamin D;, calcitonin is a peptide hormone, synthesized, by thyroid gland., Calorimetry; colorimetry – Calorimetry deals with, the measurement of heat production by, organism; colorimetry is concerned with the, measurement of colour compounds., , Apoenzyme; coenzyme – Apoenzyme is the protein, part of the functional enzyme (holoenzyme);, coenzyme is the non-protein organic part, associated with enzyme activity., , Carboxyl; carbonyl – These two are functional, groups found in organic substances; carboxyl, , Bile pigments; bile salts – Bile pigments (biliverdin,, bilirubin) are the breakdown products of, , Carnitine; creatine; creatinine – Carnitine, transports activated fatty acids (acyl CoA) from, , group, , 759, , COOH; carbonyl, , C .
Page 771 :
761, , Appendix III : COMMON CONFUSABLES IN BIOCHEMISTRY, , Lipoproteins; lipotropic factors – Lipoproteins are, molecular complexes composed of lipids and, proteins; lipotropic factors are the substances, (e.g. choline, betaine), the deficiency of which, causes accumulation of fat in liver., E-Lipoprotein; E-lipotropin – E-Lipoprotein refers, to the low density lipoproteins; E-lipotropin is, a peptide hormone derived from proopiomelanocortin (POMC) peptide., Lyases; ligases – Lyases are the enzymes that, catalyse the addition or removal of water,, ammonia, CO2 etc.; ligases catalyse the, synthetic reactions where two molecules are, joined together., Malate; malonate; mevalonate – Malate is an, intermediate in the citric acid cycle; malonate, is a competitive inhibitor of the enzyme, succinate dehydrogenase; mevalonate is an, intermediate in cholesterol biosynthesis., Melanin; melatonin – Melanin is the pigment of, skin and hair; melatonin is a hormone, synthesized by pineal gland., Maltose; maltase – Maltose is a disaccharide;, maltase is an enzyme that cleaves maltose to, two molecules of glucose., Methyl, methenyl; methylene – All the three are, one-carbon fragments as shown in brackets,, methyl ( CH3); methenyl ( CH ); methylene, ( CH2 )., Molarity; molality – Molarity is defined as the, number of moles of a solute per liter solution;, molality represents the number of moles of a, solute per 1,000 g of solvent., Nicotinic acid; nicotine – Nicotinic acid is a, B-complex vitamin; nicotine is an alkaloid, present in tobacco leaves., Nucleoside; nucleotide – A nucleoside is composed, of a nitrogen base and a sugar; nucleotide, contains one or more phosphate groups bound, to nucleoside., Oncogens; oncogenes – Oncogens are the, chemicals that cause cancer; oncogenes are, the genes causing cancer., Osmolarity; osmolality – Osmolarity represents, osmotic pressure exerted by the number of, moles (milli moles) per liter solution;, osmolality refers to the osmotic pressure, exerted by the number of moles (milli moles), per kg solvent., Oxidase; Oxygenase – Oxidase accepts O2 but, oxygen atoms are not incorporated into, substrate; oxygenase incorporates one or both, oxygen atoms into substrate., , Palmitate; palmitoleate – Both are even chain, (16-carbon) fatty acids. Palmitate is a saturated, fatty acid; palmitoleate is a monounsaturated, fatty acid., Phosphatase; phosphorylase – Phosphatase uses, water to remove phosphoryl group; phosphorylase utilizes Pi to break a bond and produce, a phosphorylated compound., Phosphatidyl ethanolamine; phosphatidal ethanolamine – Both are phospholipids. In, phosphatidyl ethanolamine, the fatty acid is, bound by an ester linkage. The fatty acid is, held by an ether linkage in phosphatidal, ethanolamine., Phytic acid; phytanic acid – Phytic acid is formed, by the addition of six phosphate molecules to, inositol, it is an inhibitor of the intestinal, absorption of calcium and iron; phytanic acid, is an unusal fatty acid derived from phytol, a, constituent of chlorophyll., Prokaryotes; eukaryotes – Prokaryotes are the cells, that lack a well defined nucleus; eukaryotes, possess a well-defined nucleus., Prolamines; protamines – Both are simple proteins., Prolamines are soluble in alcohol; protamines, are basic protein soluble in NH4OH., Pyridine; pyrimidine; pteridine – All the three are, heterocyclic rings containing nitrogen, as, depicted below., N, , N, , N, N, N, Pyridine, , N, Pyrimidine, , N, Pteridine, , Pyridine ring is found in niacin and, pyridoxine; pyrimidine is present in thiamine, (vitamin B1), thymine, cytosine and uracil;, folic acid contains pteridine ring., Pyridoxine; pyridoxal – Pyridoxine is the primary, alcohol form of vitamin B6; pyridoxal is the, aldehyde form of B6., RDA; SDA – RDA (recommended dietary/daily, allowance) represents the quantities of, nutrients to be provided in the diet daily for, maintenance of good health and physical, efficiency; specific dynamic action (SDA) is, the extra heat produced by the body over and, above the caloric value of foodstuffs.
Page 772 :
762, Renin; Rennin – Renin is synthesized by the kidneys, and is involved in vasoconstriction causing, hypertension; rennin is an enzyme found in, gastric juice responsible for coagulation of, milk., Ribosomes; ribozymes – Ribosomes are the sites, of protein biosynthesis; ribozymes refer to, the RNA molecules which function as, enzymes., Retinol; retinal – Retinol is the alcohol form of, vitamin A; retinal is the aldehyde form, obtained by the oxidation of retinol., Scleroproteins; selenoproteins – Scleroproteins are, a group of fibrous proteins; selenoproteins, contain the amino acid selenocysteine., Serotonin; melatonin – Serotonin is a neurotransmitter synthesized from tryptophan;, melatonin is a hormone derived from, serotonin in the pineal gland., Somatotropin; somatostatin; somatomedin –, Somatotropin is the other name for growth, hormone (GH); growth hormone release, inhibiting hormone (GRIH) is also called, somatostatin; somatomedin refers to the, insulin-like growth factor -I (IGF-I), produced, by liver in response to GH action., Sucrose; sucrase – Sucrose is a disaccharide;, sucrase is an enzyme that cleaves sucrose to, glucose and fructose., , BIOCHEMISTRY, , Synthase; synthetase – Both the enzymes are, concerned with biosynthetic reactions., Synthase does not require ATP; synthetase is, dependent on ATP for energy supply., (Note : This distinction between synthase and, synthetase however, is not maintained strictly, by most authors)., Thiamine; thymine – Thiamine is a vitamin (B1);, thymine is a pyrimidine base found in DNA, structure., Thiokinase; thiolase – Thiokinase activates fatty, acids to acyl CoA; Thiolase catalyses the final, reaction in E-oxidation to liberate acetyl CoA, from acyl CoA., Transcription; translation – Transcription refers, to the synthesis of RNA from DNA; translation, involves the protein synthesis from the, RNA., Uric acid; uronic acid – Uric acid is the, end product of purine metabolism; uronic, acids are formed by the oxidation of aldehyde, group of monosaccharides (e.g. glucuronic, acid)., Ureotelic; uricotelic – Ureotelic organisms (e.g., mammals) convert NH3 to urea; uricotelic, organisms (e.g. reptiles) convert NH3 to uric, acid., Vitamin A; coenzyme A – Vitamin A is fat soluble, vitamin; coenzyme A is derived from water, soluble vitamin, pantothenic acid.
Page 773 :
Appendix IV : Practical Biochemistry—Principles, , QUALITATIVE EXPERIMENTS, Several laboratory qualitative experiments are, performed to indentify the compounds of biochemical, importance (carbohydrates, proteins/amino acids,, non-protein nitrogenous substances) and to detect, the abnormal constituents of urine. The principles of, the reactions pertaining to the most widely employed, qualitative tests are described here., , I. REACTIONS OF CARBOHYDRATES, The carbohydrates used in the laboratory for, the qualitative tests include glucose and fructose, (monosaccharides), sucrose, lactose and maltose, (disaccharides) and starch (polysaccharide). The, principles of the reactions of carbohydrates are, given :, 1. Molisch test : It is a general test for the detection, of carbohydrates. The strong H2SO4 hydrolyses, carbohydrates (poly- and disaccharides) to liberate, monosaccharides. The monosaccharides get, dehydrated to form furfural (from pentoses) or, hydroxy methylfurfural (from hexoses) which, condense with D-naphthol to form a violet coloured, complex., 2. Iodine test : Polysaccharides combine with, iodine to form a coloured complex. Thus, starch, gives blue colour while dextrins give red colour, with iodine., 3. Benedict’s test : This is a test for the identification, of reducing sugars , which form enediols, (predominantly under alkaline conditions). The, enediol forms of sugars reduce cupric ions (Cu2+) of, copper sulfate to cuprous ions (Cu+) which form a, yellow precipitate of cuprous hydroxide or a red, precipitate of cuprous oxide., 4. Barfoed’s test : The principle of this test is the, same as that of Benedict’s test except that the, , reduction is carried out in mild acidic medium. Since, acidic medium is not favourable for reduction, only, strong reducing sugars (monosaccharides) give this, test positive. Thus, Barfoed’s test serves as a key, reaction to distinguish monosaccharides form, disaccharides., 5. Seliwanoff’s test : This is a specific test for, ketohexoses . Concentrated hydrochloric acid, dehydrates ketohexoses to form furfural derivatives, which condense with resorcinol to give a cherry red, complex., 6. Foulger’s test : This is also a test for ketohexoses., The furfural derivatives formed from ketohexoses, condense with urea in the presence of stannous, chloride to give a blue colour., 7. Rapid furfural test : Ketohexoses are converted, to furfural derivatives by HCl which form a purple, colour complex with D-naphthol., 8. Osazone test : Phenylhydrazine in acetic acid,, when boiled with reducing sugars forms osazones., The first two carbons (C1 and C2) are involved in, this reaction. The sugars that differ in their, configuration on these two carbons give the same, type of osazones, since the difference is marked by, binding with phenylhydrazine. Thus, glucose,, fructose and mannose give the same type (needle, shaped) of osazones. However, the osazones, of reducing disaccharides differ — maltose gives, sunflower-shaped while lactose powder-puff, shaped., 9. Sucrose hydrolysis test : Sucrose is a nonreducing sugar, hence it does not give Benedict’s, and Barfoed’s tests. Sucrose can be hydrolysed by, concentrated HCl, to be converted to glucose, and fructose (reducing monosaccharides) which, answer the reducing reactions. However, after, sucrose hydrolysis, the medium has to be, made alkaline (by adding Na2CO3) for effective, reduction process., , 763
Page 774 :
764, , BIOCHEMISTRY, , II. REACTIONS OF PROTEINS, The proteins employed in the laboratory for the, qualitative tests include albumin, globulins, casein,, gelatin and peptones. The principle of the most, common reactions of proteins / amino acids, performed in the laboratory are given hereunder., , A. PRECIPITATION REACTIONS, Proteins exist in colloidal solution due to, hydration of polar groups (—COO–, —NH+3, —OH)., They can be precipitated by dehydration or, neutralization of polar groups. Several methods are, in use to achieve protein precipitation., 1. Precipitation by neutral salts : The process of, protein precipitation by the addition of neutral salts, such as ammonium sulfate or sodium sulfate is, referred to as salting out. This phenomenon is, explained on the basis of dehydration of protein, molecules by salts. This causes increased proteinprotein interaction, resulting in molecular aggregation, and precipitation., The amount of salt required for protein, precipitation depends on the size (molecular weight), of the protein molecule. In general, the higher is the, protein molecular weight, the lower is the salt, required for precipitation. Thus, serum globulins are, precipitated by half saturation with ammonium sulfate, while albumin is precipitated by full saturation., 2. Precipitation by salts of heavy metals : Heavy, metal ions like Pb2+, Hg2+, Fe2+, Zn2+, Cd2+ cause, precipitation of proteins. These metals being, positively charged, when added to protein solution, (negatively charged) in alkaline medium result in, precipitate formation., 3. Precipitation by anionic or alkaloid reagents :, Proteins can be precipitated by trichloroacetic acid,, sulphosalicylic acid, phosphotungstic acid, picric, acid, tannic acid, phosphomolybdic acid etc. By the, addition of these acids, the proteins existing as cations, are precipitated by the anionic form of acids to, produce protein-sulphosalicylate, protein-tungstate,, protein-picrate etc., The anionic reagents such as phosphotungstic, acid and trichloroacetic acid are used to prepare, protein-free filtrate of blood needed for several, estimations (e.g., urea, sugar) in the laboratory., 4. Precipitation by organic solvents : Organic, solvents such as alcohol are good protein, , precipitating agents. They dehydrate the protein, molecule by removing that water envelope and cause, precipitation., , B. COLOUR REACTIONS, The proteins give several colour reactions which, are often useful to identify the nature of the amino, acids present in them as shown in the table., Colour reactions of proteins/amino acids, , Reaction, , Specific group or amino acid, , 1. Biuret reaction, , Two peptide linkages, , 2. Ninhydrin reaction, 3. Xanthoproteic, reaction, , D-Amino acids, Benzene ring of aromatic, amino acids (Phe, Tyr, Trp), , 4. Millons reaction, , Phenolic group (Tyr), , 5. Hopkins-Cole, reaction, , Indole ring (Trp), , 6. Sakaguchi reaction, , Guanidino group (Arg), , 7. Nitroprusside, , Sulfhydryl groups (Cys), , reaction, 8. Sulfur test, 9. Pauly’s test, 10. Folin–Coicalteau’s, test, , Sulfhydryl groups (Cys), Imidazole ring (His), Phenolic groups (Tyr), , 1. Biuret reactions : Biuret is a compound formed, by heating urea to 180qC. When biuret is treated, with dilute copper sulfate in alkaline medium, a, purple colour is obtained. This is the basis of biuret, test used for identification of proteins and peptides., Biuret test is answered by compounds containing, two or more CO—NH groups i.e., peptide bonds., All proteins and peptides possessing atleast two, peptide linkages i.e., tripeptides (with 3 amino acids), give positive biuret test. The principle of biuret test, is conveniently used to detect the presence of proteins, in biological fluids. The mechanism of biuret test is, not clearly known. It is believed that the colour is, due to the formation of a copper co-ordinated, complex., 2. Ninhydrin reaction : The D-amino acids react, with ninhydrin to form a purple, blue or pink colour, complex (Ruhemann’s purple)., Amino acid + Ninhydrin o, Keto acid + NH3 + CO2 + Hydrindantin, Hydrindantin + NH3 + Ninhydrin o, Ruhemann’s purple
Page 775 :
Appendix IV : PRACTICAL BIOCHEMISTRY—PRINCIPLES, , 3. Xanthoproteic reaction : Xanthoproteic reaction, is due to nitration of aromatic amino acids, (tryptophan, tyrosine and phenylalanine) on treatment, with strong nitric acid at high temperature., 4. Millon’s test : This test is given by the amino, acid tyrosine, or any other compound containing, hydroxyphenyl ring. A red colour or precipitate is, obtained in this reaction due to the formation of, mercury complex of nitrophenol derivative., 5. Hopkins-Cole reaction : This reaction is specific, for the indole ring of tryptophan. It combines with, formaldehyde in the presence of the oxidizing agent, (sulfuric acid with mercuric sulfate) to form a violet, or purple coloured compound., 6. Sakaguchi reaction : Arginine, containing, guanidino group, reacts with _-naphthol and alkaline, hypobromite to form a red colour complex., 7. Sulfur test : This is a test specific for sulfur, containing amino acids namely cysteine and cystine,, but not methionine. When cysteine and cystine are, boiled with sodium hydroxide, organic sulfur is, converted to inorganic sodium sulfide. This reacts, with lead acetate to form a black precipitate of lead, sulfide. Methionine does not give this test, since, sulfur of methionine is not split by alkali., 8. Pauly’s test : This reaction is specific for histidine, (imidazole ring). Diazotised sulfanilic acid reacts with, imidazole ring in alkaline medium to form a red, coloured complex., 9. Molisch test : This is a specific test for the, detection of carbohydrates. The proteins containing, carbohydrates (e.g., glycoproteins) give this test, positive. Albumin contains carbohydrate bound to, it, hence answers Molisch test., , III. REACTIONS OF NON-PROTEIN, NITROGENOUS SUBSTANCES, The non-protein nitrogenous (NPN) substances, of biochemical importance include urea, uric acid, and creatinine., 1. Sodium hypobromite test : This is a test for the, detection of urea. Sodium hypobromite decomposes, urea to liberate nitrogen. The latter can be identified, by brisk effervescence., 2. Specific urease test : The enzyme urease (sourcehorse gram) specifically acts on urea to liberate, ammonium carbonate (alkali). The latter can be, , 765, , identified by a colour change in phenophthalein, indicator (pink colour in alkaline medium)., 3. Benedict’s uric acid test : Uric acid, being a, strong reducing agent, reduces phosphotungstate to, tungsten blue in alkaline medium., 4. Murexide test : Uric acid is oxidized by nitric, acid to give purpuric acid (reddish yellow). This in, turn combines with ammonia to form purple red, colour ammonium purpurate (murexide)., 5. Jaffe’s test : Creatinine reacts with picric acid in, alkaline medium to form orange red colour complex., , IV. ABNORMAL CONSTITUENTS OF URINE, Urine is the most important excretory fluid from, the body. Some of the diseases are associated with, an excretion of abnormal constituents in urine. The, identification of such compounds in urine is of great, diagnostic importance., , Urine abnormal, constituent, Albumin, Hemoglobin, Glucose, Ketone bodies, Bile salts, Bile pigments, , Associated disorder(s), Kidney damage (glomerulonephritis), Damage to kidneys or urinary tract., Diabetes mellitus, renal glycosuria., Diabetes mellitus, starvation., Obstructive jaundice, Obstructive jaundice and hepatic, jaundice., , 1. Sulfosalicylic acid test : Proteins get precipitated, by sulfosalicylic acid by forming proteinsulfosalicylate., 2. Heat coagulation test : This is a test for the, detection of albumin and/or globulins in urine. Heat, coagulation test is based on the principle of, denaturation of proteins, followed by coagulation., (Note : Small amounts of dilute acetic acid are, added to dissolve the phosphates and sulfates that, get precipitated on heating.), 3. Benzidine test : This test detects the presence of, blood . Hemoglobin (acts like peroxidase), decomposes hydrogen peroxide to liberate nascent, oxygen (O–) which oxidises benzidine to a green or, blue coloured complex., (Note : Pus cells of urine possess peroxidase, activity which interferes in benzidine test. This can, be eliminated by boiling the urine prior to the test to, inactivate the enzyme).
Page 776 :
766, 4. Benedict’s test : This is a semiquantitative test, for the detection of urine reducing sugars (primarily, glucose). Benedict’s test is based on the principle of, reducing property of sugars (described in detail under, reactions of carbohydrates). Colour of the precipitate, formed indicates the approximate amount of glucose, present in urine. Thus, green turbidity = traces; green, precipitate = 0.5%; yellow precipitate = 1%; orange, precipitate = 1.5% brick red precipitate = 2%., (Note : Benedict’s test is not specific to, glucose, since it can be answered by any reducing, substance)., 5. Glucose oxidase test : This is a strip test for the, specific detection of glucose. The enzyme glucose, oxidase oxidizes glucose to liberate hydrogen, peroxide which in turn is converted to nascent, oxygen (O–) by peroxidase enzyme. The compound, O-diansidine combines with nascent oxygen to form, a coloured (yellow to red) complex., 6. Rothera’s test : Nitroprusside in alkaline medium, reacts with keto group of ketone bodies (acetone, and acetoacetate) to form a purple ring. This test is, not given by E-hydroxybutyrate., 7. Hay’s test : This test is based on the surface, tension lowering property of bile salts (sodium, glycocholate and sodium taurocholate). Sulfur, powder sprinkled on the surface of urine containing, bile salts sinks to the bottom., 8. Petternkofer’s test : This test is employed, for the detection of bile salts . The furfural, derivatives (by reacting sugar with concentrated, H2SO4) condense with bile salts to form a purple, ring., 9. Gmelin’s test : Nitric acid oxidizes the, bile pigment bilirubin to biliverdin (green) or, bilicyanin (blue). Gmelin’s test gives a play of, colours and is used for the identification of bile, pigments., 10. Fouchet’s test : This test is also employed for, the detection of bile pigments. Bile pigments are, adsorbed on barium sulfate. Fouchet’s reagents, (containing ferric chloride in trichloroacetic acid), oxidizes bilirubin to biliverdin (green) and bilicyanin, (blue)., , BIOCHEMISTRY, , QUANTITATIVE EXPERIMENTS, Quantitative experiments, dealing with the, determination of concentrations of several, biologically important compounds and the assay of, many enzymes, are of great significance in the, laboratory practice. Very often, the ultimate diagnosis, and prognosis of a large number of diseases are, guided by the quantitative biochemical investigations., The principles involved in some of the, quantitative experiments, commonly employed in, the biochemistry laboratory by an undergraduate, student, are briefly described here., , 1. Blood glucose estimation, The quantitative determination of blood (plasma/, serum) glucose is of great importance in the diagnosis, and monitoring of diabetes mellitus., (i) Folin Wu method : Alkaline copper (cupric, ions) is reduced by glucose when boiled, with protein free blood filtrate to cuprous, oxide. The cuprous oxide in turn reacts, with phosphomolybdic acid to form blue, coloured oxides of molybdenum. The, intensity of the colour can be measured in, a colorimeter at a wavelength 680 nm., [Folin Wu method is rather old and is not, specific for glucose determination, since, other substances (e.g., fructose, lactose,, glutathione) also bring about reduction., Consequently the blood glucose level when, estimated by Folin Wu method is higher, i.e., normal fasting is 80-120 mg/dl against, true glucose 60-100 mg/dl], (ii) O-Toluidine method : Glucose combines, with O-toluidine when boiled in acid, medium to form a green coloured complex, which can be measured in a colorimeter at, a wavelength 630 nm. (This method, determines glucose alone)., (iii) Glucose oxidase-peroxidase (GOD—POD), method : This is an enzymatic, determination of blood glucose. Glucose, gets oxidized by glucose oxidase to
Page 777 :
Appendix IV : PRACTICAL BIOCHEMISTRY—PRINCIPLES, , gluconic acid and hydrogen peroxide. The, enzyme peroxidase converts hydrogen, peroxide to water and oxygen. The oxygen, in turn reacts with 4-aminophenzone in, the presence of phenol to form a pink, coloured complex, the intensity of which, can be measured at 530 nm., , 2. Blood urea estimation, Determination of blood urea (reference range, 10-40 mg/dl) is important for the evaluation of kidney, (renal) function. Elevation of blood urea is associated, with pre-renal (diabetic coma, thyrotoxicosis), renal, (acute glomerulonephritis, polycystic kidney) and, post-renal (obstruction in the urinary tract, due to, tumors, stones) conditions., , Diacetyl monoxime (DAM) method : Urea when, heated with diacetyl monoxime forms a yellow, coloured complex of dioxime derivatives which can, be measured at 520 nm., , 3. Serum creatinine estimation, Estimation of serum creatinine (reference range, 0.5-1.5 mg/dl) is used as a diagnostic test to assess, kidney function. Serum creatinine is not influenced, by endogenous and exogenous factors, as is the case, with urea. Hence, some workers consider, serum creatinine as a more reliable indicator of renal, function., , Alkaline picrate method : This method is based, on Jaffe’s reaction. Creatinine reacts with alkaline, picrate to form creatinine picrate, an orange red, coloured complex, which can be measured in a, colorimeter at 530 nm., (Note : Urinary creatinine can also be, determined by employing the same principle given, above)., 4. Determination of serum proteins, , 767, , Biuret method : Peptide bonds ( CO NH) of, proteins react with cupric ions in alkaline medium, to form a violet colour complex which is measured, at a wavelength 530 nm. This method is suitable for, total serum proteins with estimation., Bromocresol green (BCG) dye method : This, technique is employed for the estimation of serum, albumin. BCG dye reacts with albumin to form an, intense blue-green coloured complex which can be, measured at 628 nm., , 5. Estimation of serum bilirubin, The total bilirubin concentration in serum is, 0.2-1 mg/dl (conjugated ~ 0.6 mg/dl; unconjugated, ~ 0.4 mg/dl). Elevation in serum bilirubin, concentration is observed in jaundice. Unconjugated, bilirubin is increased in hemolytic jaundice,, conjugated bilirubin in obstructive jaundice, while, both of them are increased in hepatic jaundice., , van den Bergh reaction : Serum bilirubin, estimation is based on van den Bergh reaction. The, principle of the reaction is that diazotised sulfanilic, acid (formed by mixing equal volumes of sulfanilic, acid in HCl and sodium nitrite) reacts with bilirubin, to form a purple coloured azobilirubin which can, be measured at 540 nm., , 6. Estimation of serum cholesterol, Serum cholesterol concentration (reference range, 150-225 mg/dl) is elevated in atherosclerosis, diabetes, mellitus, obstructive jaundice and hypothyroidism., Decreased levels are observed in hyperthyroidism., , Acetic anhydride method : Serum cholesterol, reacts with acetic anhydride in the presence of glacial, acetic acid and concentrated H2SO4 to form a green, coloured complex. Intensity of this colour is measured, at 560 nm., , 7. Estimation of serum uric acid, , The normal concentration of total serum proteins, is in the range 6-8 g/dl (albumin 3.5-5.0 g/dl;, globulins 2.5-3.5 g/dl; A/G ratio is 1.2 to 1.5 : 1)., The A/G ratio is lowered either due to a decrease in, albumin or an increase in globulins., , Uric acid is the end product of purine, metabolism. Its concentration in serum is increased, (reference range - men 4-8 mg/dl; women 3-6 mg/, dl) in gout., , Serum albumin concentration is decreased in, liver diseases, severe protein malnutrition, and, excretion of albumin in urine (due to renal damage)., Serum globulin concentration is elevated in chronic, infections and multiple myeloma., , Henry-Caraway’s method : Uric acid in the, protein-free filtrate when treated with, phosphotungstic acid in the presence of sodium, carbonate (alkaline solution) gives a blue coloured, complex which can be measured at 660 nm.
Page 778 :
768, , BIOCHEMISTRY, , 8. Estimation of serum calcium, Serum calcium level is elevated (reference range, 9-11 mg/dl) in hyperparathyroidism and decreased, in hypothyroidism., , O-Cresolphthalein complexone method :, Calcium reacts with the dye, O-cresolphthalein, complexone (CPC) in alkaline solution to form a, complex which can be measured at a wavelength, 660 nm., , 9. Estimation of serum phosphorus (inorganic), Serum phosphate (reference range 3-4.5 mg/dl), is increased in hypoparathyroidism, and decreased, in hyperparathyroidism and renal rickets., For the determination of serum phosphate, serum, proteins are precipitated by trichloroacetic acid. The, protein-free filtrate containing inorganic phosphate, is reacted with molybdic acid reagent to form, phosphomolybdate. The latter in turn is reduced to, molybdenum blue by treatment with 1-amino 2naphthol-4 sulfonic acid (ANSA). The intensity of, the blue colour is measured at 689 nm., , 10. Determination of SGPT and SGOT, Serum glutamate pyruvate transaminase (SGPT;, alanine transaminase) and serum glutamate, oxaloacetate transaminase (SGOT; aspartate, transaminase) are two important diagnostic enzymes., SGPT activity (reference range 5-40 IU/L) is more, specifically increased in liver diseases (hepatic, jaundice). SGOT activity is elevated (reference, range 5-45 IU/L) in heart diseases (myocardial, infarction)., , Principle of assay : SGPT catalyses the following, reaction, L-Alanine + D-ketoglutarate o, L-glutamate + pyruvate, SGOT brings about the following reaction, L-Aspartic acid + D-ketoglutarate o, L-glutamate + oxaloacetate, , The keto acid (pyruvate or oxaloacetate), formed, in the above reaction, when treated with, 2, 4-dinitrophenyl hydrazine forms dinitrophenyl, hydroazone (brown colour) in alkaline medium, which can be measured at 505 nm., , 11. Determination of serum alkaline, phosphatase, The activity of the enzyme serum alkaline, phosphatase (normal range 3-13 KA Units/dl) is, elevated in rickets and obstructive jaundice., , Principle of assay : Alkaline phosphatase, hydrolyses disodium phenylphosphate liberating, phenol. On treatment with 4-amino antipyrine in, alkaline medium, phenol gives ferricyanide (reddish, colour) which can be measured at 520 nm., , 12. Determination of serum amylase, Serum amylase activity is increased (reference, range 80-180 Somogyi Units/dl) in acute pancreatitis., , Principle of assay : Amylase acts on starch and, hydrolyses to dextrins and maltose. Starch forms blue, coloured complex with iodine, a decrease in the, colour (measured at 670 nm) is proportional to the, activity of amylase., , 13. Analysis of cerebrospinal fluid, Cerebrospinal fluid (CSF) is the aqueous medium, surrounding the brain and spinal cord. From the, biochemical perspective, estimation of proteins and, glucose in CSF is important. Increase in protein, (reference range 15-40 mg/dl) and decrease in, glucose (reference range 50-75 mg/dl) in the, cerebrospinal fluid are observed in tuberculosis, meningitis., , CSF protein estimation : Sulfosalicylic acid (in, sodium sulfate solution) precipitates CSF proteins, and the turbidity is measured at 680 nm., CSF glucose estimation : Any one of the standard, methods employed for the determination of blood, glucose (already described) can be used for CSF, glucose estimation.
Page 779 :
Appendix V : Clinical Biochemistry Laboratory, , The ultimate application of the biochemistry, subject is for the health and welfare of mankind., Clinical biochemistry (also known as clinical, chemistry or chemical pathology) is the laboratory, service absolutely essential for medical practice. The, results of the biochemical investigations carried out, in a clinical chemistry laboratory will help the, clinicians to determine the diseases (diagnosis) and, for follow-up of the treatment/recovery from the, illness (prognosis). Biochemical investigations hold, the key for the diagnosis and prognosis of diabetes, mellitus, jaundice, myocardial infarction, gout,, pancreatitis, rickets, cancers, acid-base imbalance, etc. Successful medical practice is unimaginable, without the service of clinical biochemistry, laboratory., The biological fluids employed in the clinical, biochemistry laboratory include blood , urine ,, cerebrospinal fluid and pleural fluid. Among these,, blood (directly or in the form of plasma or serum) is, frequently used for the investigations in the clinical, biochemistry laboratory., , COLLECTION OF BLOOD, Venous blood is most commonly used for a, majority of biochemical investigations. It can be, drawn from any prominent vein (usually from a, vein on the front of the elbow). Capillary blood, (<0.2 ml) obtained from a finger or thumb, is less, frequently employed. Arterial blood (usually drawn, under local anesthesia) is used for blood gas, determinations., Precautions for blood collection : Use of sterile, (preferably disposable) needles and syringes,, cleaning of patients skin, blood collection in clean, and dry vials/tubes are some of the important, precautions., , CHOICE OF BLOOD SPECIMENS, Biochemical investigations can be performed, on 4 types of blood specimens-whole blood, plasma,, serum and red blood cells. The selection of the, specimen depends on the parameter to be estimated., Whole blood (usually mixed with an anticoagulant), is used for the estimation of hemoglobin,, carboxyhemoglobin, pH, glucose, urea, non-protein, nitrogen, pyruvate, lactate, ammonia etc. (Note : for, glucose determination, plasma is prefered in recent years)., Plasma, obtained by centrifuging the whole, blood collected with an anticoagulant, is employed, for the parameters—fibrinogen, glucose, bicarbonate,, chloride, ascorbic acid etc., Serum is the supernatant fluid that can be, collected after centrifuging the clotted blood. It is, the most frequently used specimen in the clinical, biochemistry laboratory. The parameters estimated, in serum include proteins (albumin/globulins),, creatinine, bilirubin, cholesterol, uric acid,, electroylets (Na+, K+, Cl–), enzymes (ALT, AST, LDH,, CK, ALP, ACP, amylase, lipase) and vitamins., It may be noted that plasma is physiologic fluid, while serum is prepared in the laboratory., Red blood cells are employed for the, determination of abnormal hemoglobins, glucose, 6-phosphate dehydrogenase, pyruvate kinase etc., ANTICOAGULANTS, Certain biochemical tests require unclotted, blood. Anticoagulants are employed for collecting, such specimens., Heparin : Heparin (inhibits the conversion of, prothrombin to thrombin) is an ideal anticoagulant,, since it does not cause any change in blood, composition. However, other anticoagulants are, prefered to heparin, due to the cost factor., Potassium or sodium oxalate : These compounds, precipitate calcium and inhibit blood coagulation., Being more soluble, potassium oxalate (5-10 mg per, 5 ml blood) is prefered., , 769
Page 780 :
770, , BIOCHEMISTRY, , Potasium oxalate and sodium fluoride : These, anticoagulants are employed for collecting blood to, estimate glucose. Further sodium fluoride inhibits, glycolysis and preserves blood glucose concentration., Ammonium oxalate and potassium oxalate : A, mixture of these two compounds in the ratio 3 : 2 is, used for blood collection to carry out certain, hematological tests., Enthylene diaminetetracetic acid (EDTA) : It, chelates with calcium and blocks coagulation. EDTA, is employed to collect blood for hematological, examinations., HEMOLYSIS, The rupture or lysis of RBC, releasing the cellular, constituents interferes with the laboratory, investigations. Therefore, utmost care should be taken, to avoid hemolysis when plasma or serum are used, for biochemical tests. Use of dry syringes, needles, and containers, allowing slow flow of blood into, syringe are among the important precautions to avoid, hemolysis., PRESERVATION OF BLOOD SPECIMENS, Plasma or serum should be separated within 2, hours after blood collection. It is ideal and advisable, to analyse blood, plasma or serum, immediately after, the specimen collection. This however, may not be, always possible. In such a case, the samples (usually, plasma/serum) can be stored at 4°C until analysed., For enzyme analysis, the sample are preserved at –, 20°C., , TYPES OF LABORATORY TESTS, The biochemical investigations (on blood/, plasma/serum) carried out in the clinical biochemistry, laboratory may be grouped into different types., 1. Discretionary or on-off tests : Most common, clinical biochemistry tests that are designed to answer, specific questions. e.g., does the patient have, increased blood urea/glucose concentration?, Normally, these tests are useful to support the diagnosis., 2. Biochemical profiles : These tests are based on, the fact that more useful information on the patients, disease status can be obtained by analysing more, constituents rather than one e.g., plasma electrolytes, (Na+, K+, Cl–, bicarbonate, urea); liven function tests, (serum bilirubin, ALT, AST)., , 3. Dynamic function tests : These tests are designed, to measure the body’s response to external stimulus, e.g., oral glucose tolerance test (to assess glucose, homeostasis) : bromosulphthalein test (to assess liver, function)., 4. Screening tests : These tests are commonly, employed to identify the inborn errors of metabolism,, and to check the entry of toxic agents (pesticides,, lead, mercury) into the body., 5. Metabolic work-up tests : The programmed, intensive investigations carried out to identify the, endocrinological disorders come under this category., The term emergency tests is frequently used in, the clinical laboratory. It refers to the tests to be, performed immediately to help the clinician for, proper treatment of the patient e.g., blood glucose,, urea, serum electrolytes., , COLLECTION OF URINE, Urine, containing the metabolic waste products, of the body in water is the most important excretory, fluid. For biochemical investigations, urine can be, collected as a single specimen or for 24 hours. Single, specimens of urine, normally collected in the, morning, are useful for qualitative tests e.g., sugar,, proteins. Twenty four hour urine collections (done, between 8 AM to 8 AM) are employed for quantitative, estimation of certain urinary constituents e.g.,, proteins, hormones, metabolites., Preservatives for urine : For the collection of, 24 hr urine samples, preservatives have to be used, or else urine undergoes changes due to bacterial, action. Hydrochloric acid, toluene, light petroleum,, thymol, formalin etc., are among the common, preservatives used., , CEREBROSPINAL FLUID (CSF), CSF is a fluid of the nervous system. It is formed, by a process of selective dialysis of plasma by the, choroid plexuses of the ventricles of the brain. The, total volume CSF is 100-200 ml., Collection of CSF : CSF is collected by, puncturing the interspace between the 3rd and the, 5th lumbar vertebrae, under aseptic conditions and, local anesthesia.
Page 781 :
771, , Appendix V : CLINICAL BIOCHEMISTRY LABORATORY, , Biochemical investigations on CSF : Protein,, glucose and chloride estimations are, usually performed in the clinical biochemistry, laboratory., , QUALITY CONTROL, Quality control in clinical biochemistry, laboratory refers to the reliability of investigative, service. Any error in the laboratory will jeopardize, the lives of patients. It is therefore utmost important, that the laboratory errors are identified and rectified., Quality control comprises of four interrelated, factors namely precision, accuracy, specificity and, sensitivity., Precision refers to the reproducibility of the result, when the same sample is analysed on different, occasions (replicate measurements) by the same, person. For instance, the precision is good, if the, blood glucose level is 78, 80 and 82 mg/dl on, replicates., Accuracy means the closeness of the estimated, result to the true value e.g., if true blood urea level, is 50 mg/dl, the laboratory reporting 45 mg/dl is, more accurate than the one reporting 35 mg/dl., Specificity refers to the ability of the analytical, method to specifically determine a particular, parameter e.g., glucose can be specifically estimated, by enzymatic glucose oxidase method., Sensitivity deals with the ability of a particular, method to detect small amounts of the measured, constituent., METHODS OF QUALITY CONTROL, Internal quality control refers to the analysis of, the same pooled sample on different days in a, , laboratory, the results should vary within a narrow, range., External quality control deals with the analysis, of a sample received from outside, usually from a, national or regional quality control centre. The results, obtained are then compared., , AUTOANALYSERS IN, CLINICAL CHEMISTRY, The heavy work load in the clinical biochemistry, laboratory has lead to the discovery of autoanalysers., These modern equipment are useful to analyse, hundreds of samples in a short time. Single channel, and multi-channel machines (autoanalysers) based, on the principles of either continuous or discrete, analysis are available on the market., , ANALYSIS IN CLINICAL BIOCHEMISTRY, LABORATORY AND REFERENCE VALUES, As already stated, clinical biochemistry, laboratory is a service-oriented establishment for the, benefit of patient health care. The reader may refer, tools of biochemistry (Chapter 41) and principles of, practical biochemistry (Appendix-V) for a brief, knowledge on the principles of some of the, equipment used and the laboratory investigations, employed., The details on the biochemistry of health and, disease states in relation to the normal and abnormal, biochemical data are described in the text of this, book. For ready reference, the most common, reference biochemical values are given on the inside, of back cover.
Page 782 :
Appendix VI : Case Studies with, Biochemical Correlations, giving the child rice gruel, along with breast milk., The laboratory data of the child are given hereunder., , NUTRITIONAL DISORDERS, , Parameter, , CASE STUDY, , 1, , A 3-year old female child had stunted growth,, edema (particularly on legs and hands) discoloration, of skin and hair, apathy and moon-face. She also, had frequent respiratory infections and diarrhea. On, enquiring, the mother informed the physician that, the child was mostly breast-fed until 2 years of age,, and for the past one year she was being given dilute, buffalo milk and a small quantities of rice with ghee, and dhal. The following are the laboratory data of, the child, , Parameter, , Subject, , Hemoglobin, , 7 g/dl, , 13-15 g/dl, , 4 g/dl, 2 g/dl, 2.8 mEq/1, , 6-8 g/dl, 3-4.5 g/dl, 3.5-4.5 mEq/1, , Serum proteins (total), Serum albumin, Serum potassium, , Reference range, , The clinical manifestations and the nutritional, history, supported by the laboratory data clearly, indicate that the child was suffering from, kwashiorkor, a predominant nutritional disorder, in, the developing countries. Edema occurred due to, lack of adequate serum proteins to maintain water, distribution between blood and tissues. The, immunological response of the child to infections, was very low. Deficiency of serum K+ was observed, due to diarrhea., , CASE STUDY, , 2, , A one year old male baby had growth, retardation, reduced physical activity, muscle wasting, (emaciation), loose folds of skin wrapped over bones., The mother of the child, now in third month of, gestation, informed the physician that she had been, , Reference range, , Hemoglobin, , 7 g/dl, , 13-15 g/dl, , Serum proteins (total), , 6 g/dl, , 6-8 g/dl, , 3 g/dl, 3.5 mEq/1, , 3-4.5 g/dl, 3.5-5 mEq/1, , Serum albumin, Serum potassium, , Diagnosis and discussion, The child was suffering from marasmus , a, nutritional disorder, predominantly due to the, deficiency of calories. Marasmus mostly occurs in, children less than one year of age. It can be, distinguished from kwashiorkor (Case 1) by lack, of edema and almost unaltered serum albumin level., , CASE STUDY, , Diagnosis and discussion, , Subject, , 3, , A 6-year old boy had bone deformities such as, bow legs and pigeon chest. He had a history of, delayed eruption of teeth. On enquiring, the mother, informed the physician that the boy had been on a, strict vegetarian diet with low intake of milk as well, as fats and oils. The following are the laboratory, findings of this boy., , Parameter, , Subject, , Reference range, , Calcium, , 8.1 mg/dl, , 9-11 mg/dl, , Phosphate, Alkaline phosphatase, , 2.5 mg/dl, 40 KAU/dl, , 3-4.5 mg/dl, 3-13 KAU/dl, , Diagnosis and discussion, This case is classical example of rickets due to, vitamin D deficiency. As a result of low serum, calcitriol (the biochemically active form of vitamin, D), calcium and phosphate levels are not maintained, in the circulation, hence mineralization is impaired,, ultimately causing bone deformities such as bow, legs. Serum alkaline phosphatase is elevated in rickets, in a vain attempt to result in bone formation., , 772
Page 783 :
773, , Appendix VI : CASE STUDIES WITH BIOCHEMICAL CORRELATIONS, , CASE STUDY, , 4, , A 25-year old strict vegetarian woman, with, two children aged 5 years and 3 years, complained, of tiredness and appeared pale. In the recent few, months, the woman had heavy and prolonged, menstrual flow. Enquiries revealed that her, consumption of milk and milk products was, reasonably good, but leafy vegetables was low. The, laboratory investigations showed that the woman’s, hematocrit was 28% (reference range 40% to 50%),, while her hemoglobin concentration was 8 g/dl, (reference range 13-15 g/dl)., Diagnosis and discussion, The woman with a low hemoglobin, concentration and reduced hematocrit depicts a very, common nutritional disorder — iron deficiency, anemia. A strict vegetarian diet coupled with low, consumption of leafy vegetables led her to iron, deficiency., , METABOLIC DISEASES/INBORN ERRORS, CASE STUDY, , 5, , A boy, aged 12 years was given treatment for, prolonged diarrhea. After improvement, he, complained abdominal discomfort and diarrhea with, a feeling of being bloated, after consumption of milk., He was taken to a physician who advised him to, stop his intake of milk. He felt better in 3 days., Diagnosis and discussion, The boy was suffering from acquired lactose, intolerance. This was precipitated by diarrhea where, the intestinal mucosal cells were denuded faster., The brush border of the intestine houses the enzyme, lactase which is lost due to diarrhea, and hence, typical symptoms of flatulence., , CASE STUDY, , 6, , A normal one month old baby had a history of, vomiting and diarrhea that frequently occurred after, breast feeding. The urine gave a positive test for, reducing sugars (Benedict’s test) while the test was, negative by Glucostix (specific for glucose). The RBC, were found to be totally deprived of activity of the, enzyme galactose 1-phosphate uridyltransferase, (Reference range 4-30 units/g of hemoglobin)., , Diagnosis and discussion, The baby was suffering from galactosemia, a, metabolic disorder, due to the deficiency of the, enzyme galactose 1-phosphate uridyltransferase. The, disease is characterized by increase in blood, galactose level, and its excretion into urine. For the, babies suffering from galactosemia, milk has to be, removed from the diet and replaced with infant, formula containing sucrose., , CASE STUDY, , 7, , A 25-year old man was on treatment with, antimalarial drug primaquine. As the treatment was, in progress, he developed complications. This, subject’s laboratory investigative data are given, hereunder., , Parameter, , Subject, , Reference range, , Hematocrit, , 30 %, , 45-50 %, , Hemoglobin, before treatment, after treatment, Serum bilirubin, , 12 g/dl, 8 g/dl, 3.5 mg/dl, , 13-15 g/dl, < 1.2 mg/dl, , The laboratory tests also indicated increased, fragility of erythrocytes., Diagnosis and discussion, This subject obviously had the deficiency of, the glucose 6-phosphate dehydrogenase (G6PD). It, was severe in erythrocytes resulting in an impairment, in the production of NADPH, a coenzyme required, by glutathione peroxidase for the inactivation of free, radicals (superoxide and H2O2). The drugs such as, primaquine cause increased generation of free, radicals which cannot be effectively inactivated due, to G6PD deficiency. This leads to hemolysis of RBC, and consequently jaundice, as is evident from the, laboratory data-reduced hemoglobin and increased, serum bilirubin., , CASE STUDY, , 8, , A 30-year old man was admitted in a cardiology, ward after he complained chest pain. His clinical, and biochemical investigations (ECG changes,, isoenzymes of creatine phosphokinase etc.) indicated, that he suffered a mild myocardial infarction. Timely, intervention and appropriate treatment saved him, from death. The lipid profile data of this patient are, given in the next page.
Page 784 :
774, Parameter, , BIOCHEMISTRY, , Subject (mg/dl) Reference range, , Serum total cholesterol, LDL-cholesterol, HDL-cholesterol, VLDL-cholesterol, Serum triglycerides, , 450, 380, 40, 30, 150, , 150-225, 80-150, 30-60, 20-40, 75-150, , Diagnosis and discussion, This man had highly elevated LDL-cholesterol, while other lipid profile parameters are within the, normal limits. This is a case of familial, hypercholesterolemia (hyperlipoproteinemia type, IIa). The victims of this disorder have decreased, number of LDL receptors. They have very high risk, of coronary heart diseases in the 3rd and 4th decades, of life. Lipid-lowering drugs, besides low cholesterol,, low fat diet and regular exercise are useful for these, people., , CASE STUDY, , 9, , A 5-year old boy had delayed developments, and was unable to speak or walk properly. He was, found to be mentally retarded with characteristic, seizures and tremors. The boy had a plasma, phenylalanine level of 30 mg/dl (reference range of, 1-2 mg/dl). His urine gave a positive ferric chloride, test, indicating an elevated excretion of, phenylpyruvate., Diagnosis and discussion, An elevation is plasma phenylalanine, concentration with an increased excretion of, phenylpyruvate in urine, supported by the clinical, manifestations, clearly indicate that the boy was a, victim of phenylketonuria (PKU). This is an inborn, error of phenylalanine metabolism due to a defect in, the enzyme phenylalanine hydroxylase. As a result, of this, phenylalanine cannot be converted to, tyrosine, hence gets diverted to alternate pathways,, producing metabolites which are directly or indirectly, inolved in growth failure and mental retardation., The treatment for PKU is to provide diet low or, deficient in phenylalanine, so that their plasma, phenylalanine levels are kept within the normal, limits., , CASE STUDY, , 10, , A 55-year old man complained severe pain in, the joints. He was a non-vegetarian and consumed, alcohol occasionally. His laboratory findings are as, follows., , Parameter, , Subject, , Reference range, , Serum uric acid, Blood urea, Urinary uric acid, Urinary pH, , 12 mg/dl, 25 mg/dl, 2.5 g/day, 4.5, , 3-7 mg/dl, 15-40 mg/dl, 0.5-0.7 g/day, 5.5-7.0, , The subject responded to the treatment by the, drug-allopurinol., Diagnosis and discussion, Elevation of uric acid in serum and urine along, with case history, further supported by the response, of the subject to the drug allopurinol, clearly indicate, that the patient was suffering from gout. Overproduction of uric acid, the excretory end product of, purine metabolism, causes gout. Crystals of sodium, urate get deposited in the joints causing gouty arthritis., Allopurinol competitively inhibits the enzyme, xanthine oxidase and lowers the formation of uric, acid, hence the relief by administering this drug., , CASE STUDY, , 11, , A 4-year old boy showed signs of learning, disability and aggressive behaviour, besides pain in, the joints. It was observed that he had an irresistible, urge to bite his fingers and lips. The laboratory, investigations revealed that the boy had serum uric, acid concentration of 10 mg/dl (reference range, 4-6 mg/dl)., Diagnosis and discussion, The clinical manfestations along with elevated, serum uric acid level support that the boy was, suffering from Lesch-Nyhan syndrome. This is an, inborn error of purine metabolism (salvage pathway)., It is a sex-linked disorder affecting only males due to, the deficiency of the enzyme hypoxanthine-guanine, phosphoribosyl transferase (HGPRT). Lesch Nyhan, syndrome is characterized by increased production, of uric acid, often causing gouty arthritis, besides, the neurological abnormalities., , CASE STUDY, , 12, , An African negro boy (aged 12 years), studying, in an Indian High School, was admitted in a hospital, with complaints of fever and severe pain in the arms, and legs. He was found to have hepatosplenomegaly., The boy’s laboratory investigative data are given in, the next page.
Page 785 :
775, , Appendix VI : CASE STUDIES WITH BIOCHEMICAL CORRELATIONS, , Parameter, , Subject, , Hemoglobin, , 7 g/dl, , Hematocrit, , 20 %, , Reference range, 14-16 g/dl, 40-50 %, , Hemoglobin, electrophoresis, , A distinct HbS, band with slower, movement than, adult hemoglobin, (HbA) detected, , Microscopic, examination of, blood smear, , Crescent (sickle), shaped, erythrocytes, , —, , —, , ORGAN FUNCTION DISORDERS, CASE STUDY, , 14, , An 18-year old male medical student had the, complaints of loss of appetite (anorexia), nausea,, headache and malaise. On clinical examination, his, liver was found to be slightly enlarged. He was passing, pale coloured stools but dark coloured urine. The, data of his laboratory investigations are as follows., , Parameter (serum), , Subject, , Bilirubin (total), (van den Bergh reaction, , 8 mg/dl, Biphasic, , Reference range, 0.2-1 mg/dl, Negative), , Diagnosis and discussion, , Conjugated bilirubin, , 4.5 mg/dl, , <0.4 mg/dl, , The laboratory data of the body with sickleshaped erythrocytes and altered hemoglobin, electrophoretic pattern with a distinct Hbs band, supported by anemia and reduced hematocrit, along, with the clinical manifestations support the diagnosis, of sickle cell anemia. This disorder primarily occurs, in black population and the patients may die before, they reach adulthood (< 20 years). Sickle cell anemia, is caused by an abnormality in hemoglobin. It occurs, due to the replacement of glutamate by valine at the, 6th position of E-chain of hemoglobin., , Unconjugated bilirubin, , 3.5 mg/dl, , <0.6 mg/dl, , CASE STUDY, , 13, , A school boy aged 12 years complained of, abdominal pain and was admitted in a hospital. He, was weak and tired, and had a history of behavioural, disturbance and epileptic form of seizures. Clinical, examination revealed that the boy had an enlarged, liver. His cornea showed the presence of Kayser, Fleishw ring. The laboratory data of the boy are, given below., , Parameter, , Subject, , Serum copper, , 40 Pg/dl, , 100-200 Pg/dl, , 5 mg/dl, , 25-50 mg/dl, , 200 Pg/dl, , < 25 Pg/dl, , Serum ceruloplasmin, Urinary copper, , Reference range, , Diagnosis and discussion, This boy had low serum level of copper and, ceruloplasmin with an elevated copper excretion in, urine. The clinical manifestations and the, biochemical data indicate that the boy was suffering, from Wilson’s disease. This disorder is associatd, with the deposition of copper in liver, kidney and, brain., , Alanine transaminase (ALT), , 170 IU/l, , 5-40 IU/l, , Aspartate transaminase (AST) 80 IU/l, , 5-45 IU/l, , Alkaline phosphatase (ALP), , 3-13 KAU/dl, , 20 KAU/dl, , Diagnosis and discussion, A biphasic van den Bergh positive reaction, (elevated serum conjugated and unconjugated, bilirubin), along with increased activity of ALT and, AST clearly indicates that this is a case of hepatic, jaundice. This is due to impairment in liver cell, function caused by viral infection (viral hepatitis),, poisons and toxins or cirrhosis of liver. Damage to, the liver cells adversely affects the bilirubin uptake, and its conjugation (hence conjugated and, unconjugated bilirubins are elevated). The pale, coloured stools are due to the absence of, stercobilinogen while the dark coloured urine is due, to urobilinogen., , CASE STUDY, , 15, , A 20-year old man was treated for malaria a, month ago. For the past one week, he was excreting, slightly dark coloured urine and dark brown coloured, stools. His laboratory findings are given hereunder., , Parameter (serum), Bilirubin (total), (van den Bergh reaction, Conjugated bilirubin, Unconjugated bilirubin, Alanine transaminase (ALT), , Subject, , Reference range, , 7 mg/dl, 0.2-1 mg/dl, Indirect positive Negative), 0.4 mg/dl, <0.4 mg/dl, 6.6 mg/dl, <0.6 mg/dl, 30 IU/l, , 5-40 IU/l, , Aspartate transaminase (AST) 40 IU/l, , 5-45 IU/l, , Alkaline phosphatase (ALP), , 3-13 KAU/dl, , 10 KAU/dl
Page 786 :
776, , BIOCHEMISTRY, , Diagnosis and discussion, , CASE STUDY, , Following a malarial attack, the man developed, hemolytic jaundice. This was due to an excessive, breakdown of erythrocytes releasing hemoglobin. The, heme degraded to bilirubin cannot be effectively, conjugated, as a result unconjugated bilirubin in the, serum was elevated. The laboratory data — indirect, van den Bergh reaction positive with no increase in, the activities of serum ALT, AST and ALP support the, diagnosis of hemolytic jaundice. The urine was dark, coloured due to increased excretion of urobilinogen, while the feces were dark brown due to, sterocobilinogen., , CASE STUDY, , 16, , A 30-year old man had fever and abdominal, pain. His stools were pale in colour and contained, fat (steatorrhea). The man’s urine was found to be, dark in colour. His laboratory findings are given, below., , Parameter (serum), , Subject, , Reference range, , Bilirubin (total), 12 mg/dl, 0.2-1 mg/dl, (van den Bergh reaction, Direct positive, Negative), Conjugated bilirubin, 11.5 mg/dl, <0.4 mg/dl, Unconjugated bilirubin, 0.5 mg/dl, <0.6 mg/dl, Alanine transaminase (ALT) 60 IU/l, 5-40 IU/l, Aspartate transminase (AST), Alkaline phosphatase (ALP), , 65 IU/l, 180 KAU/dl, , 5-45 IU/l, 3-13 KAU/dl, , In addition, the urine gave a positive test for bile, salts (Hay’s test) and bile pigments (Fouchet’s test)., Diagnosis and discussion, A direct van den Bergh reaction reflecting an, increase in conjugated bilirubin, along with an, elevation in serum alkaline phosphatase indicates, that the man was suffering from obstructive jaundice, (cholestasis). A marginal increase in the activity of, ALT and AST is also observed in this disorder., Cholestasis is due to an obstruction (caused by gall, stones, tumors etc.) that prevents the passage of bile, into the intestine. The urine was dark in colour due, to the excretion of conjugated bilirubin while the, stools were pale in colour due to the deficiency of, stercobilinogen, a pigment derived from bilirubin., Feces contained excess fat indicating impairment in, fat digestion and absorption due to the absence of, bile sats., , 17, , A 3-day old male baby had yellow colouration, of the sclerae of the eyes. His urine was also found, to be yellow in colour. The laboratory investigations, revealed that the serum total bilirubin concentration, was 18 mg/dl (normal <1 mg/dl), most of it being, unconjugated (van den Bergh indirect positive). The, paediatrician advised phototherapy for the baby., Diagnosis and discussion, About 50% of the normal new born babies, develop physiological neonatal jaundice after 30, hours after birth, as was described in this case. This, is due to increased hemolysis coupled with immature, hepatic system for the uptake, conjugation and, secretion of bilirubin. The activity of the enzyme, UDP-glucuronyl transferase is low in the newborn., In severe forms of hyperbilirubinemia, phototherapy, is advised. The ultraviolet rays of the light isomerize, bilirubin into a non-toxic form. It may be noted that, the neonatal physiological jaundice usually, disappears within 10 days of birth., , CASE STUDY, , 18, , A 55-year old man was brought to the hospital, with severe chest pain, breathlessness and vomiting., He could be rushed to the city hospital 5 hours after, the onset of chest pain. His blood was immediately, drawn, and the laboratory data are given below., , Parameter (serum), , Subject, , Creatine phosphokinase (CPK) 410 IU/l, , Reference range, 10-50 IU/l, , Aspartate transaminase (AST), , 67 IU/l, , 5-45 IU/l, , Lactate dehydrogenase (LDH), , 315 IU/l, , 50-200 IU/l, , Further laboratory evaluation revealed that the, isoenzymes CPK 2(MB) and LDH 1 were highly, elevated., Diagnosis and discussion, The man obviously had an attack of myocardial, infarction. This is supported by the laboratory findings, — elevation in CPK (MB), LDH (isoenzyme LDH1), and aspartate transaminase. It may be noted that, CPK starts to rise at 4-6 hours and reaches a peak, value within 24-30 hours. As regards LDH and AST,, they rise from the second day onwards. Thus, CPK is, the earliest marker enzyme for the diagnosis of, myocardial infarction.
Page 787 :
777, , Appendix VI : CASE STUDIES WITH BIOCHEMICAL CORRELATIONS, , CASE STUDY, , 19, , ENDOCRINE FUNCTION DISORDERS, , A 50-year old man, an occasional alcoholic,, had the complaint of severe abdominal pain following, a large meal or alcohol intake. He also had the, symptoms of nausea and vomiting. His laboratory, data are given hereunder., , Parameter, , Subject, , Serum amylase 500 Somogyi U/dl, , Reference range, 80-180 Somogyi U/dl, , Urine amylase 1320 Somogyi U/24 hr 250-850 Somogyi U/24hr, , CASE STUDY, , 21, , A 30-year old woman, married five years ago,, had no children. She complained of tiredness, weight, gain, cold intolerance, neuromuscular pains and, constipation. She was found to be anemic. Her, laboratory data are given hereunder., , Parameter (serum) Subject, , Reference range, , Triiodothyronine (T3), , 100 ng/dl, , 70-200 ng/dl, , Diagnosis and discussion, , Thyroxine, total (T4), , 4 Pg/dl, , 4.2-12 Pg/dl, , Elevation in the amylase activity is an indication, of pancreatitis. It may be noted that serum amylase, activity is elevated on the first day of the disease and, falls rapidly due to renal clearance. Very frequently,, the urinary amylase activity is more important for, the diagnosis of pancreatitis., , Thyroid stimulating, hormone (TSH), , CASE STUDY, , 20, , A 20-year old man had generalized edema of, the body with puffiness of the face in the mornings., His laboratory findings are given below., , Parameter, Serum total proteins, , Subject, , Reference range, , 4.5 g/dl, , 6-8 g/dl, , albumin, , 1.5 g/dl, , 3.5-5 g/dl, , globulins, , 3.0 g/dl, , 2.5-3.5 g/dl, , Serum cholesterol, , 350 mg/dl, , 150-225 mg/dl, , Blood urea, , 30 mg/dl, , 15-40 mg/dl, , Serum creatinine, , 1.2 mg/dl, , 0.5-1.5 mg/dl, , Urinary proteins, , 15 g/day, , Cholesterol, , 20 PU/ml, , 0.5-4 PU/ml, , 250 mg/dl, , 150-225 mg/dl, , Diagnosis and discussion, The woman with the described clinical, manifestations, elevated serum TSH and marginally, lower T4 and T3 was a case of hypothyroidism., Serum cholesterol level is also increased in, hypothyroidism. (Note : This has no diagnositic, importance, since serum cholesterol gets elevated in, several other diseases e.g. diabetes, nephrotic, syndrome)., , CASE STUDY, , 22, , A 13-year old boy had the complaints of, increased frequency of urination, increased appetite, and thirst. On routine examination, his urine was, found to contain glucose and ketone bodies. He had, a random blood glucose concentration 190 mg/dl., His laboratory data on the oral glucose tolerance, test (OGTT) are given below., , < 100 mg/day, , Fasting, , 2 hr, , 150, , 240, , The serum electrophoresis of the subject showed, a sharp and prominent D2-globulin band., , Blood glucose (mg/dl), , < 110, , < 140), , Diagnosis and discussion, , Urine glucose, , –ve, , ++, , Ketone bodies, , +, , ++, , This is a case of nephrotic syndrome ,, characterized by generalized edema heavy, proteinuria and hypoproteinemia (particularly low, albumin). Increased serum cholesterol level in this, disorder is quite common. The serum electrophoretic, pattern (a prominent D2-globulin band) supports the, diagnosis of nephrotic syndrome. Generalized edema, is due to low plasma colloidal osmotic pressure, as, a result of reduced albumin concentration., , (Normal, , Diagnosis and discussion, The laboratory data of the boy-increase in blood, glucose coupled with urinary excretion of glucose, and ketone bodies, along with the results obtained, in the OGTT point out that this is a good example of, juvenile onset diabetes mellitus (type I diabetes or, insulin dependent diabetes mellitus, IDDM). IDDM,, which mainly occurs in childhood, is characterized
Page 788 :
778, , BIOCHEMISTRY, , by almost total deficiency of insulin due to the, destruction of E-cells of pancreas. The boy has to be, treated by insulin administration., , CASE STUDY, , 23, , A 45-year old woman visited her physician with, complaints of increased appetite and thirst with high, frequency of urination. She also had the symptoms, of diminished or impalpable pulses in the feet, besides, gangrene of the feet. Her fasting blood glucose level, was 160 mg/dl, with no presence of glucose or ketone, bodies in urine. Her laboratory findings on the oral, glucose tolerance test are as follows., , Blood glucose (mg/dl), (Normal Reference, Urine glucose, Ketone bodies, , Fasting, , 2 hr, , 155, <110, –ve, –ve, , 205, <140), ++, –ve, , Diagnosis and discussion, The woman’s fasting (elevated) blood glucose, and the data obtained on OGTT indicate that she was, suffering from non-insulin dependent diabetes mellitus, (NIDDM or type II diabetes). NIDDM mainly occurs, in adults (>30 yrs) with a strong genetic predisposition., The patients of NIDDM can be usually treated with, oral hypoglycemic drugs. Before prescribing the drugs,, diet control and exercise are tried., , MISCELLANEOUS DISORDERS, CASE STUDY, , 24, , A 55-year old man was brought to the hospital, in a confused and semiconscious state. He had low, BP and feeble pulse. His breath had fruity odour., The data of his laboratory investigations are given, below., , Parameter, Blood pH, , Subject, , Reference range, , 7.1, , 7.35-7.45, , Plasma bicarbonate, (HCO–3 ), , 12 mmol/l, , 24-30 mmol/l, , 1.2 mmol/l, , 1.2 mmol/l, , Plasma carbonic acid, (H2CO3), , Diagnosis and discussion, The blood pH was reduced due to a decrease in, plasma bicarbonate (HCO–3) concentration. This is, case of metabolic acidosis. The blood glucose level, was highly increased along with urinary excretion of, glucose and ketone bodies. The breath of the subject, was fruity due to exhalation of acetone. Obviously,, this diabetic patient was not under control. The, acidosis was due to the accumulation of ketone, bodies. This may be appropriately regarded as, diabetic ketoacidosis., , CASE STUDY, , 25, , One patient with severe vomiting and hyokalemia, was admitted in an intensive care unit. His laboratory, data and acid-base status are given below., , Parameter, Blood pH, Plasma bicarbonate, (HCO–3 ), Plasma carbonic acid, (H2CO3), (pCO2 40 mm Hg), , Subject, , Reference range, , 7.7, , 7.35-7.45, , 42 mmol/l, , 24-30 mmol/l, , 1.2 mmol/l, , 1.2 mmol/l, , Diagnosis and discussion, This patient with increased blood pH due to an, elevation in the plasma bicarbonate concentration is, a typical case of metabolic alkalosis., , CASE STUDY, , 26, , A 6-year old boy had high temperature with, sweats during sleep, marked tachycardia and loss of, weight. He also had the complaints of headache,, neck stiffness and vomiting. The liver was found to, be slightly enlarged. The following are the laboratory, findings of this boy., , Parameter, , Subject, , Reference range, , Cerebrospinal fluid (CSF), Glucose, , 30 mg/dl, , 50-75 mg/dl, , Proteins, 100 mg/dl, Chloride, 105 mEq/l, Blood glucose, 100 mg/dl, (simultaneously collected), , 15-40 mg/dl, 120-130 mEq/l, 70-100 mg/dl, , Blood glucose (random) 580 mg/dl, , <130 mg/dl, , Diagnosis and discussion, , Blood urea, , 40 mg/dl, , 15-40 mg/dl, , Serum creatinine, , 1.5 mg/dl, , 0.5-1.5 mg/dl, , Urine sugar, , 4+, , negative, , Urine ketone bodies, , 3+, , negative, , A decrease in the CSF glucose and chloride, concentrations along with an increase in protein, level indicates that the boy was a victim of, tuberculosis meningitis. This is supported by the, clinical manifestations.
Page 791 :
781, , INDEX, Arginase, 339, Arginine, 48, 336, 339,, Arginosuccinase, 339, Arginosuccinate, 337, Arginosuccinic aciduria, 340, Aromatic aminoacid decarboxylase,, 350, Aromatic amino acids, 47, 345, Arsenate, 246, Arsenite, 256, Arthritis, 7, 352, 394, 647, Ascorbic acid, 18, 132, 275, 414, Asparaginase, 106, 371, Asparagine, 46, 370, Aspartame, 67, Aspartate transaminase (aspartate, aminotransferase), 107, 112,, 144, 455, Aspartate transcarbamoylase, 102,, 399, Aspartic acid (aspartate), 46, 337,, 370, Aspirin, 640, 646, AST/ALT ratio, 455, Asymmetric carbon, 44, 706, Atherosclerosis, 280, 316, 326, Atkins diet, 517, ATP (see adenosine triphosphate), ATP-ADP cycle, 223, ATPase, 230, ATP synthase, 230, Atractyloside, 234, Atrial natriuretic factor, 472, Atropine, 640, Atriopeptin, 472, Augmentation histamine test, 464, Autoantibodies, 736, Autograft, 736, Autoimmune diseases, 736, Autoimmunity, 736, Automated DNA sequencer, 591, Autoradiography, 589, 718, Autosomal dominant, inheritance, 739, Autosomal recessive inheritance,, 740, Autosomes, 739, Avidin, 146, 667, 8-Azaguanine, 73, Azathioprine, 73, Azotemia, 341, Azure-A-resin, 465, , B, Bacteriophages, 583, Bad cholesterol, 316, Balanced diet, 514, Barbiturates, 641, Barfoed’s test, 16, Barth syndrome, 36, Basal acid output, 464, Basal metabolic rate, 504, Basal metabolism, 504, Base (s), 474, 709, Base excision repair, 537, Basic amino acids, 47, Beer-Lambert law, 726, Bence-Jones proteins, 189, 692, Benedict’s test, 16, Benedict-Roth apparatus, 504, Bent DNA, 76, Benzaldehyde, 639, Benzene, 639, Benzoic acid, 342, 639, Benzyl alcohol, 639, Bergstrom theory, 175, Beri-beri, 136, Betaine, 324, Bial’s test, 17, Bicarbonate buffer, 475, Bifunctional enzyme(s), 250, 265,, 398, Biguanides, 682, Bile, 173, Bile acids, 173, 313, Bile pigments, 214, 454, Bile salts, 173, 313, Bilirubin, 215, 454, 641, Bilirubin diglucuronide, 216, 641, Bilirubin glucuronyltransferase, 216, Bilirubin metabolism, 217, Biliverdin, 214, Biliverdin reductase, 215, Binding change model, 231, Biocatalyst, 85, Biocytin, 146, Bioenergetics, 221, Bioflavonoids, 159, Biogenic amines, 375, 667, Bioinformatics, 634, , Biological databases, 636, Biological oxidation, 221, Biological value of proteins, 512, Biomaker, 106, Biomembranes, 650, Biopterin, 345, 356, Biotin, 146, 259, 292, 298, Biotransformation, 638, 1,3-Bisphosphoglycerate, 247, 2,3-Bisphosphoglycerate, 200, 251, Bisphosphoglycerate mutase, 251, Biuret reaction, 61, Bitot’s spots, 123, Blood buffers, 475, Blood clotting, 190, Blood gas analysis, 484, Blood group antigens, 26, Blood pH, 474, Blood urea nitrogen, 341, Blotting techniques, 587, B-Lymphocytes, 733, Body fluids, 496, BMR (see basal metabolic rate),, Body mass index, 325, Bohr effect, 199, Bone marrow cells, Bovine spongiform, encephalopathy, 495, Bowman’s capsule, 459, Bradshaws test, 189, Bradykinin, 67, Branched chain amino acids, 45,, 363, Branched chain D-keto acid, dehydrogenase, 135, 365, Branched chain ketonuria, 365, Brain natriuretic peptide, 112, Brig’s-Haldane’s constant, 88, British antilewisite, 227, 642, Broad beta disease, 321, Bromosulphthalein test, 455, Bronze diabetes, 416, Brown adipose tissue, 233, 326, Brownian movement, 712, Buffering capacity, 711, Buffers, 475, 710, Buffer systems of blood, 475, Burkitt’s lymphoma, 688, Burning feet syndrome, 150, Butyric acid, 30
Page 795 :
785, , INDEX, Down’s syndrome, 741, Du Bois and Du Bois formula, 504, Duchenne muscular dystrophy,, 626, Dulcitol, 16, 277, Duodenal ulcer, 179, Dyslipidemias, 321, Dystrophin, 494, , E, ECoRI, 580, Edman’s reagent, 56, Edward’s syndrome, 741, Ehlers-Danlos syndrome, 489, Eicosanoids, 32, 644, Eicosapentanoic acid, 510, 649, Elaidic acid, 31, Elastins, 64, 689, Electrolyte balance, 470, Electron transport chain,, components, 226, inhibitors, 232, in prokaryotes, 236, organization, 225, oxiditive phosphorylation, 228, sites of ATP synthesis, 227, Electrophoresis, 724, of hemoglobins, 206, of plasma proteins, 182, of serum lipoproteins, 317, types, 725, Electroporation, 584, Electrostatic bonds, 59, ELISA (enzyme-linked, immunosorbant assay), 729, Elongation factors, 557, Embden-Meyerhof pathway, 245, Emphysema, 185, Emulsification of lipids, 173, Emulsions, 40, 712, Enantiomers, 13, Endocytosis, 172, 654, Endopeptidases, 170, Endoplasmic reticulum, 6, Endorphins, 436, Endothelium derived releasing, factor, 367, End product inhibition, 102, , Enediols, 15, Energy metabolism, 242, Energy requirements, 506, Engine driving model, 231, Enhancers, 547, 572, Enkephalin, 67, 436, 450, Enolase, 248, Enolization, 15, Enteroglucagon, 450, Enterohepatic circulation, 314, Enteropeptidase, 171, Enterokinase, 171, Enthalpy, 221, Entropy, 221, Environmental biochemistry, 662, Enzyme(s),, active site, 91, adaptive, 104, affinity, 89, analytical reagents, 106, classification, 86, clinical (diagnostic), importance, 166, commission (EC), 87, compartmentation, 103, constitutive, 104, effect of activators, 91, effect of pH, 90, effect of substrate, 88, effect of temperature, 90, extracellular, 86, immobilized, 106, induction and repression, 104, inhibition, allosteric, 95, 100, inhibition, competitive, 92, inhibition, irreversible, 94, inhibition, non-competitive, 93, inhibition, reversible, 92, intracellular, 86, kinetics, 88, Km value, 88, 92, mechanism of action, 98, monomeric, 87, oligomeric, 87, specificity, 95, substrate complex, 98, therapeutic agents, 105, units, 104, Vmax, 88, 92, Epidermal growth factor, 688, Epidermolysis bullosa, 489, Epigenetics, 576, Epimerases, 12, Epimers, 12, , Epinephrine, 144, 267, 287, 349,, 444, 678, Epoxide, 131, Epstein-Barr virus, 687, Ergocalciferol, 124, Ergosterol, 38, Erythrodextrin, 21, Erythromycin, 560, Erythropoietic protoporphyria, 214, Erythropoietin, 420, 459, Erythrose, 13, Essential amino acids, 48, 511, Essential fatty acids, 31, 509, Essential fructosuria, 280, Essential pentosuria, 276, Estimated average glucose, 683, Estradiol, 314, 447, Estriol, 447, Estrogens, 314, 442, 446, Ethanol, 263, 327, 639, Ethanolamine, 36, 303, Ethereal sulphate, 414, Ethics and human genome, 624, Ethylene, 360, Eugenics, 741, Eukaryotes, 4, 546, Eutrophication, 665, Exergonic reaction, 221, Exit site, 558, Exocytosis, 654, Exons, 548, Exopeptidases, 170, Extracellular enzymes, 86, Extracellular fluid, 468, Extracellular matrix, 487, Extrinsic factor of Castle, 153, Extrinsic pathway (of blood, coagulation), 191, Exudate, 499, Ex vivo gene therapy, 627, , F, Fab fragment, 187, Facilitated diffusion, 651, FAD, 137, 139, 225, 252, 335, Familial hypercholesterolemia, 626, Farber’s disease, 307
Page 796 :
786, Farnesyl pyrophosphate, 310, Fat(s), chemistry, 32, digestion and absoprtion, 174, nutrition, 509, Fat soluble vitamins, 118, Fatty acid(s), activation, 288, deficiency, 31, elongation, 302, essential, 31, isomerism, 31, nomenclature, 29, oxidation, 287, 381, peroxidation, 33, 128, 656, polyunsaturated, 31, 128, 316,, 509, saturated, 29, synthesis, 147, 297, unsaturated, 29, 292, Fatty acid cycle, 287, Fatty acid synthase, 298, Fatty liver, 322, Favism, 275, Fc fragment, 187, Feedback inhibition, 102, 389,, 399, Feedback regulation, 102, 312,, 392, Fehling’s test, 16, Ferritin, 415, Ferrochelatase, 210, Ferroxidase, 415, Fetal hemoglobin, 197, 201, Fetal lung maturity, 498, Fetal maturity, 498, D-Fetoprotein, 691, Feulgin staining, 18, Fiber, 22, 168, 316, 508, Fibrillin, 489, Fibrin, 190, Fibrin monomer, 190, Fibrinogen, 190, Fibrinolysis, 192, 327, Fibronectin, 489, Fibrinopeptides, 190, Fibrous proteins, 64, Fischer projections, 13, Fischer’s short hand models, 209, Fischer’s template theory, 98, Flame photometer, 727, Flatulence, 169, Flavin adenine dinucleotide, (see FAD), , BIOCHEMISTRY, Flavin mononucleotide (see FMN), Flavoproteins, 137, 227, Flocculation, 62, Fluid mosaic model, 650, Fluoridation, 421, Fluoride, 248, 420, Fluorine, 420, Fluoroacetate, 257, 420, Fluorometer, 727, Fluorosis, 420, 5-Fluorouracil, 73, FMN, 137, 139, 227, 334, Folacin, 153, Folate conjugase, 150, Folate trap, 156, Folic acid (folate), 150, Folinic acid, 151, Follicle stimulating hormone, 434,, 449, Follicular phase, 449, Food allergy, 172, Forbe’s disease, 269, Formaldehyde, 638, Formimino glutamic acid, 152,, 366, N-Formylmethionine, 557, Fouchets test, 455, Four-stranded DNA, 77, Fractional test meal, 464, Fragility test, 713, Frame shift mutations, 536, 553, Frederickson’s classification, 321, Free energy, 221, Free radicals, 66, 422, 510, 655,, 687, Friedewald formula, 315, Fructokinase, 278, 280, Fructosamine, 683, Fructosan, 20, Fructose 14, 21, 168, 278, Fructose 1,6-bisphosphatase, 261, Fructose 1,6-bisphosphate, 246,, 261, Fructose 2,6-bisphosphate, 250, Fructose 6-phosphate, 246, Fructose intolerance, 280, Fructosuria, essential, 280, FSH (see follicle stimulating, hormone), Fucose, 26, Fudge factor, 511, Fumarase, 256, , Fumaric acid, 706, Fumarate, 256, 339, Functional isomerism, 705, Furanose, 14, Furfural, 17, Futile cycles, 268, , G, Gabapentin, 51, G-proteins, 430, G-quartets, 77, G-tetraplex, 77, GABA shunt, 370, Galactitol, 277, Galactocerebroside, 307, Galactoflavin, 138, Galactokinase, 277, Galactosamine, 281, Galactose, 12, 276, Galactosemia, 277, Galactose 1-phosphate, uridyltransferase, 277, Galactose tolerance test, 457, E-Galactosidase, 167, 276, 308,, 568, Galactosuria, 277, Gall stones, 178, 314, Ganciclovir, 630, Gangliosides, 37, 308, Gas-liquid chromatography, 721, Gastric function tests, 463, Gastric HCl, 170, 463, Gastric inhibitory polypeptide, 450, Gastric juice, 170, Gastric lipase, 173, Gastric ulcers, 179, Gastrin, 450, 463, Gastrointestinal hormones, 67,, 450, 672, Gastrointestinal tract, 165, Gaucher’s disease, 308, Geiger counters, 717, Gelatin, 65, Gel electrophoresis, 725, Gel-filtration chromatography, 724, Gene(s), amplification, 574, 688, chip, 600, constitutive, 567
Page 797 :
787, , INDEX, expression, 571, inducible, 567, library, 597, mutations, 535, rearrangement, 575, regulation of expression, 566, therapy, 625, Gene augmentation, 625, Gene augmentation therapy, 625, Gene cloning, 578, Gene delivery by viruses, 629, Gene family, 202, Gene inhibition therapy, 625, Gene libraries, 596, Gene linkage map, 620, Gene therapy, 625, Genetic code, 551, Genetic engineering, 578, 585, Genetic immunization, 609, Genetics, 737, Genome, 542, 619, Genomic library, 596, Genu valgum, 421, Geometric isomerism, 31, 706, Geranyl pyrophosphate, 311, Germ cell gene therapy, 625, Gibbs-Thomson principle, 716, Gigantism, 434, Gilbert’s disease, 219, Glibenclamide, 682, Globin(s), 64, 196, 202, Globular protein, 64, Globulins, 64, 185, Glomerular filtration rate, 459, Glomerulus, 459, Glossitis, 138, Glucagon, 262, 267, 288, 312,, 674, 678, Glucan, 20, Glucocorticoids, 312, 441, 678, Glucogenic amino acids, 48, 261,, 372, Glucokinase, 246, Gluconeogenesis, 147, 258, 381,, 675, Gluconic acid, 16, Glucosamine, 281, Glucosan, 20, Glucose, absorption, 168, alanine cycle, 262, , blood level, 244, 674, homeostasis, 674, insulin secretion, 670, metabolism, 244, monitor-liver, 244, structure, 13, tolerance test, 679, toxicity, 682, uptake by tissues, 671, Glucose 6-phosphatase, 261, 266,, 269, 395, Glucose 6-phosphate, 246, 266,, 271, Glucose 6-phosphate, dehydrogenase, 109, 271, 274, Glucose transporters, 245, E-Glucosidase, 308, Glucosuria (see glycosuria), Glucuronic acid, 16, 275, 640, E-Glucuronidase, 216, Glucovanillin, 18, Glutamate dehydrogenase, 274,, 334, Glutamic acid (glutamate), 46,, 150, 333, 365, 369, 479, Glutaminase, 336, 369, 479, Glutamine, 336, 369, 398, 479,, 641, Glutamine synthetase, 336, J�Glutamyl cycle, 66, 172, J�Glutamyl transpeptidase, 107,, 113, 454, Glutaric acid, 92, Glutathione, 65, 172, 342, 369,, 641, Glutathione peroxidase, 274, 422,, 659, Glutathione reductase, 138, 274,, 396, 659, Glutelins, 64, Glycans, 20, Glycated hemoglobin, (see glycosylated hemoglobin), Glyceraldehyde, 10, 44, 707, Glycemic index, 507, Glyceraldehyde 3-phosphate, dehydrogenase, 246, 252, Glycerol, 32, 259, 287, Glycerokinase, 287, Glycerol-phosphate shuttle, 234, Glycerophospholipids, 29, 34, Glycine, 45, 210, 341, 641, , Glycinuria, 344, Glycine oxidase, 343, Glycocalyx, 650, Glycocholic acid, 173, 313, 641, Glycine synthase, 342, Glycogen, 21, 263, 382, Glycogenesis, 263, Glycogenin, 264, Glycogenic amino acids, 48, (see glucogenic amino acids), Glycogenolysis, 265, 675, Glycogen phosphorylase, 103,, 145,265, Glycogen storage diseases, 269, Glycogen synthase, 264, Glycolipids, 29, 37, 307, Glycolysis, 245, 381, Glycoprotein hormones, 434, Glycoproteins, 25, 64, 190, Glycosaminoglycans, 22, 281, Glycosidases, 166, Glycosides, 17, Glycosidic bonds, 17, Glycosphingolipids, 37, Glycosuria, 674, 681, Glycosylated hemoglobin, 197,, 683, Glycosylation, 562, Glyoxylate cycle, 281, Glyoxysomes, 7, 282, Gmelin’s test, 455, Goiter, 418, 440, Goitrogens, 440, 667, Golgi apparatus, 6, Gonadal hormones, 445, Gonadotropin releasing hormone,, 431, Gonadotropins, 434, Good cholesterol, 316, Gramicidin, 67, Gratuitous inducers, 569, Greying of hair, 349, Gout, 394, 396, Gouty arthritis, 270, 394, Grave’s disease, 440, Growth factors, 689, Growth hormone, 433, 678, Growth hormone releasing, hormone, 431, Guanidoacetate, 343
Page 798 :
788, Guanine, 70, 391, Guanosine, 72, 393, diphosphate, (GDP), 391, monophosphate (GMP), 390, triphosphate (GTP), 391, L-Gulonate, 275, L-Gulonolactone, 276, L-Gulonolactone oxidase, 132, 275, Gusten, 419, Guthrie test, 352, Gyrase, 528, , H, Hagemen factor, 191, Hair waving, 490, Half-maximal velocity, 89, Hapten, 729, Haptoglobin, 185, Haptoglobin-hemoglobin complex,, 185, Hartnup’s disease, 173, 358, 654, HAT medium, 731, Haworth projections, 15, Hay’s test, 716, Heat shock protein, 560, Heat stroke, 663, Heat syncope, 663, Heavy meromyosin, 493, Helicobacter pylori, 179, D-Helix, 56, Helix-loop-helix motif, 574, Helix-turn-helix motif, 573, Hematocrit, 182, Heme, 197, 210, 214, 342, 414, Heme oxygenase, 214, Heme synthase, 210, Hemicellulose, 508, Hemin, 210, Hemochromatosis, 416, Hemocuprein, 417, Hemocyanin, 417, Hemoglobin(s), abnormal, 202, as buffer, 476, Hemoglobin(s) contd., biochemical functions, 197, CO2 transport, 199, derivatives, 202, glycated, 683, O2 transport, 198, , BIOCHEMISTRY, oxygen dissociation curve, 200, structure, 196, T and R forms, 198, types, 197, Hemoglobin A1c, 197, Hemoglobin C, 206, Hemoglobin D, 206, Hemoglobin E, 206, Hemoglobin H disease, 207, Hemoglobinopathies, 203, Hemolysis, 275, 714, Hemolytic jaundice, 216, 457, Hemophilia, 193, Hemoproteins, 414, Hemosiderin, 415, Hemosiderosis, 416, Hemostasis, 190, Henderson-Hasselbach equation,, 475, Heparin, 24, 191, Hepatic jaundice, 217, 457, Hepatitis, 107, Hepatitis B, 609, Hepatitis B vaccine, 609, Hepatocuprein, 417, Hepatoflavin, 137, Hepatolenticular degeneration, 417, Heptose, 11, Hereditary coproporphyria, 214, Hereditary fructose intolerance,, 280, Hereditary persistence of fetal, hemoglobin, 197, Heredity, 738, Her’s disease, 269, Heterocyclic amino acids, 48, Heterocyclic rings, 704, Heteroduplex DNA, 533, Heterogeneous nuclear RNA, 81,, 547, Heteropolysaccharides, 10, 20, 22, Hexose monophosphate shunt,, 270, 381, Hexokinase, 246, 250, Hexoses, 11, HGPRTase, 391, 396, High density lipoproteins, (see HDL), High-energy bonds, 223, High-energy compounds, 222, High-energy phosphates, 223, High-fructose corn syrups, 278,, 507, High performance liquid, , chromatography, 624, High-sensitive CRP, 186, Hippuric acid, 342, 458, Hirudin, 599, Histamine, 144, 366, 377, 464, Histamine stimulation test, 464, Histidase, 335, 366, Histidine, 46, 366, Histidinemia, 366, Histone acetylation, 571, Histone code, 576, Histone deacetylase, 576, Histones, 64, 79, HIV (human immunodeficiency, virus), 695, HMG CoA, 294, 310, HMG CoA reductase, 102, 312, Hogness box, 546, Hollander’s test, 465, Holliday model, 532, Holoenzyme, 87, Homeostasis of plasma calcium,, 407, Homeostasis of blood glucose, 674, Homocyclic rings, 703, Homocysteine, 154, 360, Homocysteine methyltransferase,, 156, Homocystinuria(s), 362, Homogentisate, 346, 352, Homogentisate oxidase, 352, Homologous recombination, 532, Homopolysaccharides, 10, 20, Homoserine, 52, 145, Hoogsteen hydrogen bonds, 76, Hopkins-Cole test, 61, Hormone(s), 427, adrenal cortex, 441, adrenal medulla, 444, anterior pituitary, 432, classification, 427, gastrointestinal, 449, gonads, 445, hypothalamic, 431, mechanism of action, 428, ovarian, 446, pancreatic, 670, 674, posterior pituitary, 437, receptors, 428, second messengers, 430, thyroid, 437, vitamin D, 127, Hormone sensitive lipase, 287, Host cells in cloning, 581, House keeping genes, 567, Human artificial chromosome, 583
Page 806 :
796, Radioactive pollution, 666, Radiation therapy, 657, Radioimmunoassay, 729, Raffinose, 169, Rancidity, 33, Rapaport-Leubering cycle, 200,, 251, Reactive oxygen species, 656, Recombinant DNA technology,, 578, Recombinant insulin, 607, Recombinant ribozymes, 82, Recombinant vaccines, 688, Recommended dietary (daily), allowance, 514, Redox potential, 224, Reducing equivalents, 234, Reducing sugars, 16, Reduction, 224, Refsum’s disease, 293, Regulation of,, citric acid cycle, 257, fatty acid synthesis, 301, gene expression, 566, gluconeogenesis, 262, glycogenesis, 266, glycogenolysis, 266, heme synthesis, 210, ketogenesis, 296, purine synthesis, 392, pyrimidine synthesis, 398, urea cycle, 339, Release factors, 558, Reichert-Meissl number, 34, Renal function tests, 459, Renal glycosuria, 460, 681, Renal plasma flow, 459, Renal regulation of pH, 477, Renal rickets, 128, 408, Renal threshold substances, 460, Renaturation of DNA, 79, Renaturation of protein, 62, Renin, 459, 472, Rennin, 170, Renin-angiotensin, 472, Replication,, in eukaryotes, 527, in prokaryotes, 524, Replication bubbles, 524, Replication factor C, 528, Replication fork, 526, Replication protein A, 528, Repression, 566, , BIOCHEMISTRY, Repressor protein, 566, Reserpine, 356, Resistin, 679, Respiratory acidosis, 482, Respiratory alkalosis, 482, Respiratory burst, 657, Respiratory chain (see electron, transport chain), Respiratory distress syndrome, 37, Respiratory regulation of pH, 476, Repiratory quotient, 503, Resting metabolic rate, 504, Restriction endonucleases, 579, Restriction fragment length, polymorphisms, 603, Restriction fragment map, 620, Retina, 121, Retinal, 119, Retinoic acid, 119, Retinol, 119, Retinol binding protein, 119, Retrotransposition, 534, Retroviruses, 550, 688, Reverse transcriptase, 550, 688, Reverse transcription, 550, Reverse triidothyronine, 438, Rf value, 721, Reye’s syndrome, 400, Rhamnohexose, 9, Rheumatoid arthritis, 647, Rhodopsin, 121, Rhodopsin cycle, 121, Rho factor, 544, Ribitol, 137, Riboflavin, 137, Ribonuclease P, 105, Ribonucleases, 179, Ribonucleic acid (see RNA), Ribonucleosides, 71, Ribonucleotide reductase, 392, Ribonucleotides, 72, 79, 387, Ribose, 71, Ribose 5-phosphate, 272, 387, Ribosomal RNA, 79, 82, Ribosomes, 553, 557, Ribozymes, 105, 558, Ribulose, 11, 14, Ribulose 5-phosphate, 271, Richner-Hanhart syndrome, 352, Rickets, 127, 408, Rifampin, 549, RNA, 79, 523, 542, 589, RNA editing, 549, , RNA polymerase, 543, 546, RNA primer, 524, RNA viruses, 550, 688, Rods, 121, Rotary motor model, 230, Rotenone, 227, Rothera’s test, 295, Rous sarcoma virus 687, Ruhemann’s purple, 51, Ryle’s tube, 646, , S, Sakagauchi reaction, 61, Salicylic acid, 640, Salivary amylase,166, Salkowski’s test, 38, Salting in, 60, Salting out, 60, 182, Salvage pathways, 303, 391, 400, Sanfilippo syndrome, 281, Sanger’s reagent, 56, Saponification, 33, Saponification number, 33, Sarcopenia, 492, Schiff base, 143, 333, Scleroproteins, 64, Scurvy, 134, Secondary messengers, 428, 430, Secondary structure of protein, 56, Secretin, 170, 450, Sedimentation coefficient, 728, Sedoheptulose, 14, Sedoheptulose 7-phosphate, 273, Selenium, 49, 128, 421, 659, Selenocysteine, 48, 422, Selenoproteins, 48, Selenosis, 422, Sequenator, 56, Serine, 45, 361,371, Serine proteases, 95, 171, Serotonin, 144, 356, Serotonin pathway, 354, Severe combined, immunodeficiency, 397, Sex chromosomes, 738, Sex hormones, 445, Sex-linked inheritance, 740, SGOT (see aspartate transaminase), SGPT (see alanine transaminase), Shine-Dalgarno sequence, 557
Page 807 :
797, , INDEX, Short interspersed nuclear, elements, 622, Short (simple) tandem repeats,, 603, 620, SI units, 104, Sialic acid, 18, Sickle-cell anemia, 203, 601, Sickle-cell hemoglobin, 203, Sickle-cell trait, 204, Sigma factor, 543, Silent mutations, 536, Simple tandem repeats, 603, Single nucleotide polymorphisms,, 606, 622, Single-stranded DNA binding, proteins, 525, Small dense LDL, 316, Site-directed mutagenesis, 597, Sitosterol, 39, Small nuclear ribonucleoprotein, particles, 548, Small nuclear RNA, 81, 550, Snake venom, 306, Soaps, 40, Sodium, 411, Sodium fluoride, 284, 421, Soluble RNA, 81, Solutions, 711, Somatic cell gene therapy, 625, Somatomedin C, 434, Somatostatin, 433, Somatotropin, 433, Sorbitol, 16, Southern blot technique, 587, Specific dynamic action, 505, Specific optical rotation, 14, Spectrophotometer, 726, Spermidine, 375, Spermine, 375, Sphingolipidoses, 308, Sphingolipids, 308, Sphingomyelinase, 308, Sphingomyelins, 36, Sphingosine, 36, Splicing of introns, 548, Squalene, 310, Standard free energy, 221, Starch, 20, Starvation, 383, Stearic acid, 30, Steatorrhoea, 117, Stercobilin, 216, Stereoisomerism, 10, 705, Stereoisomers, 10, 705, Stereospecificity, 95, , Steroid (s), 37, Steroid derivative(s), 37, Steroid hormones, 38, 314, Sterols, 38, Stigmasterol, 39, Streptokinase, 105, 193, Streptomycin, 18, 560, Subcellular organelles, 727, Substrate level phosphorylation,, 224, Substrate strain theory, 99, Subunit vaccines, 608, Succinate (succinic acid), 93, 255, Succinate dehydrogenase, 93, 227,, 255, Succinyl CoA, 149, 210, 255, 292, Sucrase,167, Sucrose, 19, Sudden infant death syndrome, 291, Sugars, 10, Suicide enzyme, 645, Suicide inhibition, 95, Suicide substrate, 257, Sulfate, 362, 413, 642, Sulfatides, 37, Sulfhydryl groups, 46, 59, 659, Sulfite, 362, Sulfonamides, 94, 153, 159, 390,, 642, Sulfonylureas, 682, Sulfur, 413, Sulfur amino acids, 46, 357, Sulfur dioxide, 663, Sun-shine vitamin, 124, Superoxide, 655, Superoxide dismutase, 659, Surface tension, 716, Surfactants, 716, Svedberg units, 728, Symport system, 653, Synovial fluid, 716, , T, T3 (see triiodothyronine), Tangier’s disease, 322, Tanning of leather, 61, Taq DNA polymerase, 594, Tartarate labile ACP, 107, TATA box, 544, 546, Taurine, 313, 362, , Tauri’s disease, 269, Taurocholic acid, 173, 313, Tautomeric forms, 70, Tautomerism, 705, Tautomerization, 15, TCA cycle (see citric acid cycle), T-cells, 734, Teeth, 405, Telomerase, 530, Telomeres, 530, Temperature coefficient, 90, Template strand, 543, Termination,, of transcription, 544, of translation, 558, Termination codons, 551, 558, Tertiary structure of protein, 58, Testosterone, 446, Tetany, 408, Tetracycline, 560, Tetrahydrobiopterin, 346, 357, Tetrahydrofolate, 150, 363, 389, Tetramer, 58, Tetroses, 11, Thalassemia(s), 206, Theobromine, 71, Theophylline, 71, Therapeutic diets, 518, Thermodynamics, 221, Thermogenesis, 327, Thermogenic action, 505, Thermogenin, 327, Thiamine, 135, Thiamine pyrophosphate, 135,, 252, 272, Thiazole ring, 135, Thin layer chromatography, 721, Thiobarbituric acid reactive, substances, 657, Thiocyanate, 642, Thioether group, 46, Thiokinase, 177, 288, Thiolase, 289, 296, 311, Thiophene ring, 146, Thiophorase, 296, Thioredoxin, 98, 392, Thiosulfate, 642, Thiouracil, 438, Thiourea, 438, Three Ds, 141, Threonine, 45, 372, Threshold substances, 460, Thirst centre, 469, Thrombin, 190, Thromboplastin, 191, Thrombosis, 648
Page 809 :
799, , INDEX, Vanillyl mandelic acid, 445, Variable number tandem repeats,, 604, 620, Variegate porphyria, 214, Vasoactive intestinal peptide, 450, Vasopressin, 66, 437, Vectors, 582, Viral RNA, 688, Viscosity, 715, Visual cycle, 121, Vital force theory, 703, Vitamers, 118, 143, Vitamin A, 118, Vitamin B1 (see thiamine), Vitamin B2 (see riboflavin), Vitamin B6 (see pyridoxine), Vitamin B12 (see cobalamin), Vitamin C (see ascorbic acid), Vitamin D, 123, Vitamin E, 128, Vitamin E and selenium, 129, Vitamin K, 129, Vitamin P, 159, Vitamins, 116, classification, 117, definition, 116, fat soluble-general, 118, in TCA cycle, 256, nomenclature, 116, synthesis, 117, water soluble-general, 118, Vitellin, 65, Vitiligo, 353, Vitreous body, 715, VLDL (very low density, lipoproteins), 315, von Gierke’s disease, 269, Von Willenbrand’s disease, 193, , W, Wald’s visual cycle, 129, Warfarin, 131, Water, balance, 468, dissociation, 709, distribution, 46, endogenous, 469, exogenous, 469, functions, 468, intake, 469, output, 469, structure, 709, Water pollution, 664, Water tank model, 473, Watson and Crick model, 74, Waxes, 28, Wernicke-Korsakoff syndrome, 137,, 275, Western blot technique, 589, Whey proteins, 497, White adipose tissue, 326, Williams syndrome, 489, Wilson’s disease, 417, Wobble hypothesis, 552, World wide web, 636, , X, Xanthine, 71, 393, Xanthine oxidase, 393, Xanthine stone, 398, Xanthinuria, 398, , Xanthoproteic reaction, 61, Xanthurenate, 145, 354, X-chromosome, 397, 740, Xeaxanthin, 659, Xenobiotics, 638, Xenograft, 736, Xeroderma pigmentosum, 538, Xerophthalmia, 123, Xylitol, 273, Xylitol dehydrogenase, 273, Xylose, 11, Xylulose, 11, , Z, Zak’s test, 38, Zein, 65, Zellweger syndrome, 8, Zidovudine, 699, Zinc, 419, Zinc finger motif, 573, Zn-proteases, 171, Zona fasciculata, 441, Zona glomerulosa, 441, Zona reticularis, 441, Zollinger-Ellison syndrome, 465, Zone electrophoresis, 725, Zoo blot, 588, Zymogens, 102, 170, 562, Zymosterol, 310, Zwitterion, 49, 60