Page 1 :
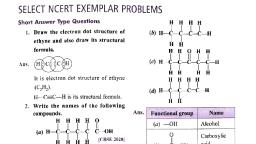
Catenation: The property due to which an atom can form stable covalent bond with the atoms of same element., , EXERCISE 1.1, , I. Multiple Choice Questions (1 Mark), Choose the correct answer from the given options., , 1., , 2., , 3., , 4., , 5., , A molecule of ammonia (NH,) has, (a) only single bonds (b) only double bonds, , (c) only triple bonds (d) two double bonds and one single bond, Which of the following is the correct representation of electron dot structure of nitrogen?, (a) Ni Ne (b) (c) sh (d):, Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of, , (a) Helium (b) Neon (c) Argon (d) Krypton, , , , The correct electron dot structure of a water molecule is, (a) (b) -H:0- He 6: H: (d) H:0: H:, ‘The molecular formula of ethene and its electron dot structure is, , , , , , , , , , , HH, @ CH, HCHC-H () C,H, HC, HH, (©) C,H,, H: (d) C,H, H:, . 2 HH, Il. Assertion-Reason Type Questions (1 Mark), , For question numbers 1 and 2 two statements are given-one labeled as Assertion (A) and the other labeled, Reason (R). Select the correct answer to these questions from the codes (a), (b), (¢) and (d) as given below:, , 1., , (@ Both ‘A’ and‘R’ are true and ‘R’ is correct explanation of the assertion., (b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion., , (c) ‘A’ is true but ‘R’ is false., , (@)‘A’is false but ‘R’ is true., , Assertion: Carbon forms covalent compound with chlorine of formula CCl,., , Reason: Carbon has 4 valence electrons, valency 4, chlorine has 7 valence electrons, valency 1., , , , 2. Assertion: Carbon forms very large number of compounds., Reason: _ It is due to property of catenation and tetravalency, IIL. Very Short Answer Type Questions (1 Mark), 1. Draw electron dot structure of NH, molecule. Predict the total no. of bonds around N-atom., [Delhi 2016], OR, A molecule of ammonia has the formula NH,,. Predict the total number of bonds present around nitrogen, atom. [CBSE 2016], 2. Why covalent compounds are poor conductors of electricity? [CBSE 2020}, 3. What would be the electron dot structure of carbon dioxide which has the formula, CO,? (NCERT], 4. Which element exhibits the property of catenation to maximum extent and why? [Delhi 2016], 5. State two characteristic features of carbon which when put together give rise to large number of carbon, compounds., 6. Explain why carbon generally forms compounds by covalent bonds or do not form ionic compounds., OR, Give reason why carbon neither forms C**cations nor C* anions but form covalent compounds which, are bad conductors of electricity and have low melting and boiling points. [Delhi 2013], OR, Carbon has four electrons in its valence shell. How does carbon attain stable configuration?, [Delhi 2015], 7. Why are most carbon compounds poor conductors of electricity? (CBSE 2018], 8. Define catenation. [CBSE Sample Paper 2019-2020], 9. Covalent compounds have low melting and boiling point. Why? [CBSE 2020], 10. How are covalent bonds formed? [CBSE 2020], IV. Short Answer Type Questions-I (2 Marks), , 1., , Carbon, group 14 element in the periodic table, is known to form compounds with many elements., Write an example of a compound formed with () Chlorine, (ii) Oxygen. (NCERT Exemplar], , . In electron dot structure, the valence shell electrons are represented by crosses or dots., , (i) The atomic number of chlorine is 17. Write its electronic configuration., , (ii) Draw the electron dot structure of chlorine molecule. (NCERT Exemplar], , 3. Compare the ability of catenation of carbon and silicon. Give reasons. (NCERT Exemplar], 4. Give a test that can be used to confirm the presence of carbon in a compound. With a valency of 4, how, is carbon able to attain noble gas configuration in its compounds? — [CBSE Sample Paper 2020-21], , 5. The number of carbon compounds is more than those formed by all other elements put together. Justify, the statement by giving two reasons. (CBSE Sample Paper 2020-21], , V. Short Answer Type Questions-II (3 Marks), 1. What are covalent compounds? Why are they different from ionic compounds? List their three, characteristic properties. (Delhi 2016], , 2. (a) Explain why carbon forms covalent bond? Give two reasons for carbon forming a large number of, , compounds., (b) Explain the formation of ammonia molecule. [CBSE Sample Paper 2018-19], 3. Carbon, a member of group 14, forms a large number of carbon compounds estimated to be about three, , >, , 5s, , million. Why is this property not exhibited by other elements of this group? Explain. — [CBSE 2020], Atoms of an element contain five electrons in its valence shell. This element is the major component of, air. It exists as a diatomic molecule., (i) Identify the element., Gi) Show the bond formed between two atoms of this element., (iii) Write the nature of bond between the two atoms., (@) Explain the formation of calcium chloride with the help of electron dot structure. (At numbers: Ca, =20;Cl=17), (ii) Why do ionic compounds not conduct electricity in solid state but conduct electricity in molten and, aqueous state?. [CBSE Sample Paper 2020-21]