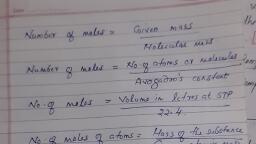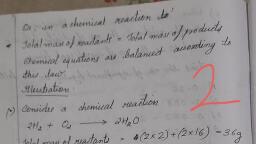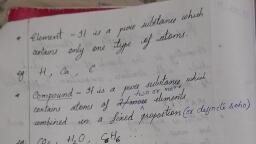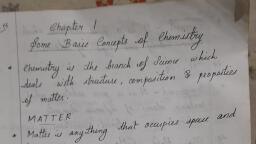Page 1 :
‘Mass and Weight, Mass is the amount of matter present in a body. It is a constant quantity. Its SI unit is kilogram (kg). Weight is the, gravitational force acting on a body. Itis a variable quantity. i.. it changes with place. Its SI unit is newton (N).., Volume (V), itis the space occupied by a body. Its S unit is m3. Other units are em3, mL, Lete., , 1m*=10%m’, , tem? = 1 mt, , 1L= 10" em’ (mL), , dm? = 10° em?, , , , , , Density (d), itis the amount of mass per unit volume., {.e. density = mass/volume. Its SI unit is kg/m?. But it is commonly expressed in g/cm., Temperature (T), Itis the degree of hotness or coldness of a body. It is commonly expressed in degree Celsius (°C). Other units are, degree Fahrenheit (°F), Kelvin (K) etc. its SI unit is Kelvin (K)., Degree Celsius and degree Fahrenheit are related as:, "F =9/5(°C) +32, Degree celsius and Kelvin are related as:, K=C+273.15, Precision and Accuracy, Precision refers to the closeness of various measurements for the sa, particular value to the true value of the result., Significant Figures, Every experimental measurement has some amount of unce‘ainty associated with it. The uncertainty in the, experimental or the calculated values is indicated by mentioning thé number of significant figures. Significant, {figures are meaningful digits which are known with certainty. Ve uncertainty is indicated by writing the certain, digits and the last uncertain digit., There are certain rules for determining the number of, 1. All non-zero digits are significant. For example, there are two significant figures., 2. Zeros preceding to first non-zero digit ire Fct significant. Such zero indicates the position of decimal point., Thus, 0.03 has one significant figure-and (0.0052 has two significant figures., 3. Zeros between two non-zero digit« ars significant. Thus, 2.005 has four significant figures., 4. Zeros at the end or right of a nuimber are significant if they are on the right side of the decimal point;, otherwise, they are not significar Hor example, 0.200 g has three significant figures., 5. Exact numbers have an infinite umber of significant figures. For example, in 2 balls or 20 eggs, there are, infinite significant figures since these are exact numbers and can be represented by writing infinite number, of zeros after placing a decimal ie., 2 = 2.000000 or 20 = 20.000000, 6. When numbers are written in scientific notation, the number of digits between 1 and 10 gives the number, of significant figures. For e.g. 4.0110" has three significant figures, and 8.256 x 10” has four significant, figures., , / But, accuracy is the agreement of a, , , , , , , , Significant figures. These are:, “285 cm, there are three significant figures and in 0.25 mL,