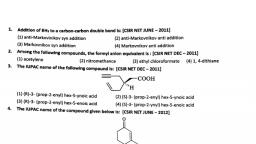
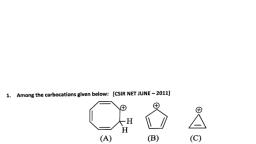
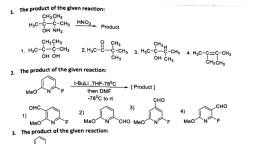
Page 1 :
Qu., , Q.2., , Q.3., , Q4., , Q5., , Q.6., , Q7., , Q.8., , Q9., , Q.10., , The no. of metal- metal bonds in dimmers [ CpFe(CO)(NO)]2 and [CpMo(CO);}2, , respectively are:, , (a) 2and2 (b) 2and3, , (c) land 2 (d) Oand!, , Which of the following has NOT three bridging carbonyl group?, , (a) Fex(CO)y (b) = Cos(CO):2, , (c) Rhg(CO):2 (d) — Fex(CO):2, , An example of species having quaderpole bond is : ., , (a) Mnx(CO)y (b) Cr,07~, , (c) — [ReCls]” (a) Hgx(CH;COO),, (A) (Cp2Z(COKC)]° (B) [Cp2Zr(CO)2], , Which of the following statement is correct?, , (a) Vo of A is greater than that of B, , (b) —_ vo Of B is greater than that of A, , (c) Veo Of A is equal to v.. of B, , (d) The metal Zr having +4 oxidation state in (A) whereas in (B) Zr is present in Zero, , oxidation state., Which of the following complex has highest v,.. stretching frequency?, (a) Fe(CO),(PF3) (b) — Fe(CO),(PCls), (c) Fe(CO)4(PMe;) (d) — Fe(CO)s4P(OMe);, , On reducing Fe;(CO),2with an excess of Na, a carbonylate ion is formed. The Iron is, isoelectronic with:, , (a) [ Mn(CO)s} (b) ~~ [ Ni(CO).], , (c) [Mn(CO)s} (d) — [V(CO)6], , Which of the following oxidation state stabilizes the metal atom in carbonyl compounds?, (a) 1 (b) 2, , (c) 3 (dd) oO, , Which of the following statement is false about ferrocene?, , (a) It obeys 18-electron rule. (b) _ It is diamagnetic., , (c) It is an Orange solid. (d) It resists electrophilic reaction., Arrange the following in increasing order of Vc.ostretching frequency:, , (1) [Mo(CO)3(PF3)s] (I) [Mo(CO);(PCl3)s], , (I) = [Mo(CO);P{CI(Ph)2}3] (IV) [Mo(CO);(PMe3)3], , (a) IV<II<II<I (b) Il<IV<II<I, , (c) I<I<II<IV (d) I<Ml<I<Iv, , The complex having highest Vy.c frequency:, , (a) Mo(Co). (b) — Mo(CO)s(PMe;)2, , Scanned by CamScanner
Page 2 :
Ql., , Q.12., , Q.13., , Q.14., , Q.15., , Q.16., , Q.17., , Q.18., , Q.19., , Q.20., , Q.21., , (c) Mo(CO)4(P(OMes)2) (d), The cluster having arachano type structure:, , (a) [Os3(CO);6} (b), (c) — [Ir(CO),2] (d), , Mo(CO),(PF3)2, , [Os3(CO);2], [Rhe(CO) 6], , Which of the following statement is incorrect about metal carbonyls?, (a) In metal carbonyls metal present in zero oxidation state., , (b), carbonatoms., , (c) In metal carbonyl CO act as a neutral ligand., , In metal carbonyls o-bond as well as x-bonds are formed between metal and, , Co(CO),, Fe(CO),?, , Epoxidation, , (d) | Only o-bond is formed between metal atom and carbon atom., Which of the following has lowest stretching frequency?, , (a) V(CO)." (b), , (c) Ni(CO), (d), , Wilkinson's catalyst is used for:, , (a) Hydrogenation (b), , (c) Polymerization (d), , Which of the following is most easily reduced?, (a) V(CO)s (b), {c) Fe(CO)s (d), , Metathesis reaction, , Cr(CO)s, Ni(CO),, , Reaction of Fe(CO)s with OH leads to a complex A which on oxidation with MnO> gives, , B. Compound A and B respectively are:, (a) [HFe(CO),] and Fes(CO))2, (©) [Fe(CO)4J*_ and Mnx(CO)i0, , (b), (d), , [Fe(CO)s(OH)] and Fex(CO), [HFe(CO)s} and Fe2O3, , Which of the following metal carbonyls cannot be formed by the direct combination, , with CO?, , (a) CriCO)s (b) MnxCO) io, {c) Fe(CO)s (d) CoxCO)s, Wilkinson's catalyst is:, , (a) (Ph3P)sRhCl (b) = (PhsP)RhC!, (c) PhyPsRhCl (d) — (PhsP)2RhCl>, Which of the following IR frequencies is the closest to that of the triply bridged CO, group?, , (a) 1700 cm" (b) —:1810.cm", , (c) —1920.cm" (d) 2140 cm", Which of the following does not obey 18 e'rules?, , (a) CrCO)s (b) MnxCO)10, {c) Fe(CO)s (d) V(CO)., , For the reaction,, , trans- [IrC(CO)(PPh3)2] + Cl, ————>, the correct observations:, , trans- [ IrCl;(CO)(PPh3)2] ., , Scanned by CamScanner
Page 3 :
Q.22., , Q.23., , Q.24., , Q.25., , Q.26., , Q.27., , Q.28., , Q.29., , Q.30., , Q31., , (a) Oco(product) >vco (reactant) (b) Uco (product) < dco (reactant), (c) _ Deo (product) = dco (reactant) (4) — Oco (product) = vco (Free CO), In the reaction,, , 4C\HsMgBr + CrCl; + 2CO ——® = A(unstable) iH, = CKCO + 2Cr? + 12CHy + 3H,, The unstable intermediate formed is:, , (a) Cr(CO)AC6Hs)s (b) — Cr(CO)3(CsHs)s, (c) Cr(CO)(CeHs)2 (d) — Cr(COCsHs)s, Which of the following compounds show normal spinal structure?, (a) NaCrO4 (b) ZnO, , (c) FesO4 (d) — Fe(CO)s, Metal-Metal quadruple bonds are well known for metal:, , (a) Ni (b) Co, , (c) Fe (d) Re, , The no. of Metal-Metal bonds present in Irs(CO),2 are:, , (a) 4 (b) 5, , (cc) 6 (dd) 8, , Na in liq. NH; reacts with Fe,(CO), to give:, , (a) [Fe(CO)s] (b) — [HFe(CO),s}, (c) (H2Fe(CO),]" (4) [Fe(CO).}”, , The correct statement regarding terminal/ bridging CO groups in solid Co,(CO),; and, Irg(CO),2 is:, , (a) Both have equal no. of bridging CO groups., , (b) | Number of bridging CO groups in Co,(CO))> is 4., , (c) The no. of terminal CO groups in Co(CO))> is 8., , (d) — The no. bridging CO groups in Ir,(CO),> is 0., , Which of the following is paramagnetic in nature?, , (a) Cr(CO). (b) V(CO)s, , (c) Fe(CO)s (d) —_Ni(CO)s, , Regarding the catalytic cycle of hydrogenation of alkenes involving (Ph3P);RhCI as the, catalyst, the correct statements is:, , (a) Only 18-electron Rh complex is involved., , (b) 14-,16- and 18- electron Rh complexes are involved., , (c) 14- and 16- electron Rh complexes are involved., , (d) 16- and 18- electron Rh complexes are involved., , The value of x in Fe3(CO), is:, (a) 8 (b) 9, (c) 10 (d) 12, , Mention the incorrect statement about poly nuclear carbonyls:, (a) They have the general formula M,(CO),., , (b) These are generally insoluble in organic solvent., , (c) They decompose at or below their melting point., , Scanned by CamScanner
Page 4 :
Q.32., , Q.33., , Q.34., , Q.35., , Q.36., , Q.37., , Q.38., , Q.39., , Q.40., , Q41., , (d) These carbonyls are more volatile than the others., , [Co(CO),] is isolobal with:, , (a) CH, (b) CH;, , (c) CH, (d) CH, , The final product of the reaction [Mn(CO)s] + MeLi — is:, , (a) [Mn(CO).]"Me" (b) — [Mn(CO)sMe], , (c) — [Mn(CO).] (d) — [(MeCO)Mn(CO)s}, The oxidative addition and reductive elimination are favored by:, , (a) Electron rich metal centre., , (b) —_ Electron deficient metal centre., , (c) Electron deficient and electron rich metal centers respectively., , (d) _ Electron rich and electron deficient metal centers respectively., , The hepticities ‘x’ and ‘y’ of the arenes moieties in the diamagnetic complex, [(}"-CoH,)Ru(I}’-CeH,)] are:, , (a) 6 and 6 (b) 4and4, , (c) 4and6 (d) 6 and2, , Arrange the following in increasing order of CO stretching frequencies :, , L Cr(CO). Il. [V(CO)s} IIL [Mo(CO).]* IV.co, (a) I>0>II>IV (b) U<I<II<IV, , (ce) IV<I< <I (d) I< I<IV<Il, , What are the favorable conditions for a ligand to be pi-acceptor?, , (a) The ligand must have vacant orbital., , (b) The metal atom should be of low oxidation state., , (c) The electron density should be high on metal atom., , (d) All are correct., , Intense band generally observed for a carbonyl group in the IR spectrum is due to:, (a) The force constant of CO bond is large., , (b) —_ The force constant of CO bond is small., , (c) There is no change in dipole moment for CO bond stretching., , (d) The dipole moment changes due to CO bond stretching is large., , The product of the reaction of propene, CO and H: in presence of Co>(CO)gis:, , (a) Butanoic acid (b) —_ Butanal, , (c) 2-butanone (d) Methyl propanoate, , Among the metals Mn, Fe, Co and Ni the one those would react in its native form directly, with CO giving metal carbonyl compound are:, , (a) Co and Mn (b) Mnand Fe, , (c) Fe and Ni (d) NiandCo, , Carbonylate ions are formed by the action of:, , (a) Reaction of Lewis acid such as AICI; with CO and carbonyl halide., , (b) Reaction of Halogen with Fe(CO)s., , Scanned by CamScanner
Page 5 :
Q. 42., , Q.43., , Q.44,, , Q45., , Q.46., , Q47., , Q.48., , Q.49,, , Q.50., , Q51., , (c) Reaction of alkali with simple Carbonyl., , (d) By reduction of carbonyls with alkali metals., , Total number of vertices in metal clusters [Rug(C) (CO)\7}, [Oss(C) (CO),s} and, [Rus(CXKCO)i6] are 6, 5 and 5 respectively. The predicted structures of these, complexes respectively are:, , (a) _—_Closo, nido and nido (b) —_Closo, nido and arachano, (c) Arachano, closo and nido (d) — Arachano, nido and closo, Which of the following is an example of poly nuclear carbonyl?, , (a) Cr(CO), (b) — Fe(CO)s, , (c) W(CO). (d) Fex(CO),, , The no. of Metal-Metal bonds in the dimers, [CpFe(CO\NO)]2 and [CpMo(CO)s}>, respectively are, , (a) 2and2 (b) 2and3, , (c) 1 and2 (d) Oand!, , In the hydroformylation reaction, the intermediate CH;CH»CH»Co(CO),:, , (a) Forms acyl intermediate CH;CHyCH,Co(CO),;, , (b) Forms an adduct with an olefin reagent., , (c) Reacts with H2, , (d) _ Eliminates propane., , Oxidation occurs very easily in case of:, , (a) (n° -CsHs )2Fe (b) — (n°-CsHs )Co, (©) ((°-CsHs Ru (d) (n° -CsHs )xCo*, The color of Fe(CO) is:, , (a) White (b) Black, , (c) Yellow (d) Brown, , The no. of anti bonding electrons in NO and CO according to molecular orbital theory, are:, , (a) 1.0 (b) 2,2, , (c) 3,2 qd) 2,3, , The no. of terminal CO groups present in Fe(CO), is, , (—) 2 (bt) 5, , (c) 6 (d) 3, , What are the oxidation states of metal ion in the following compounds?, , 1. PdCl, 2. Pd(PPh;), 3. Pd(OAc), 4. Pd(Ar)Br, (a) 2,4,2,2, (b) =. 2,0,2,1, , (c) —_2,0,2,2, (d) —-0,0,0,2, , The refluxing ofRhCl;.3H,O with an excess of PPh; in ethanol gives a complex A., Complex A and valence electron count on Rhodium are, respectively,, (a) [RhCI(PPhs)3], 16 (b) — [RhCI(PPhs)s}, 16, (c) — [RhCI(PPhs)3], 18 (d) — [RhCI(PPhs)s}, 18, , Scanned by CamScanner