Question 4 :
<img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ead5f37381c2135355c76db"> is strong field ligand. This is due to the fact that
Question 6 :
The coordination compounds,<br> <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ead5ffe86ab83696376398d"><br> <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ead5ffe381c2135355c7831"> are example of
Question 7 :
The effective atomic number rule is less likely to apply if the metal-ligand bond:
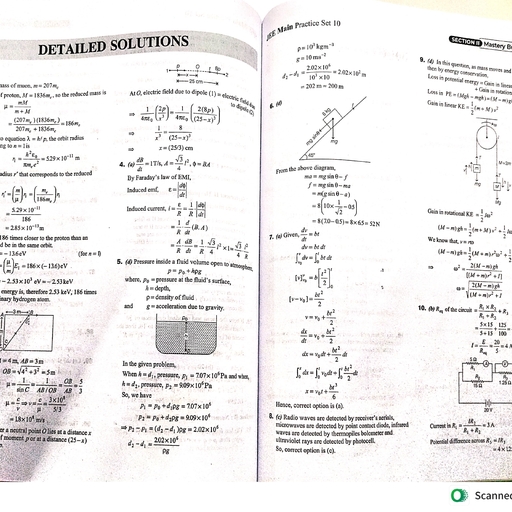
Question 10 :
In any ferric salt, on adding potassium ferrocyanide a Prussian blue colour is obtained which is due to the formation of
Question 14 :
What is the EAN of nickel in <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ead5f3986ab836963763854"> ?
Question 16 :
Considering {tex} \mathrm { H } _ { 2 } \mathrm { O } {/tex} as a weak field ligand, the number of unpaired electrons in {tex} [ \mathrm { Mn } ( \mathrm { H } _ { 2 } \mathrm { O } ) _ { 6 }] ^ { 2 + } {/tex} will be <br> (atomic number of Mn = 25 )
Question 17 :
The {tex} d {/tex} -electron configurations of {tex} \mathrm { Cr } ^ { 2 + } , \mathrm { Mn } ^ { 2 + } , {/tex} {tex} \mathrm { Fe } ^ { 2 + } {/tex} and {tex} \mathrm { Co } ^ { 2 + } {/tex} are {tex} d ^ { 4 } , d ^ { 5 } , d ^ { 6 } {/tex} and {tex} d ^ { 7 } {/tex} respectively. Which one of the following will exhibit minimum paramagnetic behaviour?<br>{tex} \text { (At. nos. } \mathrm { Cr } = 24 , \mathrm { Mn } = 25 , \mathrm { Fe } = 26 , \mathrm { Co } = 27 ) {/tex}
Question 18 :
Crystal field splitting energy for high spin {tex} d ^ { 4 } {/tex} octahedral complex is
Question 19 :
The complex given is<br><img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5dfdac64a66aa960ca736c20"><br>(i) non-superimposable on its mirror images <br>(ii) optically active <br>(iii) rotate plane polarised light<br>(iv) planar
Question 20 :
In which of the following octahedral complex species the magnitude of {tex} \Delta _ { 0 } {/tex} will be maximum?
Question 21 :
In isolated condition {tex} \mathrm { C } - \mathrm { C } {/tex} bond length of {tex} \mathrm { C } _ { 2 } \mathrm { H } _ { 4 } {/tex} is {tex} \mathrm { x } {/tex}, than the bond length of a {tex} \mathrm { C } - \mathrm { C } {/tex} bond of {tex} \mathrm { C } _ { 2 } \mathrm { H } _ { 4 } {/tex} in Zeise's salt is
Question 22 :
Which of these statements about {tex} \left[ \mathrm { Co } ( \mathrm { CN } ) _ { 6 } \right] ^ { 3- } {/tex} is true?
Question 23 :
Which of the following statements related to crystal field splitting in octahedral coordination entities is incorrect?
Question 24 :
The anion of acetylacetone (acac) forms {tex} \mathrm { Co } ( \mathrm { acac } ) _ { 3 } {/tex} chelate with {tex} \mathrm { Co } ^ { 3 + } {/tex} . The rings of the chelate are
Question 25 :
Consider the coordination compound, {tex} \left[ \mathrm { Co } \left( \mathrm { NH } _ { 3 } \right) _ { 6 } \right] \mathrm { Cl } _ { 3 } . {/tex} In the formation of this complex, the species which acts as the Lewis acid is:
Question 26 :
Among the following complexes {tex} ( \mathrm { K } - \mathrm { P } ) {/tex}<br>{tex} \mathrm { K } _ { 3 } \left[ \mathrm { Fe } ( \mathrm { CN } ) _ { 6 } \right] ( \mathrm { K } ) , \left[ \mathrm { Co } \left( \mathrm { NH } _ { 3 } \right) _ { 6 } \right] \mathrm { Cl } _ { 3 } ( \mathrm { L } ), {/tex} {tex} \mathrm { Na } _ { 3 } \left[ \mathrm { Co } \left( \text { oxalate) } _ { 3 } \right] ( \mathrm { M } ) , \left[ \mathrm { Ni } \left( \mathrm { H } _ { 2 } \mathrm { O } \right) _ { 6 } \right] \mathrm { Cl } _ { 2 } ( \mathrm { N } ) ,\right. {/tex} {tex} \mathrm { K } _ { 2 } \left[ \mathrm { Pt } ( \mathrm { CN } ) _ { 4 } \right] ( \mathrm { O } ) {/tex} and {tex} \left[ \mathrm { Zn } \left( \mathrm { H } _ { 2 } \mathrm { O } \right) _ { 6 } \right] \left( \mathrm { NO } _ { 3 } \right) _ { 2 } ( \mathrm { P } ) {/tex} the<br>diamagnetic complexes are
Question 28 :
Which of the following has longest {tex} \mathrm { C } - \mathrm { O } {/tex} bond length? ( Free {tex} \mathrm { C } - {/tex} O bond length in {tex} \mathrm { CO } {/tex} is {tex}1.128\space {\text{Å}} {/tex} .)
Question 29 :
Crystal field stabilization energy for high spin {tex} d ^ { 4 } {/tex} octahedral complex is
Question 30 :
{tex} \left[ \mathrm { Co } \left( \mathrm { NH } _ { 3 } \right) _ { 4 } \left( \mathrm { NO } _ { 2 } \right) _ { 2 } \right] \mathrm { Cl } {/tex} exhibits