Page 1 :
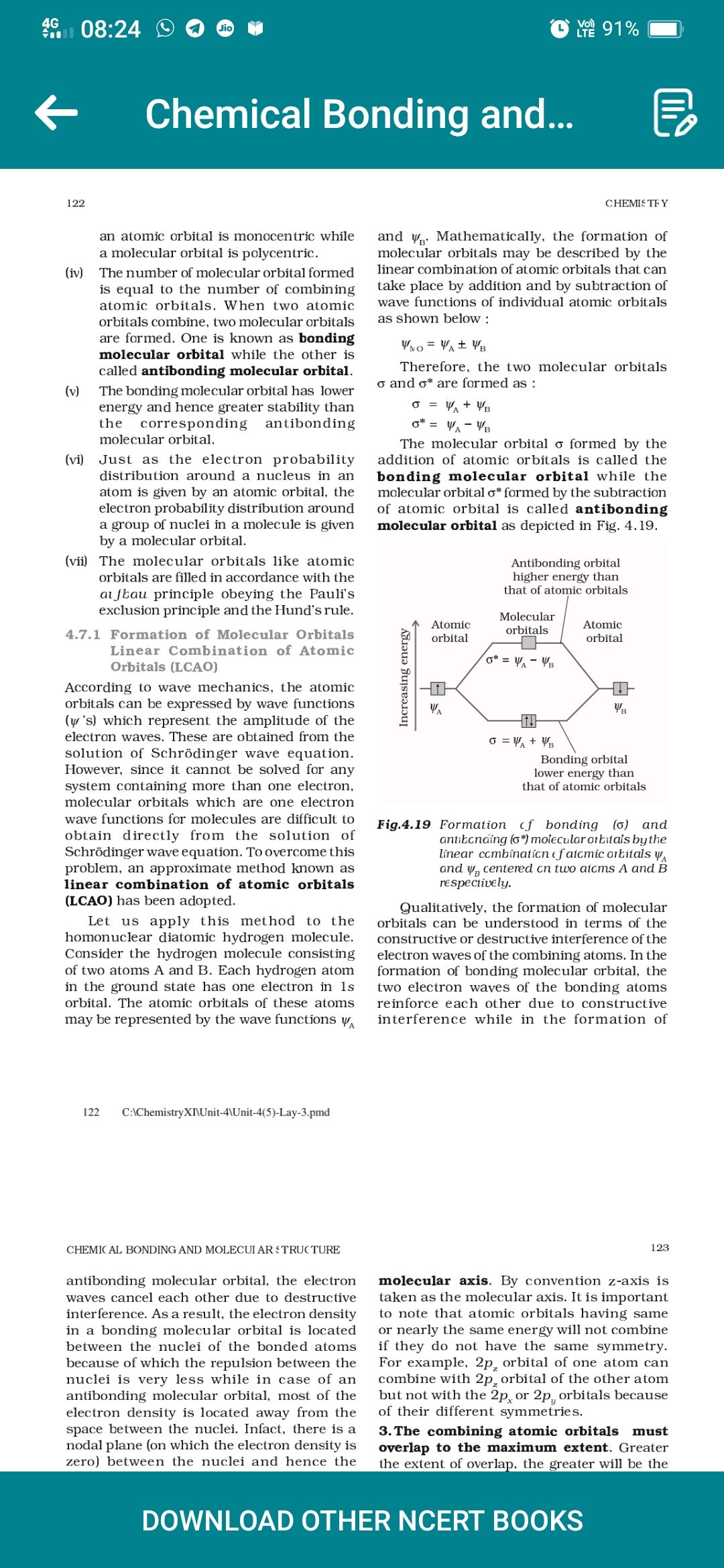
Chemical Bonding and..., , , , 122, , an atomic orbital is monocentric while, a molecular orbital is polycentric., , (iv) The number of molecular orbital formed, is equal to the number of combining, atomic orbitals. When two atomic, orbitals combine, two molecular orbitals, are formed. One is known as bonding, molecular orbital while the other is, called antibonding molecular orbital., , (vy) The bonding molecular orbital has lower, energy and hence greater stability than, the corresponding antibonding, molecular orbital., , (vi) Just as the electron probability, distribution around a nucleus in an, atom is given by an atomic orbital, the, electron probability distribution around, a group of nuclei in a molecule is given, by a molecular orbital., , The molecular orbitals like atomic, orbitals are filled in accordance with the, atfbau principle obeying the Pauli’s, exclusion principle and the Hund's rule., , (vii), , 4.7.1 Formation of Molecular Orbitals, Linear Combination of Atomic, Orbitals (LCAO), , According to wave mechanics, the atomic, orbitals can be expressed by wave functions, (y's) which represent the amplitude of the, electron waves. These are obtained from the, solution of Schrédinger wave equation., However, since it cannot be solved for any, system containing more than one electron,, molecular orbitals which are one electron, wave functions for molecules are difficult to, obtain directly from the solution of, Schrédinger wave equation. To overcome this, problem, an approximate method known as, linear combination of atomic orbitals, (LCAO) has been adopted., , Let us apply this method to the, homonuclear diatomic hydrogen molecule., Consider the hydrogen molecule consisting, of two atoms A and B. Each hydrogen atom, in the ground state has one electron in 1s, orbital. The atomic orbitals of these atoms, may be represented by the wave functions y,, , 122 C\ChemistryXNUnit-4\Unit-4(5)-Lay-3.pmd, , CHEMIC AL BONDING AND MOLECUI AR £ TRUCTURE_, , antibonding molecular orbital, the electron, waves cancel each other due to destructive, interference. Asa result, the electron density, in a bonding molecular orbital is located, between the nuclei of the bonded atoms, because of which the repulsion between the, nuclei is very less while in case of an, antibonding molecular orbital, most of the, electron density is located away from the, space between the nuclei. Infact, there is a, nodal plane (on which the electron density is, zero) between the nuclei and hence the, , O1% =, , 2, , CHEMISTY, , and y,. Mathematically, the formation of, molecular orbitals may be described by the, linear combination of atomic orbitals that can, take place by addition and by subtraction of, wave functions of individual atomic orbitals, as shown below :, , Wo= Vat Ys, , Therefore, the two molecular orbitals, o and o* are formed as :, C= Wt Vy, = YAM, The molecular orbital o formed by the, addition of atomic orbitals is called the, bonding molecular orbital while the, molecular orbital o* formed by the subtraction, of atomic orbital is called antibonding, molecular orbital as depicted in Fig. 4.19., , Antibonding orbital, higher energy than, that of atomic orbitals, , , , , , Molecular, , Atomic orbitals, , orbital, , Atomic, orbital, , , , |, , , , Yo, , Increasing energy, Ss, , Bonding orbital, lower energy than, that of atomic orbitals, , Fig.4.19 Formation cf bonding (6) and, antikcnaing (6*) molecular orbitals by the, linear ccmbinaticn ¢f atcmic arkitals y,, and y, centered cn two atcms A and B, respectively., , Qualitatively, the formation of molecular, orbitals can be understood in terms of the, constructive or destructive interference of the, electron waves of the combining atoms. In the, formation of bonding molecular orbital, the, two electron waves of the bonding atoms, reinforce each other due to constructive, interference while in the formation of, , 123, , molecular axis. By convention z-axis is, taken as the molecular axis. It is important, to note that atomic orbitals having same, or nearly the same energy will not combine, if they do not have the same symmetry., For example, 2p, orbital of one atom can, combine with 2p, orbital of the other atom, but not with the 2p, or 2p, orbitals because, of their different symmetries., , 3.The combining atomic orbitals must, overlap to the maximum extent. Greater, the extent of overlap, the greater will be the, , DOWNLOAD OTHER NCERT BOOKS