Page 1 :
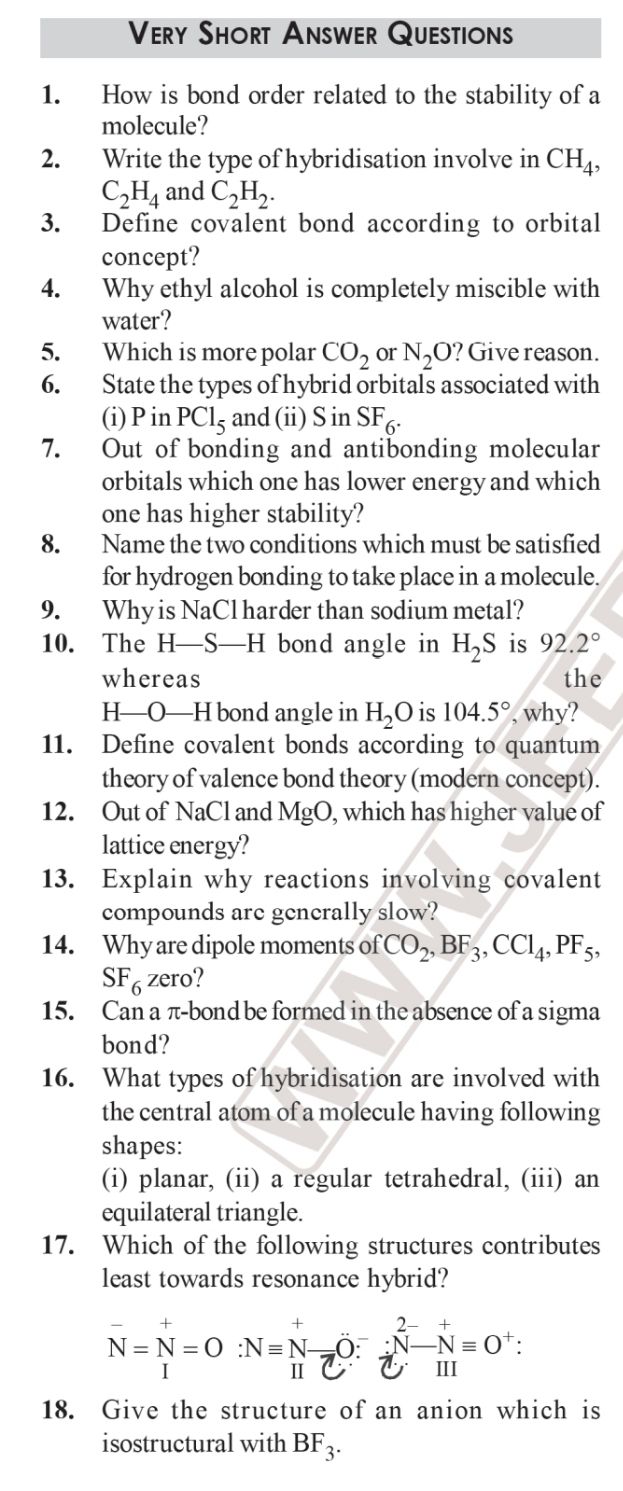
il., , 12., , 13., , 14,, , 16., , 17., , 18., , VerY SHorT ANSWER QUESTIONS, , How is bond order related to the stability of a, molecule?, , Write the type of hybridisation involve in CH,, C,H, and C,H)., , Define covalent bond according to orbital, concept?, , Why ethyl alcohol is completely miscible with, water?, , Which is more polar CO, or N,O? Give reason., State the types of hybrid orbitals associated with, (i) Pin PCI, and (ii) Sin SF,., , Out of bonding and antibonding molecular, orbitals which one has lower energy and which, one has higher stability?, , Name the two conditions which must be satisfied, for hydrogen bonding to take place in a molecule., Why is NaCl harder than sodium metal?, , The H—S—H bond angle in H,S is 92.2°, whereas the, H—O—H bond angle in H,O is 104.5°, why?, Define covalent bonds according to quantum, theory of valence bond theory (modern concept)., Out of NaCl and MgO, which has higher value of, lattice energy?, , Explain why reactions involving covalent, compounds are gencrally slow?, , Why are dipole moments of CO5, BF3, CCl, PFs,, SF, zero?, , Can a z-bond be formed in the absence ofa sigma, bond?, , What types of hybridisation are involved with, the central atom ofa molecule having following, shapes:, , (i) planar, (11) a regular tetrahedral, (iii) an, equilateral triangle., , Which of the following structures contributes, least towards resonance hybrid?, , = + + . yaa, N=N=O :N=N<0: jN—N=0°:, I 1G G, , Give the structure of an anion which is, isostructural with BF.