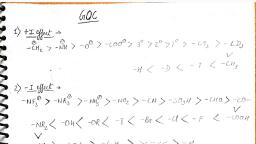Page 1 :
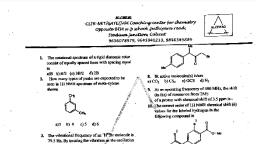
QUIZ -4UV AND IR SPECTROSCOPY, , 1-Select the wavelength range corresponding to UV-visible region., (A) 400-800 nm, (B) 200-800 nm, (C) 25 pm-2.5 pm, (D) 2.5 pm —- Imm, , 2-The types of transitions possible in UV-visible region for a compound with molecular, formula C 2H 4O are, , (P) nz * (Qha—a*, (R)n>o" (S) nn *, a) P,Q, b) P.QR, c) PS, d) P,QRS, , 3. If the above compound was completely reduced, what will be the possible transitions it can, undergo?, , (A) n—p*, (B) s—+s*, (C) pp*, (D) ns*, s—s*, , 4-At alkaline pH, phenol loses one proton and exists as phenoxide anion as . Select the, correct statement regarding their absorption., , A) Phenol shows more absorption than phenoxide anion, , (B) Phenoxide anion shows more absorption than phenol, (C) Phenol has equal absorption as phenoxide anion
Page 2 :
(D) Phenol shows absorption but phenoxide anion doesn’t., , 5-On increasing pH, phenol showsOn increasing pH, phenol shows, (p) bath chromic shift (Q) Hypos chromic Shift, (R) Blue shift (SO Red shift, , (QR, (B)P.R, (C)Q,S, , (D) PS., 6-The base value for tech following compound is, (CH2=CH-CH=CH-CH3, , (A) 246 nm, , (B) 250 nm, , (C) 230 nm, , (D) 217 nm., , 7. The approximate lambda max for the above compound will be, (A) 214 nm, , (B) 217 nm, , (C) 222 nm, , (D) 227 nm, , 8. The possible transitions for water molecule in UV-visible region are, (A)o 0°, , (B) na "jan", , (C) 6 “,n>1*, , (D)n>o°
Page 3 :
9-Energies required for the following transitions in increasing order, (P)n-z* (Q)a—a*, , (R)ynoo (S)xon”, , (A) P<QeR<S, , (B) Q<S<R<P, , (C) S<R<QeP, , (D) P<S<R<Q, , 10 Base value for the p-chlorobenzaldehyde compound will be, (A) 230, , (B) 250, (D) 246, (D) 217, , 11- Which is the correct order of increasing wave number of the stretching vibrations of (1), C-H (alkane), (2) O-H (alcohol), (3) C=O (ketone), and (4) C=C (alkyne)?, , c, a) (4) <G)<(Q)<(), c, b 3) <4) <<), , c, ¢) (3) < (4) < (1) < (2), , c, d) (4) < (3) < (1) < (2), , 12-Which of the following statements is wrong?, , © a) UV absorption is attributable to electronic transitions., c b) UV spectra provide information about valence electrons., , Cc ¢) IR absorption is attributable to transitions between rotational energy levels of whole, , molecules., , © d) NMR spectrometers use radiofrequency electromagnetic radiation.
Page 4 :
13-Which of the following statements regarding IR spectroscopy is wrong?, , c a) Infrared radiation is higher in energy than UV radiation., , o b) Infrared spectra record the transmission of IR radiation., , Cc ¢) Molecular vibrations are due to periodic motions of atoms in molecules, and include, bond stretching, torsional changes. and bond angle changes., , c d) Infrared spectra give information about bonding features and functional groups in, , molecules, , 14-Absorption of radiation in the UV range attributable to n—+-n* electronic transitions 1s, characteristic of which of the following types of compounds?, , © a) Aromatic hydrocarbons., , © b) Unsaturated carbony! compounds., , Cc ¢) Non-conjugated polyenes., , c d) Conjugated polyenes., , 15-Which is the correct order of increasing wave number of the stretching vibrations of (1), C-H (alkane), (2) C-H (alkene), (3) C-H (alkyne), and (4) C-H (arene)?, , © ay(1)<(2)=8)<(4), , b) (4) <= (3) =(2)<(), ©) (3) <= (4) =(2)<(1), d) (1) <(4) = (2) <(3), , 00 0, , 16-Which of the following statements is wrong., , c a) The wavenumber of a band in an IR spectrum is proportional to the frequency of the, , associated molecular vibration., , c b) Water is a good solvent for recording UV spectra of water-soluble compounds., , c, c, , ¢) Water is a good solvent for recording IR spectra of water-soluble compounds.*, , d) Hydrogen bonding in hydroxy compounds leads to broadening of spectral bands, attributable to O-H stretching vibrations., , 17- The IR band for C= N group is observed in the range of
Page 5 :
A. 3000 — 2800 cm-1, B. 3400 ~ 3300 cm-!, C. 2260 ~ 2200 cm-1, D. 1750-1650 cm-I, , 18- The IR band for -NH2 group is observed in the range of, , A. 3000 — 2800 cm-1, , B. 3400 — 3300 cm-1, , C. 2200 — 2100 cm-1, , D. 1750 — 1650 cm20., , 19-In IR spectroscopy, the wavenumber for carbonyl group is observed in the range of, A.3500-3300 cm-I, , B. 2200-2100 cm-1, , C.1740-1650 cm-1, , D.3000-2800 cm-1, , 20. The increment for double bond extending conjugation on 1, 3 butadiene is?, ALS, B.10, Cc., D.15, , 21. The increment for a alkyl substitution on a, B unsaturated ketone is?, A.12, , B.18, , c.10, , D.5 4., , 22-IR spectrum is a plot of:, , A. % Transmittance versus lime