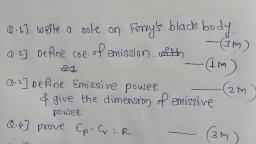Question 1 :
<p class="wysiwyg-text-align-left">A ballon filled with air at 47$^{0}$C has volume of 3 litre. If ballon is kept in a room its volume becomes 2.7 litre, the temperature of the room is :<br/></p>
Question 2 :
$O_2$ is filled in a cylinder. When pressure is increased $2$ times, temperature becomes four times, then how much times their density will become
Question 3 :
A vessel has 6 g of oxygen of pressure P and temperature 400 K, a small hole is made in it so that oxygen leaks out. How much oxygen leaks out if the final pressure is P/2 and temperature is 300 K?
Question 4 :
Which of the following statements is the most accurate with regard to the significance of Avogadro's number, $6.02 \times 10^{23}$ ?
Question 5 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">The parameter that determine the physical state of gas are :<br/></p><p class="wysiwyg-text-align-left">a) Pressure b) Volume</p><p>c) Number of moles d) Temperature</p>
Question 8 :
What happens to the density of the gas if the volume of an ideal gas is reduced to half its original volume:
Question 9 :
Under which of the following conditions is the law $pV=RT$ obeyed most closely by a real gas?
Question 10 :
Which term describes the mass of $6.022\times { 10 }^{ 23 }$ representative particles?
Question 11 :
The value of gas constant $R$ (in cal/mol-K) for hydrogen is :
Question 12 :
<p class="wysiwyg-text-align-left">16 gm of $O_{2}$ gas and x gm of $H_{2}$ gas occupy the same volume at the same temperature and pressure. Then x is :<br/></p>
Question 13 :
<p>How many times is the weight of the air filling a room in winter ($7^{0}C$) greater than weight in summer $(37^{0}C)$ ? Assume that pressure is constant.</p>
Question 14 :
<span>A box has two compartments of equal volume separated by a divider each compartment filled with a random sample of $'n\ '$ </span><span>moles of a certain gas, but the pressure in compartment $A$ </span><span>is twice the pressure in compartment $B$. Identify w</span><span>hich of the following statements is true?</span>
Question 15 :
All gases have the same number of moles in the <span>same volume at constant T and P is stated by :</span>
Question 17 :
The nature of the graph of pressure 'P' against reciprocal of volume 'V' of an ideal gas at constant temperature is
Question 18 :
Which one of the following is not an assumption of kinetic theory of gases?
Question 19 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">If the temperature of a gas is increased by 1 K at constant pressure, its volume increases by 0.0035 of the initial volume. The temperature of the gas is :</p>
Question 20 :
The volume of a mole of a perfect gas at NTP is
Question 21 :
For any gas the pressure coefficient ($\beta $) is equal to the volume coefficient ($\alpha $). This can be proved by:<p></p>
Question 22 :
A large glass container $A$ is filled with 1 mole of hydrogen gas ($2\, g/mol$) and other container $B$ is filled with 1 mole of helium gas ($4 \,g/mol$). If the volume and pressure of containers are equal then which of the following is true?
Question 23 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">If the volume of the gas is to be increased by 4 times :</p>
Question 24 :
Which of the following statements is correct regarding one mole of helium gas and one mole of neon gas ,both are at <i>STP</i><span>. </span>
Question 25 :
The lowest pressure (the best vaccum) that can be created in laboratory at $20^{\circ}C$ is $10^{-10}\ mm$ of $Hg$. At this pressure, the number of ideal gas molecules per $cm^{3}$ will be
Question 26 :
An ideal gas is enclosed in a sealed container. Upon heating, which property of the gas does not change?
Question 27 :
The adiabatic and isothermal volume elasticities B$_{\phi}$ and B$_{\theta}$ are related as :
Question 28 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">The temperature of a gas contained in a closed vessel is increased by 2 K when the pressure is increased by 2%. The initial temperature of the gas is :<br/></p>
Question 29 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">Select the correct formula :</p><p class="wysiwyg-text-align-left">(where k=Boltzmann's constant, R= gas constant, n= moles, r = density, M= molecular weight, p= pressure, T= kelvin temperature, V= volume)<br/></p><p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">a) k=RN$_{av}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span></p><p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"></span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">b)$r=\dfrac{nM}{V}$</span></span></p><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">c)$\dfrac{p}{r}=\dfrac{RT}{M}$</p><p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">d) R=kN$_{av}$</span></span></p>
Question 30 :
Two gases A and B are taken in same volume containers under similar conditions of temperature and pressure. In container A, there are '2N' molecules of gas A. How many number molecules does container B have? <br/>
Question 31 :
A vessel has 6g of hydrogen at pressure P and temperature 500K. A small hole is made in it so that hydrogen leaks out. How much hydrogen leaks out if the final pressure is $P/2$ and temperature falls to 300K?
Question 32 :
If the pressure of a gas remains constant and the temperature is doubled as a result of it the volume of the gas also gets doubled. Identify by which of the following law this can be explained ?
Question 33 :
The gas law $\left [ \dfrac{PV}{T} \right ]=$ constant is true for<br/>
Question 34 :
Which of the following property is used to identify whether a substance is a solid, liquid or gas ?
Question 35 :
Which of the following gives evidence to kinetic theory of gases?
Question 37 :
A gas behaves more closely as an ideal gas at :
Question 38 :
A cycle tube has volume $2000 cm^3$. Initially the tube is filled to 3/4th of its volume by air at pressure of $105 N/m^{2}$. It is to be inflated to a pressure of $6 \times 10^{5} N/m^{2}$ under isothermal conditions. The number of strokes of pump, which gives $500 cm^3$ air in each stroke, to inflate the tube is
Question 39 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">An enclosure of volume 3 litre contains 16 gms of <span>oxygen, 7 gms of nitrogen and 11 gms of carbon </span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">di-oxide at </span></span><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">27$^{0}$</span></span><i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">C </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">. The pressure exerted by the </span></span><span>mixture is approximately</span></p>
Question 40 :
The temperature of the sun, if pressure is $1.4 \times 10^9$ atm, density is $1.4 gcm^{-3}$ and average molecular weight is 2, will be<br/>$[Given\ R = 8.4 J mol^{-1} K^{-1}]$
Question 41 :
One gram mol of helium at 27$^{0}$ C is mixed with three gram mols of oxygen at 127$^{0}$ C at constant pressure. If there is no exchange of heat with the atmosphere then the final temperature will be
Question 42 :
If T represents the absolute temperature of an ideal gas, the volume coefficient of thermal expansion at constant pressure is proportional to:
Question 43 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">Assertion:At constant pressure when a gas is </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">heated from 40 to 41$^{0}$</span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C, the increase in volume </span></span>is $\dfrac{1}{273}$ of its initial volume at 273 K<br/></span></span><p class="wysiwyg-text-align-left">Reason:Volume coefficient of gas is $\dfrac{1}{273}^{0}C$</p>
Question 44 :
A balloon contains $500m^3$ of He at $27^o$C and $1$ atmospheric pressure. The volume of He at $-3^o$C and $0.5$ atmospheric pressure will be.
Question 45 :
<p><span>In an experiment, 1.35 mol of oxygen (O</span><span>2</span><span>) are heated at constant pressure starting at 11.0ºC. How much heat must be added to the gas to double its volume?</span><br/></p>
Question 46 :
The temperature of an open room of volume $30 m^3$ increases form $17^oC$ to $27^oC$ due to the sunshine. The atmospheric pressure in the room remains $1\times 10^5$ Pa. If $n_i$ and $n_f$ are the number of molecules in the room before and after heating, then $n_f-n_i$ will be :
Question 47 :
Pressure of an ideal gas is increased by keeping temperature constant. The kinetic energy of molecules.
Question 48 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">Equation of gas in terms of pressure $p$ absolute temperature $T$ and density $d$ is :</p>
Question 49 :
The density of a polyatomic gas in standard conditions is 0.795 kgm$^{-3}$. The specific heat of the gas at constant volume is
Question 50 :
Two identical glass spheres filled with air are connected by a horizontal glass tube. The glass tube contains a pellet of mercury at its mid-point. Air in one sphere is at $0^o$C and the other is at $20^o$C. If both the vessels are heated through $10^o$C, then neglecting the expansions of the bulbs and the tube.
Question 51 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">Match List I and List II</p><b><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span></b><p class="wysiwyg-text-align-left">List-I List-II</p><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">a) Barometer e) Charles law</p><p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">b) specific gas constant f) </span></span><i>Jmole</i>$^{-1}$<em>K</em>$^{-1}$</p><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"></span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">c) gas thermometer g) Boyels law</p><p><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">d) universal gas constant h) </span></span><em>JKg</em>$^{-1}$<em>K</em>$^{-1}$</p>
Question 52 :
Find out the final pressure of a gas that expands from 1 litre at $10^oC$ to 10 litres at $100^oC$ . The original pressure was 3 atm.<br/>
Question 53 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">A vessel is filled with an ideal gas at a pressure <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">of 10 atmospheres and temp </span></span>27$^{0}$<i>C </i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">. Half of the </span></span>mass of the gas is removed from the vessel & the temp. of the remaining gas is increased to 87$^{0}$<i>C </i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">. Then the pressure of the gas in the </span></span>vessel will be</p>
Question 54 :
The pressure of a gas filled in a closed vessel increases by 0.4%. When temperature is increases by 1$^o$C the initial temperature of the gas is
Question 55 :
If the pressure and the volume of certain quantity of ideal gas are halved, then its temperature
Question 56 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><p class="wysiwyg-text-align-left">A given amount of gas is heated until both its pressure and volume are doubled. If <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">initial temperature is 27$^{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C, its final temperature is</span></span></p></span></span>
Question 57 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">A partially inflated balloon contains <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">500 m<span class="wysiwyg-font-size-medium">$^{3}$</span></span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">of helium at 27<span class="wysiwyg-font-size-medium">$^{0}$</span></span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C and 1 atm pressure. </span></span><span>The volume of the helium at an altitude of 6000 </span><span>m, where the pressure is 0.5atm and the </span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">temperature is -</span></span><span>3$^{0}$</span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C is</span></span></p>
Question 58 :
<p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">The volume of a gas at $27^o$</span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C and 2 </span></span><span>atmospheric pressure is 2 litres. If the pressure </span><span>is doubled and absolute temperature is made </span><span>half, the new volume of gas is</span></p>
Question 59 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">A closed vessesl contains 8 gms of oxygen and <span>7gm of Nitrogen. Total pressure at a certain </span><span>temperature is 10 atm. When all the oxygen is </span><span>removed from the system without chage in </span><span>temperature then the pressure will be</span></p>
Question 60 :
Statement - I:<br/>Two vessels A and B of equal capacity are connected to each other by a stopcock. Vessel A contains a gas at $ 0^{0}C$ and 1 atmosphere pressure and vessel B is evacuated. If the stopcock is suddenly opened, the final pressure in A and B will be 0.5 atmosphere.<br/>Statement - 2:<br/>If the temperature is kept constant, the pressure of a gas is inversely proportional to its volume<br/>
Question 63 :
During an experiment an ideal gas is found to obey an additional law $V{p}^{2}=$ constant. The gas is initially at temperature $T$ and volume $V$, when it expands to volume $2V$, the resulting temperature is $T_2$:
Question 64 :
64 g of sulphur dioxide occupies $22.4\ L$ volume at STP.<br/>
Question 65 :
A gas in a flexible container initially has volume of 320 L. If we decrease the pressure of the gas from 4.0 atm to 1.0 atm and decrease the temperature form 273 degrees Celsius to 0 degrees Celsius, what is the new volume of the gas?<br>
Question 66 :
The number of oxygen molecules in a cylinder of volume $1 m^3$ at a temperature of $27^o C$ and pressure $13.8 Pa$ is:(Boltzmaans constant $k=1.38\times10^{-23} J{K^-1}$:
Question 67 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">One litre of Helium gas at a pressure of 76 cm - <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">Hg and temperature </span></span><span>27$^{0}$</span><i>C </i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">is heated till its </span></span><span>pressure and volume are doubled. The final </span><span>temperature attained by the gas is</span></p>
Question 68 :
<p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">A gas is heated through $1^{o}\ C$</span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"> in a closed vessel. </span></span>Its pressure is increased by $0.4$%. The initial emperature of the gas is</p>
Question 69 :
The volume of an ideal gas is 1 L and its pressure is equal to 72 cm of mercury column. The volume of gas is made $900\, cm^3$ by compressing it isothermally. The stress of the gas will be
Question 70 :
If pressure of a gas contained in a closed vessel is increased by 0.4% when heated by $1^{0}C$, the initial temperature must be
Question 71 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">A horizontal uniform glass tube of 100cm length <span>is sealed at both ends contains 10 cm mercury </span><span>column in the middle the temperature and </span><span>pressure of air on either side of mercury column </span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">are respectively </span></span><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">31$^{0}$<i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large"></span></span></i></span></span><i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">C </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">and 76cm of mercury if </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">the air column at one end is kept at </span></span><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">0$^{0}$</span></span><i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">C </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">and </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">the other end at </span></span><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">273$^{0}$<i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large"></span></span></i></span></span><i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">C </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">the pressure of air </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">which is at </span></span><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">0$^{0}$<i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large"></span></span></i></span></span><i><span class="wysiwyg-font-size-large"><span class="wysiwyg-font-size-large">C </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">is (in cm of Hg )</span></span></p>
Question 72 :
A vessel is filled with an ideal gas at a pressure of $10$ atm and temperature $27^{\circ}C$. Half of the mass is removed from the vessel and temperature of the remaining gas is increased to $87^{\circ}C$. Then the pressure of the gas in the vessel will be:
Question 73 :
The air density at Mount Everest is less than that at the sea level. It is found by mountaineers that for one trip lasting a few hours, the extra oxygen needed by them corresponds to 30,000cc at sea level (pressure 1 atmosphere, temperature 27$^o$C). Assume that the temperature around Mount Everest is -$73^o$C and that the oxygen cylinder has a capacity of 5.2 litters. The pressure at which oxygen be filled (at site) in the cylinder is
Question 74 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">A cylinder contains gas at a pressure of 2.5 atm. <span>Due to leakage, the pressure falls to 2 atm, after </span><span>sometime. The percentage of the gas which is </span><span>leaked out is</span></p>
Question 75 :
<span>An ideal gas is filled in a perfectly closed container, Now the gas is heated at constant volume. Identify which of the following properties of the gas increases as the temperature of the gas increases ?</span>
Question 76 :
From a disc of radius $R$ and mass $M$, a circular hole of diameter $R$, whose rim passes through the centre is cut. What is the moment of inertia of the remaining part of the disc about a perpendicular axis, passing through the centre?
Question 77 :
A box contains two compartments of equal volume separated by a divider. The two compartments each contain a random sample of n moles of a certain gas, but the pressure in compartment A is twice the pressure in compartment B . Which of the following statements is true?
Question 78 :
If 300 ml of a gas at $27^{\circ}C$ is cooled to $7^{\circ}C$ at constant pressure, then its final volume will be
Question 80 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">Assertion: In Joules bulb apparatus, as reservoir is moved up, the mercury level raises into the bulb. </p><p class="wysiwyg-text-align-left"><span>Reason: The pressure on the enclosed gas increases.</span></p>
Question 81 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><p class="wysiwyg-text-align-left">For an isochoric process the temperature at which the pressure of a gas will be double that of its <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">pressure at 270$^{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C is</span></span></p></span></span></span>
Question 82 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">At. N.T.P. 28 gm of Nitrogen occupies 22.4 liters. What is the mass of 5.6 liters of <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">nitrogen at 38cm of Hg pressure and 273$^{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C </span></span>temperature?</p>
Question 83 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"></span></span><p class="wysiwyg-text-align-left">The relation between volume V, pressure P and absolute temperature T of an ideal gas is PV = <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><i>x</i></span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">T, where x</span></span><i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"> </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">is a constant. The value of x</span></span><i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"> </span></span></i><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">depend </span></span>upon</p>
Question 84 :
A vessel contains $1$ mole of $O_{2}$ gas (molar mass$=32$) at temperature T. The pressure of the gas is $P$. An identical vessel containing one mole of He gas (molar mass$=4$) at a temperature $2T$, has a pressure of
Question 85 :
In a thermally isolated system, two boxes filled with an ideal gas are connected by a valve. When the valve is in closed position, states of the box 1 and $2$, respectively, are ($1$ atm, V.T) and $0.5$ atm, $4V$. T). When the valve is opened, the final pressure of the system is approximately
Question 87 :
A vessel of volume V $=20 L$ contains a mixture of hydrogen and helium at a temperature $t=20^\circ$ and pressure p $=$ 2.0 atm. The mass of the mixture is equal to m $=$ 5.0 g. Find the ratio of the <span>mass of hydrogen to that of helium in the given mixture.</span>
Question 88 :
A glass bulb of volume $400\, cm^3$ is connected to another of volume $200\, cm^3$ by means of a tube of negligible volume. The bulbs contain dry air and both are at a common temperature and pressure of $20^{\circ}C$ and $1.00$ $atm$ respectively. The larger bulb is immersed in steam at $10^{\circ}C$ and the smaller in melting ice at $0^{\circ}C$. Find the final common pressure.
Question 89 :
<p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">A closed container of volume 0.02 m$^3$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">contains a </span></span><span>mixture of neon and argon gases at a temperature </span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">of 27$^{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">C and at a pressure of $1\times 10^{5}N/m^{2}$</span></span><span>. </span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">The </span></span><span>total mass of the mixture is 28 g. If the gram </span><span>molecular weights of neon and argon are 20 and </span><span>40 respectively, the masses of the individual </span><span>gases in the container are respectively(assuming </span><span>them to be ideal) [R = 8.314 J/mol K]</span></p>
Question 90 :
Two gases occupy two containers A and B. The gas in A of volume $0.11\, m^3$ exerts a pressure of 1.38 MPa. The gas in B of volume $0.16\, m^3$ exerts a pressure of 0.69 MPa. Two containers are united by a tube of negligible volume and the gases are allowed to intermingle. What is the final pressure in the container if the temperature remains constant ?
Question 91 :
A perfectly conducting vessel of volume V = $0.4m^{3}$ contains an ideal gas constant temperature T = 273 K. A portion of the gas is let our and the pressure of the gas falls by $\Delta P\, =\, 0.24 atm$ (Density of the gas at STP is $\rho\, =\, 1.2 kg/m^{3}$). Find the mass of the gas which excapes from the vessels.
Question 92 :
An air bubble of volume V is released from the bottom of a lake which has a depth of 11 m. Find the volume of the bubble at the surface of the lake. The water has density $1000\, kg/m^{3}$. At the release point of the bubble and at the surface, temperature is $4^{\circ}\,C$. The atmospheric pressure is $1.01\, \times\, 10^{5}\, N/m^{2}$.
Question 93 :
A gas expands such that its initial and final temperature are equal. Also, the process followed by the gas traces a straight line on the P-V diagram:
Question 94 :
In a process the density of a gas remains constant. If the temperature is doubled, then the change in the pressure will be<br>
Question 95 :
A vessel of volume V $=$ 30 l contains ideal gas at the temperature $0^\circ$ After a portion of the gas has been let out, the pressure in the vessel decreased by $\Delta p=0.78$ atm (the temperature remaining constant). Find the mass of the released gas. The gas density under the normal conditions $\rho=1.3\:g/l$
Question 96 :
<p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">A closed vessel </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">contains a </span></span><span>mixture of two gases Neon & Argon the total </span><span>mass of mixture is 28 gm.The partial pressure due to Argon and neon are 4atm and 12atm respectively.The mass of individual </span><span>gases in vessel is</span><span>$(M_{neon}=20,M_{argon}=40,R=8.3J/mol-k)$</span></p>
Question 97 :
A gas thermometer measure the temperature from, the variation of pressure of a sample of gas. If the pressure measured at the melting point of lead is 2.20 times the pressure measured at the triple point of water find the melting point of lead.
Question 98 :
The volume of mole of a prefect gas at NTP is <span>______.</span>
Question 99 :
One mole of an ideal gas expands against a constant external pressure of 1 atm from a volume of $10d{ m }^{ 3 }$ to a volume of $30d{ m }^{ 3 }$. What would be the work done in joules?
Question 100 :
<p class="wysiwyg-text-align-left"><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">Two identical containers each of volume V$_{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">are </span></span>joined by a small pipe. The containers contain <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">identical gases at temperature T$_{0}$</span></span><span class="wysiwyg-font-size-xx-small"><span class="wysiwyg-font-size-xx-small"> </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">and pressure </span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">P$_{0}$</span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">. One container is heated to temperature 2T$_{0}$ </span></span>while maintaining the other at the same temperature. The common pressure of the gas is P and n is the number of moles of gas in <span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">container at temperature 2T$_{0}$</span></span><span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small">.</span></span></p>