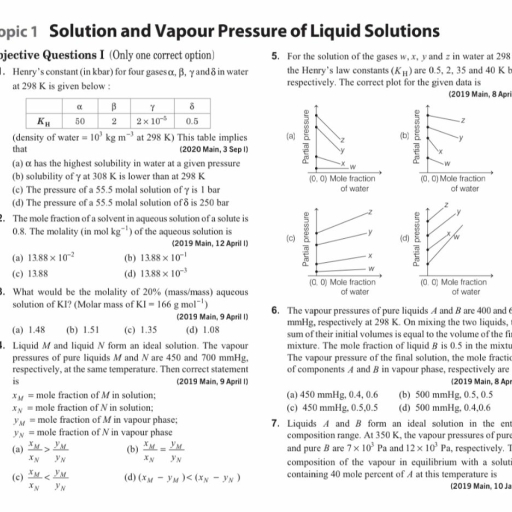
Page 1 :
D- BLOCK ELEMENTS
Page 2 :
d block contains 3-12 group, , The names transition metals and inner transition metals are often used to refer, to the elements of d-and f-blocks respectively., , 4 series in d block, 3d series (Sc to Zn),, , 4d series (Y to Cd),, 5d series (La and Hf toHg) and VL SHARMA 7703993757, , 6d series which has Ac and elements from Rf to Cn.( incomplete, , two series of the inner transition metals; 4f (Ce to Lu) and 5f (Th to Lr) are known as, lanthanoids and actinoids respectively., , transition metals are defined as metals which have incomplete d subshell either in, neutral atom or in their ions., , Zinc, cadmium and mercury are not regarded as transition metals.
Page 3 :
Position in the Periodic Table, , VL SHARMA 7703993757, , between s-— and p- blocks in the periodic table, , Electronic Configurations of the d-Block Elements, , (n-1)d 1-10ns 1-2.
Page 4 :
The d orbitals of the transition elements protrude to the periphery of an atom more than, the other orbitals hence affected generally by their surrounding, , The transition metals and their compounds also exhibit catalytic property and, paramagnetic behaviour., , There are greater similarities in the properties of the transition elements of a horizontal, row in contrast to the non-transition elements, , VL SHARMA 7703993757, , high tensile strength, ductility, malleability, high thermal and electrical conductivity and, metallic lustre., , , , exceptions of Zn, Cd, Hg and Mn, they have one or more typical structures at normal temp.
Page 5 :
The transition metals (with the exception of Zn, Cd and Hg) are very hard and have low volatilit, Their melting and boiling points are high., , The high melting points of these metals are attributed to the involvement of greater number of, electrons from (n-1)d in addition to the ns electrons in the interatomic metallic bonding., , melting points of these metals rise to a maximum at d 5 except for anomalous values of, Mn and Tc and fall regularly as the atomic number increases., , They have high enthalpies of atomnisation, , L SHARMA 7703993757, , greater the number of valence electrons, stronger is the resultant interatomic bonding, , , , occurrence of much more frequent metal — metal bonding in compounds of the heavy, transition metals. bcoz second and third series have greater enthalpies of atomisation, than the corresponding elements of the first series;