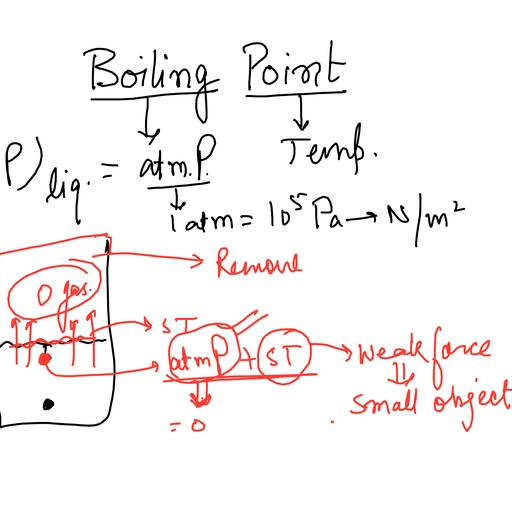
Page 1 :
Objective Questions, LEVEL – I, Choose the correct alternate. Only ONE is correct., Q 1., , Which one of the following salts would have the same value of the van‘t Hoff factor as that, of K3[Fe(CN6)] ?., (A) Al2(SO4)3, (B) NaCl, (C) Na2SO4, (D) Al (NO3)3, , Q 2., , The vapour pressure of benzene at a certain temperature is 640 mm of Hg. A non -volatile and nonelectrolytic solid, weighing 2.175g, is added to 39.08g of benzene. The vapour pressure of thesolution, is 600mm of Hg. What is the molecular mass of the solid substance ?., (A) 49.50, (B) 59.6, (C) 65, (D) 79.8, , Q 3., , A 5 % solution of cane sugar (mol. mass = 342) is isotonic with 1 % solution of a substance X, . The molecular mass of X is :, (A) 34.2, (B) 171.2, (C) 68.4, (D) 136.8, , Q 4., , The degree of dissociation ‘ ’ of a weak electrolyte is:, (A), , i 1, n 1, , (B), , i 1, n 1, , (C), , n 1, i 1, , (D), , n 1, i 1, , Q 5., , The molal boiling point constant for water is 0.513 K kg mol-1 . When 0.1 mole of sugar is, dissolved in 200 g of water , the solution boils under a pressure of 1 atm at :, (A) 100.513 ºC, (B) 100.0513 ºC, (C) 100.256 ºC, (D) 101.025 ºC, , Q 6., , The correct expression relating molality (m) , molarity (M) , density (D) and molar mass (M2) of, solute is :, (A) m =, , Q 7., , M, d M M2, , (B) m =, , M, d M M2, d M M2, (C) m =, (D) m =, d M M2, M, M, , Match the following graph, A, B, , V.P., , PºA, , PºB, C, , Mole fraction, , A (i) (+) deviation, (A) A (i) , B (ii) , C (iii), (C) A (ii) , B (iii) , C (i), Q 8., , B (ii) ideal, , C (iii) (-) deviation, (B) A (iii) , B (ii) , C (i), (D) none of these, , Human blood gives rise to an osmotic pressure of approximately 7.65 atm at body temperature,, 370C. Hence , molarity of an glucose solution to be, to have the same osmotic pressure as blood, is :, (A) 0.30 M, (B) 0.20M, (C) 0.10 M, (D) 0.50 M
Page 2 :
Q 9., , At a given temperature, total vapour pressure in Torr of a mixture of volatile components A and, B is given by p =120 -75 XB hence, vapour pressure of pure A and B respectively, (in Torr) are:, (A) 120, 75, (B) 120, 195, (C) 120, 45, (D) 75, 45, , Q 10. Total vapour pressure of mixture of 1 mole volatile component A (poA = 100 mg Hg ) and 3, moles of volatile component B (poB= 60 mm Hg ) is 75 mm . For such case:, (A) there is positive deviation from Raoult’s law, (B) boiling point has been lowered, (C) force of attraction between A and B is smaller than that between A and A or between B and, B, (D) all the above statements are correct, Q 11. Vapour pressure of pure water is 40 mm . If a non-volatile solute is added to it , vapour, pressure falls by 4 mm . Hence, molality of solution is :, (A) 6.173 molal, (B) 3.0864 molal, (C) 1.543 molal, (D) 0.772 molal, Q 12. The vapour pressure of a pure liquid A is 40 mm Hg at 310 K. The vapour pressure of this liquid, in a solution with liquid B is 32 mm Hg. Mole fraction of A in the solution, if it obeys Raoult’s law,, is :, (A) 0.8, (B) 0.5, (C) 0.2, (D) 0.4, Q 13. The total concentration of dissolved particles inside red blood cells is approximately 0.30 M, and the membrane surrounding the cells is semipermeable. What would be the osmotic pressure, (in atmosphere) inside the cells become if the cells were removed from the blood plasma and placed, in pure water at 298 K?, (A) 7.34 atm, (B) 1.78 atm, (C) 2.34 atm, (D) 0.74 atm, Q 14. Which one of the following pairs of solutions will be expected to be isotonic under the same, temperature ?, (A) 0.1 M urea and 0.1 M NaCl, (B) 0.1 M urea and 0.2 M MgCl2, (C) 0.1 M NaCl and 0.1 M Na2SO4, (D) 0.1 M Ca(NO3)2 and 0.1 M Na2SO4, LEVEL – II, Choose the correct alternate. Only ONE is correct., Q 1., , Equimolal solutions of A and B show depression in freezing point in the ratio of 2 : 1., A remains in normal state in solution. B will be in ... state in solution., (A) normal, (B) associated, (C) hydrolysed, (D) dissociated, , Q 2., , The values of observed and calculated molecular mass of Ca(NO3)2 are 65.4 and 164, respectively. The degree of ionisation of the salt will be :, (A) 0.25, (B) 0.50, (C) 0.60, (D) 0.75, , Q 3., , Assuming the salts to be unionised in solution, which of the following has highest osmotic, pressure?, (A) 1 % CsCl, (B) 1 % RbCl, (C) 1 % KCl, (D) 1 % NaCl, , Q 4., , The vapour pressure of a solvent decreased by 10 mm of mercury when a non-volatile solute was, added to the solvent. The mole fraction of the solute in the solution is 0.2 What should be the, mole fraction of the solvent, if the decrease in the vapour pressure is to be 20 mm of mercury?, (A) 0.8, (B) 0.6, (C) 0.4, (D) 0.2
Page 3 :
Q 5., , An aqueous solution of sucrose, C12H22O11 containing 34.2 g/L has an osmotic pressure of 2.38, atmospheres at 17 ºC . For an aqueous solution of glucose C6H12O6 , to be isotonic with this, solution, it would have :, (A) 34.2 g/L, (B) 17.1 g/L, (C) 18.0 g/L, (D) 36.0 g/L of glucose, , Q 6., , The expression relating to mole fraction of solute (x2) and molarity (M) of the solution is :, (where is the density of solution & M1 & M2 are the molar masses of solvent and solute, respectively), , Q 7., , (A) x2 =, , M M1, M ( M1 M 2 ) , , (B) x2 =, , M M1, M ( M1 M 2 ) , , (C) x2 =, , M ( M1 M 2 ) , M M1, , (D) x2 =, , M ( M1 M 2 ) , M M1, , Elevation in b.p.of a molar glucose solution (d = 1.2 gmL-1) is:, (A) 0.98 Kb, (B) Kb, (C) 1.20 Kb, , (D) 1.02 Kb, , Q 8., , Elevation in b.p.of an aqueous urea solution is 0.520C. (Kb = 0.52 K mol -1 kg). Hence, mole, fraction of urea in this solution is :, (A) 0.982, (B) 0.0567, (C) 0.943, (D) 0.018, , Q 9., , An aqueous solution of a solute AB has b.p of 101.080C (AB is 100% ionised in boiling point of, the, solution ) and freezes at -1.800C. Hence, AB (K b / K f = 0.3 ), (A), is 100% ionised at the f.p. of the solution, (B), behaves as non-electrolyte at the f.p. of the solution, (C), forms dimer, (D), none of these, , Q 10. In the following equilibrium N2O4(g), 2NO2.NO2 is 50 % of the total volume. Hence,, degree of dissociation (x) and van`t Hoff factor (i) respectively are:, (A) 0.5,1.5, (B) 0.25,1.25, (C) 0.33,1.33, (D) 0.66,1.66, Q 11. Depression of freezing point of 0.01 mole aq. CH3COOH solution is 0.02046°C. 1 molal urea, solution freezes at – 1.86°C. Assuming molality equal to molarity, pH of CH3COOH solution is, :, (A) 2, (B) 3, (C) 3.2, (D) 4.2, Q 12. Mole fraction of A vapour above the solution in mixture of A and B (XA = 0.4) will be, [ PA0 = 100 mm PB0 = 200 mm ], (A) 0.4, (B) 0.8, (C) 0.25, (D) none of these, Q 13. If relative decrease in V.P. is 0.4 for a solution containing 1 mole NaCl in 3 moles H2O, NaCl is, ... % ionized:, (A) 60%, (B) 50%, (C) 100%, (D) 40%, Q 14. 12.2 g of benzoic acid ( m.w.= 122) in 100 g benzene has depression in freezing point 2.6K;, Kf = 5.2 K kg/mol. If there is 100% polymerization, number of molecules of benzoic acid in, associated state is :, (A) 1, (B) 2, (C) 3, (D) 4
Page 4 :
LEVEL – III, Q.1, , For an ideal binary liquid solution with PA > PB , which relation between XA (mole fraction of A, in liquid phase) and YA(mole fraction of A in vapour phase) is correct?, (A) YA < YB, , Q.2, , (B) XA > XB, , YA X A, (C) Y X, B, B, , Mole fraction of A vapours above the solution in mixture of A and B (XA = 0.4) will be, [Given : PA = 100 mm Hg and PB = 200 mm Hg], (A) 0.4, (B) 0.8, (C) 0.25, , Q.3, , (D) none of these, , The exact mathematical expression of Raoult’s law is, , P 0 Ps n, , (A), N, P0, Q.4, , YA X A, (D) Y X, B, B, , P 0 Ps N, , (B), n, P0, , P 0 Ps n, , (C), Ps, N, , (D), , P 0 Ps, =n×N, P0, , A mixture contains 1 mole of volatile liquid A ( PA =100 mm Hg) and 3 moles of volatille liquid, B ( PB = 80 mm Hg). If solution behaves ideally, the total vapour pressure of the distillate is, (A) 85 mm Hg, (B) 85.88 mm Hg, (C) 90 mm Hg, (D) 92 mm Hg, , Q.5, , Which of the following aqueous solution will show maximum vapour pressure at 300 K?, (A) 1 M NaCl, (B) 1 M CaCl2, (C) 1 M AlCl3, (D) 1 M C12H22O11, , Q.6, , The Van’t Hoff factor for a dilute aqueous solution of glucose is, (A) zero, (B) 1.0, (C) 1.5, , (D) 2.0, , Q.7, , The correct relationship between the boiling points of very dilute solution oif AlCl3 (T1K) and, CaCl2 (T2K) having the same molar concentration is, (A) T1 = T2, (B) T1 > T2, (C) T2 > T1, (D) T2 T1, , Q.8, , A 0.001 molal solution of a complex [MA8] in water has the freezing point of –0.0054°C. Assuming, 100% ionization of the complex salt and Kf for H2O = 1.86 km–1, write the correct representation, for the complex, (A) [MA8], (B) [MA7]A, (C) [MA6]A2, (D) [MA5]A3, , Q.9, , The vapour pressure of a solution of a non-volatile electrolyte B in a solvent A is 95% of the, vapour pressure of the solvent at the same temperature. If the molecular weight of the solvent is, 0.3 times the molecular weight of solute, the weight ratio of the solvent and solute are, (A) 0.15, (B) 5.7, (C) 0.2, (D) 4.0, , Q.10, , At a given temperature, total vapour pressure in Torr of a mixture of volatile components A and B, is given by, PTotal = 120 – 75 XB, hence, vapour pressure of pure A and B respectively (in Torr) are, (A) 120, 75, (B) 120, 195, (C) 120, 45, (D) 75, 45, , Q.11, , Assuming each salt to be 90 % dissociated, which of the following will have highest boiling, point?, (A) Decimolar Al2(SO4)3, (B) Decimolar BaCl2, (C) Decimolar Na2SO4, (D) A solution obtained by mixing equal volumes of (B) and (C)
Page 5 :
Q.12, , The vapour pressure of a solvent decreased by 10 mm of Hg when a non-volatile solute was, added to the solvent. The mole fraction of solute in solution is 0.2, what would be mole fraction, of the solvent if decrease in vapour pressure is 20 mm of Hg, (A) 0.2, (B) 0.4, (C) 0.6, (D) 0.8, , Q.13, , Elevation of boiling point of 1 molar aqueous glucose solution (density = 1.2 g/ml) is, (A) Kb, (B) 1.20 Kb, (C) 1.02 Kb, (D) 0.98 Kb, , Q.14, , What will be the molecular weight of CaCl2 determined in its aq. solution experimentally from, depression of freezing point?, (A) 111, (B) < 111, (C) > 111, (D) data insufficient, , Q.15, , 1.0 molal aqueous solution of an electrolyte A2B3 is 60% ionised. The boiling point of the solution, 1, at 1 atm is ( K b( H 2O) 0.52 K kg mol ), , (A) 274.76 K, Q.16, , (B) 377 K, , (C) 376.4 K, , (D) 374.76 K, , Which of the following plots represents an ideal binary mixture?, (A) Plot of Ptotal v/s 1/XB is linear (XB = mole fraction of 'B' in liquid phase)., (B) Plot of Ptotal v/s YA is linear (YB = mole fraction of 'A' in vapour phase), , 1, (C) Plot of P, v/s YA is linear, total, 1, (D) Plot of P, v/s YB is non linear, total, Q.17, , Pressure over ideal binary liquid mixture containing 10 moles each of liquid A and B is gradually, decreased isothermally. If PAo =200 mm Hg and PBo =100 mm Hg, find the pressure at which half, of the liquid is converted into vapour., (A) 150 mm Hg, (B) 166.5 mm Hg, , (C) 133 mm Hg, , (D) 141.4 mm Hg, , Q.18, , The lowering of vapour pressure in a saturated aq. solution of salt AB is found to be 0.108 torr. If, vapour pressure of pure solvent at the same temperature is 300 torr. Find the solubility product of, salt AB, (A) 10–8, (B) 10–6, (C) 10–4, (D) 10–5, , Q.19, , Which of the following represents correctly the changes in thermodynamic properties during the, formation of 1 mol of an ideal binary solution., (A), , Q.20, , (B), , (C), , FeCl3 on reaction with K4[Fe(CN)6] in aqueous solution gives blue, colour. These are separated by a semipermeable membrane AB as, shown. Due to osmosis there is, (A) blue colour formation in side X., (B) blue colour formation in side Y., (C) blue colour formation in both of the sides X and Y., (D) no blue colour formation., , (D)
Page 6 :
LEVEL – IV, Choose the correct alternate(s). ONE or MORE than one may be correct, Q.1, , When a non-volatile solute is added to a pure solvent, the, (A), vapour pressure of the solution becomes lower than that of the pure solvent, (B), rate of evaporation of the pure solvent is reduced, (C), solute does not affect the rate of condensation, (D), rate of evaporation of the solution is equal to the rate of condensation of the solution at, a lower, vapour pressure than that in the case of the pure solvent., , Q.2, , According to Raoult’s law the relative decrease in the solvent vapour pressure over the solution, is equal to, (A), the mole fraction of the solvent, (B), the mole fraction of the solute, (C), the number of moles of the solute, (D), i times the mole fraction of the solute which undergoes dissociation or association in, the solvent, [ i = van’t Hoff factor ], , Q.3, , Which of the following combinations are correct for a binary solution, in which the solute as, well as the solvent are liquid?, (A), C6H6 and C6H5CH3;, Hsoln > 0;, Vsol = 0, (B), CH3COCH3 and CHCl3;, Hsoln < 0;, Vsol < 0, (C), H2O and HCl;, Hsoln > 0;, Vsol < 0, (D), H2O and C2H5OH;, Hsoln > 0;, Vsol > 0, , Q.4, , Which of the following statements are correct for a binary solution which shows negative deviation from Raoult’s law?, (A), The negative deviation from linearity diminishes and tends to zero as the concentration, of the, solution component approaches unity., (B), When solutions from, their volumes are smaller than the sum of the volumes of their, components, (C), Heat is released during the formation of the solution., (D), Heat is absorbed during the formation of the solution., , Q.5, , A binary liquid (AB) shows positive deviation from Raoult’s law wen, (A) pA > pA0 XAliq > pB0 XBliq, (B) intermolecular forces: A–A, B–B > A–B, (C) Vmix > 0, (D) Hmix > 0, , Q.6, , The azeotropic solutions of two miscible liquids, (A), can be separated by simple distillation, (B), may show positive or negative deviation from Raoult’s law, (C), are supersaturated solutions, (D), behave like a single component and boil at a constant temperature, , Q.7, , In which of the following pairs of solutions will the values of the van’t Hoff factor be the same?, (A), 0.05 M K4[Fe(CN)6] and 0.10 M FeSO4, (B), 0.10 M K4[Fe(CN)6] and 0.05 M FeSO4(NH4)2SO4.6H2O, (C), 0.20 M NaCl and 0.10 M BaCl2, (D), 0.05 M FeSO4(NH4)2SO4.6H2O and 0.02 M KCl. MgCl2.6H2O
Page 7 :
Q.8, , 0, 1 mol benzene ( P0benzene = 42 mm) and 2 mol toluene (Ptoluene, = 36 mm) will have:, (A) total vapour pressure 38 mm, (B) mol fraction of vapours of benzene above liquid mixture is 7/19, (C) positive deviation from Raoult’s law, (D) negative deviation from Raoult’s law., , Q.9, , At 40 ºC, the vapour pressure in torr. of methanol and ethanol solutions is , P = 199x + 135, where x is the mol fraction of methanol . Hence :, (A) vapour pressure of pure methanol is 119 torr., (B) vapour pressure of pure ethanol is 135 torr., (C) vapour pressure of equimolar mixture of each is 127 mm, (D) mixture is completely immiscible., Q.10 Consider following cases:, I :, 2 M CH3COOH solution in benzene at 27ºC where there is dimer formation to the extent of, 100%, II :, 0.5 M KCL aq. solution at 27ºC, which ionises 100%; which is/are true statement(s), (A) both are isotonic, (B) I is hypertonic, (C) II is hypertonic, (D) none is correct, Q.11, , Consider following solutions:, I : 1 M aq. glucose, III : 1 M benzoic acid in benzene, Select correct statement (s), (A) all are isotonic solutions, (C) I, II, IV are hypertonic of III, , II : 1 M aq. sodium chloride, IV : 1 M ammonium phosphate, (B) III is hypotonic of I, II, IV, (D) IV is hypertonic of I, II, III, , Q.12 If PA is the vapour pressure of a pure liquid A and the mol fraction of A in the mixture of two, liquids A and B is x, the partial vapour pressure of A is:, (A) (1– x)PA, , (B) xPA, , (C), , x, P, 1 x A, , (D), , 1 – x , X, , PA, , Q.13 When mercuric iodide is added to the aqueous solution of potassium iodide, the, (A) Freezing point is increase, (B) freezing point is lowered, (C) freezing point does not change, (D) boiling point does not change, Q.14 Which is/are correct statement(s)?, (A) when mixture is less volatile, there is positive deviation from Raoult’s law., (B) when mixture is more volatile, there is negative deviation from Raoult’s law., (C) when mixture is less volatile, there is negative deviation from Raoult’s law., (D)when mixture is less volatile, there is positive deviation from Raoult’s law., Q.15 At 35ºC, the vapour pressure of CS2 is 512 mm Hg, and of acetone is 344 mm Hg. A solution of, CS2 and acetone in, which the mol fraction of CS2 is 0.25, has a total vapour pressure of 600 mm, Hg. Which of the following statement is/are correct?, (A) a mixture of 100 mL of acetone and 100 mL of CS2 has a volume of 200 mL, (B) when acetone and CS2 are mixed at 35º, heat must be absorbed in order to produce a, solution at 35ºC, (C) when acetone and CS2 are mixed at 35ºC heat is released, (D) there is negative deviation from Raoult’s law
Page 8 :
LEVEL – V, Match the followng columns, Q.1, , Column I, Symbols of concentration terms, , Column II, formula, , (a), , % w/w, , (i), , Mass of solute, x 106, Mass of solution, , (b), , ppm, , (ii), , Number of moles of solute, Volume (of solution in Litre), , (c), , m (molality), , (iii), , Mass of solute, x 102, Mass of solution, , (d), , N (normality), , (iv), , Number of moles of solute, Volume of solution in litres, , (e), , M (molarity), , (v), , Number of moles of solute, Weight (of solvent in kg), , Q.2, , Column I, Examples of solution, (a), Acetone + Aniline, (b), Water + Methanol, (c), Benzene + Toluene, (d), n-Hexane + N-heptane, (e), Water + HCl, , Column II, Types of solution, (i), Positive deviation from ideal behaviour, (ii), Negative deviation from ideal behaviour, (iii), Ideal solution, , Q.3, , Column I, Column II, Condition for various solutions, Type of solutions, (a), PA + PB < PAº xA + PºB xB, (i), Positive deviation from ideal behaviour, (b), A–B attractive forces should be, (ii), Negative deviation from ideal, behaviour, weaker than B–B attractive forces. (iii), Ideal solution, (c), Vmix > 0 & Endothermic dissolution, (d), Hmix < 0 & volume decreased, during dissolution, (e), Raoult’s law is obeyed at every range, of temperatre, , Q.4, , Column I, Example of electrolyte, (a), K3[Fe(CN)3], (b), Benzoic acid in benzene, (c), NaCl, , Column II, Value of Van’t Hoff factor., (i), 2, (ii), 1, (iii) 1 + , , (d), , CH3COOH, , (iv), , (e), , Urea, , (v), , 1, 1 – 1 , n, 1 + 3
Page 9 :
Q.5, , Q.6, , Column I, Colligative properties., (a), , , Column II, Their formula, (i), P0xB, , (b), , P0–Ps, , (ii), , (c), , Tf, , (iii), , (d), , Tb, , (iv), , Column I, Properties, , nRT, V, Kb.m, , 1000 K f WA, m A WB, , Column II, Formula, , Molal depression constant (Kf), , (b), , degree of dissociation, , (ii), , (c), , degree of association, , (iii), , M solute (normal), M solute (observed), , (d), , Van’t Hoff factor, , (iv), , i 1, n 1, , (v), , R Tf 2, 1000 L f, , (e), , Msolute (observed), , (i), , 1000 K f W, W Tf, , (a), , i 1, 1, 1, n, , Subjetive Questions, LEVEL – I, Q.1, , At 50 ºC the vapour pressure of pure water and ethyl alcohol are 92.5 and 219.9 mm of Hg, respectively. If 6 g of non-volatile solute of m. wt. 120 is dissolved in 150 g of each of these, solvent, what will be the ratio of relative vapour pressure lowering in two solvents ?, , Q.2, , Benzene and toluene form two ideal solution A and B at 313 K. Solution “A” contains, 4 mole of toluene and one mole of C6H6. Solution B contains equal masses of toluene and, Benzene . Calculate total pressure in each case . The vapour pressure of C6H6 and toluene are, 160 and 60 mm respectively at 313 K., , Q.3, , A solution of 1 - propanol and 2 - propanol having 34 by weight of 2 - propanol has an equilibrium, vapour pressure of 88.8 mm Hg . Another solution having 13 by weight of 2 - propanol has an, equilibrium vapour pressure of 68.3 mm Hg. Calculate vapour pressure of pure alcohols at 40 ºC, assuming ideal solution mixtures prepared at 40ºC., , Q.4, , A liquid mixture containing 26 g C6H6 and 46 g C7H8 at 50 ºC has a vapour pressure of 163.75, mm of Hg. When another 52 g of C6H6 are added , vapour pressure of mixture is increased to, 211.57 mm of Hg. Calculate the vapour pressure of pure components . Also find the values of A, and B if vapour pressure of mixture is represented by P = A + B XT , XT is mole fraction of, toluene.
Page 10 :
Q.5, , The boiling point of a solution of 0.1050 g of a substance in 15.84 g of ether was found to be 0.1, ºC higher than that of pure ether . What is molecular weight of solute ?, Molal elevation constant of ether = 21.6 K mol-1 100 g ., , Q.6, , Calculate the molecular weight of a substance , 1.3 g of which is dissolved in 169 g of H2O gave, a solution boiling at 100.025 ºC. Kb for H2O is .52 K kg mol–1., , Q.7, , Calculate the density of glycol solution whose 2.976 litre on addition to 5 litre of water produce, an antifreeze which protects automobile radiator down to - 20 ºC . Also calculate the temperature, at which the solution will boil . Kf and Kb for water are 1.86 and 0.51 K mol–1 kg respectively., , Q.8, , Two solvents A and B have Kf values 1.86 and 2.72 K mol–1 kg respectively . A given amount of, substance when dissolved in 500 g of A , it completely dimerizes and when same amount of, substance is dissolved in 500 g of B , the solute undergoes trimerization . What will be the ratio, of observed lowering of freezing points in two cases ., , Q.9, , 2.8 g of cadmium iodide (CdI2) in 20 g of water boiled at 0.20 K higher temperature than the, boiling point of pure water . Calculate the molar mass of CdI2 and comment on result . Kb, for H2O = 0.52 K molality -1 ., , Q.10 What approximate proportion by volume of water (d = 1 g mL–1) and ethylene glycol (d = 1.2 g mL1, ) must be mixed to ensure protection of an automobile radiator to cooling – 10 ºC., LEVEL – II, Q.1, , Vapour pressure of C6H6 and C7H8 mixture at 50 ºC are given by P = 179 XB + 92 , where XB is, mole fraction of C6H6 . Calculate (in mm) :, (a), Vapour pressure of pure liquids, (b), Vapour pressure of liquid mixture obtained by mixing 936 g C6H6 and 736 g toluene ., (c), If the vapours are removed and condensed into liquid and again brought to the temperature, of, 50 ºC, what would be mole fraction of C6H6 in vapour state ?, , Q.2, , 2 g of benzoic acid dissolved in 25 g of C6H6 shows a depression in freezing point equal to, 1.62 K. Molal depression constant of C6H6 is 4.9 K mol-1 kg . What is the percentage, association of acid, if it forms double molecule in solution ., , Q.3, , 1.1 g CoCl3. 6 NH3 (molecular weight = 267.5) was dissolved in 100 g of water . The freezing, point of solution was - 0.306 ºC . How many mol of solute particles exist in solution for each, mole of solute introduced if 100 % ionisation of complex is noticed ., [ Kf for H2O = 1.86 K, mol-1 kg ], , Q.4, , A metal M of molar mass 96 g mol-1 reacts with fluorine to form a salt that can be represented, as MFx . In order to determine ‘ x ‘ , a 9.18 g of the sample of the salt is dissolved in 100 g of, water and its boiling point was determined to be 374.38 K . What is the chemical formula of the, salt ?, [ Given Kb (water) = 0.52 K kg mol–1 ] Assume complete dissociation of salt., , Q.5, , An aqueous solution of cane sugar (molecular weight = 342) has an osmotic pressure 1.5 atm, at 18 ºC . What will be its osmotic pressure at 40 ºC ? If 100 g of this solution is cooled to - 3, ºC , will it freeze out . If so , what weight of ice will be separated out ?, [ Kf = 1.86 K mol-1 kg ] Assume molality and molarity same.
Page 11 :
ANSWERS, OBJECTIVE, LEVEL I, 1. D, , 2. C, , 8. A, , 3. C, 9. C, , 4. B, , 10. D, , 11. A, , 5. C, , 6. B, , 7. A, , 12. A, , 13. A, , 14. D, , LEVEL II, 2. D, , 3. D, , 9. B, , 4. B, , 10. C, , 5. C, , 11. B, , 6. A, , 12. C, , 7. A, , 13. A, , 8. D, , 14. B, , LEVEL III, Q.1, Q.8, Q.15, , C, C, D, , Q.2, Q.9, Q.16, , C, B, C, , Q.3, Q.10, Q.17, , C, C, D, , Q.4, Q.11, Q.18, , B, A, C, , Q.5, Q.12, Q.19, , D, C, C, , Q.6, Q.13, Q.20, , B, D, D, , Q.7, Q.14, , 6. BD, , 7. BD, , LEVEL IV, 1. ABCD, 8. AB, , 2. BD, 9. B, , 3. BD, 10. A, , 4. ABC, 11. BCD, , 15. BD, , LEVEL V, Q.1, , (a)-(iii), (b)-(i), (c) -(v), (d) -(ii), e-(iv), , Q.2, , (a), (e) - (ii), (b)-(i), (c), (d)-(iii), , Q.3, , (a), (d) - (ii), (b),(c)-(i), (e)-(iii), , Q.4, , (a)-(v), (b)-(iv), (c) -(i), (d)-(iii), (e)-(ii), , Q.5, , (a)-(ii), (b)-(i), (c)-(iv), (d)-(iii), , Q.6, , (a)-(v), (b)-(iv), (c)-(ii), (d)-(iii), (e)-(i), , 5. ABC, 12. B, , 13. B, , 14. CD, , B, B
Page 12 :
SUBJETIVE, LEVEL I, 1., , 0.3949, , 2., , 3., , 2 - propanol = 101.10 mm; 1 - propanol = 51.9 mm, , 4., , PB° = 271.35 , PT° = 92.02 mm , A = 271.35 , B = – 179.33, , 5., , 143.18, , 6., , 160, , 8., , 1 : 1 :: A : B, , 9., , 364.0 ; CdI2 is not dissociated, , 7., , P°A = 80 mm, P°B = 114.117 mm, , 1.12 g mL-1 , 105.48 ºC, 10., , 36 : 10, , LEVEL II, 1., , (a) 271 mm , 92 mm, , (b) 199.4 mm, , (c) 0.072, , 2., , 99.2 %, , 3., , i = 4 ; [Co (NH3)6] Cl3 Co (NH3)63+ + 3 Cl¯, , 4., , x = 4, MF4, , 5., , 1.613 atm , Yes , 93.89 g